Graphene-Infused Hybrid Biobattery–Supercapacitor Powered by Wastewater for Sustainable Energy Innovation
Abstract
1. Introduction
2. Results and Discussion
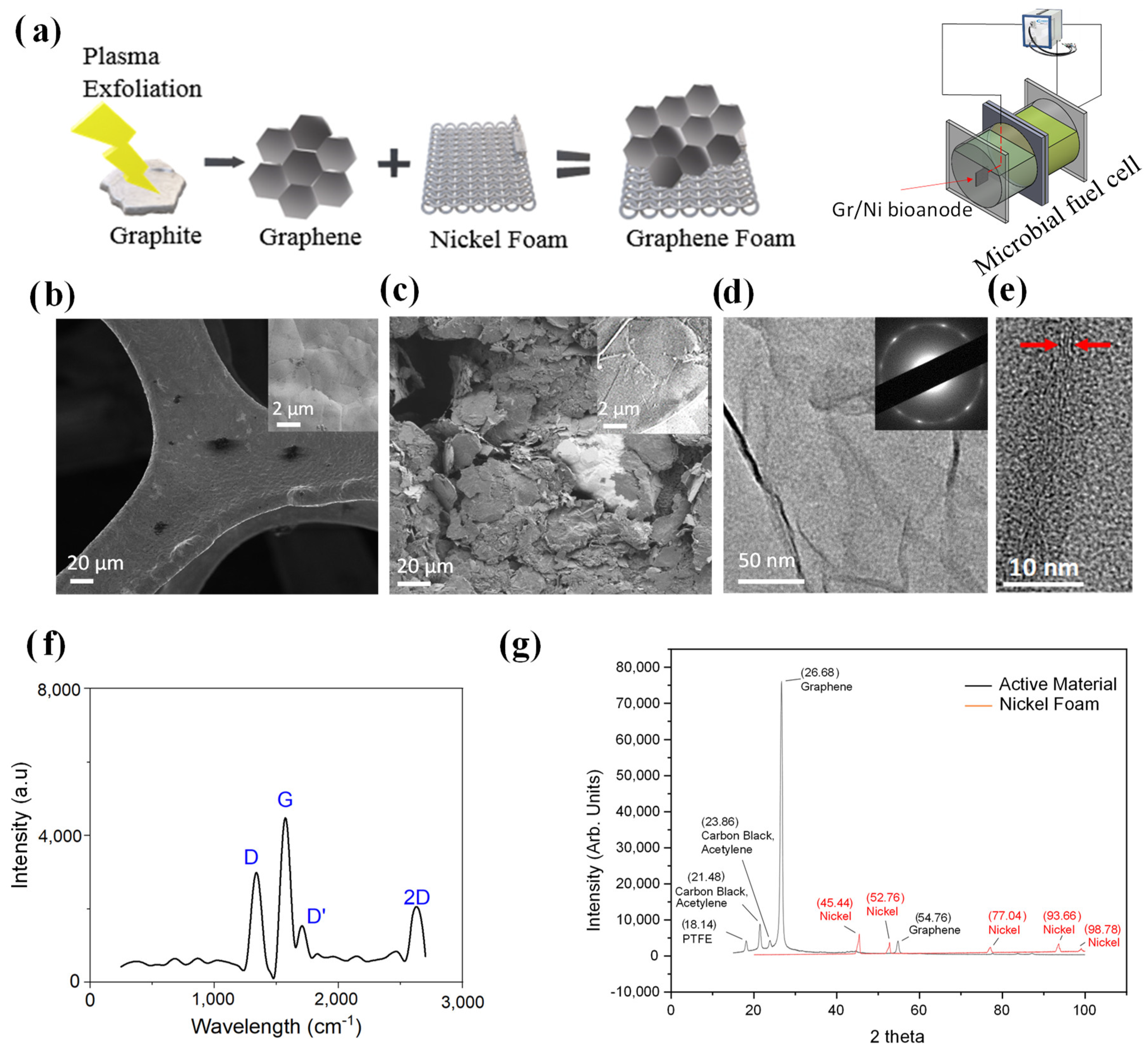
2.1. Facile Strategy for the Synthesis of a 3D Bioanode
2.2. Physical Characteristics of Bioanode
2.3. TEM, Raman and XRD Characterization
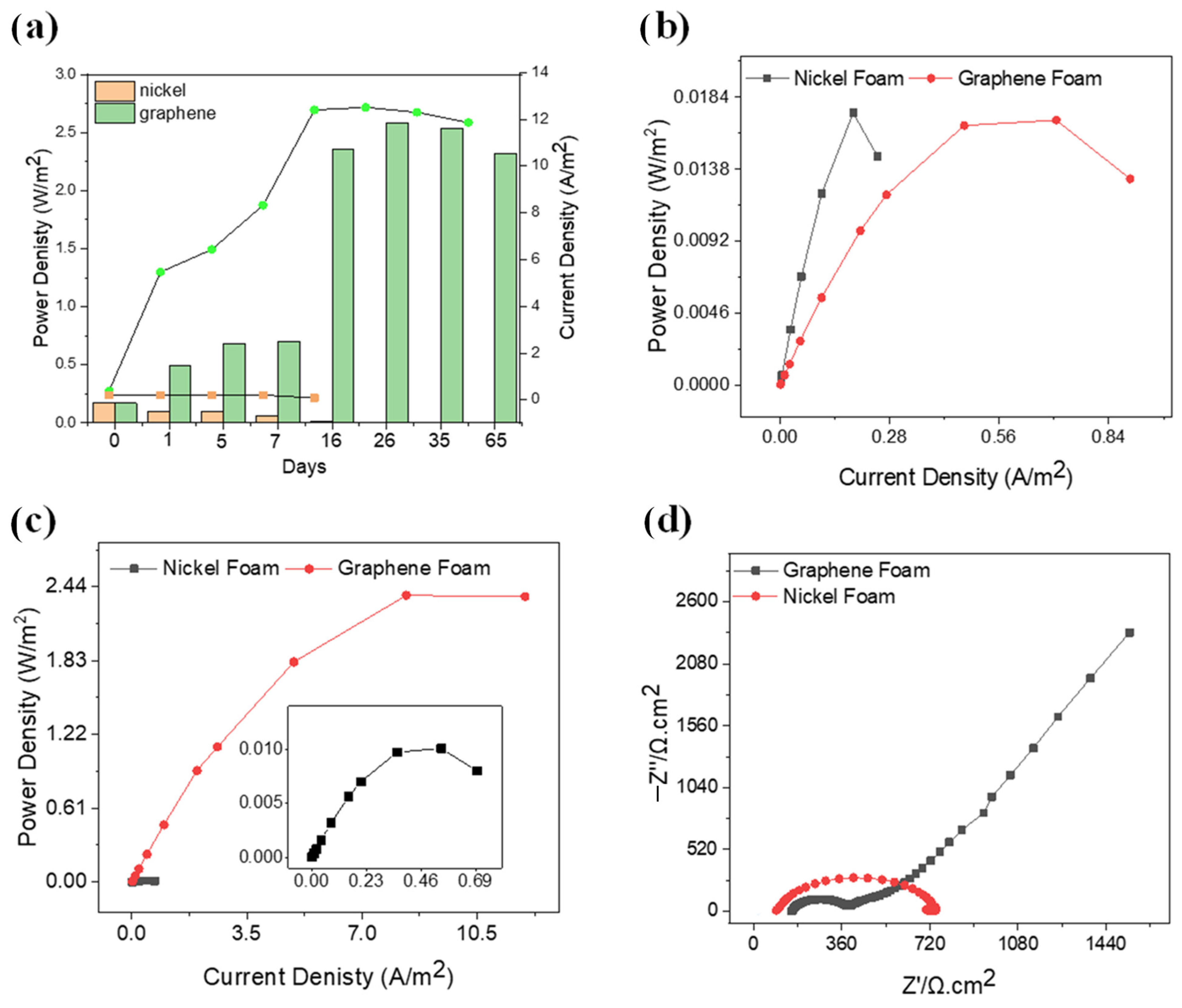
2.4. Exceptional Electrochemical Power Output
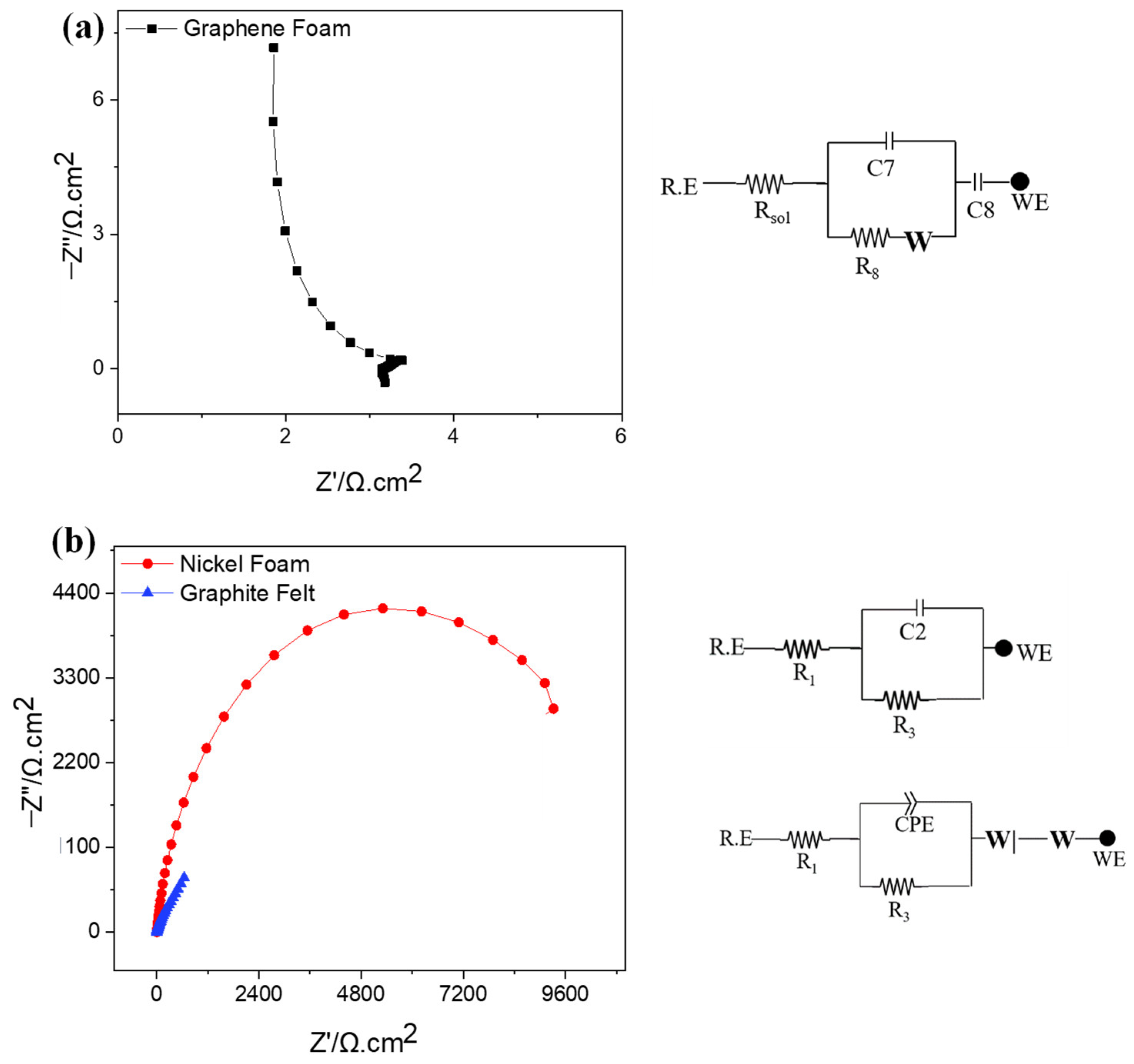
2.5. A Unique Impedance Signature of Capacitive Behavior
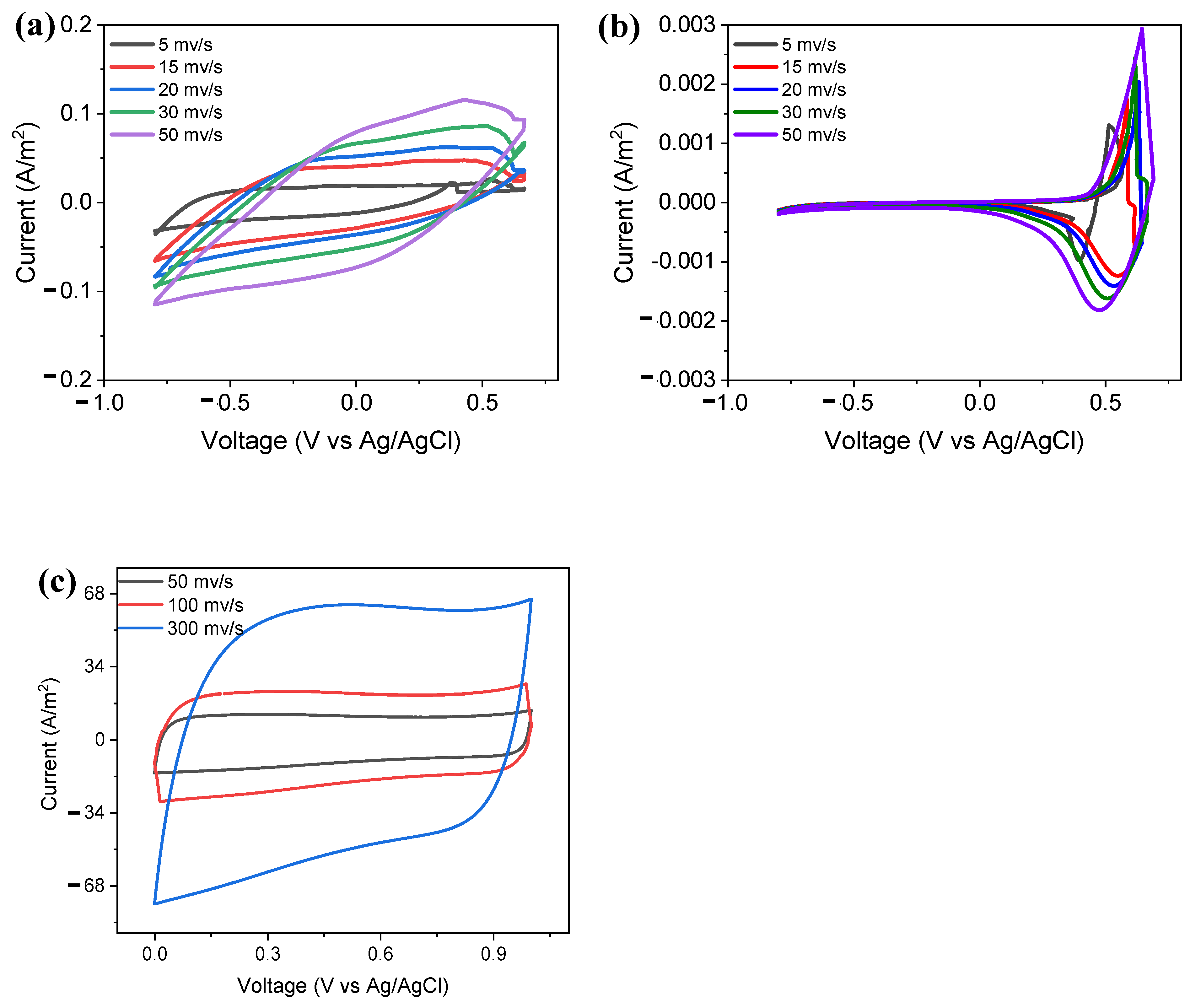
2.6. Cyclic Voltammetry Confirms Capacitive Properties of Gr/Ni Bioanode
2.7. Charge/Discharge Properties of Gr/Ni Bioanode
3. Experimental Section
3.1. Source of Wastewater
3.2. Graphene Exfoliation with Low-Temperature Discharge Plasma
3.3. Fabrication of Graphene Electrodes
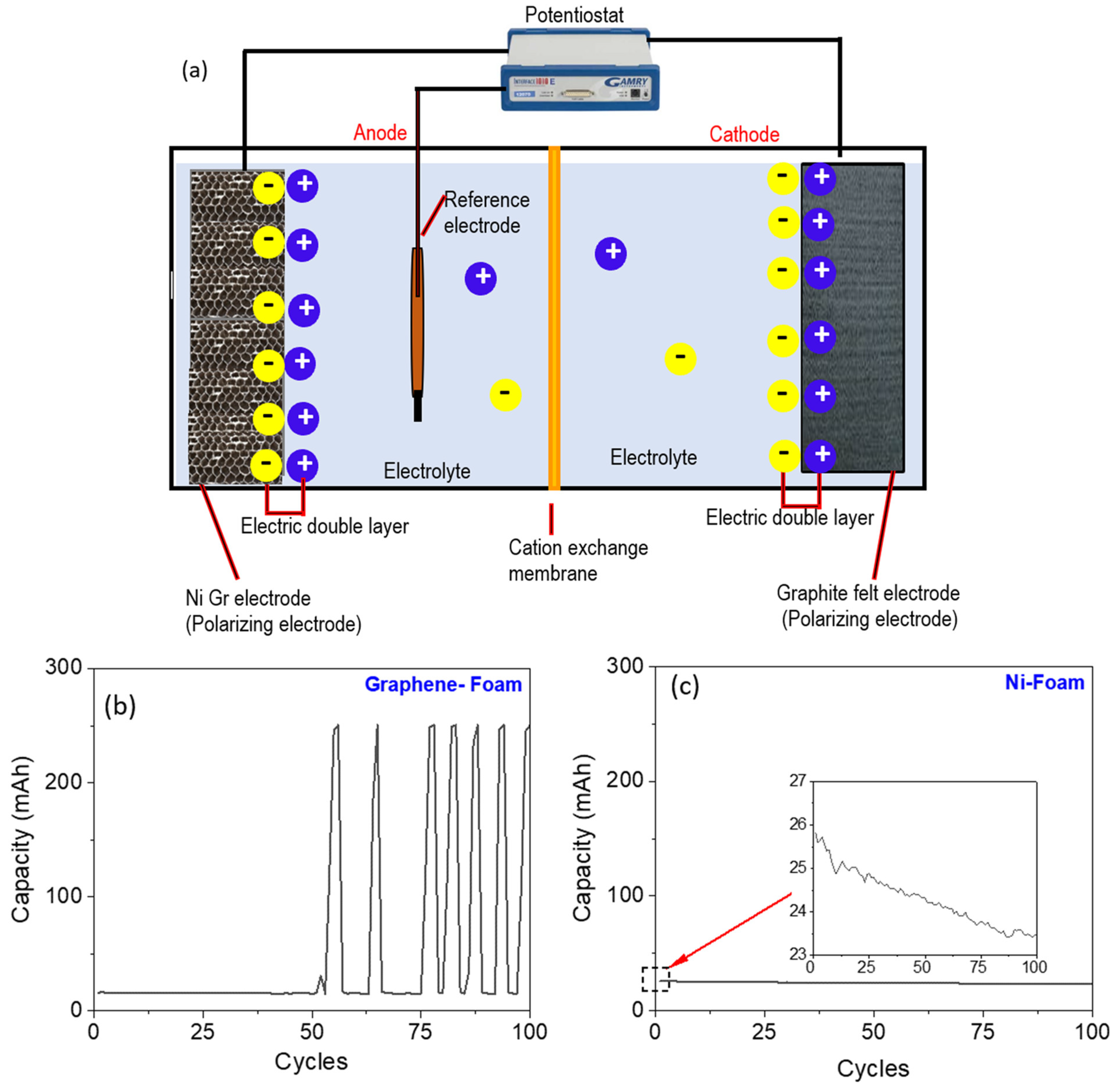
3.4. A Hybrid Battery–Supercapacitor Device
3.5. Charging/Discharging Cycles and Capacitive Behavior of the Device
3.6. Surface Analysis and Chemical Composition
3.7. Analytical Methods
3.8. Electrochemical Methods
4. Outlook
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bhatt, R.P. Achievement of SDGS globally in biodiversity conservation and reduction of greenhouse gas emissions by using green energy and maintaining forest cover. GSC Adv. Res. Rev. 2023, 17, 001–021. [Google Scholar] [CrossRef]
- Environmental Protection Agency. Energy Efficiency for Water Utilities. Available online: https://www.epa.gov/sustainable-water-infrastructure/energy-efficiency-water-utilities (accessed on 25 October 2023).
- Environmental Protection Agency. Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ghgemissions/global-greenhouse-gas-emissions-data (accessed on 15 January 2024).
- Malcolm Pirnie, Inc.; New York State Energy Research and Development Authority. Statewide Assessment of Energy Use by the Municipal Water and Wastewater Sector: Final Report; New York State Energy Research and Development Authority: Albany, NY, USA, 2008. [Google Scholar]
- Sayed, D.M.; El-Deab, M.S.; Elshakre, M.E.; Allam, N.K. Nanocrystalline cellulose confined in amorphous carbon fibers as capacitor material for efficient energy storage. J. Phys. Chem. C 2020, 124, 7007–7015. [Google Scholar] [CrossRef]
- Wang, K.; Xu, M.; Gu, Y.; Gu, Z.; Liu, J.; Fan, Q.H. Low-temperature plasma exfoliated n-doped graphene for symmetrical electrode supercapacitors. Nano Energy 2017, 31, 486–494. [Google Scholar] [CrossRef]
- Pumera, M. Electrochemistry of graphene: New horizons for sensing and energy storage. Chem. Rec. 2009, 9, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.I.; Sunarso, J.; Wong, B.T.; Lin, H.; Yu, A.; Jia, B. Towards enhanced energy density of graphene-based supercapacitors: Current status, approaches, and future directions. J. Power Sources 2018, 396, 182–206. [Google Scholar] [CrossRef]
- Yu, M.; Feng, X. Thin-film electrode-based supercapacitors. Joule 2019, 3, 338–360. [Google Scholar] [CrossRef]
- Huang, Y.; Sutter, E.; Shi, N.N.; Zheng, J.; Yang, T.; Englund, D.; Gao, H.-J.; Sutter, P. Reliable exfoliation of large-area high-quality flakes of graphene and other two-dimensional materials. ACS Nano 2015, 9, 10612–10620. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Peng, Y.; Wang, X.; Ran, F. Emerging Design Strategies Toward Developing Next-Generation Implantable Batteries and Supercapacitors. Adv. Funct. Mater. 2023, 33, 2301877. [Google Scholar] [CrossRef]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical property and structure of covalent functionalised graphene/epoxy nanocomposites. Sci. Rep. 2014, 4, 4375. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman spectroscopy as a versatile tool for studying the properties of graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Chilkoor, G.; Sarder, R.; Islam, J.; ArunKumar, K.; Ratnayake, I.; Star, S.; Jasthi, B.K.; Sereda, G.; Koratkar, N.; Meyyappan, M. Maleic anhydride-functionalized graphene nanofillers render epoxy coatings highly resistant to corrosion and microbial attack. Carbon 2019, 159, 586–597. [Google Scholar] [CrossRef]
- Chilkoor, G.; Shrestha, N.; Kutana, A.; Tripathi, M.; Herna, F.C.R.; Yakobson, B.I.; Meyyappan, M.; Dalton, A.B.; Ajayan, P.M.; Rahman, M.M. Atomic layers of graphene for microbial corrosion prevention. ACS Nano 2020, 15, 447–454. [Google Scholar] [CrossRef]
- Krishnamurthy, A.; Gadhamshetty, V.; Mukherjee, R.; Chen, Z.; Ren, W.; Cheng, H.-M.; Koratkar, N. Passivation of microbial corrosion using a graphene coating. Carbon 2013, 56, 45–49. [Google Scholar] [CrossRef]
- Anagbonu, P.; Ghali, M.; Allam, A. Low-temperature green synthesis of few-layered graphene sheets from pomegranate peels for supercapacitor applications. Sci. Rep. 2023, 13, 15627. [Google Scholar] [CrossRef]
- Kulyk, B.; Freitas, M.A.; Santos, N.F.; Mohseni, F.; Carvalho, A.F.; Yasakau, K.; Fernandes, A.J.S.; Bernardes, A.; Figueiredo, B.; Silva, R. A critical review on the production and application of graphene and graphene-based materials in anti-corrosion coatings. Crit. Rev. Solid State Mater. Sci. 2022, 47, 309–355. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Tang, L.; Lu, J.; Li, J. Application of graphene-modified electrode for selective detection of dopamine. Electrochem. Commun. 2009, 11, 889–892. [Google Scholar] [CrossRef]
- Prina-Mello, A.; Diao, Z.; Coey, J. Internalization of ferromagnetic nanowires by different living cells. J. Nanobiotechnol. 2006, 4, 9. [Google Scholar] [CrossRef][Green Version]
- Tian, Z.Q.; Wang, X.L.; Zhang, H.M.; Yi, B.L.; Jiang, S.P. Microwave-assisted synthesis of PTFE/C nanocomposite for polymer electrolyte fuel cells. Electrochem. Commun. 2006, 8, 1158–1162. [Google Scholar] [CrossRef]
- Xie, X.; Yu, G.; Liu, N.; Bao, Z.; Criddle, C.S.; Cui, Y. Graphene–sponges as high-performance low-cost anodes for microbial fuel cells. Energy Environ. Sci. 2012, 5, 6862–6866. [Google Scholar] [CrossRef]
- Yong, Y.-C.; Dong, X.-C.; Chan-Park, M.B.; Song, H.; Chen, P. Macroporous and monolithic anode based on polyaniline hybridized three-dimensional graphene for high-performance microbial fuel cells. ACS Nano 2012, 6, 2394–2400. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.M.; Mangadlao, J.; Ahmed, F.; Leon, A.; Advincula, R.C.; Rodrigues, D.F. Graphene nanocomposite for biomedical applications: Fabrication, antimicrobial and cytotoxic investigations. Nanotechnology 2012, 23, 395101. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan, A.; Panchakarla, L.S.; Chandran, P.; Menon, D.; Nair, S.; Rao, C.N.R.; Koyakutty, M. Differential nano-bio interactions and toxicity effects of pristine versus functionalized graphene. Nanoscale 2011, 3, 2461–2464. [Google Scholar] [CrossRef]
- Barbolina, I.; Woods, C.R.; Lozano, N.; Kostarelos, K.; Novoselov, K.S.; Roberts, I.S. Purity of graphene oxide determines its antibacterial activity. 2D Materials 2016, 3, 025025. [Google Scholar] [CrossRef]
- Lin, H.; Buerki-Thurnherr, T.; Kaur, J.; Wick, P.; Pelin, M.; Tubaro, A.; Carniel, F.C.; Tretiach, M.; Flahaut, E.; Iglesias, D.; et al. Environmental and Health Impacts of Graphene and other Two-Dimensional Materials: A Graphene Flagship Perspective. ACS Nano 2024, 18, 6038–6094. [Google Scholar] [CrossRef]
- Islam, J.; Obulisamy, P.K.; Upadhyayula, V.K.; Dalton, A.B.; Ajayan, P.M.; Rahman, M.M.; Tripathi, M.; Sani, R.K.; Gadhamshetty, V. Graphene as Thinnest Coating on Copper Electrodes in Microbial Methanol Fuel Cells. ACS Nano 2022, 17, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, G.; Ling, Y.; Qian, F.; Song, Y.; Lu, X.; Chen, S.; Tong, Y.; Li, Y. High power density microbial fuel cell with flexible 3D graphene–nickel foam as anode. Nanoscale 2013, 5, 10283–10290. [Google Scholar] [CrossRef]
- Karthikeyan, R.; Krishnaraj, N.; Selvam, A.; Wong, J.W.-C.; Lee, P.K.; Leung, M.K.; Berchmans, S. Effect of composites based nickel foam anode in microbial fuel cell using Acetobacter aceti and Gluconobacter roseus as a biocatalysts. Bioresour. Technol. 2016, 217, 113–120. [Google Scholar] [CrossRef]
- Zhang, Y.; He, Q.; Xia, L.; Li, Y.; Song, S. Algae cathode microbial fuel cells for cadmium removal with simultaneous electricity production using nickel foam/graphene electrode. Biochem. Eng. J. 2018, 138, 179–187. [Google Scholar] [CrossRef]
- Song, T.; Fei, K.; Zhang, H.; Yuan, H.; Yang, Y.; Ouyang, P.; Xie, J. High efficiency microbial electrosynthesis of acetate from carbon dioxide using a novel graphene–nickel foam as cathode. J. Chem. Technol. Biotechnol. 2018, 93, 457–466. [Google Scholar] [CrossRef]
- Zhang, Z.; Deng, X.; Sunarso, J.; Cai, R.; Chu, S.; Miao, J.; Zhou, W.; Shao, Z. Two-step fabrication of Li4Ti5O12-coated carbon nanofibers as a flexible film electrode for high-power lithium-ion batteries. ChemElectroChem 2017, 4, 2286–2292. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Zhao, X.; Tan, Y.; Liu, Y.; Wang, Z.; Ran, F. A novel hierarchical porous 3D structured vanadium nitride/carbon membranes for high-performance supercapacitor negative electrodes. Nano-Micro Lett. 2018, 10, 63. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.-M. Facile treatment of wastewater produced in Hummer’s method to prepare Mn3O4 nanoparticles and study their electrochemical performance in an asymmetric supercapacitor. RSC Adv. 2013, 3, 2398–2403. [Google Scholar] [CrossRef]
- Schönleber, M.; Uhlmann, C.; Braun, P.; Weber, A.; Ivers-Tiffée, E. A consistent derivation of the impedance of a lithium-ion battery electrode and its dependency on the state-of-charge. Electrochim. Acta 2017, 243, 250–259. [Google Scholar] [CrossRef]
- Shrestha, N.; Chilkoor, G.; Wilder, J.; Ren, Z.J.; Gadhamshetty, V. Comparative performances of microbial capacitive deionization cell and microbial fuel cell fed with produced water from the Bakken shale. Bioelectrochemistry 2018, 121, 56–64. [Google Scholar] [CrossRef]
- Somogyi, P.; Takagi, H. A note on the use of picric acid-paraformaldehyde-glutaraldehyde fixative for correlated light and electron microscopic immunocytochemistry. Neuroscience 1982, 7, 1779–1783. [Google Scholar] [CrossRef]
- Zhao, Y.; Duan, L.; Hermanowicz, S.W. Influence of water transport characteristics on membrane internal conductive structure in forward osmosis microbial fuel cell. J. Mol. Liq. 2023, 380, 121704. [Google Scholar] [CrossRef]
- Shrestha, N.; Chilkoor, G.; Xia, L.; Alvarado, C.; Kilduff, J.E.; Keating, J.J.; Belfort, G.; Gadhamshetty, V. Integrated membrane and microbial fuel cell technologies for enabling energy-efficient effluent Re-use in power plants. Water Res. 2017, 117, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Mohammadifar, M.; Choi, S. From microbial fuel cells to biobatteries: Moving toward on-demand micropower generation for small-scale single-use applications. Adv. Mater. Technol. 2019, 4, 1900079. [Google Scholar] [CrossRef]
| Materials Used | Graphene Deposition Method | OCV (V) | Power Density (mW/m2) | Current Density (A/m2) | Inoculum | Ohmic Loss (Ω) | Reference |
|---|---|---|---|---|---|---|---|
| Graphene | Plasma deposition | 0.65 | 2582 | 12.9 | Wastewater | 28 | This study |
| Graphene oxide | Autoclaved and annealed | 0.62 | 721 | 1.76 | Shewanella oneidensis MR-1 | 10 | [19] |
| 3D graphene | Chemical vapor deposition | n/a | 768 | 1.8 | Shewanella oneidensis MR-1 | 9 | [23] |
| Graphene sponge | Chemical vapor deposition | 0.33 | 1110 | 1.32 | Wastewater | 14 | [22] |
| Test | # | Reactor | Working Electrode | Test | Electrolyte | Purpose |
|---|---|---|---|---|---|---|
| Biotic (65 days) | Graphene on nickel foam (referred to as graphene foam) | Biobattery | Gr/Ni (or Graphene Foam) | EIS, CV | Wastewater anolyte | Measure current, power output, and biofilm growth |
| 3D nickel foam | Biobattery | Bare Ni (or Ni foam) | EIS, CV | Wastewater anolyte | Control | |
| Graphite felt | Biobattery | Graphite felt | EIS | Wastewater anolyte | Control for EIS studies | |
| 3D graphene | Capacitor | Gr/Ni | Charging/discharging cycle | Wastewater anolyte | Establish charge storage properties of graphene foam | |
| 3D Ni | Capacitor | Bare Ni | Charging/discharging | Wastewater anolyte | Control | |
| Abiotic | Coin cell | Super Capacitor | Gr/Ni | CV | KOH (anolyte and catholyte) | Establish bifunctional device |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapkota, S.; Hummel, M.; Zahan, M.; Karanam, S.P.; Bathi, J.; Shrestha, N.; Gu, Z.; Gadhamshetty, V. Graphene-Infused Hybrid Biobattery–Supercapacitor Powered by Wastewater for Sustainable Energy Innovation. Inorganics 2024, 12, 84. https://doi.org/10.3390/inorganics12030084
Sapkota S, Hummel M, Zahan M, Karanam SP, Bathi J, Shrestha N, Gu Z, Gadhamshetty V. Graphene-Infused Hybrid Biobattery–Supercapacitor Powered by Wastewater for Sustainable Energy Innovation. Inorganics. 2024; 12(3):84. https://doi.org/10.3390/inorganics12030084
Chicago/Turabian StyleSapkota, Sambhu, Matthew Hummel, Mahzuzah Zahan, Sushma P. Karanam, Jejal Bathi, Namita Shrestha, Zhengrong Gu, and Venkataramana Gadhamshetty. 2024. "Graphene-Infused Hybrid Biobattery–Supercapacitor Powered by Wastewater for Sustainable Energy Innovation" Inorganics 12, no. 3: 84. https://doi.org/10.3390/inorganics12030084
APA StyleSapkota, S., Hummel, M., Zahan, M., Karanam, S. P., Bathi, J., Shrestha, N., Gu, Z., & Gadhamshetty, V. (2024). Graphene-Infused Hybrid Biobattery–Supercapacitor Powered by Wastewater for Sustainable Energy Innovation. Inorganics, 12(3), 84. https://doi.org/10.3390/inorganics12030084