Comparative Solution Equilibrium Studies on Anticancer Estradiol-Based Conjugates and Their Copper Complexes
Abstract
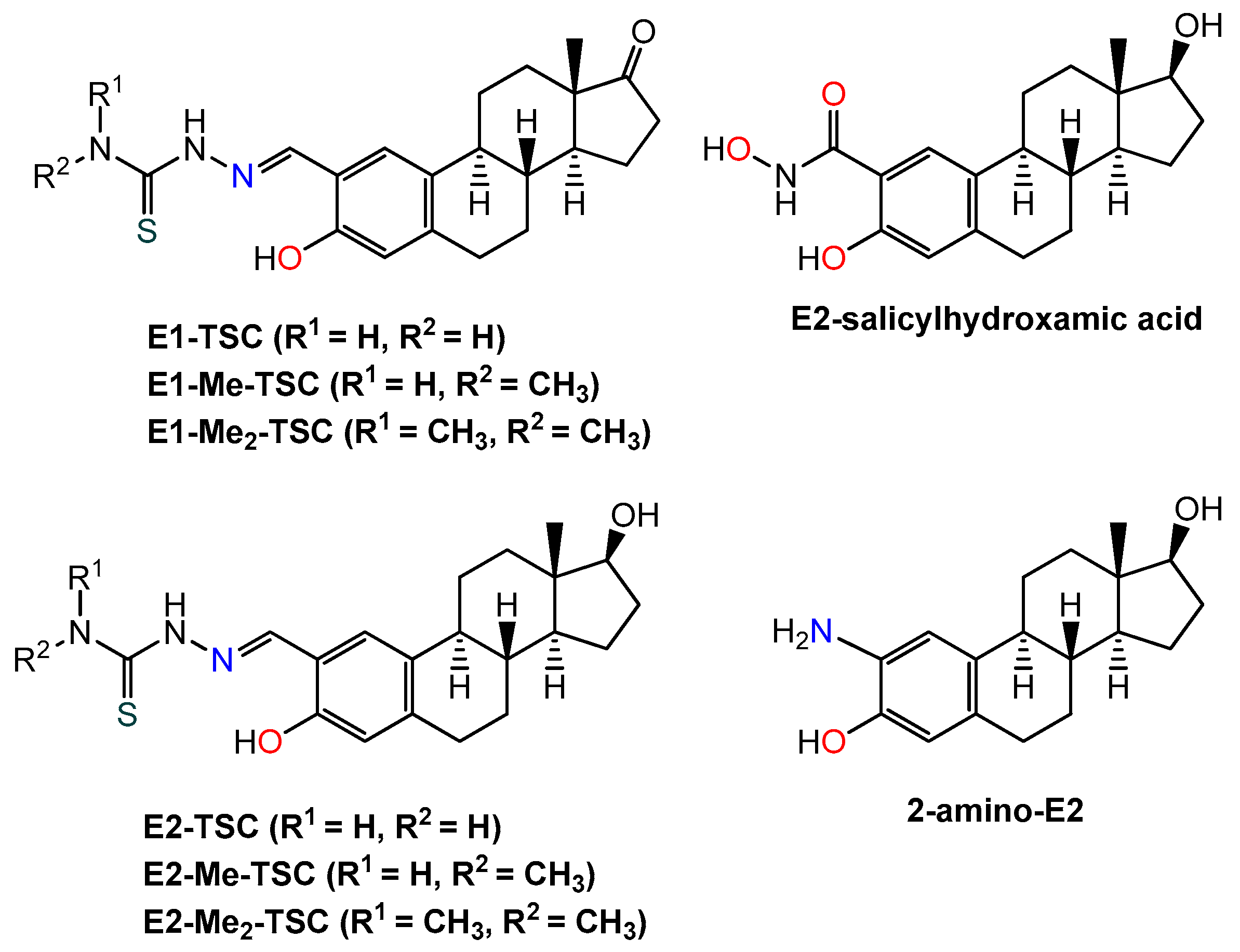
1. Introduction
2. Results and Discussion
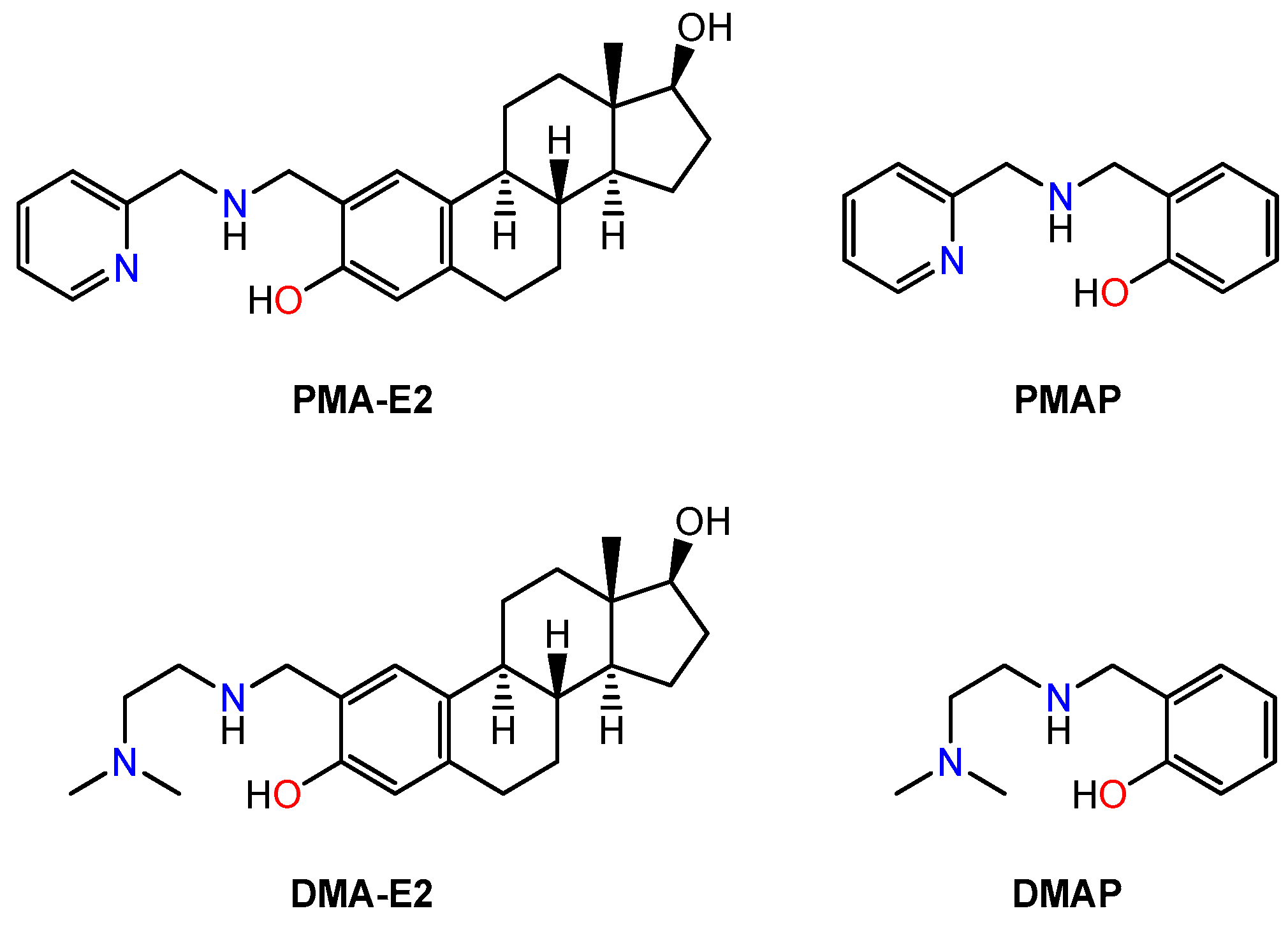
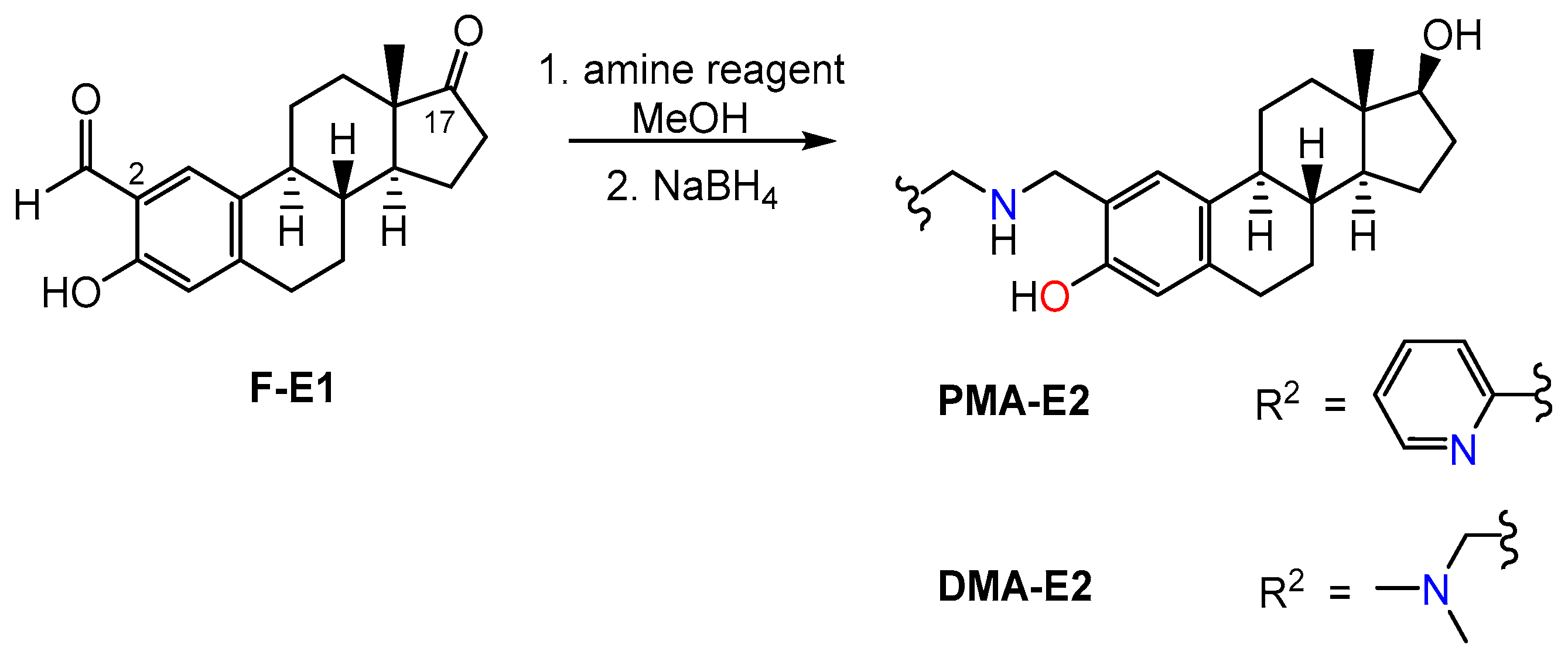
2.1. Synthesis and Characterization of PMA-E2, DMA-E2 and Their Model Compounds (PMAP, DMAP)
2.2. Solution Chemical Properties of PMA-E2, DMA-E2, and the Model Ligands PMAP and DMAP
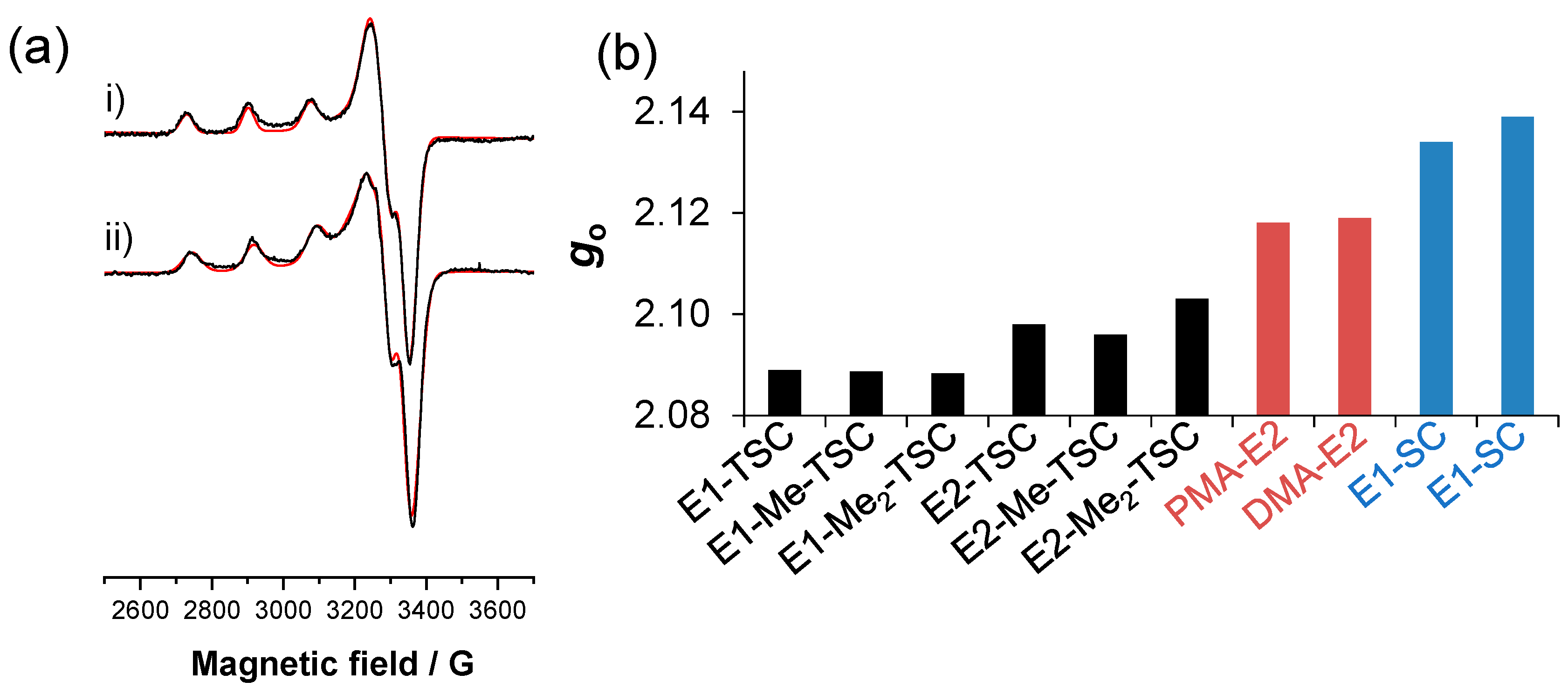
2.3. Complex Formation Equilibria of the Ligands with Cu(II) Ions in Solution
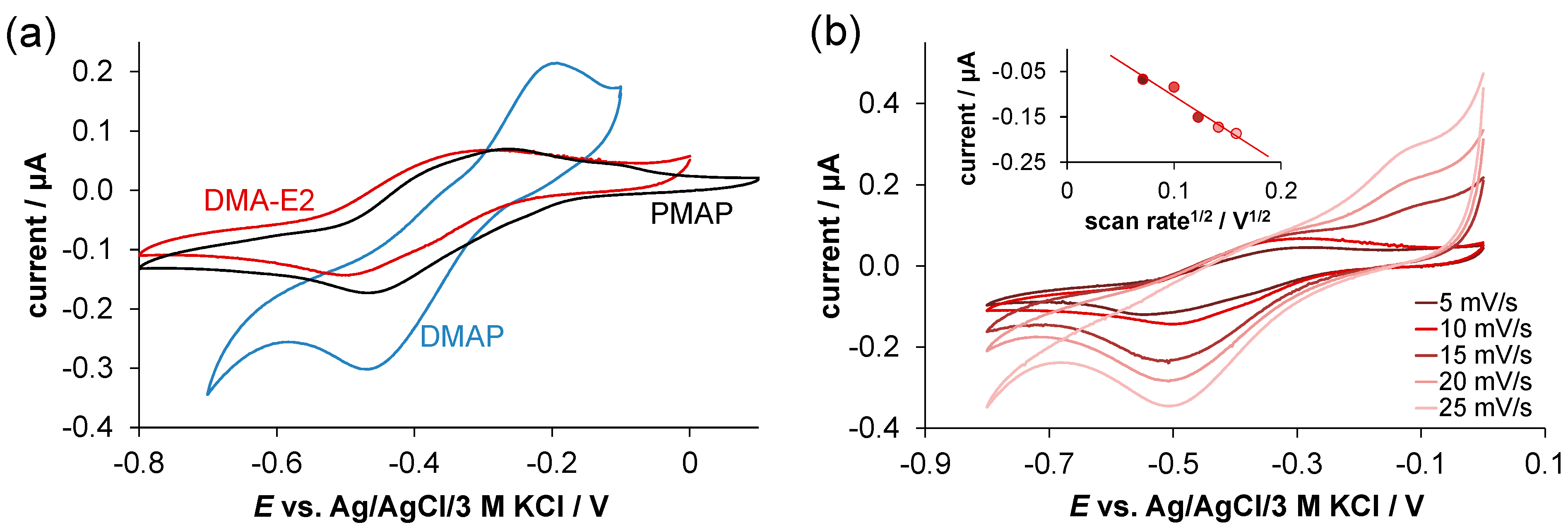
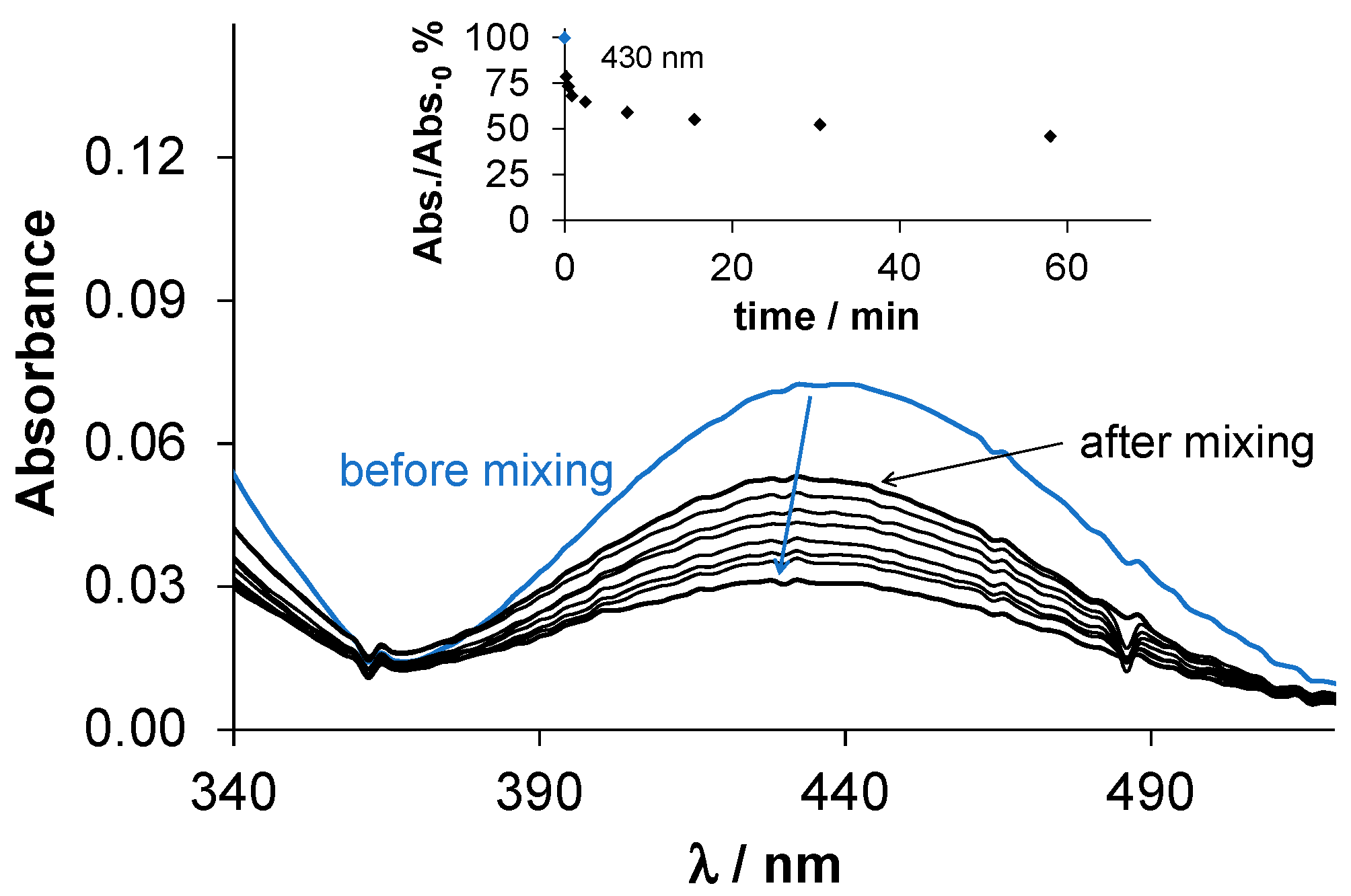
2.4. Redox Properties of the Complexes Formed with Cu(II) Ions
2.5. Synthesis and Characterization of Cu(II) Complexes of PMA-E2 and DMA-E2
2.6. In Vitro Cytotoxicity of the Compounds
3. Materials and Methods
3.1. Chemicals
3.2. General Procedure for the Synthesis of Secondary Amines (PMAP, DMAP, PMA-E2, and DMA-E2) via Reductive Amination
3.2.1. 2-((((Pyridin-2-yl)methyl)amino)methyl)phenol (PMAP)
3.2.2. 2-(((2-(Dimethylamino)ethyl)amino)methyl)phenol (DMAP)
3.2.3. 2-((((2-Pyridin-2-yl)methyl)amino)methyl)estradiol (PMA-E2)
3.2.4. 2-(((2-(Dimethylamino)ethyl)amino)methyl)estradiol (DMA-E2)
3.3. Synthesis and Characterization Cu(II) Complexes of PMA-E2 and DMA-E2
3.4. pH-Potentiometry
3.5. UV-Vis Spectrophotometry
3.6. 1H NMR Spectroscopic Titrations
3.7. EPR Spectroscopic Measurements and Evaluation of the Spectra
3.8. Cyclic Voltammetry
3.9. In Vitro Cell Studies: Cell Lines and Culture Conditions and MTT Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bansal, R.; Acharya, P.C. Man-made cytotoxic steroids: Exemplary agents for cancer therapy. Chem. Rev. 2014, 114, 6986–7005. [Google Scholar] [CrossRef]
- Minorics, R.; Zupkó, I. Steroidal anticancer agents: An overview of estradiol-related compounds. Anticancer Agents Med. Chem. 2018, 18, 652–666. [Google Scholar] [CrossRef]
- Marchesi, E.; Perrone, D.; Navacchia, M.L. Molecular hybridization as a strategy for developing artemisinin-derived anticancer candidates. Pharmaceutics 2023, 15, 218. [Google Scholar] [CrossRef]
- Bansal, R.; Surya, A. A comprehensive review on steroidal bioconjugates as promising leads in drug discovery. ACS Bio Med Chem Au 2022, 2, 340–369. [Google Scholar] [CrossRef] [PubMed]
- Zolottsev, V.A.; Latysheva, A.S.; Pokrovsky, V.S.; Khan, I.I.; Misharin, A.Y. Promising applications of steroid conjugates for cancer research and treatment. Eur. J. Med. Chem. 2021, 210, 113089. [Google Scholar] [CrossRef]
- Petrasheuskaya, T.V.; Kiss, M.A.; Dömötör, O.; Holczbauer, T.; May, N.V.; Spengler, G.; Kincses, A.; Gašparović, A.Ĉ.; Frank, É.; Enyedy, É.A. Salicylaldehyde thiosemicarbazone copper complexes: Impact of hybridization with estrone on cytotoxicity, solution stability and redox activity. New J. Chem. 2020, 44, 12154–12168. [Google Scholar] [CrossRef]
- Petrasheuskaya, T.V.; Wernitznig, D.; Kiss, M.A.; May, N.V.; Wenisch, D.; Keppler, B.K.; Frank, É.; Enyedy, É.A. Estrone–salicylaldehyde N-methylated thiosemicarbazone hybrids and their copper complexes: Solution structure, stability and anticancer activity in tumour spheroids. J. Biol. Inorg. Chem. 2021, 26, 775–791. [Google Scholar] [CrossRef] [PubMed]
- Petrasheuskaya, T.V.; Kovács, F.; Igaz, N.; Rónavári, A.; Hajdu, B.; Bereczki, L.; May, N.V.; Spengler, G.; Gyurcsik, B.; Kiricsi, M.; et al. Estradiol-based salicylaldehyde (thio)semicarbazones and their copper complexes with anticancer, antibacterial and antioxidant activities. Molecules 2023, 28, 54. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, J.P.; Kovács, H.; Spengler, G.; Kovács, F.; Frank, É.; Enyedy, É.A. A comparative study on the metal complexes of an anticancer estradiol-hydroxamate conjugate and salicylhydroxamic acid. J. Inorg. Biochem. 2023, 244, 112223. [Google Scholar] [CrossRef] [PubMed]
- Petrasheuskaya, T.V.; Kovács, F.; Spengler, G.; May, N.V.; Frank, É.; Enyedy, É.A. A comparative study on the complex formation of 2-aminoestradiol and 2-aminophenol with divalent metal ions: Solution chemistry and anticancer activity. J. Mol. Struct. 2022, 1261, 132858. [Google Scholar] [CrossRef]
- Molnár, B.; Kinyua, N.I.; Mótyán, G.; Leits, P.; Zupkó, I.; Minorics, R.; Balogh, G.T.; Frank, É. Regioselective synthesis, physicochemical properties and anticancer activity of 2-aminomethylated estrone derivatives. J. Steroid Biochem. Mol. Biol. 2022, 219, 106064. [Google Scholar] [CrossRef] [PubMed]
- Biersack, B.; Schobert, R. Metallodrug conjugates with steroids and selective estrogen receptor modulators (SERM). Curr. Med. Chem. 2009, 16, 2324–2337. [Google Scholar] [CrossRef] [PubMed]
- Le Bideau, F.; Dagorne, S. Synthesis of transition-metal steroid derivatives. Chem. Rev. 2013, 113, 7793–7850. [Google Scholar] [CrossRef] [PubMed]
- Gust, R.; Beck, W.; Jaouen, G.; Schönenberger, H. Optimization of cisplatin for the treatment of hormone dependent tumoral diseases: Part 1: Use of steroidal ligands. Coord. Chem. Rev. 2009, 253, 2742–2759. [Google Scholar] [CrossRef]
- Altman, J.; Castrillo, T.; Beck, W.; Bernhardt, G.; Schoenenberger, H. Metal complexes with biologically important ligands. 62. Platinum (II) complexes of 3-(2-aminoethoxy) estrone and-estradiol. Inorg. Chem. 1991, 30, 4085–4088. [Google Scholar] [CrossRef]
- Kitteringham, E.; Andriollo, E.; Gandin, V.; Montagner, D.; Griffith, D.M. Synthesis, characterisation and in vitro antitumour potential of novel Pt (II) estrogen linked complexes. Inorg. Chim. Acta 2019, 495, 118944. [Google Scholar] [CrossRef]
- Seroka, B.; Łotowski, Z.; Wojtkielewicz, A.; Bazydło, P.; Dudź, E.; Hryniewicka, A.; Morzycki, J.W. Synthesis of steroidal 1, 2-and 1, 3-diamines as ligands for transition metal ion complexation. Steroids 2019, 147, 19–27. [Google Scholar] [CrossRef]
- Kvasnica, M.; Budesinsky, M.; Swaczynova, J.; Pouzar, V.; Kohout, L. Platinum(II) complexes with steroidal esters of L-methionine and L-histidine: Synthesis, characterization and cytotoxic activity. Bioorg. Med. Chem. 2008, 16, 3704–3713. [Google Scholar] [CrossRef]
- Ruiz, J.; Rodríguez, V.; Cutillas, N.; Espinosa, A.; Hannon, M.J. Novel C, N-chelate platinum (II) antitumor complexes bearing a lipophilic ethisterone pendant. J. Inorg. Biochem. 2011, 105, 525–531. [Google Scholar] [CrossRef]
- Huxley, M.; Sanchez-Cano, C.; Browning, M.J.; Navarro-Ranninger, C.; Quiroga, A.G.; Rodger, A.; Hannon, M.J. An androgenic steroid delivery vector that imparts activity to a non-conventional platinum (II) metallo-drug. Dalton Trans. 2010, 39, 11353–11364. [Google Scholar] [CrossRef]
- Liang, Z.; Liu, L.; Zhou, Y.; Liu, W.; Lu, Y. Research Progress on Bioactive Metal Complexes against ER-Positive Advanced Breast Cancer. J. Med. Chem. 2023, 66, 2235–2256. [Google Scholar] [CrossRef] [PubMed]
- Koch, V.; Meschkov, A.; Feuerstein, W.; Pfeifer, J.; Fuhr, O.; Nieger, M.; Schepers, U.; Bräse, S. Synthesis, characterization, and biological properties of steroidal ruthenium (II) and iridium (III) complexes based on the androst-16-en-3-ol framework. Inorg. Chem. 2019, 58, 15917–15926. [Google Scholar] [CrossRef]
- Lv, G.; Qiu, L.; Li, K.; Liu, Q.; Li, X.; Peng, Y.; Wang, S.; Lin, J. Enhancement of therapeutic effect in breast cancer with a steroid-conjugated ruthenium complex. New J. Chem. 2019, 43, 3419–3427. [Google Scholar] [CrossRef]
- Khan, S.A.; Asiri, A.M. Synthesis and spectroscopic studies of Ru (II) complexes of steroidal thiosemicarbazones by multi step reaction: As anti-bacterial agents. Steroids 2017, 124, 23–28. [Google Scholar] [CrossRef]
- Barrett, S.; De Franco, M.; Kellett, A.; Dempsey, E.; Marzano, C.; Erxleben, A.; Gandin, V.; Montagner, D. Anticancer activity, DNA binding and cell mechanistic studies of estrogen-functionalised Cu(II) complexes. J. Biol. Inorg. Chem. 2020, 25, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kong, E.; Zhan, J.; Chen, S.; Gan, C.; Liu, Z.; Pang, L.; Cui, J. Synthesis and cytotoxic evaluation of steroidal copper (Cu (II)) complexes. Bioinorg. Chem. Appl. 2017, 2017, 4276919. [Google Scholar] [CrossRef] [PubMed]
- Borges, L.J.H.; Bull, E.S.; Fernandes, C.; Horn, A., Jr.; Azeredo, N.F.; Resende, J.A.L.C.; Freitas, W.R.; Carvalho, E.C.Q.; Lemos, L.S.; Jerdy, H.; et al. In vitro and in vivo studies of the antineoplastic activity of copper (II) compounds against human leukemia THP-1 and murine melanoma B16-F10 cell lines. Eur. J. Med. Chem. 2016, 123, 128–140. [Google Scholar] [CrossRef]
- Neves, A.; Verani, C.N.; de Brito, M.A.; Vencato, I.; Mangrich, A.; Oliva, G.; Souza, D.D.H.F.; Batista, A.A. Copper(II) complexes with (2-hydroxybenzyl-2-pyridylmethyl)amine–Hbpa: Syntheses, characterization and crystal structures of the ligand and [Cu(II)(Hbpa)2](ClO4)2·2H2O. Inorg. Chim. Acta 1999, 290, 207–212. [Google Scholar] [CrossRef]
- Kumar, P.; Baidya, B.; Chaturvedi, S.K.; Khan, R.H.; Manna, D.; Mondal, B. DNA binding and nuclease activity of copper(II) complexes of tridentate ligands. Inorg. Chim. Acta 2011, 376, 264–270. [Google Scholar] [CrossRef]
- Peters, R.H.; Chao, W.-R.; Sato, B.; Shigeno, K.; Zaveri, N.T.; Tanabe, M. Steroidal oxathiazine inhibitors of estrone sulfatase. Steroids 2003, 68, 97–110. [Google Scholar] [CrossRef]
- Prosser, K.E.; Xie, D.; Chu, A.; MacNeil, G.A.; Varju, B.R.; Kadakia, R.T.; Que, E.L.; Walsby, C.J. Copper(II) pyridyl aminophenolates: Hypoxia-selective, nucleus-targeting cytotoxins, and magnetic resonance probes. Chem. Eur. J. 2021, 27, 9839–9849. [Google Scholar] [CrossRef] [PubMed]
- Sheoran, M.; Bhar, K.; Khan, T.A.; Sharma, A.K.; Naik, S.G. Phosphatase activity and DNA binding studies of dinuclear phenoxo-bridged zinc(II) complexes with an N,N,O-donor ligand and halide ions in a rare cis-configuration. Polyhedron 2017, 129, 82–91. [Google Scholar] [CrossRef]
- Choi, K.-Y. Synthesis and crystal structure of phenolato-bridged dinuclear copper(II) complex with (2-hydroxybenzyl)(2-pyridylmethyl)amine. J. Chem. Crystallogr. 2010, 40, 1016–1020. [Google Scholar] [CrossRef]
- Biswas, A.; Drew, M.G.B.; Song, Y.; Ghosh, A. Effect of anionic co-ligands on structure and magnetic coupling of bis(μ-phenoxo)-bridged dinuclear copper(II) complexes. Inorg. Chim. Acta 2011, 376, 422–427. [Google Scholar] [CrossRef]
- Biswas, A.; Drew, M.G.B.; Gómez-García, C.J.; Ghosh, A. Use of a reduced Schiff-base ligand to prepare novel chloro-bridged chains of rare Cu(II) trinuclear complexes with mixed azido/oxo and chloro/oxo bridges. Inorg. Chem. 2010, 49, 8155–8163. [Google Scholar] [CrossRef] [PubMed]
- Dömötör, O.; May, N.V.; Gál, G.T.; Spengler, G.; Dobrova, A.; Arion, V.B.; Enyedy, É.A. Solution Equilibrium Studies on Salicylidene Aminoguanidine Schiff Base Metal Complexes: Impact of the Hybridization with L-Proline on Stability, Redox Activity and Cytotoxicity. Molecules 2022, 27, 2044. [Google Scholar] [CrossRef]
- Enyedy, É.A.; May, N.V.; Pape, V.F.S.; Heffeter, P.; Szakács, G.; Keppler, B.K.; Kowol, C.R. Complex formation and cytotoxicity of Triapine derivatives: A comparative solution study on the effect of the chalcogen atom and NH-methylation. Dalton Trans. 2020, 49, 16887–16902. [Google Scholar] [CrossRef]
- Nagy, N.V.; Doorslaer, S.V.; Szabó-Plánka, T.; Rompaey, S.V.; Hamza, A.; Fülöp, F.; Tóth, G.K.; Rockenbauer, A. Copper (II)-binding ability of stereoisomeric cis-and trans-2-aminocyclohexanecarboxylic acid–l-phenylalanine dipeptides. A combined cw/pulsed EPR and DFT study. Inorg. Chem. 2012, 51, 1386–1399. [Google Scholar] [CrossRef]
- Liu, R.-P.; Duan, M.-Y.; Li, J.; Su, Z.-P.; Zhang, J.-H.; Zhang, F.-X. Crystal structures of [Zn(SALIMP)(CH3CO2)]2 and [Cu(SALIMP)Cl] with 2-[[(2-pyridinylmethyl) imino]methyl]phenol (HSALIMP) as a ligand. J. Struct. Chem. 2011, 52, 935–940. [Google Scholar] [CrossRef]
- Enyedy, É.A.; Petrasheuskaya, T.V.; Kiss, M.A.; Wernitznig, D.; Wenisch, D.; Keppler, B.K.; Spengler, G.; May, N.V.; Frank, É.; Dömötör, O. Complex formation of an estrone-salicylaldehyde semicarbazone hybrid with copper(II) and gallium(III): Solution equilibria and biological activity. J. Inorg. Biochem. 2021, 220, 111468. [Google Scholar] [CrossRef]
- Irving, H.M.; Miles, M.G.; Pettit, L.D. A study of some problems in determining the stoicheiometric proton dissociation constants of complexes by potentiometric titrations using a glass electrode. Anal. Chim. Acta 1967, 38, 475–488. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef] [PubMed]
- Zékány, L.; Nagypál, I. PSEQUAD. In Computational Methods for the Determination of Stability Constants; Leggett, L., Ed.; Plenum Press: New York, NY, USA, 1985; p. 291. [Google Scholar]
- Enyedy, É.A.; Hollender, D.; Kiss, T. Lipophilicity of kinetically labile metal complexes through the example of antidiabetic Zn(II) and VO(IV) compounds. J. Pharm. Biomed. Anal. 2011, 54, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Rockenbauer, A.; Szabó-Plánka, T.; Árkosi, Z.; Korecz, L. A two-dimensional (magnetic field and concentration) electron paramagnetic resonance method for analysis of multispecies complex equilibrium systems. Information content of EPR spectra. J. Am. Chem. Soc. 2001, 123, 7646–7654. [Google Scholar] [CrossRef]
- Rockenbauer, A.; Korecz, L. Automatic computer simulations of ESR spectra. Appl. Magn. Reson. 1996, 10, 29–43. [Google Scholar] [CrossRef]
- GraphPad Prism Version 7.00 for Windows, Graph Pad Software, La Jolla, CA, USA. 2018. Available online: http://www.graphpad.com (accessed on 29 December 2023).

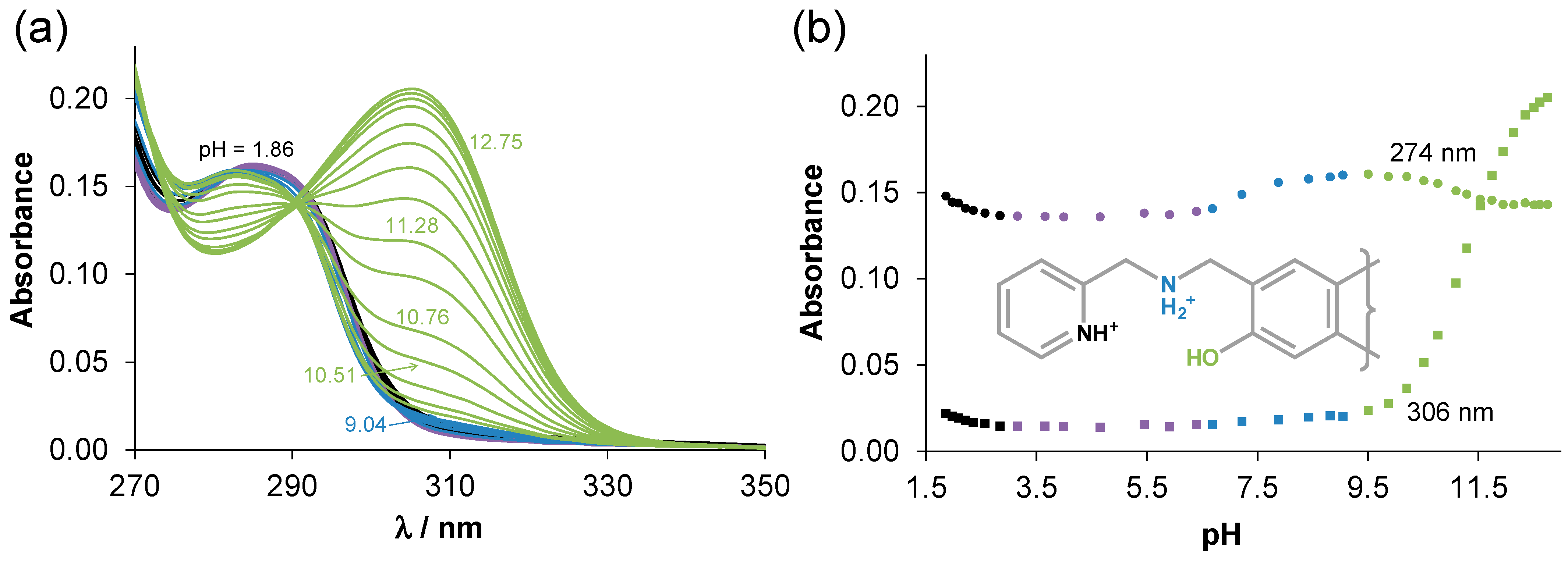
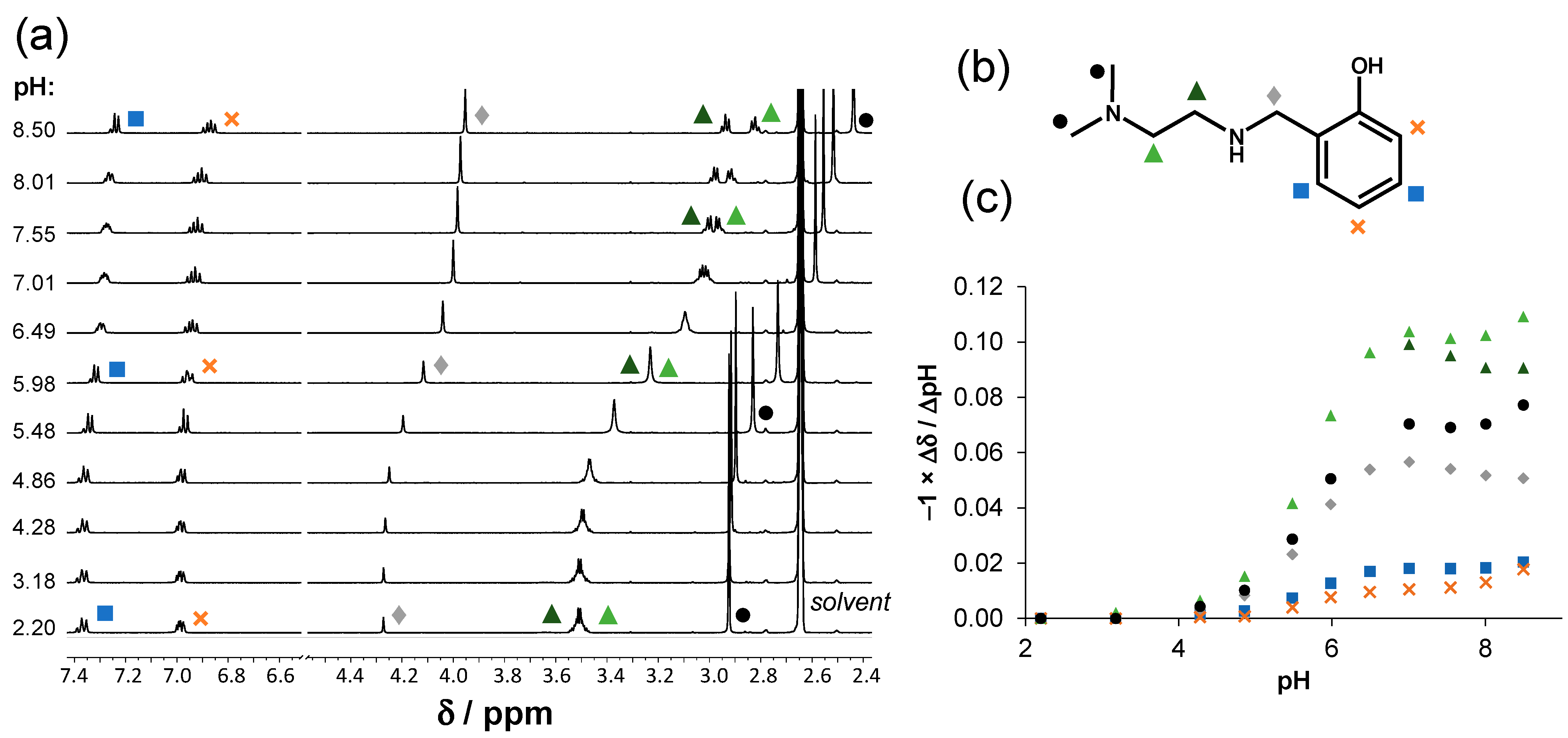
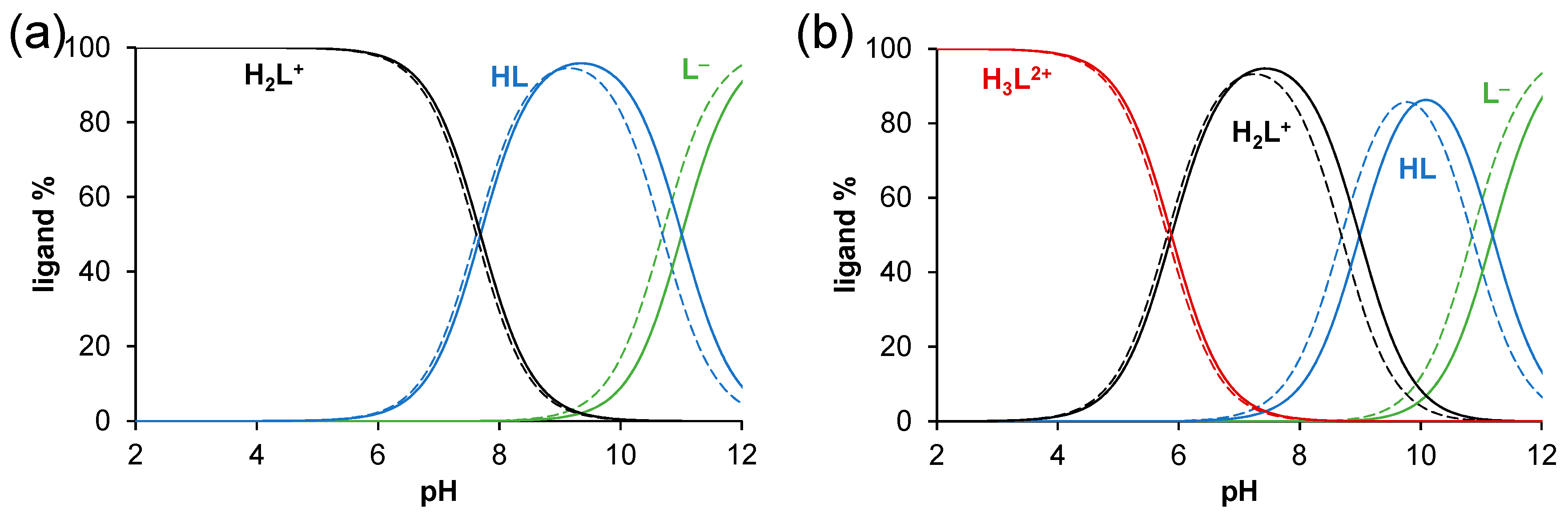
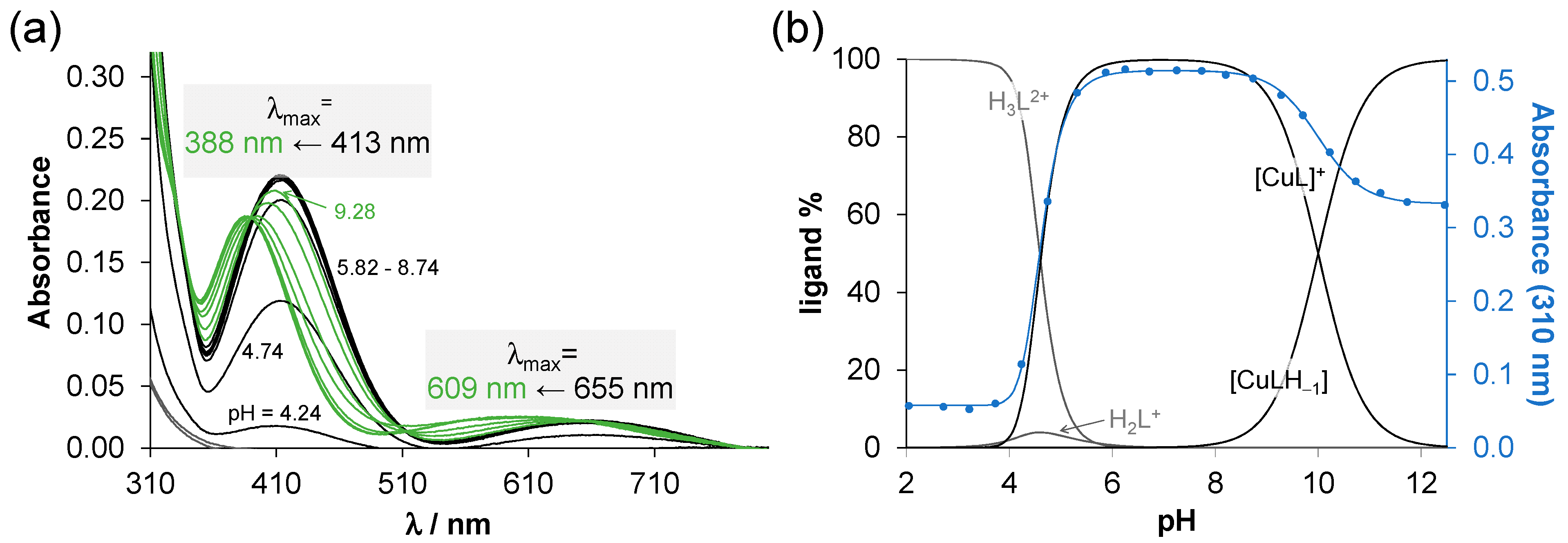
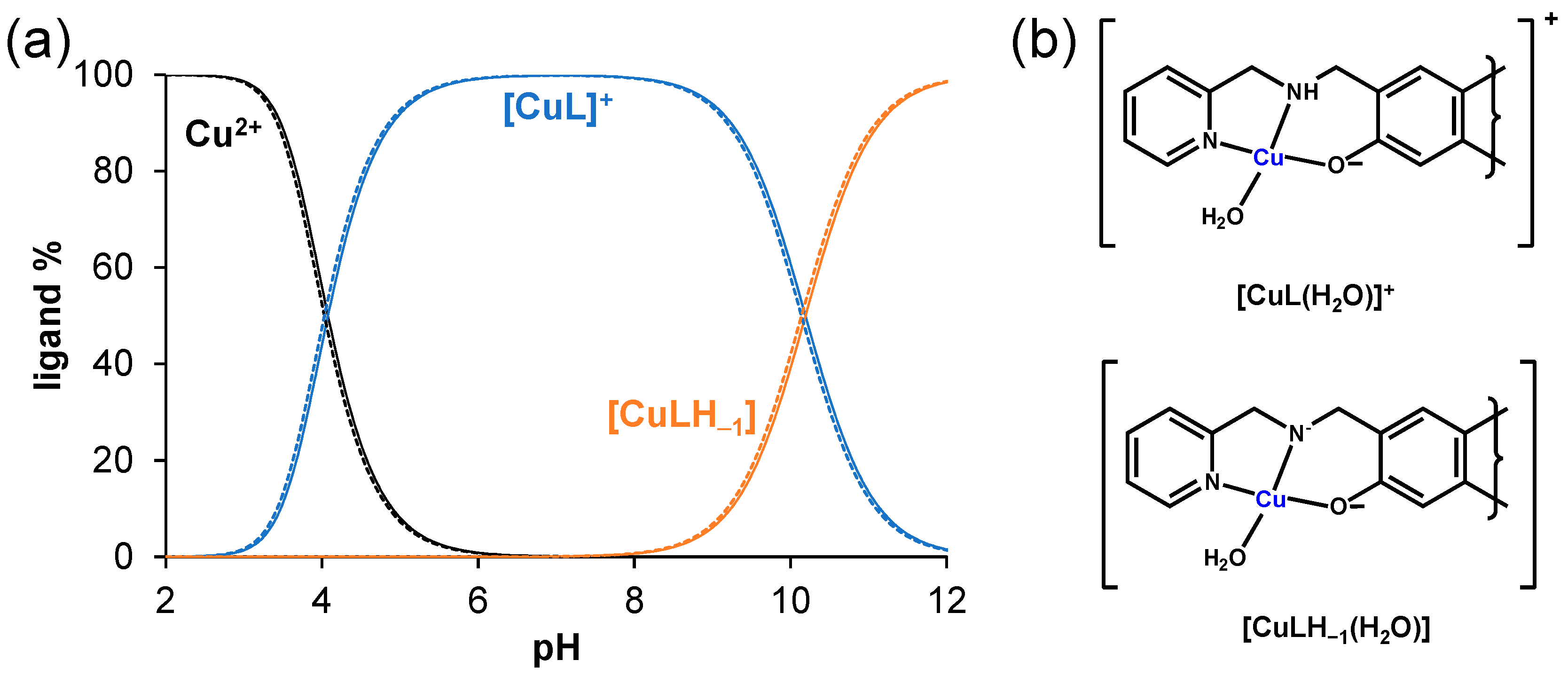

| Method | Constant | PMA-E2 | DMA-E2 | PMAP | DMAP |
|---|---|---|---|---|---|
| UV-vis | pK1 (H3L2+) | <1.5 | 5.77 ± 0.07 | <1.5 | 5.69 ± 0.03 |
| pK2 (H2L+) | ~7.6 | 8.81 ± 0.03 | 7.62 ± 0.03 | 8.68 ± 0.04 | |
| pK3 (HL) | 11.21 ± 0.08 | 11.26 ± 0.03 | 10.78 ± 0.03 | 10.90 ± 0.05 | |
| pH-pot. | pK1 (H3L2+) | <1.5 | 5.88 ± 0.06 | <1.5 | 5.81 ± 0.07 |
| pK2 (H2L+) | 7.69 ± 0.06 | 8.99 ± 0.04 | 7.62 ± 0.03 | 8.69 ± 0.05 | |
| pK3 (HL) | 11.01 ± 0.04 | 11.19 ± 0.03 | 10.69 ± 0.03 | 10.85 ± 0.04 |
| Constant | Method | PMA-E2 | DMA-E2 | PMAP | DMAP |
|---|---|---|---|---|---|
| logβ [CuL]+ | UV-vis | 15.15 ± 0.03 | 15.58 ± 0.03 | 14.94 ± 0.03 | 15.30 ± 0.03 |
| pH-pot. | - | - | 15.06 ± 0.09 | 15.38 ± 0.06 | |
| logβ [CuLH−1] | UV-vis | 4.96 ± 0.03 | 5.63 ± 0.03 | 4.80 ± 0.03 | 5.30 ± 0.03 |
| pH-pot. | - | - | 5.13 ± 0.09 | 5.48 ± 0.08 | |
| pKa [CuL]+ | UV-vis | 10.19 | 9.95 | 10.14 | 10.00 |
| pH-pot. | - | - | 9.93 | 9.90 | |
| pCu a | 8.1 | 7.7 | 8.3 | 7.8 |
| DMAP | DMA-E2 | |||
|---|---|---|---|---|
| [CuL]+ | [CuLH−1] | [CuL]+ | [CuLH−1] | |
| Isotropic Data | ||||
| g0 | 2.116 | 2.108 | 2.117 | 2.108 |
| A0 | 70 | 72 | 69 | 72 |
| a0N | 10 | 10 | 10 | 10 |
| α | 27 | 24 | 46 | 39 |
| β | −14 | −13 | −25 | −21 |
| γ | 3 | 3 | 3 | 3 |
| Anisotropic Data | ||||
| g┴ | 2.050 | 2.047 | 2.049 | 2.047 |
| g‖ | 2.248 | 2.242 | 2.248 | 2.244 |
| A┴ | 24 | 23 | 21 | 24 |
| A‖ | 181 | 176 | 181 | 179 |
| a0N | 11 | 12 | 12 | 11 |
| Calculated Data b | ||||
| g0,calcd | 2.116 | 2.112 | 2.116 | 2.113 |
| IC50/μM | MCF-7 | Colo-205 | MRC-5 |
|---|---|---|---|
| PMA-E2 | 62.56 ± 0.62 | 42.4 ± 1.1 | 34.7 ± 1.7 |
| DMA-E2 | 60.2 ± 1.1 | 29.9 ± 1.0 | 14.95 ± 0.22 |
| [Cu(PMA-E2H−1)Cl] | 31.89 ± 0.61 | 15.25 ± 0.48 | 24.46 ± 0.31 |
| [Cu(DMA-E2H−1)Cl] | 45.36 ± 0.31 | 18.27 ± 0.28 | 13.85 ± 0.55 |
| CuCl2 | 54.4 ± 1.8 | 32.79 ± 0.83 | 39.9 ± 1.3 |
| E1-TSC a | 6.42 ± 0.40 | 20.3 ± 1.4 | 22.6 ± 3.5 |
| [Cu(E1–TSCH−2)] a | 0.57 ± 0.03 | 1.99 ± 0.19 | 1.59 ± 0.13 |
| E2-salicylhydroxamic acid b | 25.2 ± 0.8 | 58.6 ± 1.4 | >100 |
| 2-amino-E2 c | ˃100 | 17.3 ± 0.1 | ˃100 |
| doxorubicin b | 0.21 ± 0.03 | 0.64 ± 0.06 | 1.16 ± 0.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enyedy, É.A.; Giricz, A.; Petrasheuskaya, T.V.; Mészáros, J.P.; May, N.V.; Spengler, G.; Kovács, F.; Molnár, B.; Frank, É. Comparative Solution Equilibrium Studies on Anticancer Estradiol-Based Conjugates and Their Copper Complexes. Inorganics 2024, 12, 49. https://doi.org/10.3390/inorganics12020049
Enyedy ÉA, Giricz A, Petrasheuskaya TV, Mészáros JP, May NV, Spengler G, Kovács F, Molnár B, Frank É. Comparative Solution Equilibrium Studies on Anticancer Estradiol-Based Conjugates and Their Copper Complexes. Inorganics. 2024; 12(2):49. https://doi.org/10.3390/inorganics12020049
Chicago/Turabian StyleEnyedy, Éva A., Anett Giricz, Tatsiana V. Petrasheuskaya, János P. Mészáros, Nóra V. May, Gabriella Spengler, Ferenc Kovács, Barnabás Molnár, and Éva Frank. 2024. "Comparative Solution Equilibrium Studies on Anticancer Estradiol-Based Conjugates and Their Copper Complexes" Inorganics 12, no. 2: 49. https://doi.org/10.3390/inorganics12020049
APA StyleEnyedy, É. A., Giricz, A., Petrasheuskaya, T. V., Mészáros, J. P., May, N. V., Spengler, G., Kovács, F., Molnár, B., & Frank, É. (2024). Comparative Solution Equilibrium Studies on Anticancer Estradiol-Based Conjugates and Their Copper Complexes. Inorganics, 12(2), 49. https://doi.org/10.3390/inorganics12020049










