1. Introduction
Oxygen, essential for human life activities, changes into a highly reactive oxygen species (ROS) due to various stimuli. While ROS is a cellular transmitter and an immune function, excessive production damages cells and tissues. ROSs resulting from oxidative stress are one of the major causes of biological disorders and are known to be closely related to the onset of lifestyle-related diseases such as cancer, aging, atherosclerosis, and diabetes, and their complications. Those that play a protective role against ROSs and free radicals are collectively called antioxidants [
1,
2]. On the other hand, in the event of an accidental and unspecified number of radiation exposure accidents, treatment strategies to alleviate strong radiation-induced oxidative stress become extremely important, but there are currently no pharmaceutical countermeasures or treatments that can reduce radiation damage.
Hydroxyl radicals (•OH), the most reactive and cytotoxic of ROSs, are selectively reduced by molecular hydrogen (H
2). Yanagihara et al. reported that the ingestion of neutral hydrogen-rich water produced by water electrolysis alleviated liver damage in rats induced by chemical oxidants [
3]. Similarly, in rat hearts in vivo, the administration of molecular hydrogen led to a significant increase in superoxide dismutase, and molecular hydrogen alleviated lipid peroxidation caused by radiation [
4]. Ohsawa et al. reported that the inhalation of hydrogen gas markedly suppressed brain injury by buffering the effects of oxidative stress. They demonstrated that hydrogen selectively reduced the hydroxyl radical, the most cytotoxic of ROSs, and effectively protected cells; however, hydrogen did not react with other ROSs, which possess physiological roles [
5]. Recently, Hirano et al. also reported that hydrogen is easily applicable because it has no adverse effects and shows marked efficacy for many diseases, including oxidative stress-related diseases and chronic inflammatory diseases [
6,
7]. Furthermore, several papers reported the radioprotective effects of hydrogen. They discussed the mechanisms of hydrogen, not only as an antioxidant but also in intracellular responses including anti-inflammation, anti-apoptosis, and the regulation of gene expression, which demonstrated the prospects of hydrogen as a novel and clinically applicable radioprotective agent [
8]. However, for easy ingestion of hydrogen, it is desirable to consume it as hydrogen water. Still, there are various issues regarding hydrogen concentration, storage methods after production, and long-term stability.
We have developed a method to produce more stable hydrogen water by dispersing hydrogen in water using ultra-fine bubbles (UFBs) with a bubble diameter of less than 1 μm [
9]. According to the International Organization for Standardization (ISO)’s terminology, fine bubbles are defined as bubbles with a volume-equivalent diameter of less than 100 μm [
10,
11]. Additionally, microbubbles are defined as fine bubbles with a volume-equivalent diameter in the range from equal or greater than 1 μm to less than 100 μm, whereas UFBs are fine bubbles in the range less than 1 μm. In this study, it was also described as UFB hydrogen water. The present study reported on the functional characteristics of antioxidant long-life UFB hydrogen water. UFB hydrogen water with excellent storage stability is expected to be needed in the future for further fine bubbles in academic and business fields such as medicine, zoology, botany, life sciences, inorganic chemistry, electronics, atomic physics, and various manufacturing and cleaning industries.
3. Discussion
The methods for manufacturing microbubbles include the following: a simple ejector method, a venturi tube method, a Shirasu porous glass membrane pass method, a pressurization–depressurization method, an ultrasonic vibration method, a gas–liquid swirling two-phase method, and a cavitation method (including the cavitation generated in the back of a screw) [
12,
13,
14,
15,
16]. Among the abovementioned methods, the pressurization–depressurization method, the ultrasonic vibration method, the gas–liquid swirling two-phase method, and the cavitation method can generate UFBs. In the present study, we reported on the characteristics of long-term stable UFB hydrogenated water using our original resonant foaming and vacuum cavitation production system. The size of the bubbles is particularly problematic in the preparation. Bubbles above 10 μm cannot perform their radical function because the bubbles are buoyant and cannot float above the water and float in the water; UFBs below 1 μm are not buoyant and will disperse in the water for a long period. The bubble mean particle size of the UFB hydrogen water used in this study was approximately 60 nm (
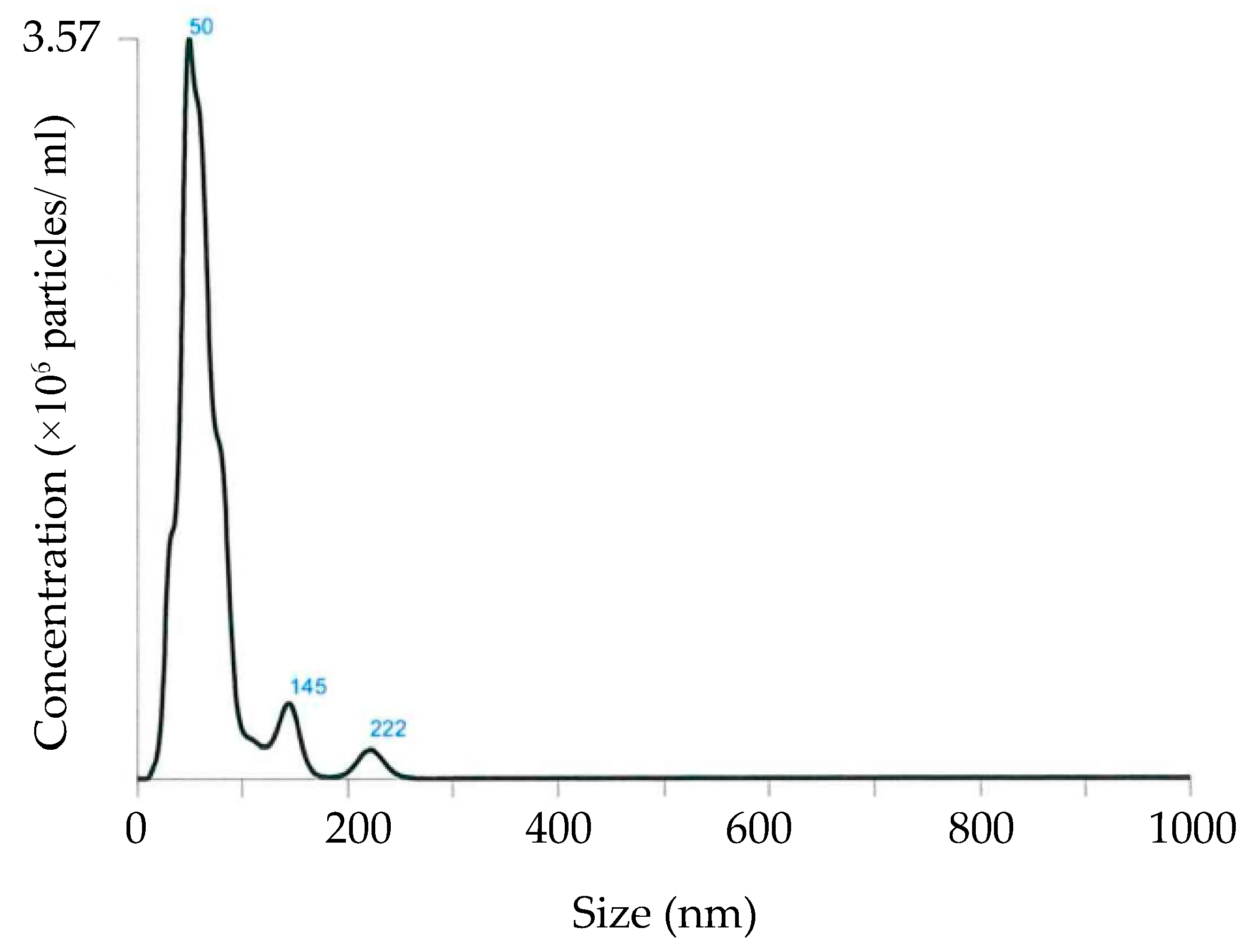
Figure 3), which likely contributed to the high functionality. Due to its extremely small molecule size, hydrogen rapidly penetrates glass or plastic walls of any container, while aluminum walls can retain hydrogen gas for a relatively longer time [
17]. Furthermore, under normal atmospheric pressure, H
2 gas can be slightly dissolved in water for up to 0.8 mM (around 1.6 ppm, wt/vol), but it can only be stored as hydrogen water for a few days. There was no significant difference in the H
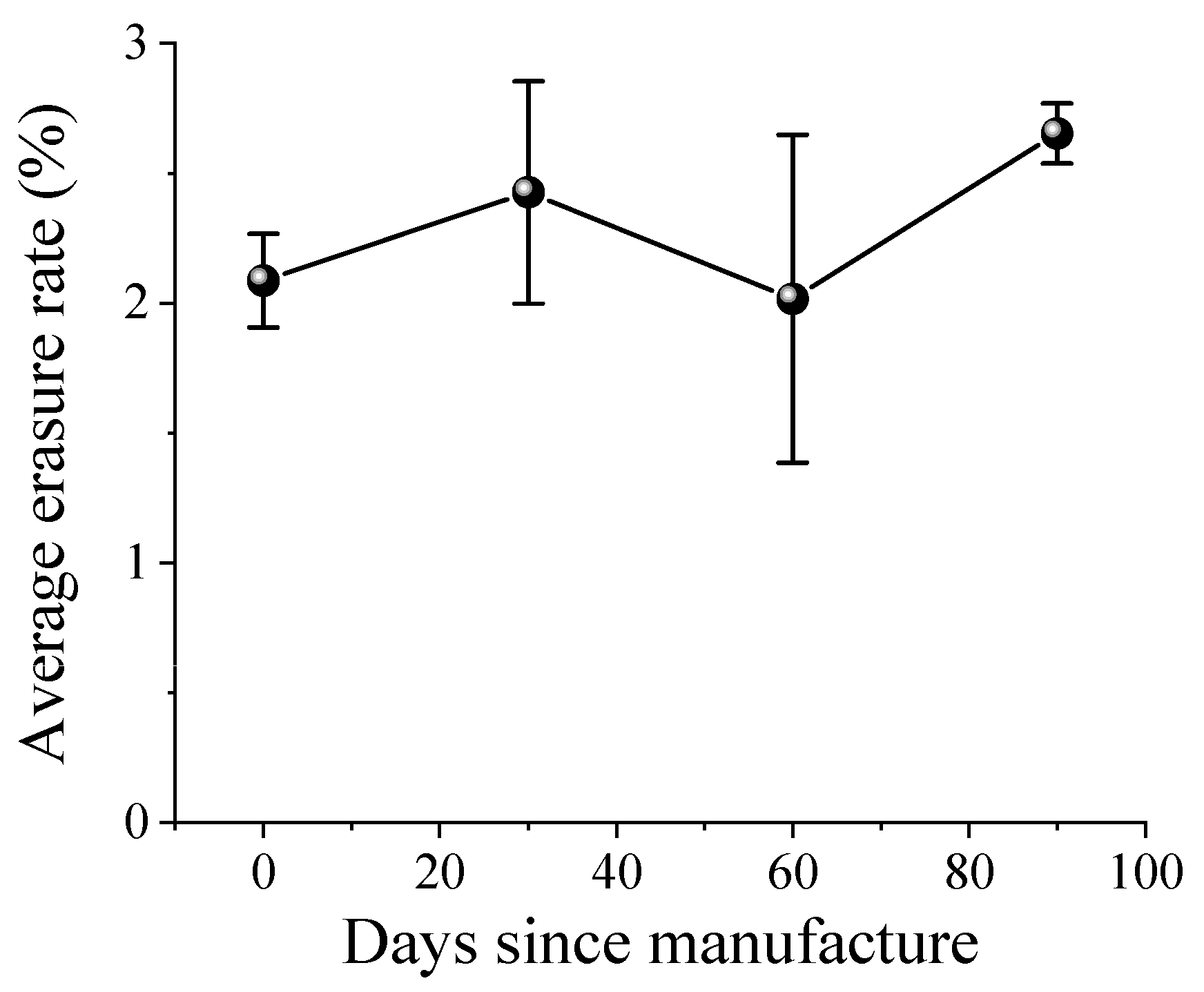
2 concentration of UFB water prepared by this method, but it was found to be stable over a long period of time even when stored at room temperature in polyethylene terephthalate containers, and its radical scavenging capacity was maintained for three months. This feature enables stable storage of UFB hydrogen water for a certain period of time in an ordinary container, which is extremely innovative. It is expected to be used for various applications in the future.
The study on microbubbles and nanobubbles just started 25 to 30 years ago. In EXPO 2005 Aichi, Mr. Masayoshi Takahashi of the National Institute of Advanced Industrial Science and Technology demonstrated that sea bream, which is saltwater fish, and carp, which is freshwater fish, can cohabit in the same fish tank filled with water containing microbubbles, which aroused the global interest in microbubbles. In the reports on international standardization promotion activities for microbubbles and nanobubbles technologies in fiscal 2012, which was sponsored by the Japanese Ministry of Economy, Trade and Industry, microbubbles and nanobubbles are provisionally defined based on the bubble size such that the air bubble having the size of 0.8 mm–1 mm or more is called a bubble, the air bubble having the size of 0.05 mm–0.1 mm or more and smaller than the “bubble” is called a submillimeter bubble, the air bubble having the size of 20 μm–1 μm and smaller than the “submillimeter bubble” is called a microbubble, and the air bubble having the size of 20 μm–1 μm or less is called an ultra-fine bubble. In addition, ISO defines fine bubbles as bubbles with a volume-equivalent diameter of less than 100 μm, microbubbles as fine bubbles with a volume-equivalent diameter of 1 μm or more and less than 100 μm, and UFBs as fine bubbles with a volume-equivalent diameter of less than 1 μm [
10,
11]. Most (90%) of the water bubble sizes obtained with this method were less than 1 μm (
Figure 3). At this time, the total bubble density was 1.94 × 10
9 bubbles/mL, and this concentration was maintained even after 3 months. A lot of reports and research applications regarding microbubbles and hydrogen water have been filed to date. In the prior art, fine bubbles were generated such that gas and liquid are mixed, and the mixture thereof is sheared by cavitation of an ejector or an agitator. The method using shearing inevitably generates various sizes of fine bubbles. The technique of resonance bubble-forming to generate fine bubbles has not been proposed. To make the size of the fine bubbles uniform, generating a reduced pressure state in a resonance device and performing resonance bubble-forming therein can realize the generation of almost uniform-sized fine bubbles. However, simply using the technique of resonance bubble-forming generates micro-sized fine bubbles, and therefore, subjecting the generated fine bubbles to vacuum cavitation under an appropriate reduced pressure state can expand the fine bubbles to be crushed so that the fine bubbles become nano-sized fine bubbles (<1 μm). This method can form almost uniform-sized, ultra-fine bubbles. This UFB has no buoyancy, so it remains dispersed in water for a long period of time.
Kato et al. prepared nano-bubble hydrogen-dissolved water (nano-hydrogen water) which contained hydrogen nano-bubbles of <717 nm diameter for 54% of total bubbles and evaluated the reducing activity of nano-hydrogen water using the 5,5-Dimethyl-1-pyrroline N-oxide (DMPO)-spin–trap electron spin resonance method (ESR) and spectrophotometric 2,2′-bipyridyl methods [
18]. They concluded that nano-hydrogen water has improved antioxidant activity as compared to hydrogen water of similar dissolved hydrogen levels, indicating the more marked importance of nano-bubbles rather than the concentration of hydrogen in terms of OH scavenging.
As molecular hydrogen is generally not very reactive, it also selectively reduces the hydroxyl radical, the most cytotoxic of ROSs, and effectively protects cells; however, hydrogen does not react with other ROSs, which possess physiological roles [
5]. Therefore, hydrogen has some advantages as a potential antioxidant: it effectively neutralizes hydroxyl radicals in living cells, and, unlike most known antioxidants, which are unable to successfully target organelles [
19], it has favorable distribution characteristics: it can penetrate biomembranes and diffuse into the cytosol, mitochondria, and nucleus. Thus, hydrogen can be used as an effective antioxidant therapy. Ionizing radiation acts on water, a constituent of cells, to produce radicals and molecular products such as hydroxyl radicals, hydrogen radicals, hydrated electrons, and hydrogen peroxide [
20]. Approximately 60–70% of DNA damage is induced by indirect free radical action induced by free radicals [
21]. Therefore, hydrogen molecules, a selective hydroxyl radical scavenger, have potential medical applications as a radioprotective agent. Many studies on the radiation damage reduction effect of hydrogen have been reported [
22,
23,
24,
25]. For example, Zhao et al. evaluated the radioprotective effects of hydrogen on the immune system in mice, demonstrating that pre-treatment with hydrogen could increase the spleen index and attenuate the radiation damage on splenic structure, and hydrogen also inhibited radiation-induced apoptosis in splenocytes and down-regulated pro-apoptotic proteins in living mice [
22]. They suggested that hydrogen is an effective radioprotective agent on the immune system by scavenging ROS. Zhang et al. examined whether the consumption of hydrogen-rich water could ameliorate hematopoietic stem cell (HSC) injury in mice with total body irradiation (TBI) [
23]. Finally, they demonstrated that hydrogen-rich water alleviated TBI-induced HSC injury concerning cell number alteration and to the self-renewal and differentiation of HSC. In addition, Liu et al. indicated that hydrogen-rich water has a protective effect on radiation-induced cognitive dysfunction and that the possible mechanisms mainly involve anti-oxidative and anti-inflammatory reactions, and its protection of newborn neurons by regulating the signaling pathway via brain-derived neurotrophic factor and its receptor, tropomyosin receptor kinase B [
24]. Hirano et al. examined whether hydrogen gas treatment improves intensity-modulated radiation therapy (IMRT)-induced bone marrow damage in cancer patients [
25]. Their results demonstrated that hydrogen gas inhalation therapy alleviated IMRT-induced bone marrow damage without compromising the anti-tumor effects of IMRT, suggesting that hydrogen gas inhalation therapy may apply to IMRT-induced bone marrow damage in cancer patients. These are expected to potentially offer a cheap and easy way to take against strong oxidative stress reduction caused by radiation in radiation accidents and nuclear disasters. Indeed, in our preliminary study, we observed a strong radio mitigative effect in UFB hydrogen water.
Recently, Xiao et al. tested the effects of hydrogen-bubbling water named Hexa Z Hydro-nano-bubble water on hydrogen peroxide (H
2O
2)- or phorbol-myristate-acetate (PMA; an inducer of endogenous superoxide anion and hydrogen peroxide)-induced ROS generation, lipid accumulation, and interleukin-6 (IL-6) secretion in OP9 adipocytes and three-dimensional subcutaneous adipose equivalents [
26]. They demonstrated that Hexa Z Hydro-nano-bubble water exhibits excellent stability and can protect HaCaT cells from harmful H
2O
2-induced injuries. HW significantly suppressed H
2O
2- or PMA-enhanced adipogenesis, ROS generation, and IL-6 secretion in OP9 adipocytes and 3D subcutaneous adipose tissue. Therefore, Hexa Z Hydro-nano-bubble water can be applied as a potential healthcare candidate for preventing metabolic disorders. Hexa Z Hydro-nano-bubble water showed a possibility of repressing oxidative stress, inflammatory response, and adipogenesis at cellular/tissue levels. It can be used to prevent the development of metabolic disorders among obese people. In addition, their research group reported the effects of the Hexa Z Hydro-nano-bubble water bath on the oxygen radical absorption-based antioxidant capacity and the inflammatory indicator, C-reactive protein (CRP), in serum between healthy volunteers and inflammatory/collagen disease patients. The Hexa Z Hydro-nano-bubble water bath apparatus supplied nano-bubbles with a diameter of 110 ± 10 nm and 338–682 μg/L of dissolved hydrogen after 120 min of electrolysis, and nano-bubbles increased to 9.91 × 10
7 particles/mL along with the increase in correlative dissolved hydrogen. A ten-minute Hexa Z Hydro-nano-bubble water bath increased the oxygen radical absorption-based antioxidant capacity to 110.9 ± 9.2% at post-bathing 120 min, although unaltered with a 10 min normal water bath at 40 °C in healthy subjects. The CRP level was repressed to 70.2 ± 12.1% at 120 min after the Hexa Z Hydro-nano-bubble water bath, although rather increased for the normal water bath. In patients with connective tissue diseases, the CRP level was repressed to 3–24% upon 9 days to 4 months of Hexa Z Hydro-nano-bubble water bathing. In another six patients with diverse autoimmune-related diseases, upon daily Hexa Z Hydro-nano-bubble water bathing for as long as 2–25 months, the pre-bathing CRP level of 5.31 mg/dL decreased to 0.24 mg/dL, being within the standard range, with relief of visible inflammatory symptoms for some cases. Thus, the Hexa Z Hydro-nano-bubble water bath with high-density nano-bubbles has beneficial effects on serum antioxidant capacity, inflammation, and skin appearance.
UFB water exhibits reductive activities and generates antioxidative radicals so that the water can be effective in the treatment of atopic dermatitis and the prevention of lifestyle-related diseases including diabetes and cancer. The UFB hydrogen water has an anti-oxidation function, and therefore, it is possible to use the water for the prevention of so-called lifestyle-related diseases including high blood pressure, hyperlipidemia, diabetes, heart disease, and cerebral infarction as well as cancer that have been increasing in the aging society of today. UFB hydrogen water with excellent storage stability is expected to be needed in the future for further fine bubbles in academic and business fields such as medicine, zoology, botany, life sciences, inorganic chemistry, electronics, atomic physics, and various manufacturing and cleaning industries.
4. Materials and Methods
Preparation of UFB hydrogen water: UFB hydrogen water was prepared from deionized water according to previous reports using a production system with resonant foaming and vacuum cavitation [
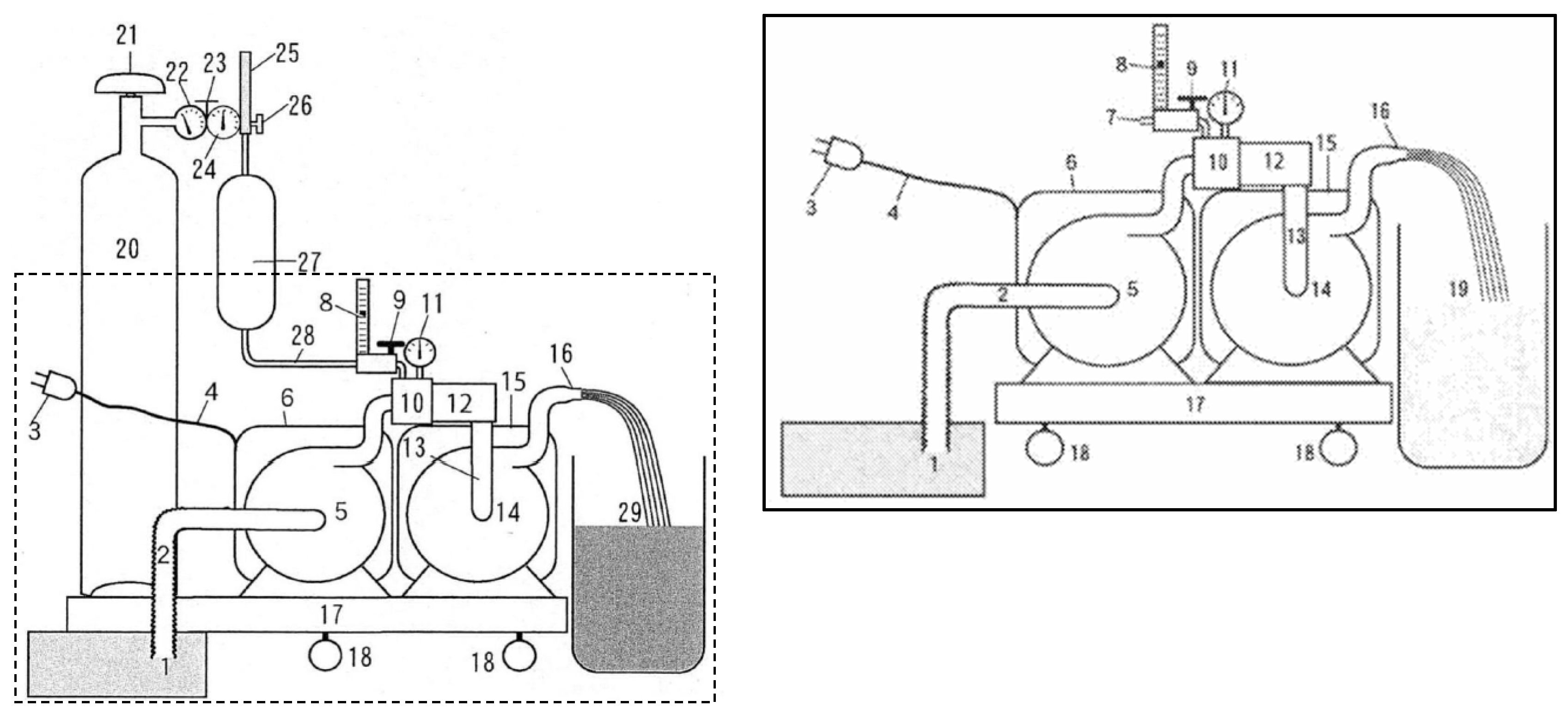
9]. The following shows an efficient method of a fine bubble water production method by resonance foaming and vacuum cavitation (
Figure 4). Water is pressurized by primary pump 5 and sent to resonance ejector 10, the reduced pressure in resonance ejector 10 and resonance foaming device 12 is adjusted by resonance adjustment needle valve 9 while watching decompression gauge 11 and gas flow meter 8, and the gas–liquid mixture mixed in resonance ejector 10 is mixed in resonance foaming device 12 by resonance similar to the principle of a flute. Foaming “resonance foaming” is carried out to generate microbubbles that instantly turn white and expand the bubbles by creating a vacuum in the entire water system after resonance ejector 10 by the suction strength of secondary pump 14 with a suction force greater than the volume of water discharged from primary pump 5 and resonance ejector 10. Vacuum cavitation ultra-fine bubble water was produced by further breaking the primary fine bubbles generated by resonance ejector 12 into smaller bubbles by vacuum and cavitation. To produce a UFB of hydrogen water, the use of resonance bubble-forming and vacuum cavitation is required. The resonance bubble-forming is performed such that water from the primary pump is transferred to the resonance ejector, gas is sucked to be mixed with the water, the pressure conditions are adjusted using a vacuum gauge and a needle valve, and the gas–liquid mixture is subjected to resonance in the resonance bubble-forming device to form fine bubbles. The vacuum cavitation is carried out by using two pumps, the primary pump and the secondary pump, in which the performance of the secondary pump is superior to that of the primary pump, so that an appropriate reduced pressure state is generated. Typical measurements of the comparative results of air content in bubbles from resonance foaming alone and from the combination of resonance foaming and vacuum cavitation are shown in
Table 3. The whole surface of the material is cloudy in the resonance foaming treatment alone, whereas it is colorless and transparent when combined with the vacuum cavitation treatment. This is because microbubbles are generated in resonance foaming treatment, but by immediately performing vacuum cavitation treatment, the micro-bubbles are crushed and become UFBs. Furthermore, a comparison of the gas dispersion as bubbles, the dispersion rate of injected gas in water, and the gas content of water after 1 h of treatment showed that the value dropped to about 40–25% with the resonance foaming treatment alone, while the value immediately after treatment was maintained with the combination treatment method.
The concentration of UFB particles was analyzed by NANOX (Kitakyushu, Fukuoka, Japan).
Measurement of ORP and antioxidant radicals: To measure the oxidation–reduction potential (ORP) of hydrogen water, using a hydrogen radical water circulation stirrer with a strong magnetic force of 0.8 to 1.0 Tesla, UFB hydrogen water was generated by adding hydrogen gas to pure water at a flow rate of 50 mL/min with stirring at high-speed rotation (3000 rpm) for 10 min. Furthermore, as a control, pure water and simple UFB hydrogen water were also created without adding a magnetization function, and the value of ORP was measured using an Eh meter (Five Go F1, Mettler Toledo, Greifensee, Switzerland).
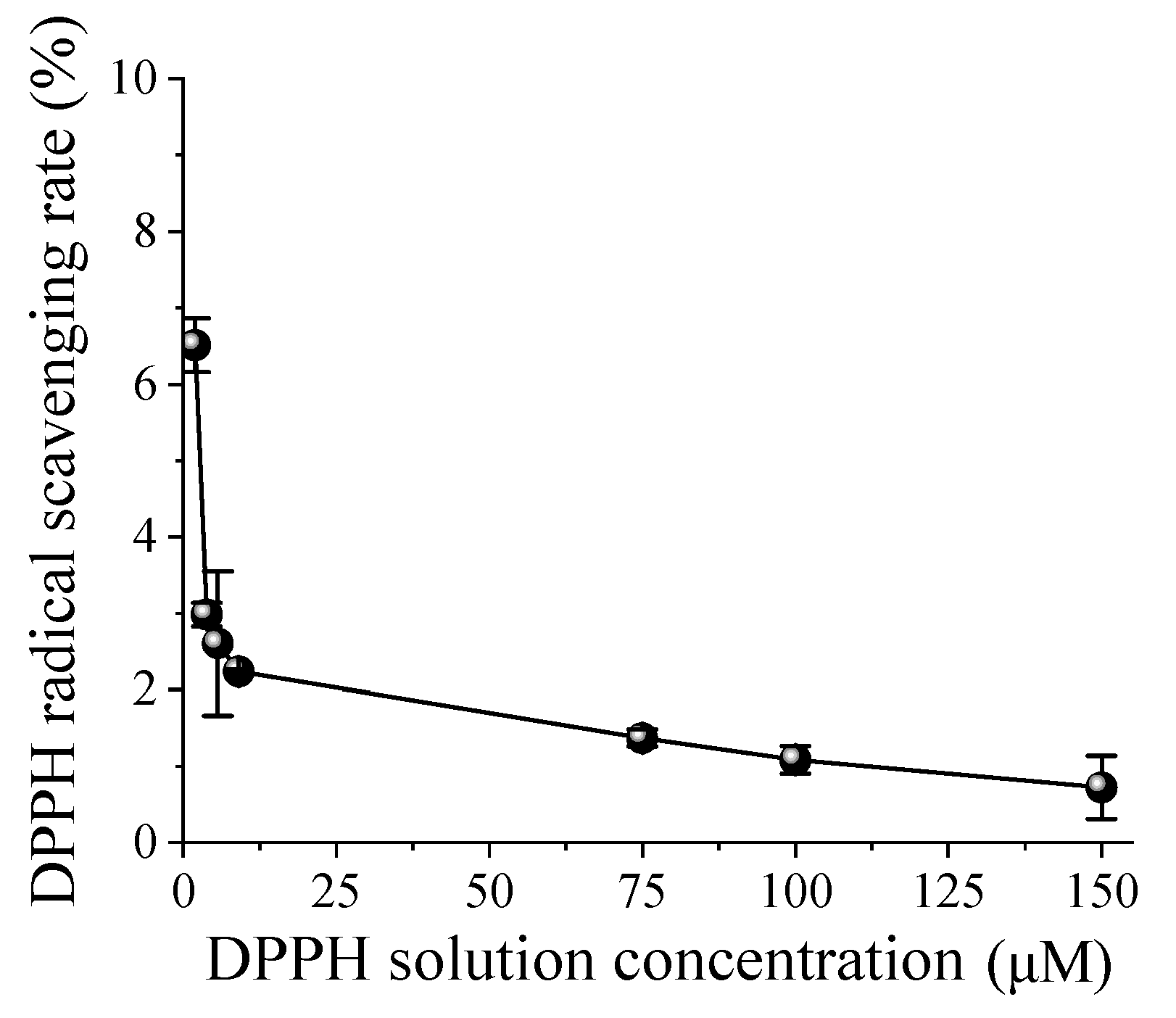
Antioxidant radicals were measured using the DPPH (Nacalai Tesque, Inc., Kyoto, Japan) radical scavenging activity assay according to the previous report [
27,
28]. Briefly, the elimination capability of the oxidative radical was measured by colorimetric determination using a spectrophotometer measuring absorbance at a wavelength of 520 nm (U3010, Hitachi, Tokyo, Japan), which uses the reaction that purple oxidative DPPH reacts with ultra-fine hydrogen-bubbled water to form colorless reductive DPPH. The above UFB hydrogen water and DPPH solution were mixed to give a final concentration of DPPH of 1.96, 3.85, 5.67, 9.09, 75, 100, and 150 μM, respectively. The radical scavenging rate (%) was determined as the ratio of the control to the measured value of the sample.
In addition, aqueous citric acid solutions at pH 3 and pH 4 were prepared using deionized water, and UFB hydrogen water was generated using the method described above. A DPPH radical solution was prepared to have a final concentration of 9.09 μM, and the radical scavenging ability of UFB hydrogen water generated from pH 3 and pH 4 citric acid solutions or ordinary deionized water was measured.
Regarding the thermal stability test of UFB hydrogen water, UFB hydrogen water was heated in boiling water for 10, 20, 30, and 40 min, respectively, and after cooling in water, the DPPH radical scavenging ability was analyzed.