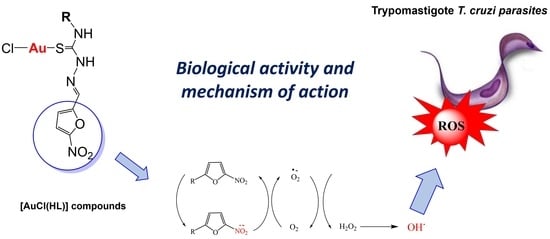
Mechanism of Anti-Trypanosoma cruzi Action of Gold(I) Compounds: A Theoretical and Experimental Approach
Abstract
1. Introduction
2. Results and Discussion
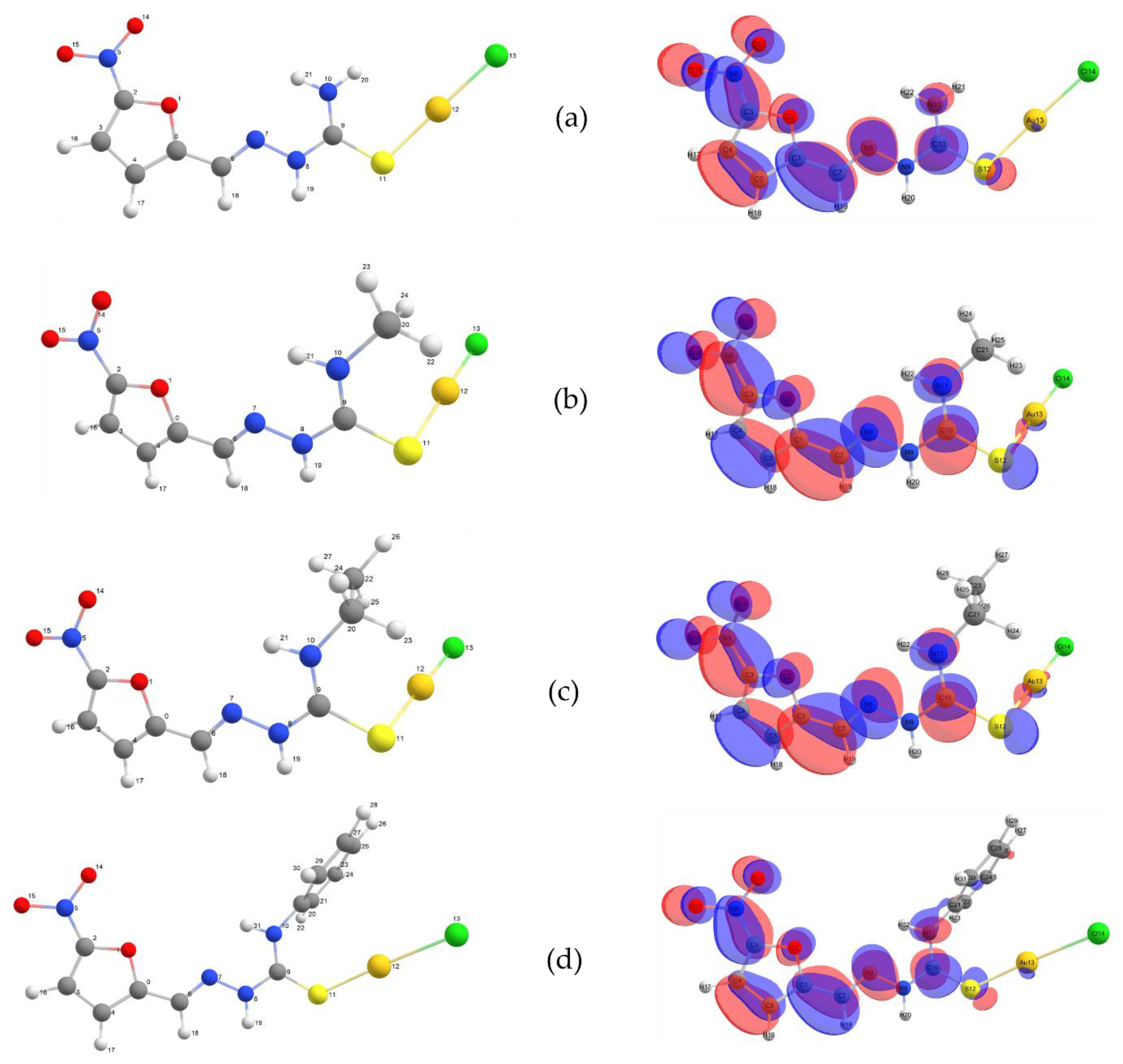
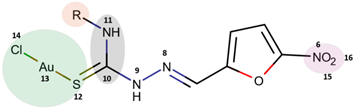
2.1. Theoretical Calculations
Natural Bond Orbital (NBO) Analysis
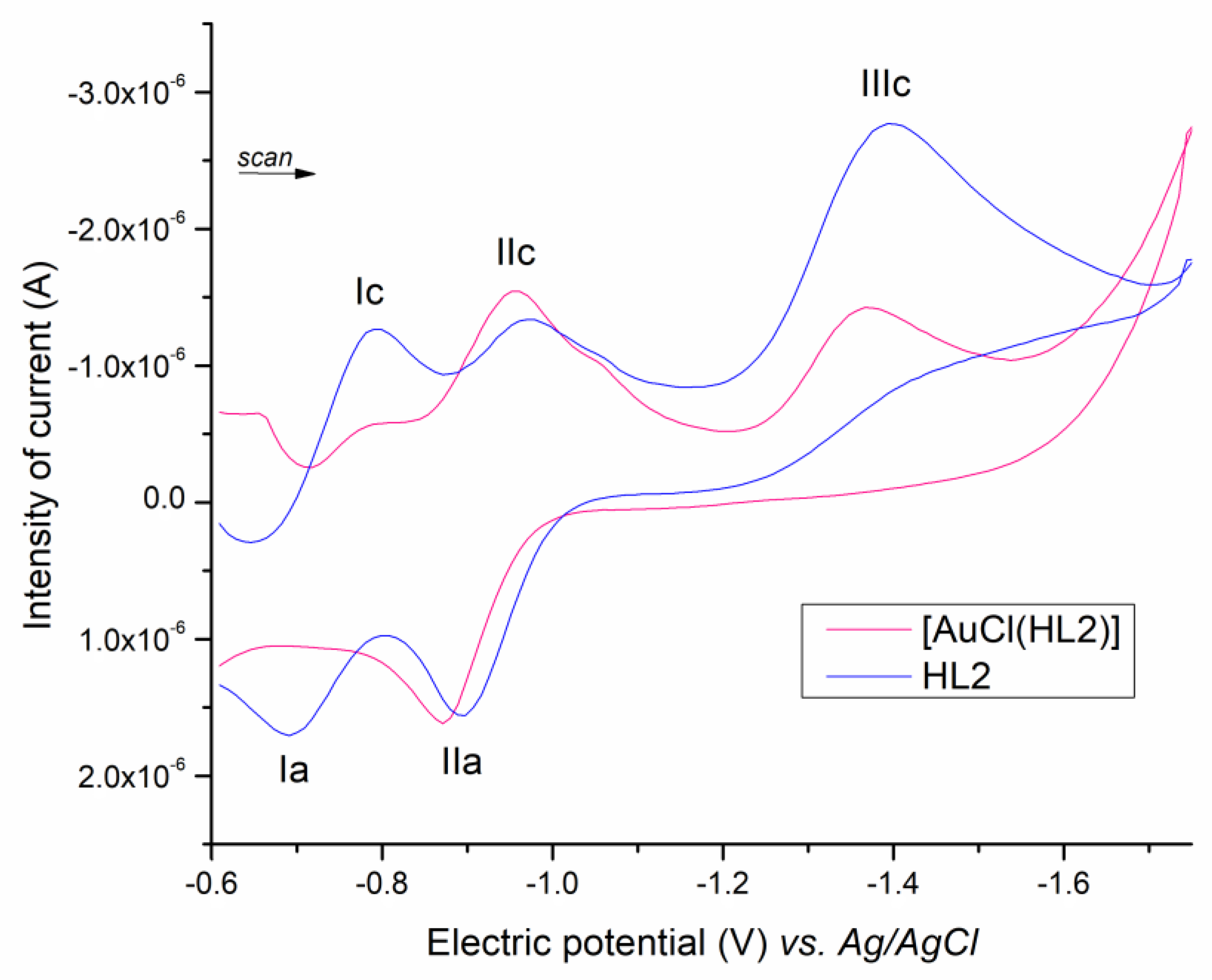
2.2. Cyclic Voltammetry
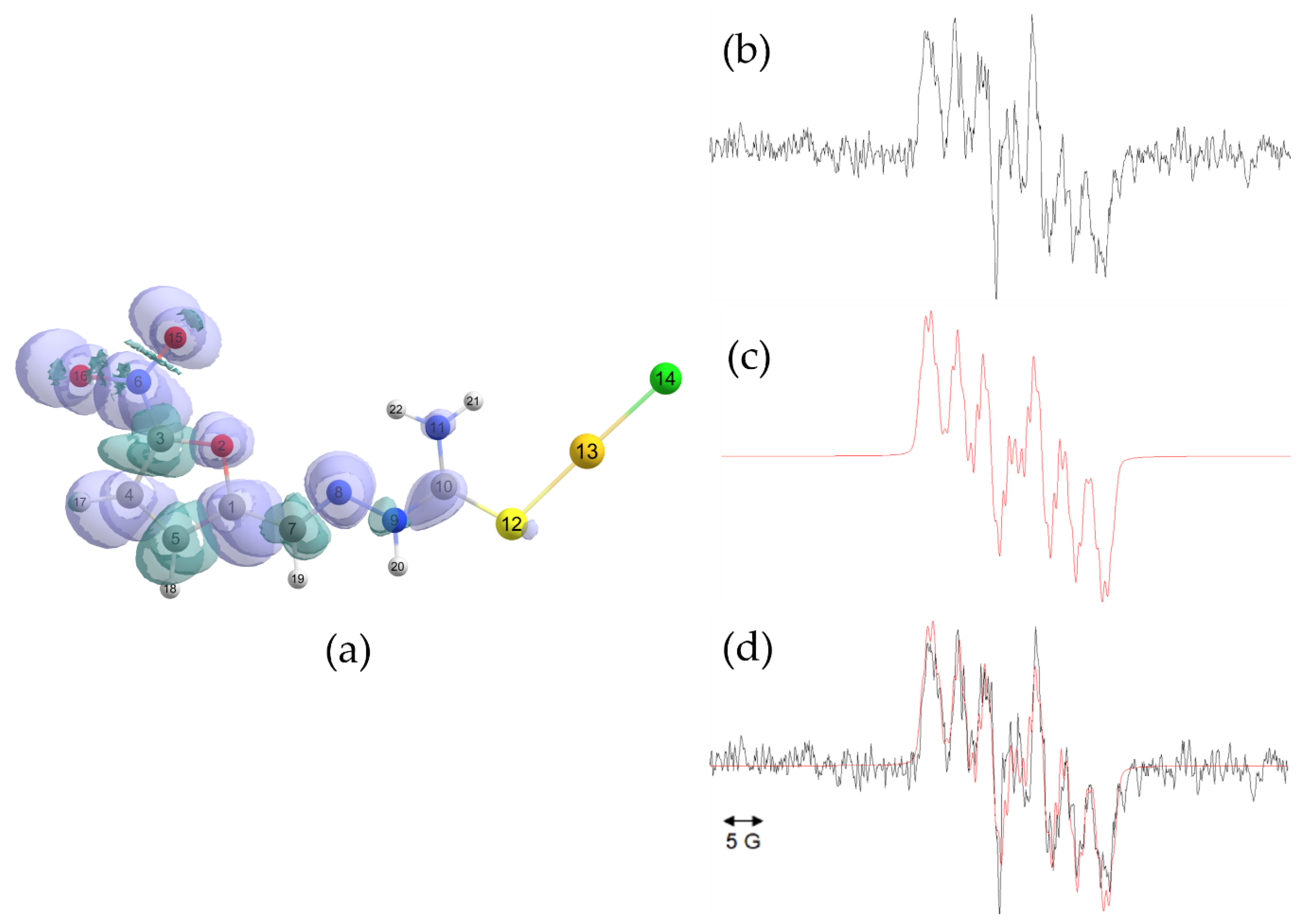
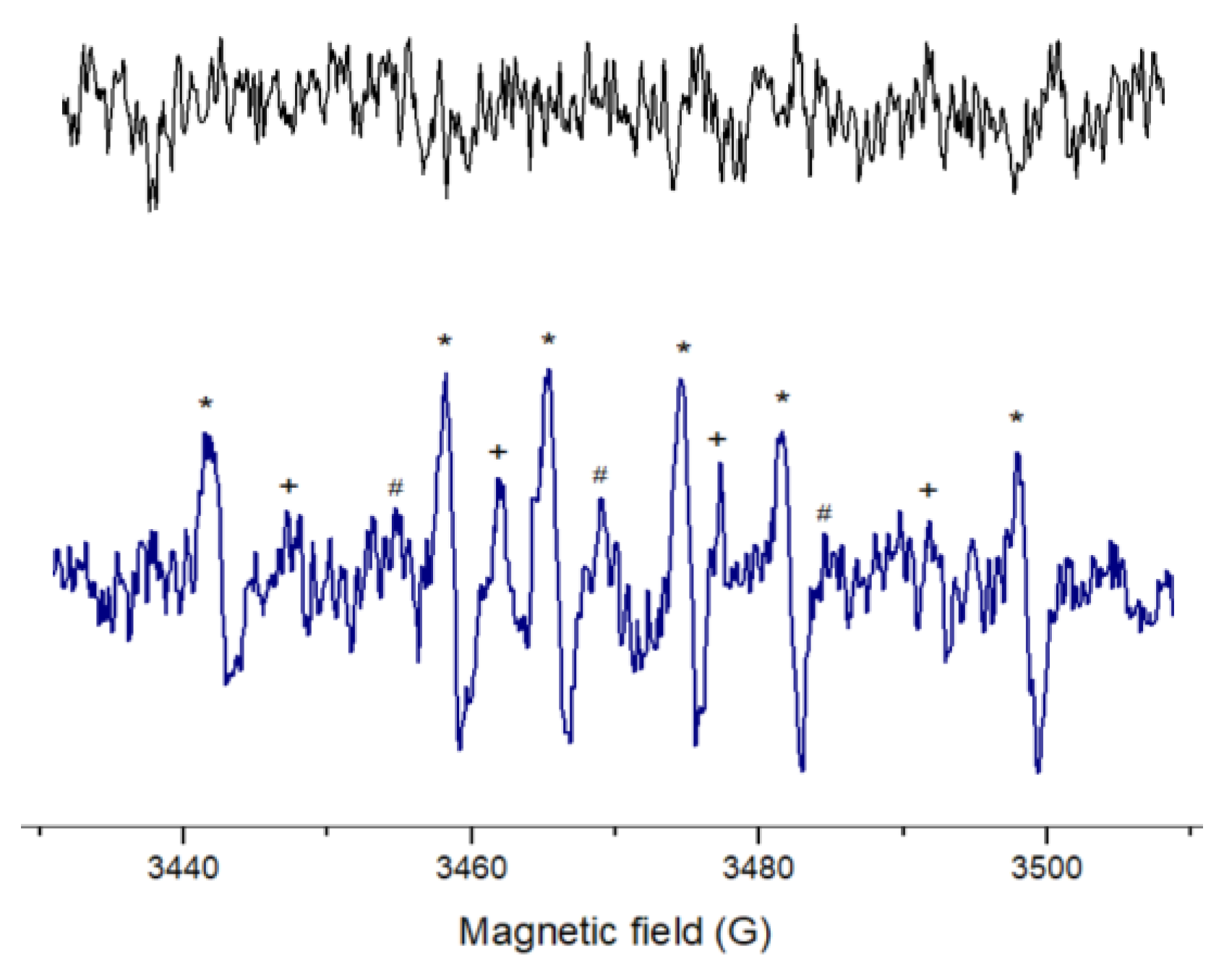
2.3. Electron Spin Resonance
2.4. Lipophilicity
2.5. Biological Studies: Anti-Trypanosoma Cruzi Activity and Cytotoxicity on Mammalian Cells Models
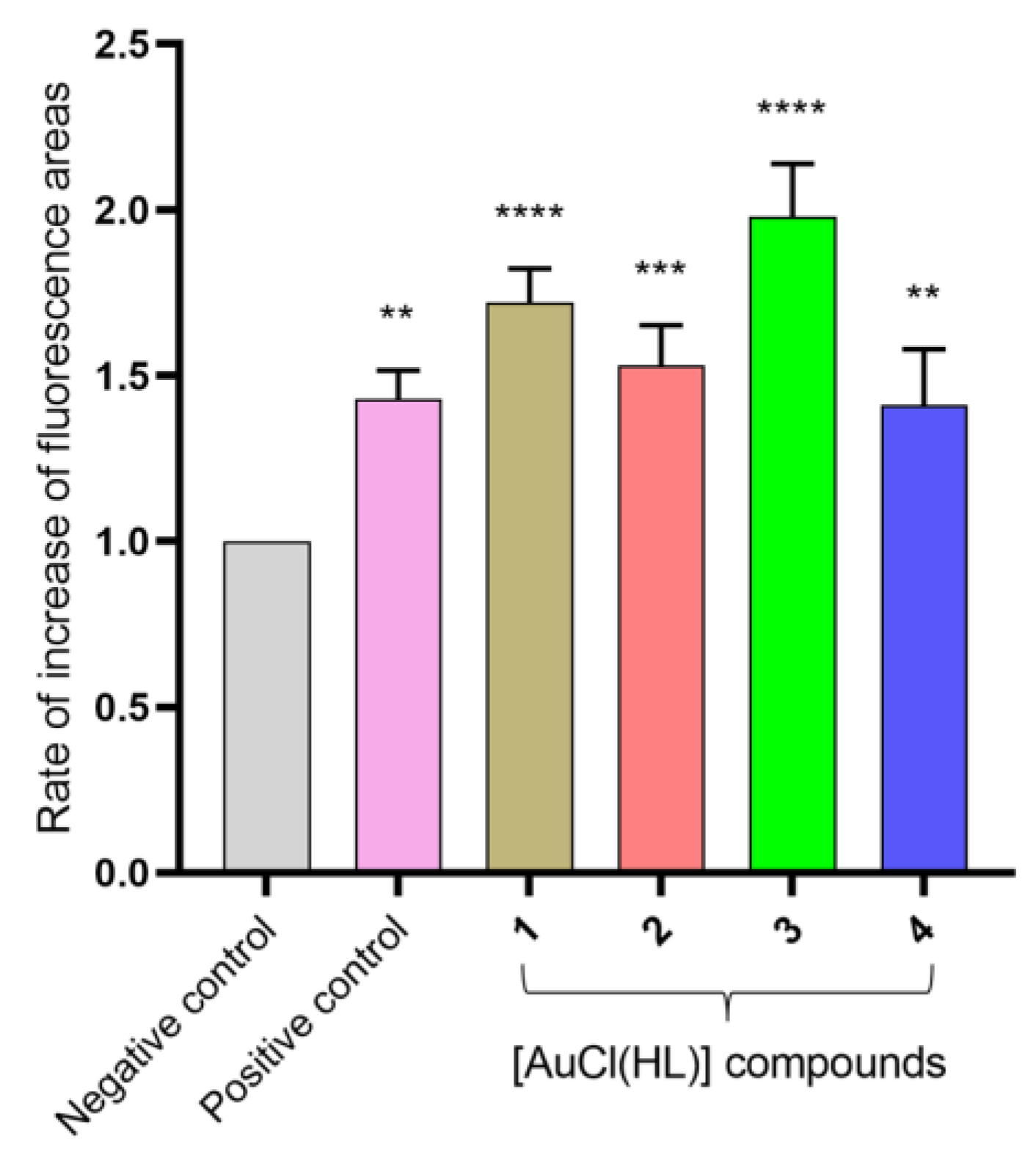
2.6. Insights into the Mechanism of Action: Production of Free Radical Species and Reactive Oxygen Species on Trypanosoma cruzi
3. Materials and Methods
3.1. Theoretical Calculations
3.2. Electrochemical Studies
3.3. ESR Spectroscopy
3.4. Lipophilicity
3.5. Biological Studies
3.5.1. Viability on T. cruzi (Dm28c) Trypomastigotes
3.5.2. Cytotoxicity on Endothelial Mammalian Cells
3.6. Insight into the Mechanism of Action
3.6.1. Generation of Free Radical Species in T. cruzi
3.6.2. Intraparasitic Reactive Oxygen Species (ROS)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Available online: www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 5 April 2024).
- Available online: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis) (accessed on 5 April 2024).
- Bermudez, J.; Davies, C.; Simonazzi, A.; Pablo Real, J.; Palma, S. Current Drug Therapy and Pharmaceutical Challenges for Chagas Disease. Acta Trop. 2016, 156, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Scarim, C.B.; Jornada, D.H.; Chelucci, R.C.; de Almeida, L.; dos Santos, J.L.; Chung, M.C. Current Advances in Drug Discovery for Chagas Disease. Eur. J. Med. Chem. 2018, 155, 824–838. [Google Scholar] [CrossRef]
- Francisco, A.F.; Jayawardhana, S.; Olmo, F.; Lewis, M.D.; Wilkinson, S.R.; Taylor, M.C.; Kelly, J.M. Challenges in Chagas Disease Drug Development. Molecules 2020, 25, 2799. [Google Scholar] [CrossRef] [PubMed]
- Brindha, J.; Balamurali, M.M.; Chanda, K. An Overview on the Therapeutics of Neglected Infectious Diseases—Leishmaniasis and Chagas Diseases. Front. Chem. 2021, 9, 622286. [Google Scholar]
- Brown, R.W.; Hyland, C.J.T. Medicinal Organometallic Chemistry—An Emerging Strategy for the Treatment of Neglected Tropical Diseases. MedChemComm 2015, 6, 1230–1243. [Google Scholar] [CrossRef]
- Gambino, D.; Otero, L. Design of Prospective Antiparasitic Metal-Based Compounds Including Selected Organometallic Cores. Inorg. Chim. Acta 2018, 472, 58–75. [Google Scholar] [CrossRef]
- Ong, Y.C.; Roy, S.; Andrews, P.C.; Gasser, G. Metal Compounds against Neglected Tropical Diseases. Chem. Rev. 2019, 119, 730–796. [Google Scholar] [CrossRef]
- Aguirre, G.; Boiani, L.; Cerecetto, H.; Fernández, M.; González, M.; Denicola, A.; Otero, L.; Gambino, D.; Rigol, C.; Olea-Azar, C.; et al. In Vitro Activity and Mechanism of Action against the Protozoan Parasite Trypanosoma cruzi of 5-Nitrofuryl Containing Thiosemicarbazones. Bioorg. Med. Chem. 2004, 12, 4885–4893. [Google Scholar] [CrossRef]
- Gambino, D.; Otero, L. Facing Diseases Caused by Trypanosomatid Parasites: Rational Design of Pd and Pt Complexes with Bioactive Ligands. Front. Chem. 2022, 9, 816266. [Google Scholar] [CrossRef]
- Rodríguez Arce, E.; Putzu, E.; Lapier, M.; Maya, J.D.; Olea Azar, C.; Echeverría, G.A.; Piro, O.E.; Medeiros, A.; Sardi, F.; Comini, M.; et al. New Heterobimetallic Ferrocenyl Derivatives Are Promising Antitrypanosomal Agents. Dalton Trans. 2019, 48, 7644–7658. [Google Scholar] [CrossRef]
- Glišić, B.Đ.; Djuran, M.I. Gold Complexes as Antimicrobial Agents: An Overview of Different Biological Activities in Relation to the Oxidation State of the Gold Ion and the Ligand Structure. Dalton Trans. 2014, 43, 5950–5969. [Google Scholar] [CrossRef] [PubMed]
- Proetto, M.T.; Alexander, K.; Melaimi, M.; Bertrand, G.; Gianneschi, N.C. Cyclic (Alkyl)(Amino)Carbene (CAAC) Gold(I) Complexes as Chemotherapeutic Agents. Chem.—Eur. J. 2021, 27, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Cisneros-Fajardo, E.J.; Lehmann, T.; Sánchez-Delgado, R.A.; Atencio, R.; Silva, P.; Lira, R.; Urbina, J.A. Toward a Novel Metal-Based Chemotherapy against Tropical Diseases. Synthesis and Characterization of New Copper(II) and Gold(I) Clotrimazole and Ketoconazole Complexes and Evaluation of Their Activity against Trypanosoma cruzi. Inorg. Chem. 2001, 40, 6879–6884. [Google Scholar] [CrossRef] [PubMed]
- Vieites, M.; Smircich, P.; Guggeri, L.; Marchán, E.; Gómez-Barrio, A.; Navarro, M.; Garat, B.; Gambino, D. Synthesis and Characterization of a Pyridine-2-Thiol N-Oxide Gold(I) Complex with Potent Antiproliferative Effect against Trypanosoma cruzi and Leishmania Sp. Insight into Its Mechanism of Action. J. Inorg. Biochem 2009, 103, 1300–1306. [Google Scholar] [CrossRef] [PubMed]
- Massai, L.; Messori, L.; Micale, N.; Schirmeister, T.; Maes, L.; Fregona, D.; Cinellu, M.A.; Gabbiani, C. Gold Compounds as Cysteine Protease Inhibitors: Perspectives for Pharmaceutical Application as Antiparasitic Agents. BioMetals 2017, 30, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Arce, E.; Gavrilov, E.; Alvite, X.; Nayeem, N.; León, I.E.; Neary, M.C.; Otero, L.; Gambino, D.; Olea Azar, C.; Contel, M. 5-Nitrofuryl-Containing Thiosemicarbazone Gold(I) Compounds: Synthesis, Stability Studies, and Anticancer Activity. ChemPlusChem 2023, 88, e202300115. [Google Scholar] [CrossRef] [PubMed]
- Dorosti, Z.; Yousefi, M.; Sharafi, S.M.; Darani, H.Y. Mutual Action of Anticancer and Antiparasitic Drugs: Are There Any Shared Targets? Future Oncol. 2014, 10, 2529–2539. [Google Scholar] [CrossRef]
- Sullivan, J.A.; Tong, J.L.; Wong, M.; Kumar, A.; Sarkar, H.; Ali, S.; Hussein, I.; Zaman, I.; Meredith, E.L.; Helsby, N.A.; et al. Unravelling the Role of SNM1 in the DNA Repair System of Trypanosoma brucei. Mol. Microbiol. 2015, 96, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.J.; Fuertes, A.M.; Nguewa, A.P.; Castilla, J.; Alonso, C. Anticancer Compounds as Leishmanicidal Drugs: Challenges in Chemotherapy and Future Perspectives. Curr. Med. Chem. 2008, 15, 433–439. [Google Scholar] [CrossRef]
- Guerini, A.E.; Triggiani, L.; Maddalo, M.; Bonù, M.L.; Frassine, F.; Baiguini, A.; Alghisi, A.; Tomasini, D.; Borghetti, P.; Pasinetti, N.; et al. Mebendazole as a Candidate for Drug Repurposing in Oncology: An Extensive Review of Current Literature. Cancers 2019, 11, 1284. [Google Scholar] [CrossRef]
- Son, D.-S.; Lee, E.-S.; Adunyah, S.E. The Antitumor Potentials of Benzimidazole Anthelmintics as Repurposing Drugs. Immune Netw. 2020, 20, e29. [Google Scholar] [CrossRef] [PubMed]
- Otero, L.; Vieites, M.; Boiani, L.; Denicola, A.; Rigol, C.; Opazo, L.; Olea-Azar, C.; Maya, J.D.; Morello, A.; Krauth-Siegel, R.L.; et al. Novel Antitrypanosomal Agents Based on Palladium Nitrofurylthiosemicarbazone Complexes: DNA and Redox Metabolism as Potential Therapeutic Targets. J. Med. Chem. 2006, 49, 3322–3331. [Google Scholar] [CrossRef] [PubMed]
- Rigol, C.; Olea-Azar, C.; Mendizábal, F.; Otero, L.; Gambino, D.; González, M.; Cerecetto, H. Electrochemical and ESR Study of 5-Nitrofuryl-Containing Thiosemicarbazones Antiprotozoal Drugs. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2005, 61, 2933–2938. [Google Scholar] [CrossRef]
- Pagano, M.; Demoro, B.; Toloza, J.; Boiani, L.; González, M.; Cerecetto, H.; Olea-Azar, C.; Norambuena, E.; Gambino, D.; Otero, L. Effect of Ruthenium Complexation on Trypanocidal Activity of 5-Nitrofuryl Containing Thiosemicarbazones. Eur. J. Med. Chem. 2009, 44, 4937–4943. [Google Scholar] [CrossRef] [PubMed]
- Vieites, M.; Otero, L.; Santos, D.; Toloza, J.; Figueroa, R.; Norambuena, E.; Olea-Azar, C.; Aguirre, G.; Cerecetto, H.; González, M.; et al. Platinum(II) Metal Complexes as Potential Anti-Trypanosoma cruzi Agents. J. Inorg. Biochem. 2008, 102, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Hagiwara, T.; Murakami, A. A Mini Review: Fundamental Aspects of Spin Trapping with DMPO. Int. J. Radiat. Appl. Instrum. Part C Radiat. Phys. Chem. 1991, 37, 657–665. [Google Scholar] [CrossRef]
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An Advanced Semantic Chemical Editor, Visualization, and Analysis Platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. The ORCA Program System. WIREs Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal Solvation Model Based on Solute Electron Density and on a Continuum Model of the Solvent Defined by the Bulk Dielectric Constant and Atomic Surface Tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Dolg, M.; Stoll, H.; Preuss, H. Energy-adjusted Ab Initio Pseudopotentials for the Rare Earth Elements. J. Chem. Phys. 1989, 90, 1730–1734. [Google Scholar] [CrossRef]
- Andrae, D.; Häußermann, U.; Dolg, M.; Stoll, H.; Preuß, H. Energy-Adjustedab Initio Pseudopotentials for the Second and Third Row Transition Elements. Theor. Chim. Acta 1990, 77, 123–141. [Google Scholar] [CrossRef]
- Peterson, K.A.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically Convergent Basis Sets with Relativistic Pseudopotentials. II. Small-Core Pseudopotentials and Correlation Consistent Basis Sets for the Post-d Group 16–18 Elements. J. Chem. Phys. 2003, 119, 11113–11123. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Foster, J.P.; Weinhold, F. Natural Hybrid Orbitals. J. Am. Chem. Soc. 1980, 102, 7211–7218. [Google Scholar] [CrossRef]
- Zapata-Torres, G.; Fierro, A.; Barriga-González, G.; Salgado, J.C.; Celis-Barros, C. Revealing Monoamine Oxidase B Catalytic Mechanisms by Means of the Quantum Chemical Cluster Approach. J. Chem. Inf. Model. 2015, 55, 1349–1360. [Google Scholar] [CrossRef] [PubMed]
- Muelas-Serrano, S.; Nogal-Ruiz, J.J.; Gómez-Barrio, A. Setting of a Colorimetric Method to Determine the Viability of Trypanosoma cruzi Epimastigotes. Parasitol. Res. 2000, 86, 999–1002. [Google Scholar] [CrossRef]
- Faundez, M.; Pino, L.; Letelier, P.; Ortiz, C.; López, R.; Seguel, C.; Ferreira, J.; Pavani, M.; Morello, A.; Maya, J.D. Buthionine Sulfoximine Increases the Toxicity of Nifurtimox and Benznidazole to Trypanosoma cruzi. Antimicrob. Agents Chemother. 2005, 49, 126–130. [Google Scholar] [CrossRef]


 | |||||
|---|---|---|---|---|---|
| Spin | Donor (L) NBO 1 | Acceptor (NL) NBO 2 | E(2) 3 kcal/mol | ||
| α | 32. | LP (1) N 11 | 93. | BD*(1) C 10–N 9 | 73.13 |
| α | 34. | LP (2) S 12 | 93. | BD*(1) C 10–N 9 | 33.07 |
| α | 43. | LP (4) Cl 14 | 99. | BD*(1) S 12–Au 13 | 75.17 |
| β | 30. | LP (1) N 6 | 99. | BD*(1) O 15–O 16 | 1075.57 |
| β | 32. | LP (1) N 9 | 93. | BD*(1) C 10–N 11 | 68.87 |
| β | 34. | LP (2) S 12 | 93. | BD*(1) C 10–N 11 | 35.94 |
| β | 43. | LP (4) Cl 14 | 98. | BD*(1) S 12–Au 13 | 75.13 |
| Compound | Couple I | Couple II | Couple III | ||
|---|---|---|---|---|---|
| Epc 1 | Epa 2 | Epc 1 | Ep 2 | Epc | |
| [AuCl(HL1)] | −0.80 | - | −0.96 (−0.92) | −0.89 (−0.80) | −1.38 |
| [AuCl(HL2)] | −0.80 | −0.73 | −0.95 (−0.98) | −0.89 (−0.85) | −1.39 |
| [AuCl(HL3)] | −0.79 | −0.72 | −0.93 (−0.95) | −0.87 (−0.84) | −1.35 |
| [AuCl(HL4)] | −0.75 | −0.74 | −0.92 (−0.92) | −0.86 (−0.81) | −1.38 |
| Nifurtimox | - | - | −1.18 | −1.12 | - |
| [AuCl(HL1)] | [AuCl(HL2)] | [AuCl(HL3)] | [AuCl(HL4)] | |||||
|---|---|---|---|---|---|---|---|---|
| Spin Density | a (G) | Spin Density | a (G) | Spin Density | a (G) | Spin Density | a (G) | |
| N6 | 0.202404 | 8.55 | 0.199949 | 9.16 | 0.200306 | 9.05 | 0.196242 | 8.20 |
| H17 | 0.000468 | 5.20 | 0.000456 | 5.42 | 0.000462 | 5.43 | 0.00043 | 5.10 |
| H18 | −0.000346 | 3.28 | −0.000343 | 3.35 | −0.000348 | 3.30 | −0.000338 | 3.40 |
| H19 | −0.000119 | 0.83 | −0.000103 | 0.53 | −0.000107 | 0.96 | −0.000087 | 0.90 |
| N8 | 0.149215 | 1.09 | 0.15112 | 1.07 | 0.150759 | 1.05 | 0.152195 | 1.07 |
| N9 | 0.003226 | 0.88 | 0.002067 | 0.98 | 0.002081 | 0.94 | 0.000466 | 0.90 |
| H20 | −0.000051 | 0.67 | −0.000031 | 0.46 | −0.000034 | 0.80 | −0.000061 | 0.90 |
| N11 | 0.00880 | - | 0.012168 | - | 0.012004 | - | 0.00945 | - |
| H21 | 0.000103 | - | −0.000003 | - | −0.000012 | - | 0.000026 | - |
| Compound | T. cruzi IC50/μM | EA.hy926 IC50/μM | SI 1 | RM 3 |
|---|---|---|---|---|
| [AuCl(HL1)] (1) | 24.5 ± 1.4 | 108.2 ± 6.6 | 4 | −0.22 |
| HL1 | 9.8 ± 1.5 2 | >100 2 | >10 | −0.31 |
| [AuCl(HL2)] (2) | 10.3 ± 1.0 | 94.0 ± 1.7 | 9 | −0.01 |
| HL2 | 17.4 ± 1.9 2 | >100 2 | >6 | −0.04 |
| [AuCl(HL3)] (3) | 9.9 ± 1.9 | 46.7 ± 0.7 | 5 | 0.13 |
| HL3 | 18.5 ± 1.7 2 | >100 2 | >5 | 0.12 |
| [AuCl(HL4)] (4) | 49.1 ± 7.3 | 78.4 ± 2.1 | 2 | 0.17 |
| HL4 | 22.7 ± 1.6 2 | >100 2 | >4 | 0.29 |
| Nifurtimox | 10.0 ± 0.4 | >200 | >20 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Órdenes-Rojas, J.; Risco, P.; Ortega-Campos, J.; Barriga-González, G.; Liempi, A.; Kemmerling, U.; Gambino, D.; Otero, L.; Olea Azar, C.; Rodríguez-Arce, E. Mechanism of Anti-Trypanosoma cruzi Action of Gold(I) Compounds: A Theoretical and Experimental Approach. Inorganics 2024, 12, 133. https://doi.org/10.3390/inorganics12050133
Órdenes-Rojas J, Risco P, Ortega-Campos J, Barriga-González G, Liempi A, Kemmerling U, Gambino D, Otero L, Olea Azar C, Rodríguez-Arce E. Mechanism of Anti-Trypanosoma cruzi Action of Gold(I) Compounds: A Theoretical and Experimental Approach. Inorganics. 2024; 12(5):133. https://doi.org/10.3390/inorganics12050133
Chicago/Turabian StyleÓrdenes-Rojas, Javiera, Paola Risco, José Ortega-Campos, Germán Barriga-González, Ana Liempi, Ulrike Kemmerling, Dinorah Gambino, Lucía Otero, Claudio Olea Azar, and Esteban Rodríguez-Arce. 2024. "Mechanism of Anti-Trypanosoma cruzi Action of Gold(I) Compounds: A Theoretical and Experimental Approach" Inorganics 12, no. 5: 133. https://doi.org/10.3390/inorganics12050133
APA StyleÓrdenes-Rojas, J., Risco, P., Ortega-Campos, J., Barriga-González, G., Liempi, A., Kemmerling, U., Gambino, D., Otero, L., Olea Azar, C., & Rodríguez-Arce, E. (2024). Mechanism of Anti-Trypanosoma cruzi Action of Gold(I) Compounds: A Theoretical and Experimental Approach. Inorganics, 12(5), 133. https://doi.org/10.3390/inorganics12050133