1. Introduction
The problem regarding the alimentary deficiency of trace elements, including germanium, is relevant for modern mankind. This element provides the most efficient oxygen uptake in the cells of the body, has an antioxidant and anti-stress effect, normalizes the conduction of nerve impulses, and eliminates or reduces the effects of impaired cell conduction within the inflammation focus. Previously, the oncotherapeutic effect of the organic derivative of germanium compound sesquioxide has also been described. In nature, the element occurs mainly in the form of complex derivatives of thiogermanic acid (these are the minerals rennyerite and argyrodite).
In medicine, germanium has found application in the form of organogermanium compounds. At the same time, in the literature [
1], a close analogy is noted in the biochemical behavior of germanium and silicon; silicon has long been recognized as a biomicroelement, and its organic derivatives are used to create medicines. At the same time, the metabolism of silicon and germanium compounds in living organisms has a subtle mutual influence.
Since the positive medicinal properties of germanium organometallic compounds have been confirmed by many studies, further study of the methods of synthesis, as well as the physicochemical and pharmacological properties of these compounds, is a promising task [
2]. This paper presents theoretical and experimental material on the methods of synthesis, and the determination of the composition, structure, and biological activity of the complex compound of germanium (IV) with 2-amino-3-hydroxybutanoic acid, which is an important part of modern coordination and supramolecular chemistry.
The authors of the patent [
3] obtained complex compounds of germanium with amino acids and carboxylic acids of the general formula, Ge[OH]
a[AA]
b[CA]
c, where AA is an amino acid, CA is a carboxylic acid, a = 0 ÷ 3, b = 1 ÷ 3, s = 0 ÷ 3 and 1 ≤ b + c ≤ 4. In this case, all AA and CA in a complex compound can be either the same or different. The authors obtained stable complex compounds with a controlled composition and ratio of germanium to amino acid or carboxylic acid. The resulting complex compounds are chemically stable in solid form and can be used in medicine.
Later, the authors of [
4] proposed germanium complex compounds having the general structural formula: Ge
x[AD][CA]
y[AA]
z, where AD is a derivative of a nitrogenous base of the purine series with antiviral activity; CA—hydroxycarboxylic acid; AA is an amino acid selected from α-amino acids, where x = 1 ÷ 2, y = 2 ÷ 4, z = 0 ÷ 2, while all CA and AA in the complex compound may be the same or different.
The technique makes it possible to obtain germanium complex compounds with high antiviral and immunostimulatory activity, and also relates to the development of drugs intended for the prevention and/or treatment of viral diseases caused, in particular, by herpes viruses.
The main direction of the search for new effective and safe drugs is the targeted synthesis of biologically active substances with predetermined pharmacological properties [
5].
When creating new compounds, structures containing coordination bonds are often used [
6]. Often, biometals act as complexing agents, and natural metabolites of the body—vitamins, proteins, etc.—are bioligands. At the Department of General Chemistry and Polymers of the Odessa National University, I.I. Mechnikov under the guidance of Ph.D.Sc. Professor Seifullina I.I. synthesized biologically active additives-coordination compounds of germanium with bioligands (germanium with nicotinic acid and germanium hydroxyethylidene diphosphonate with nicotinic acid). Along with low toxicity (LD
50 = 1475 mg/kg and LD
50 = 339.0 mg/kg), germanium coordination compounds exhibited pronounced pharmacological activity. Both biologically active substances are characterized by antihypoxic, membrane- and cardioprotective effects [
7]. These promising compounds have been studied for their pharmacokinetic properties and compared for pharmacokinetic constants.
Previously, carboethylgermsesquioxane was synthesized in Japan, which had a wide range of biological and pharmacological effects, including antitumor activity.
In Russia, the development of the synthesis of water-soluble complexes of germanium with carboxylic acids was carried out by a team led by Bashkirova S.A. The synthesized germanium complexes are carboxylates of 1-hydroxy germatran based on carboxylic acids of the Kreps cycle (citric, malic, fumaric, succinic, malic). The greatest therapeutic effect of the complex of germanium with citric acid (“Eniogerm”) [
8] was a pronounced autoprotective effect with the stimulation of tissue respiration.
Previously, the radioprotective properties and antihypoxant activity of germanium biologically active complexes in aqueous and aqueous-organic solutions were studied.
This work provides the results of studies of 1-hydroxylates-german carboxylates and German complexes with lemon (citrate 1-hydroxyermatran C
6H
13GeNO
4·C
6H
8O
7—“Eniogerm”) and pyrovinaric acids (Pyruvat 1-Hydroxyermatrane C
6H
13GeNO
4·C
3H
4O
3): SPU German: 1). Germranol (Germatranol)-2-[bis (2-hydroxyethyl) amino] ethanol; Germane Hydrate, C
6H
1 3GeNO
4 [
9].
To confirm the protective properties and antihypoxic activity of germanium complexes with carboxylic acids, [Ge...ROOH], which manifest themselves under stressful conditions, physicochemical methods of studying reactions were used. To elucidate the mechanism of the reduction reactions of molecular oxygen in the presence of Ge-carbon complexes, the inversion polarography method was used, occurring in model systems under the influence of ionizing radiation. The radiation–chemical method makes it possible to generate active intermediate particles, including active forms.
Pharmaceutical aspects of the study of biological objects include substances containing active oxygen and reactive oxygen species, which are responsible for redox processes in living organisms under normal conditions and pathology. The samples were irradiated with 60Co γ-rays using the RKhM-γ-20 installation at a dose rate of 0.11 Gy/sec, determined by ferrosulfate dosimetry. Absorbed doses were selected in a range from 0.2 kGy to 25 kGy. The optical absorption spectra of aqueous solutions of [Ge...ROOH] complexes, initial and irradiated, were measured spectrophotometrically in a wavelength range from 190 nm to 900 nm (Spectrophotometer Hitachi U-3310) in the presence of atmospheric oxygen. The optical path length of the quartz cell is 10 mm.
The work [
10] presents methods for the synthesis and reaction of organic silicon and germanium peroxides. The use of these compounds in the development of antiparasitic drugs is considered.
The article [
11] provides a comparative description of the pharmacokinetics of new biologically active substances—coordination compounds of germanium with nicotinic acid and germanium oxyethylidene diphosphonate with nicotinic acid in the heart muscles. It was revealed that the coordination compound of germanium oxyethylidene diphosphonate with nicotinic acid penetrates the heart muscles faster and better, reaches a maximum concentration, but is eliminated faster compared to the germanium compound with nicotinic acid. The data obtained will help develop rational pharmacotherapy for the consequences of a heart attack.
The work [
12] compared the effects of various concentrations of 1-(germatran-1-yl)-1-hydroxyethylamine (germatran) on the bioenergetic characteristics of mitochondria of animal and plant origin. It was shown that the introduction of 10
−5–10
−11 M of the drug into the medium incubation of mitochondria led to an increase in the efficiency of oxidative phosphorylation, as well as an increase in the maximum rates of oxidation of NAD-dependent substrates. At the same concentrations, germatrane reduced the intensity of lipid peroxidation compared to control values in the membranes of rat liver mitochondria.
Previously, organic germanium compounds were synthesized, in particular Ge-132 (antiviral immunomodulatory agent), spirogermanium (antitumor agent), sanumgermanium, etc. [
13].
Bis(2-carboxyethylgermanium)sesquioxide-(O
1,5GeCH
2CH
2COOH)
n, known as Ge-132 and widely used in cancer therapy, has become widely known. It should be noted that the conversion of biologically active organic acids into their triethanol ammonium salts—protatranes—significantly expands the spectrum of their physiological action. At the same time, employees of M.G. Voronkov researched tricyclic silicon and germanium ethers of triethanolamine with the general formula XM(OCH
2CH
2)
3N, where Si and Ge were synthesized, and their biological activity was studied [
14,
15]. Intracomplex tricyclic compounds of silicon and germanium-silatranes and germatranes, respectively, as a rule, have almost the same biological activity, which is probably due to the similarity of the elements Si and Ge in atomic radius of electronegativity. However, germatranes, having the same or higher biological activity, are less toxic than their silicon analogues. Unlike silatranes, which are widely used in medicine and agriculture, germatranes have received much less attention. However, it has been shown in animals that germatranes are broad-spectrum drugs with immunocorrective and biostimulating properties. They activate the macrophage and cellular immune systems, and also increase the body’s natural resistance.
Thus, it is of interest to synthesize, as well as study the composition, structure, and physicochemical properties of new complex germanium-containing compounds with biologically active ligands [
16,
17], as well as modify them in order to enhance and/or expand the spectrum of pharmacological activity for possible use in practical healthcare.
2. Experimental Part
At the first stage, a precursor was synthesized for the further preparation of germanium coordination compounds. We used hydrated germanium dioxide as a precursor. It should be noted that germanium dioxide itself is used in some dietary supplements [
18,
19,
20,
21].
The synthesis was carried out using the hydrolysis of germanium tetrachloride. As a result, the following chemical reaction occurred:
Hydration was carried out at a molar ratio between germanium tetrachloride and water of 1:7. The purity of the water was previously monitored using a conductometer. It was found that the distilled water used had a resistivity of at least 15 mOhm cm, which corresponds to a high degree of deionization. Since germanium tetrachloride is insoluble in water, the hydrolysis reaction was carried out with continuous stirring to ensure contact of the reagents. The reaction is exothermic, so germanium tetrachloride was added in small portions, preventing the water from boiling.
Next, the mixture was filtered, and the germanium (IV) oxide on the filter was washed with cold distilled water. After washing, germanium dioxide was freed from excess moisture by moderate heating (not higher than 70 °C) in a drying cabinet.
The ligand used was 2-amino-3-hydroxybutane (α-amino-β-hydroxybutyric or α-amino-β-hydroxybutyric) amino acid (threonine, Thr, Thr, T) with the chemical formula HO2CCH(NH2)CH(OH)CH3. Threonine is an essential α-amino acid that is part of many proteins (pepsin, gliadin, fibrin, etc.), and has polar and neutral side chains.
The synthesis of the complex compound was carried out in a three-neck flask equipped with a stirrer, a dropping funnel, and a reflux condenser. First, 2 g of hydrated germanium dioxide GeO2 was dissolved in 250 mL of distilled water. The specific electrical conductivity of distilled water was at least 5 μS/cm, which corresponds to a resistivity of more than 0.2 MΩ cm, which was determined using a MARK-601 conductometer. The suspension was heated (95 °C) for 1 h and stirred. Next, 5 g of 2-amino-3-hydroxybutanoic acid dissolved in 50 g of distilled water was added dropwise to the resulting clear solution, and the resulting solution was stirred with heating for four hours. Then, the solution was cooled and the resulting suspension was separated from the solution by centrifugation on a laboratory OPN-302 Dastan centrifuge.
The resulting precipitate was identified using IR spectroscopy.
The study using infrared spectroscopy included three stages: sample preparation, recording of its spectrum, and its interpretation. The most acceptable methods of preparing samples for obtaining IR absorption spectra of solid substances (and in particular, of our compound) is the method of pressing the test substance with alkali metal halides. This method involves strong grinding of the sample. A small amount of the test solid is thoroughly ground into powder and thoroughly mixed with an alkali metal halide (with KBr, which serves as a “filler”) in an agate mortar, and the resulting mixture is pressed by a special holder (mold) into tablet disks for 1–3 min under high pressure of the order of 10−12 t/cm2. Then, after pressing, the resulting transparent KBr tablet with the mineral under study is mounted in a special holder and placed in the main radiation beam of the spectrophotometer, where the IR absorption spectrum is recorded in the usual way.
The clear solution obtained after separating the precipitate was dried in a ShS-80–01-SPU drying cabinet at a temperature of 80–90 °C.
3. Results and Its Discussion
The choice of ligand is due to the fact that—being a proteinogenic amino acid—threonine is a part of almost all proteins in the human body. Threonine is not synthesized in the human body; for normal life, this amino acid must be supplied in sufficient quantities from food.
Threonine performs a number of functions: structural, digestive, antitoxic, immunostimulating, neurotransmitting, and takes part in amino acid and fat metabolism. The alcohol OH group in threonine is attached to a carbon atom in the β position, which gives the amino acid both hydrophilic and lipophilic properties, and this allows the molecule to dissolve in both water and fats. It should be noted that such neutral amino acids slightly change the pH of the aqueous solution and do not impart electrical conductivity to it. Being bipolar in an acidic environment adds a proton to the carboxyl group and acquires a positive charge, while in an alkaline environment, the NH
3+ group loses a proton, which binds to water, and the bipolar ion acquires a negative charge. In this case, the isoelectric point of trianine lies in the weakly acidic region and is:
X-ray phase analysis of hydrated germanium dioxide was carried out. It was possible to recognize unknown phases from the diffraction pattern through comparison with the diffraction patterns of known phases (
Figure 1).
It should be noted that we are talking about the tetragonal modification of GeO2 (PDF card No. 00-056-1273 explains the peak at 2θ ≈ 23°).
In the tetragonal state of α-GeO
2, germanium has a coordination number of 6, unit cell parameters: a = 0.4395 nm, c = 0.2860 nm, d
20 = 6.24 g/cm³ [
22].
According to the patent [
3], germanium dioxide of both the α-modification, which is insoluble in water, and the β-modification, which is soluble in water, can be used as a precursor for the synthesis of coordination compounds. However, it is indicated that it is preferable to use α-modified germanium dioxide, which forms a suspension when mixed with water.
The resulting hydrated germanium dioxide GeO
2·nH
2O was also identified by IR spectroscopy on a Shimadzu IR Prestige-21 device (
Figure 2).
In the IR spectrum of this complex, in comparison to the standard IR spectrum of germanium dioxide (519, 553, 586, 2924, 2953, 3018, 3069, 3071, 3097 and 3116 cm
−1), a band at 877 cm
−1 was detected, associated with the appearance of a band of deformation vibrations δ(Ge-OH) in its molecule [
23].
The presence of absorption bands for the Ge-O and Ge-O-H bonds in the resulting IR spectrum proves the receipt of a precursor suitable for the next stage of the synthesis associated with the preparation of coordination organogermanium compounds.
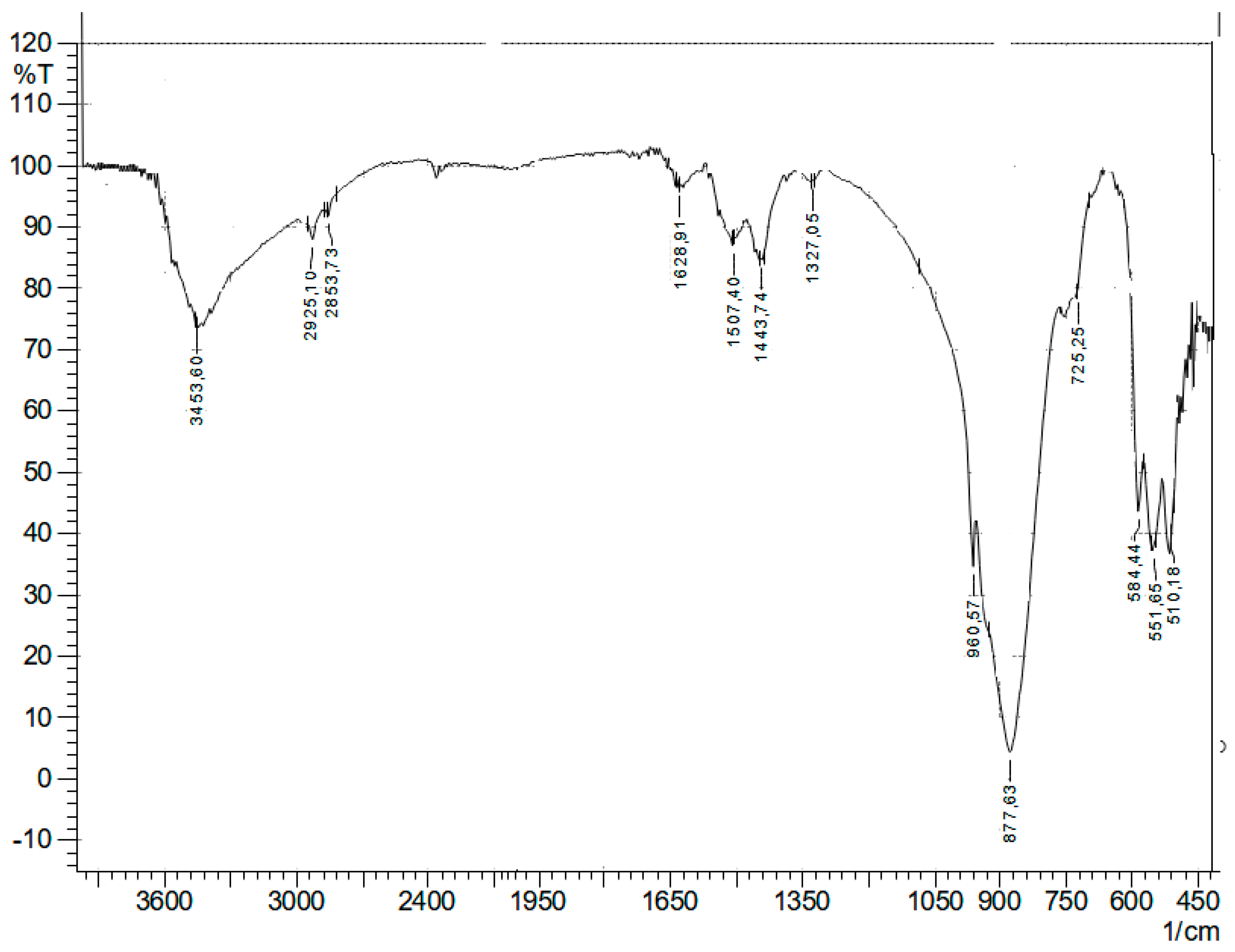
Figure 3 shows the IR spectrum of the product of the synthesis of hydrated germanium dioxide and 2-amino-3-hydroxybutanoic acid.
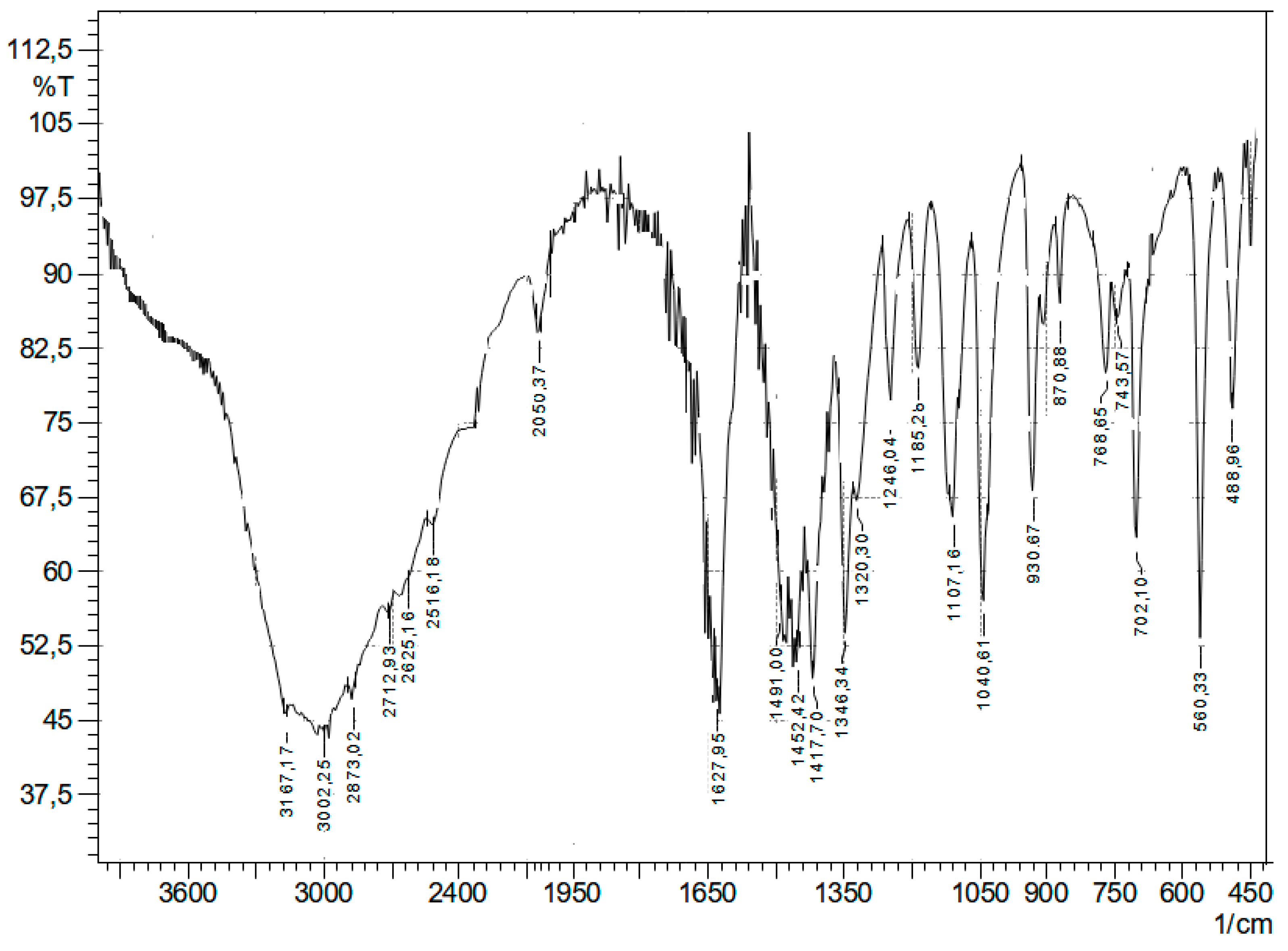
In the IR spectrum of the reaction product (
Figure 3), compared to 2-amino-3-hydroxybutanoic acid (
Figure 4), there are no absorption bands of bending vibrations of -OH and COOH groups at 1491, 1452, 1417, 1346, 1320, and 1246 cm
−1. In addition, a shift of the υ(C=O) vibration band is observed in the region of 1610–1611 cm
−1, and an increase in its intensity is noted. It is assumed that these changes may indicate equivalent binding of the amino group to GeO
2·nH
2O. This is also indicated by the appearance of new absorption bands, asymmetric and symmetric stretching vibrations of carboxylate ions and υ(Ge-O).
The absence of the band at 878 cm
−1 (υ Ge-O-Ge) indicates the rupture of the bridging group [
24].
In the IR spectrum of the resulting compound, in comparison with the original IR spectrum, a new band (1610 cm
−1) was discovered, associated with the appearance of an NH
+ group in its molecule, due to which the charge of the complex anion is compensated. There is also a band of bending vibrations at δ(NH
2+) ~ 1576 cm
−1, indicating protonation of one of the NH-exo-ligand groups [
25,
26,
27].
The deprotonation of the OH group of hydrated germanium dioxide, and therefore its connection with the amino acid, is indicated by the disappearance of characteristic vibrations in the IR spectrum in the region of ~3453 cm−1. This is confirmed by the presence of bands υ(Ge-O) = 668.643 and 555 cm−1, which indicates the inequality of Ge-O bonds.
The formation of a bond between hydrated germanium dioxide and the ligand can be judged by the appearance of a Ge-O stretching vibration band (668 cm−1) in the IR spectra; along with this, an absorption band responsible for vibrations of the Ge-OH bond ~838 cm−1 was detected.
Thus, we can assume the formation of a coordination bond between the nitrogen and germanium atoms (N→Ge).
The compound contains a hydrolyzed form of hydrated germanium dioxide and coordinated water molecules, as indicated by the bands of Ge-O-H, as well as pendulum and fan H2O vibrations.
The hydroxy groups of 2-amino-3-hydroxybutanoic acid are deprotonated and bonded to germanium Ge (IV), which is confirmed by the appearance of stretching vibration bands at 666 and 643 cm−1.
Thus, the values of the main vibration frequencies of atomic groups in the range of 400–4000 cm−1 made it possible to conduct a qualitative analysis of the IR spectra of the product of the synthesis of hydrated germanium dioxide and 2-amino-3-hydroxybutanoic acid.
For a quantum-chemical study of the structure of a complex compound, a modern method of the theory of density functionality was used (DFT, B3lyp/6–311 ++ G (2D, 2P)). For all obtained molecular structures, a complete optimization of geometry was carried out with the subsequent calculation of oscillatory frequencies, as well as thermodynamic functions in the framework of harmonic approximation. Calculations are made using the Gaussian03 program. To analyze the results and thermodynamic calculations, the Moltran program was used.
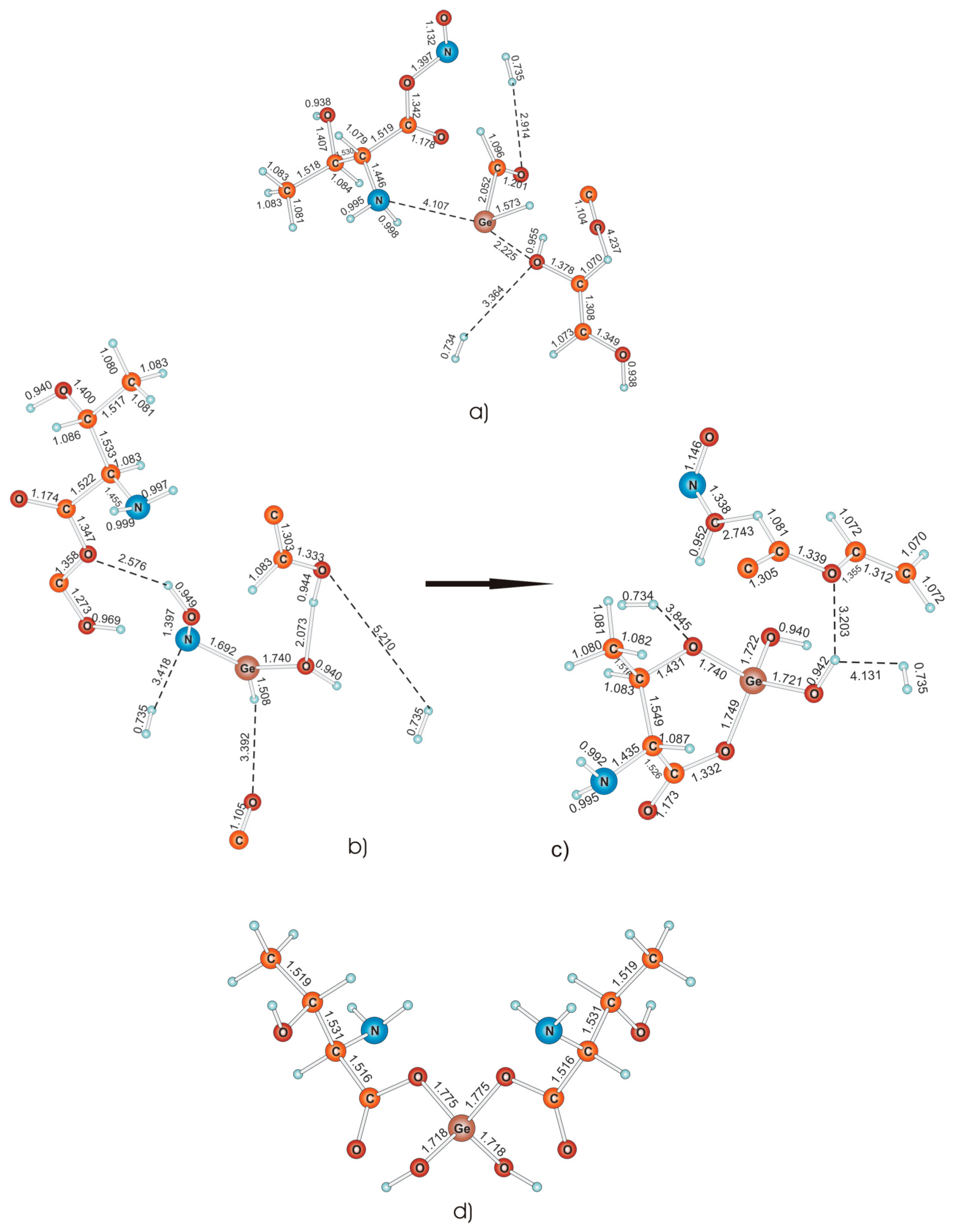
A comparison of the structure obtained using the quantum chemical method was carried out using IR spectra, experimental and theoretical. During the study, four different structures of the complex compound were obtained, the spectrum of one of which is almost identical to the experimental one (
Figure 5).
The first DFT-modeled reaction complex of germanium dioxide with 2-amine-3-hydroxybutane acid is shown in
Figure 5a. Here we see a Ge–C bond, which was not detected in the spectrum obtained experimentally.
The second and third intermediates are shown as (b) and (c). When comparing the IR spectra of the obtained complexes with the experimental one, a large number of coinciding peaks were found, but none of the IR spectra obtained by quantum chemical modeling could be identified as an experimentally obtained compound. However, these compounds may be present in the mixture as impurities, as evidenced by the presence of common peaks in the spectrum. It is assumed that the second reaction complex passes into the third reaction complex and represents one of the final stages of the reaction forming a complex of germanium dioxide with 2-amine-3-hydroxybutanoic acid, as shown in the figure.
Letter (d) represents a compound whose spectrum turned out to be almost identical to the experimentally obtained complex. The absence of bonds indicated by a dotted line indicates that the final compound, and not the intermediate, is optimized. Therefore, with a fairly high degree of probability, we can say that the structure of the complex of germanium dioxide with 2-amine-3-hydroxybutanoic acid has been established.
A thermodynamic study of the reaction—provided that Ge(OH)4 was one of the reagents, according to the standard reaction scheme—showed that all stages are characterized by a positive and very high Gibbs energy, about seven thousand, which indicates the impossibility of a spontaneous reaction even at high temperatures, which does not correspond to the experiment. This can be explained by the fact that the experiment describes the production of hydrated germanium dioxide GeO2·nH2O, which was used in the synthesis of the complex of germanium dioxide with 2-amine-3-hydroxybutanoic acid. It was assumed that n = 2, but this calculation also gave high positive values of the Gibbs energy. This may indicate that the number of water molecules in the hydrated dioxide is more than two.
Table 1 shows the thermodynamic parameters of the proposed elementary stages of the formation of a complex of germanium dioxide with 2-amine-3-hydroxybutanoic acid at the level B3LYP/6–311++G(2d,2p), where the complex is designated as complex, intermediates 2 and 3—Int2 and Int3, respectively.
Both stages described above and shown in
Figure 5 are characterized by high negative Gibbs energies under normal conditions, which suggests that the formation of a complex from intermediates can occur spontaneously, which in principle is in good agreement with experiment.
The data obtained, simulated by the DFT method, indicate that germanium complex compounds have a general structural formula C8H18GeN2O8, where Ge is germanium, C is carbon, H is hydrogen, O is oxygen, and N is nitrogen.
Elemental analysis data for the germanium complex compound are presented in
Table 2 and confirm the composition of this compound.
To confirm the antimicrobial effect of the germanium complex compound, microbiological studies were carried out. The study was carried out on pure cultures of Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, and Candida albicans (
Table 3). To prepare a bacterial suspension, pure cultures of bacteria were used in a meat-peptone broth (MPB) after 24-h cultivation (37 °C) (“daily” culture). Sabouraud dextrose broth was used to cultivate candida. The studied microorganisms were inoculated at a concentration of 0.5 according to McFarland in 100 μL of broth in 96-well plates. Then, 100 μL of a suspension of germanium compound was added to the wells; 100 μL of a nutrient medium was added to the wells with a suspension of microorganisms as a control. The plates were thermostated for 24 h at 37 °C. At the end of incubation, the minimum inhibitory concentration of the germanium compound was determined. Sowing (50 μL) was carried out from each well of the plate without visible growth of microorganisms onto Petri dishes with meat-peptone agar, and the inoculations were incubated for 24 h at 37 °C. At the end of incubation, the minimum bactericidal/fungicidal concentration of the germanium compound was determined.
This variant of the German coordination compound shows antimicrobial action against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii, and against the micromycetes Candida albicans.
The decomposition temperature of the resulting compound was also determined, which was 150 °C.
5. Conclusions
Currently, in the development of antimicrobial complexes, the use of biometal compounds is relevant. Their interaction leads to a change in the biochemical properties of the compound and ensures antimicrobial activity in the resulting complexes. Metal ions that make up natural enzymes act as polarizing factors. In this way, compounds are synthesized that model and mimic the active sites of enzymes, and behave like activated and modified amino acids.
A coordinated biometal is less toxic than its inorganic salt. The combination of microelements and bioactive organic matter leads to synergistic action.
The presented substance has a bacteriostatic and bactericidal effect against the bacterial cultures of Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae, Staphylococcus aureus, Enterococcus faecalis, and Acinetobacter baumannii, which have antibiotic resistance.
The structure and composition of the coordination compound was established through calculation and experimental methods.
Using a modern quantum chemical method, density functional theory (DFT, B3LYP/6–311++G(2d,2p)), both stages of the process, characterized by high negative Gibbs energies under normal conditions, were studied, which suggests that the formation of the complex from intermediates can occur spontaneously. This, in principle, is in good agreement with experiment.
The comparison showed good agreement between the calculated and experimental data. The structure of the coordination compound and the structure of intermediates at all stages of the synthesis process were established by calculation.