Fluorosolvatochromism of Platinum Supramolecular Coordination Complexes: Stepwise Synthesis and Photophysical Properties of Organometallic Tetranuclear Pt(II) Squares
Abstract
1. Introduction
2. Results and Discussion
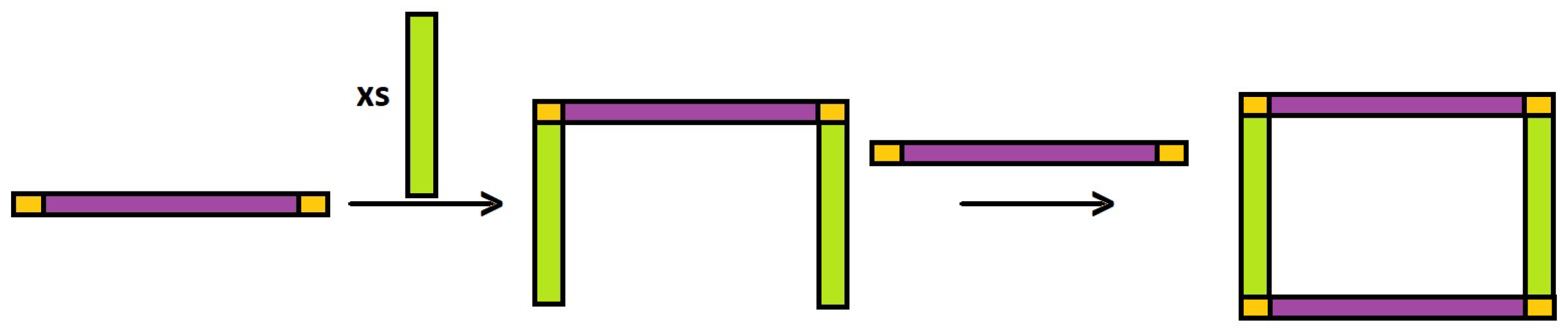
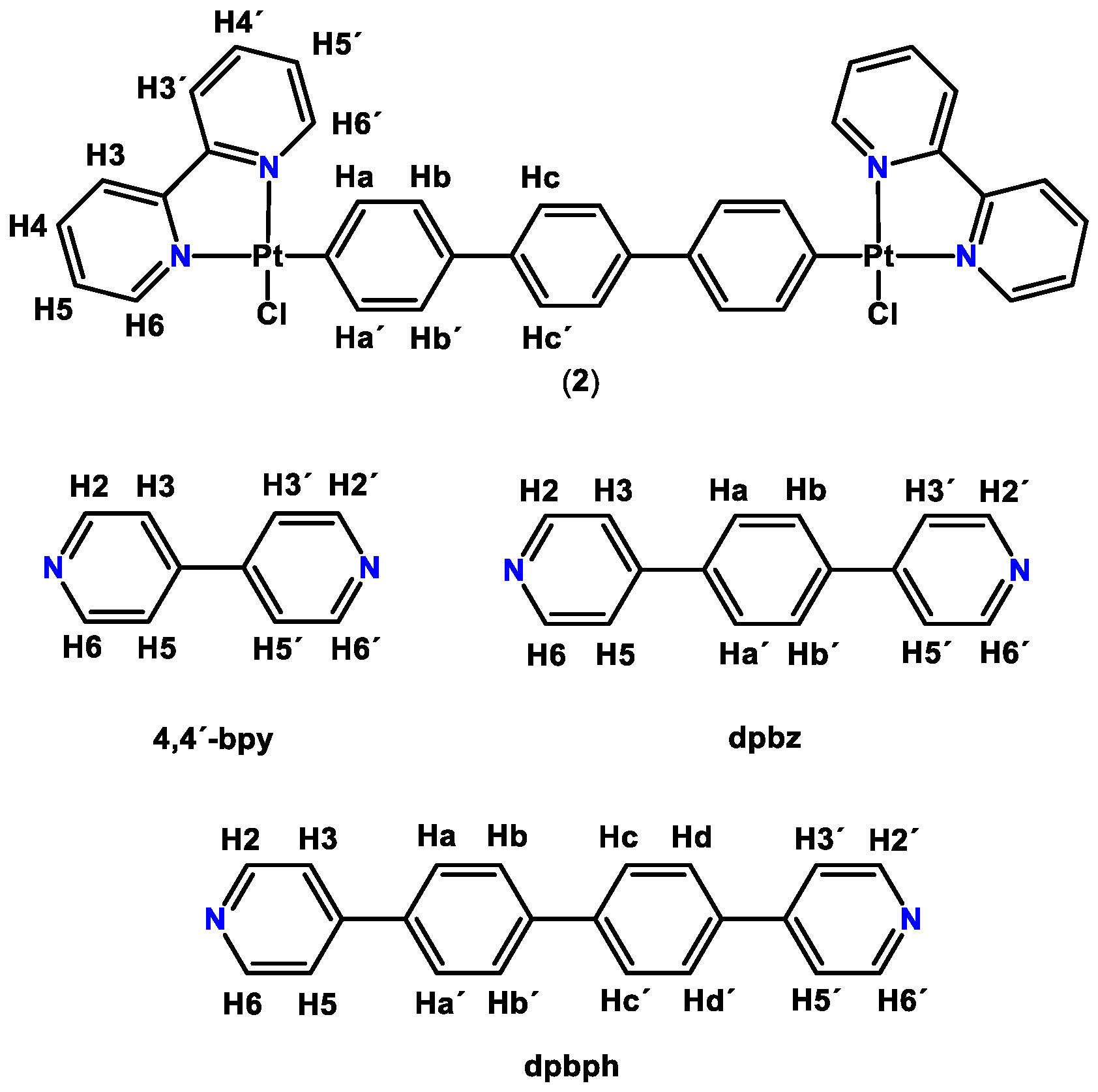
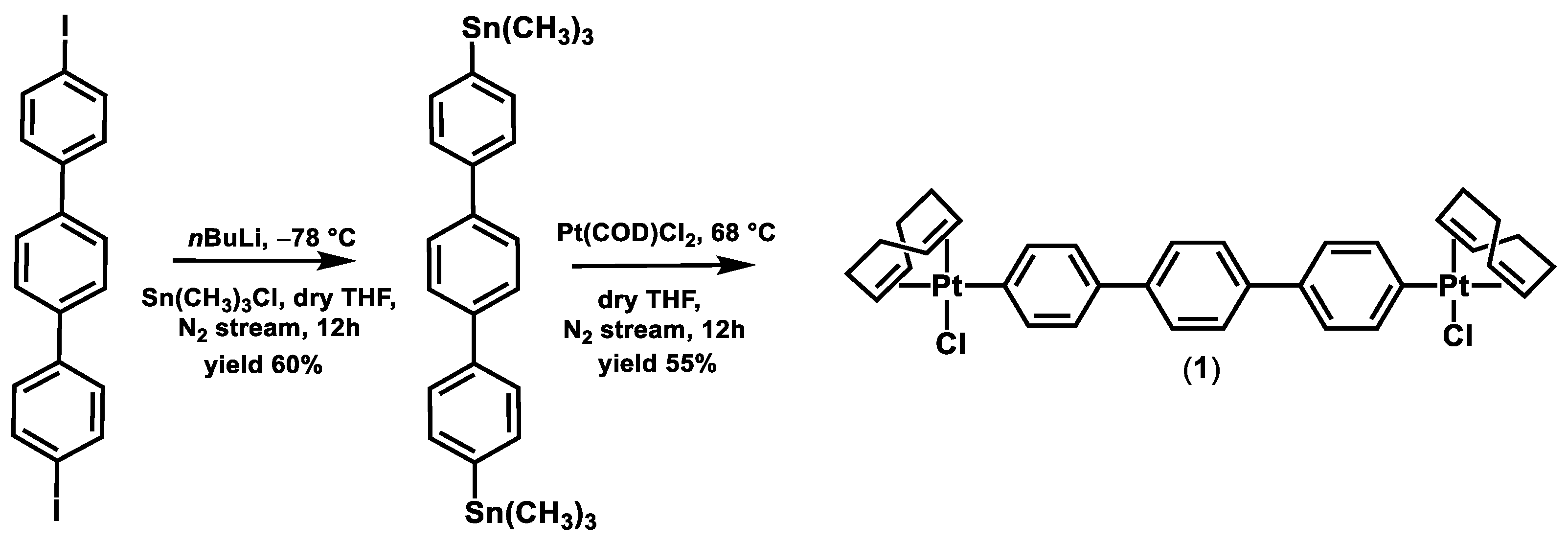
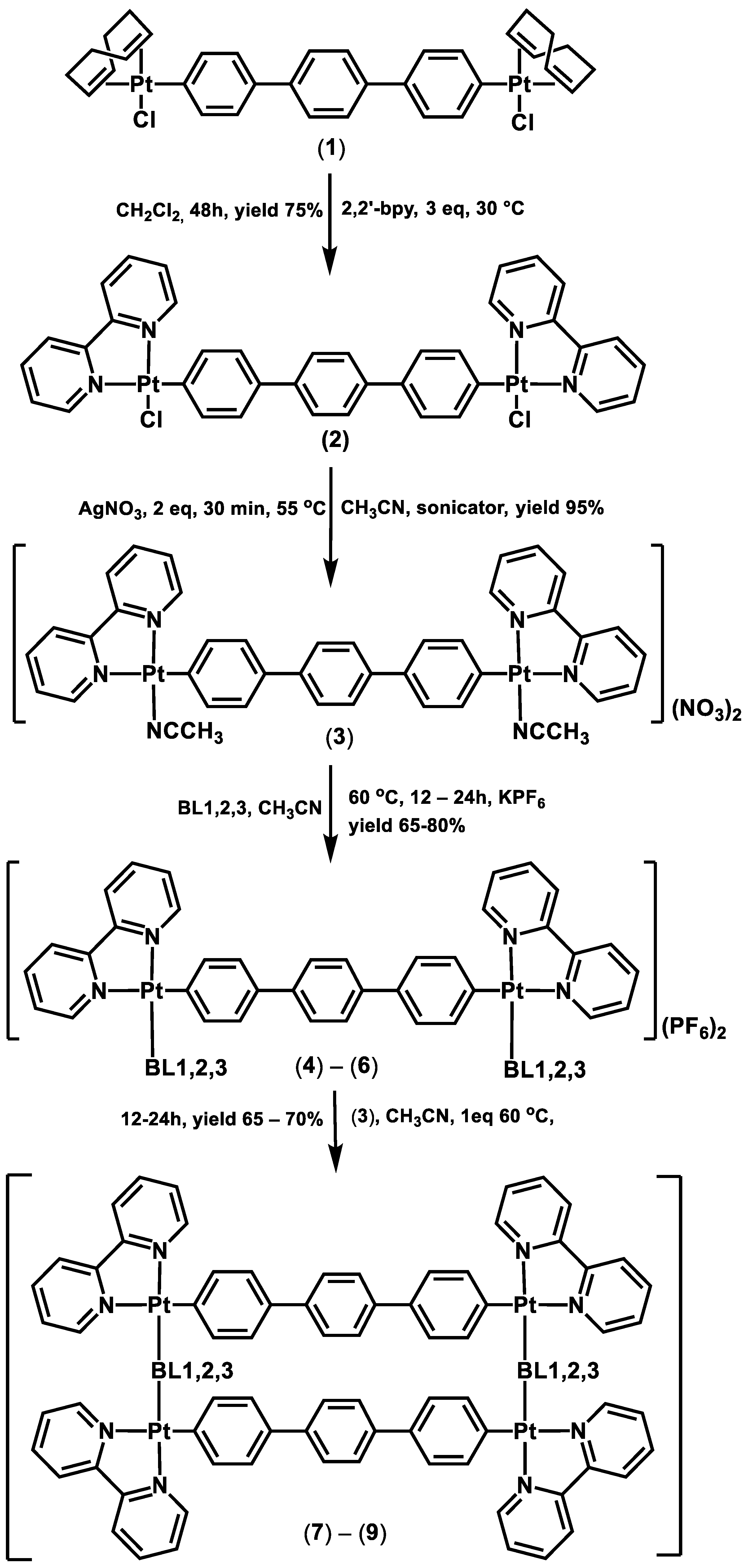
2.1. Synthesis and Characterization
2.2. Photophysical Studies
2.2.1. Absorption and Emission Spectra of Complexes (7)–(9)
2.2.2. Solvent Effect on Emission Spectra of Complexes (7)–(9)
3. Experiments
3.1. Materials and Methods
3.2. Fluorescence Emission Studies
3.3. Synthesis of Complexes (2)–(9)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iwamoto, T.; Watanabe, Y.; Sakamoto, Y.; Suzuki, T.; Yamago, S. Selective and Random Syntheses of [n]Cycloparaphenylenes (n = 8–13) and Size Dependence of Their Electronic Properties. J. Am. Chem. Soc. 2011, 133, 8354–8361. [Google Scholar] [CrossRef] [PubMed]
- Vivekananda, K.V.; Dey, S.; Maity, D.K.; Bhuvanesh, N.; Jain, V.K. Supramolecular Macrocyclic Pd(II) and Pt(II) Squares and Rectangles with Aryldithiolate Ligands and Their Excellent Catalytic Activity in Suzuki C–C Coupling Reaction. Inorg. Chem. 2015, 54, 10153–10162. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.H.; Chen, H.Y.; Tong, M.L.; Ji, L.N.; Mao, Z.W. Platinum Squares with High Selectivity and Affinity for Human Telomeric G-Quadruplexes. Chem. Commun. 2012, 48, 7607–7609. [Google Scholar] [CrossRef] [PubMed]
- Grishagin, I.V.; Pollock, J.B.; Kushal, S.; Cook, T.R.; Stang, P.J.; Olenyuk, B.Z. In Vivo Anticancer Activity of Rhomboidal Pt(II) Metallacycles. Proc. Natl. Acad. Sci. USA 2014, 111, 18448–18453. [Google Scholar] [CrossRef] [PubMed]
- Garypidou, A.; Ypsilantis, K.; Plakatouras, J.C.; Garoufis, A. Dual-Emissive Rectangular Supramolecular Pt(II)-p-Biphenyl with 4,4′-Bipyridine Derivative Metallacycles: Stepwise Synthesis and Photophysical Properties. Molecules 2023, 28, 7261. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Roelfes, F.; Hepp, A.; Hahn, F.E. Single-Step Synthesis of Organometallic Molecular Squares from NR,NR′,NR′′,NR′′′-Substituted Benzobiscarbenes. Chem. A Eur. J. 2017, 23, 5939–5942. [Google Scholar] [CrossRef] [PubMed]
- Würthner, F.; Sautter, A. Highly Fluorescent and Electroactive Molecular Squares Containing Perylene Bisimide Ligands. Chem. Commun. 2000, 2, 445–446. [Google Scholar] [CrossRef]
- Chen, J.-S.; Zhao, G.-J.; Cook, T.R.; Han, K.-L.; Stang, P.J. Photophysical Properties of Self-Assembled Multinuclear Platinum Metallacycles with Different Conformational Geometries. J. Am. Chem. Soc. 2013, 135, 6694–6702. [Google Scholar] [CrossRef]
- Goeb, S.; Bivaud, S.; Dron, P.I.; Balandier, J.Y.; Chas, M.; Sallé, M. A Bpttf-Based Self-Assembled Electron-Donating Triangle Capable of C60 Binding. Chem. Commun. 2012, 48, 3106–3108. [Google Scholar] [CrossRef]
- Schmidtendorf, M.; Pape, T.; Hahn, F.E. Molecular Rectangles from Platinum(Ii) and Bridging Dicarbene, Diisocyanide and 4,4′-Bipyridine Ligands. Dalt. Trans. 2013, 42, 16128–16141. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Yazaki, J.; Ogura, K. Preparation of a Macrocyclic Polynuclear Complex, [(En)Pd(4,4’-Bpy)]4(NO3)8 (En = Ethylenediamine, Bpy = Bipyridine), Which Recognizes an Organic Molecule in Aqueous Media. J. Am. Chem. Soc. 1990, 112, 5645–5647. [Google Scholar] [CrossRef]
- Orita, A.; Jiang, L.; Nakano, T.; Ma, N.; Otera, J. Solventless Reaction Dramatically Accelerates Supramolecular Self-Assembly. Chem. Commun. 2002, 2, 1362–1363. [Google Scholar] [CrossRef]
- Würthner, F.; You, C.-C.; Saha-Möller, C.R. Metallosupramolecular Squares: From Structure to Function. Chem. Soc. Rev. 2004, 33, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Mounir, M.; Lorenzo, J.; Ferrer, M.; Prieto, M.J.; Rossell, O.; Avilès, F.X.; Moreno, V. DNA Interaction and Antiproliferative Behavior of the Water Soluble Platinum Supramolecular Squares [(En)Pt(N–N)]4(NO3)8 (En=ethylenediamine, N–N=4,4′-Bipyridine or 1,4-Bis(4-Pyridyl)Tetrafluorobenzene). J. Inorg. Biochem. 2007, 101, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Kieltyka, R.; Englebienne, P.; Fakhoury, J.; Autexier, C.; Moitessier, N.; Sleiman, H.F. A Platinum Supramolecular Square as an Effective G-Quadruplex Binder and Telomerase Inhibitor. J. Am. Chem. Soc. 2008, 130, 10040–10041. [Google Scholar] [CrossRef] [PubMed]
- Garci, A.; Castor, K.J.; Fakhoury, J.; Do, J.L.; Di Trani, J.; Chidchob, P.; Stein, R.S.; Mittermaier, A.K.; Friščić, T.; Sleiman, H. Efficient and Rapid Mechanochemical Assembly of Platinum(II) Squares for Guanine Quadruplex Targeting. J. Am. Chem. Soc. 2017, 139, 16913–16922. [Google Scholar] [CrossRef]
- Chand, D.K.; Biradha, K.; Kawano, M.; Sakamoto, S.; Yamaguchi, K.; Fujita, M. Dynamic Self-Assembly of an M 3 L 6 Molecular Triangle and an M 4 L 8 Tetrahedron from Naked Pd II Ions and Bis(3-pyridyl)-Substituted Arenes. Chem. An Asian J. 2006, 1, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Ning, G.-H.; Yao, L.-Y.; Liu, L.-X.; Xie, T.-Z.; Li, Y.-Z.; Qin, Y.; Pan, Y.-J.; Yu, S.-Y. Self-Assembly and Host−Guest Interaction of Metallomacrocycles Using Fluorescent Dipyrazole Linker with Dimetallic Clips. Inorg. Chem. 2010, 49, 7783–7792. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Hippius, C.; Grüne, M.; Würthner, F. Light-Harvesting Metallosupramolecular Squares Composed of Perylene Bisimide Walls and Fluorescent Antenna Dyes. Chem. A Eur. J. 2006, 12, 7510–7519. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Chen, C.; Sun, Y.; Lu, S.; Huo, H.; Tan, T.; Li, A.; Li, X.; Ungar, G.; Liu, F.; et al. Luminescent Metallacycle-Cored Liquid Crystals Induced by Metal Coordination. Angew. Chem. 2020, 132, 10229–10236. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Li, Y.; Chen, Q.; Ni, Z.; Zhou, H.; Yu, J.; Qiu, H.; Yin, S. Amphiphilic Rhomboidal Metallacycles with Aggregation-Induced Emission and Aggregation-Caused Quenching Luminogens for White-Light Emission and Bioimaging. Mater. Chem. Front. 2022, 6, 633–643. [Google Scholar] [CrossRef]
- Pollock, J.B.; Schneider, G.L.; Cook, T.R.; Davies, A.S.; Stang, P.J. Tunable Visible Light Emission of Self-Assembled Rhomboidal Metallacycles. J. Am. Chem. Soc. 2013, 135, 13676–13679. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.S.; Das, N.; Kryschenko, Y.K.; Arif, A.M.; Stang, P.J. Design, Synthesis, and Crystallographic Studies of Neutral Platinum-Based Macrocycles Formed via Self-Assembly. J. Am. Chem. Soc. 2004, 126, 2464–2473. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Yuan, L.; Pei, L.; Shen, Y.; Zhang, Y.; Zhou, L.; Bian, H. Phosphorescent Pt(<scp>ii</scp>) Acetylacetonate Complexes Bearing 9-(Pyrimidin-2-Yl)-9 H -Carbazole Ligand: Syntheses, Photophysical Properties and OLED Applications. J. Mater. Chem. C 2023, 11, 16679–16688. [Google Scholar] [CrossRef]
- Pollock, J.B.; Cook, T.R.; Schneider, G.L.; Lutterman, D.A.; Davies, A.S.; Stang, P.J. Photophysical Properties of Endohedral Amine-Functionalized Bis(Phosphine) Pt(II) Complexes as Models for Emissive Metallacycles. Inorg. Chem. 2013, 52, 9254–9265. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.L.; Yan, X.; Stang, P.J. Photophysical Properties of Organoplatinum(II) Compounds and Derived Self-Assembled Metallacycles and Metallacages: Fluorescence and Its Applications. Acc. Chem. Res. 2016, 49, 2527–2539. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Li, M.; Que, W.; Stang, P.J. Self-Assembly of Metal-Ion-Responsive Supramolecular Coordination Complexes and Their Photophysical Properties. Dalt. Trans. 2017, 46, 3120–3124. [Google Scholar] [CrossRef] [PubMed]
- Acharya, N.; Upadhyay, M.; Dey, S.; Ray, D. White Light Emission Achieved by Dual-TADF in a Single Emissive Layer of Multicomponent Emitters. J. Phys. Chem. C 2023, 127, 7536–7545. [Google Scholar] [CrossRef]
- Pollock, J.B.; Cook, T.R.; Stang, P.J. Photophysical and Computational Investigations of Bis(Phosphine) Organoplatinum(II) Metallacycles. J. Am. Chem. Soc. 2012, 134, 10607–10620. [Google Scholar] [CrossRef] [PubMed]
- Schlickum, U.; Decker, R.; Klappenberger, F.; Zoppellaro, G.; Klyatskaya, S.; Auwärter, W.; Neppl, S.; Kern, K.; Brune, H.; Ruben, M.; et al. Chiral Kagomé Lattice from Simple Ditopic Molecular Bricks. J. Am. Chem. Soc. 2008, 130, 11778–11782. [Google Scholar] [CrossRef] [PubMed]
- Reichardt, C. Empirical Parameters of Solvent Polarity as Linear Free-Energy Relationships. Angew. Chem. Int. Ed. 1979, 18, 98–110. [Google Scholar] [CrossRef]
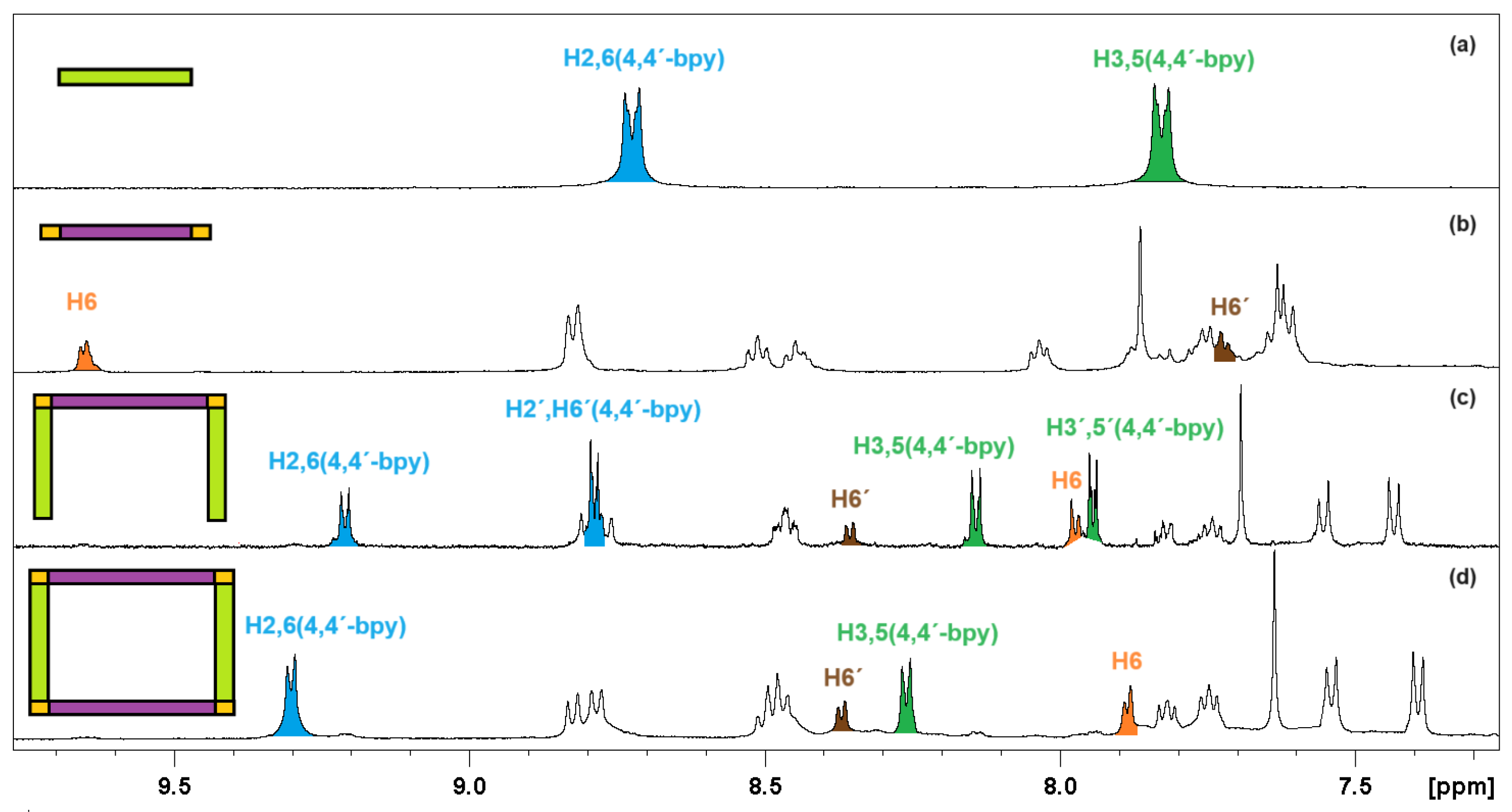



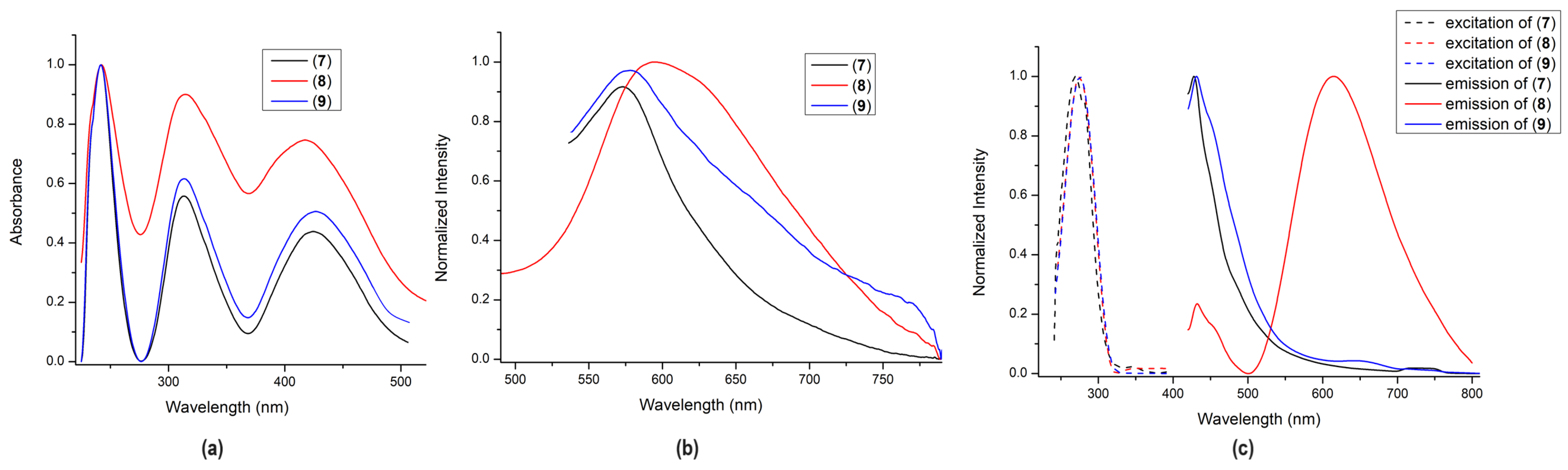
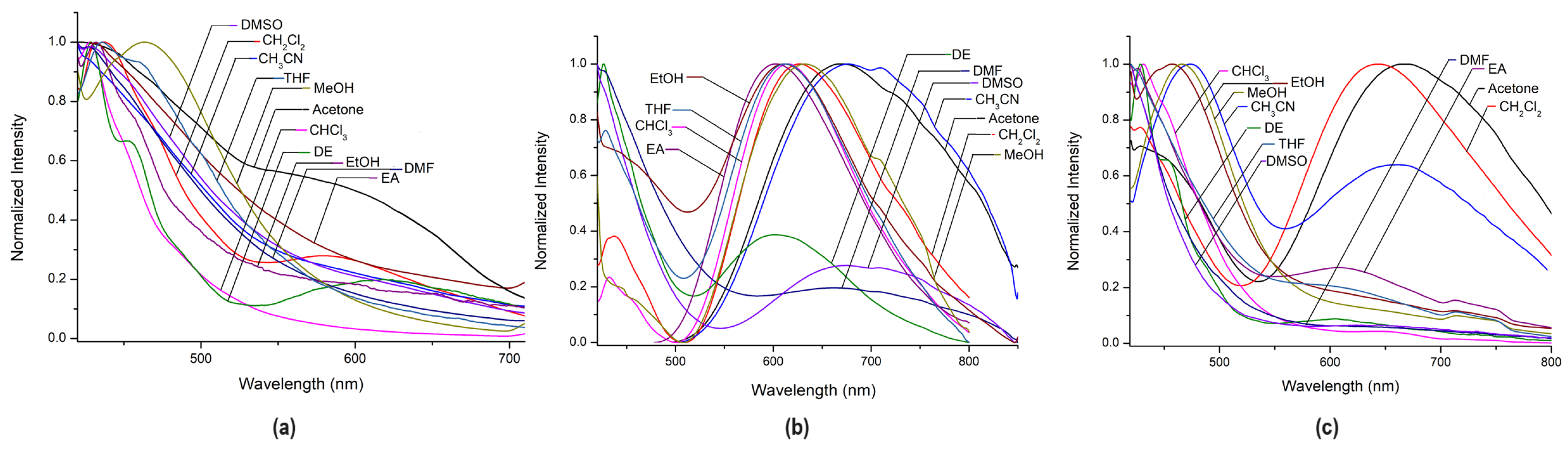
| Complex | UV/Vis Absorbance λmax [nm], (ε × 104 [M−1cm−1]) | Excitation | Emission | QY% | ||||
|---|---|---|---|---|---|---|---|---|
| Solution (CHCl3) | Solid | λexc | λem | |||||
| Solution (CHCl3) | Solid | Solution (CHCl3) | Solid | Solution (CHCl3) | Solid | |||
| (7) | 268 (15.30), 281 (12.2), 328 (5.2) | 242, 313, 420 | 365 | 400 | 427 | 574 | 1.7% | 4% |
| (8) | 276 (12.2), 345 (2.1) | 242, 315, 418 | 365 | 400 | 433, 615 | 597 | 5% | 2% |
| (9) | 278 (11.1) | 242, 313, 422 | 365 | 400 | 431 | 578 | 1% | 1% |
| Complex | Acetone | CH2Cl2 | CH3CN | CHCl3 | DE | DMF | DMSO | EA | EtOH | MeOH | THF |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (7) | 429 | 437 | 426 | 427 | 429 451 613 | 429 | 430 | 431 | 432 | 464 | 436 |
| 541 | 584 | 460 | |||||||||
| (8) | 671 | 436 | 677 | 433 | 427 | 427 | 421 | 606 | 429 | 432 | 429 |
| 626 | 616 | 605 | 680 | 690 | 610 | 632 | 614 | ||||
| (9) | 433 | 428 | 472 | 431 | 429 | 421 | 421 | 427 | 457 | 467 | 421 |
| 644 | 642 | 661 | 449 | 616 | 569 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garypidou, A.; Ypsilantis, K.; Garoufis, A. Fluorosolvatochromism of Platinum Supramolecular Coordination Complexes: Stepwise Synthesis and Photophysical Properties of Organometallic Tetranuclear Pt(II) Squares. Inorganics 2024, 12, 132. https://doi.org/10.3390/inorganics12050132
Garypidou A, Ypsilantis K, Garoufis A. Fluorosolvatochromism of Platinum Supramolecular Coordination Complexes: Stepwise Synthesis and Photophysical Properties of Organometallic Tetranuclear Pt(II) Squares. Inorganics. 2024; 12(5):132. https://doi.org/10.3390/inorganics12050132
Chicago/Turabian StyleGarypidou, Antonia, Konstantinos Ypsilantis, and Achilleas Garoufis. 2024. "Fluorosolvatochromism of Platinum Supramolecular Coordination Complexes: Stepwise Synthesis and Photophysical Properties of Organometallic Tetranuclear Pt(II) Squares" Inorganics 12, no. 5: 132. https://doi.org/10.3390/inorganics12050132
APA StyleGarypidou, A., Ypsilantis, K., & Garoufis, A. (2024). Fluorosolvatochromism of Platinum Supramolecular Coordination Complexes: Stepwise Synthesis and Photophysical Properties of Organometallic Tetranuclear Pt(II) Squares. Inorganics, 12(5), 132. https://doi.org/10.3390/inorganics12050132







