‘Charge Reverse’ Halogen Bonding Contacts in Metal-Organic Multi-Component Compounds: Antiproliferative Evaluation and Theoretical Studies
Abstract
1. Introduction
2. Results and Discussion
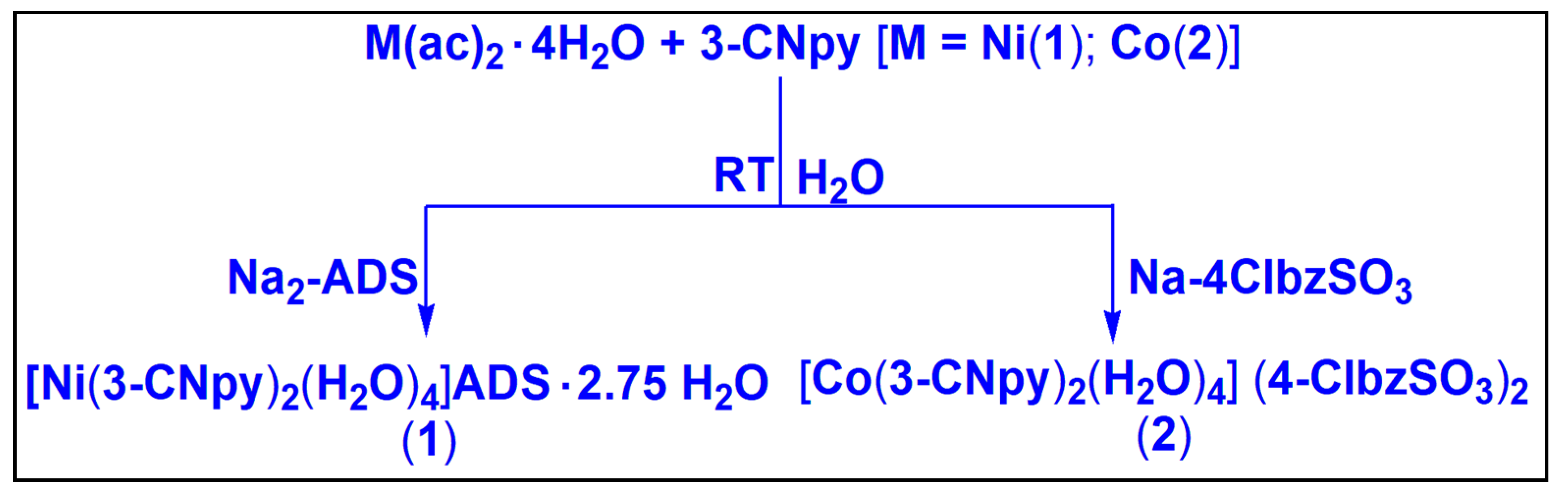
2.1. Synthesis and General Aspects
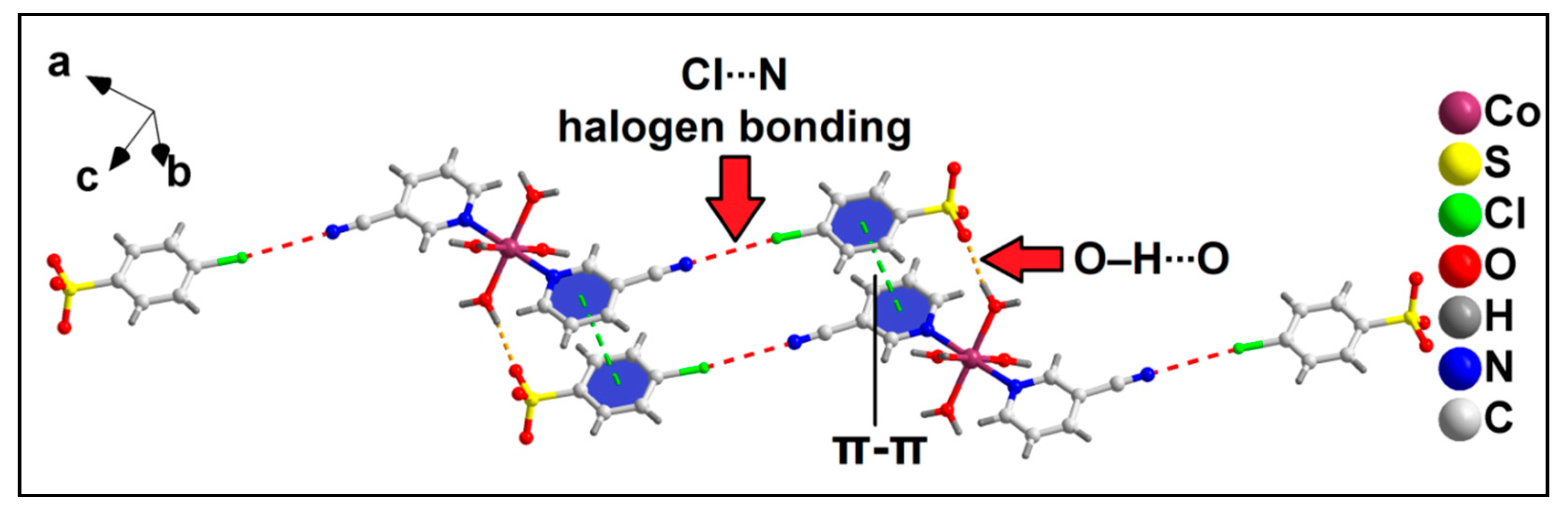
2.2. Crystal Structure Analysis
2.3. Spectral Studies
2.3.1. FT-IR Spectroscopy
2.3.2. Electronic Spectroscopy
2.4. Thermogravimetric Analyses
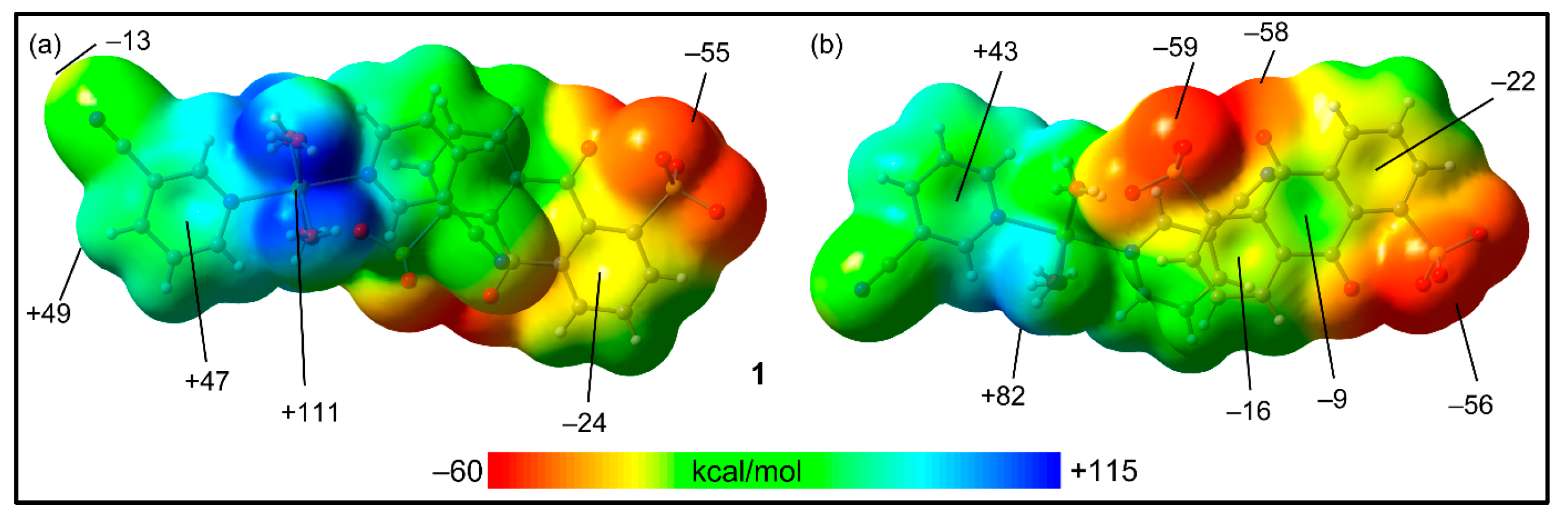
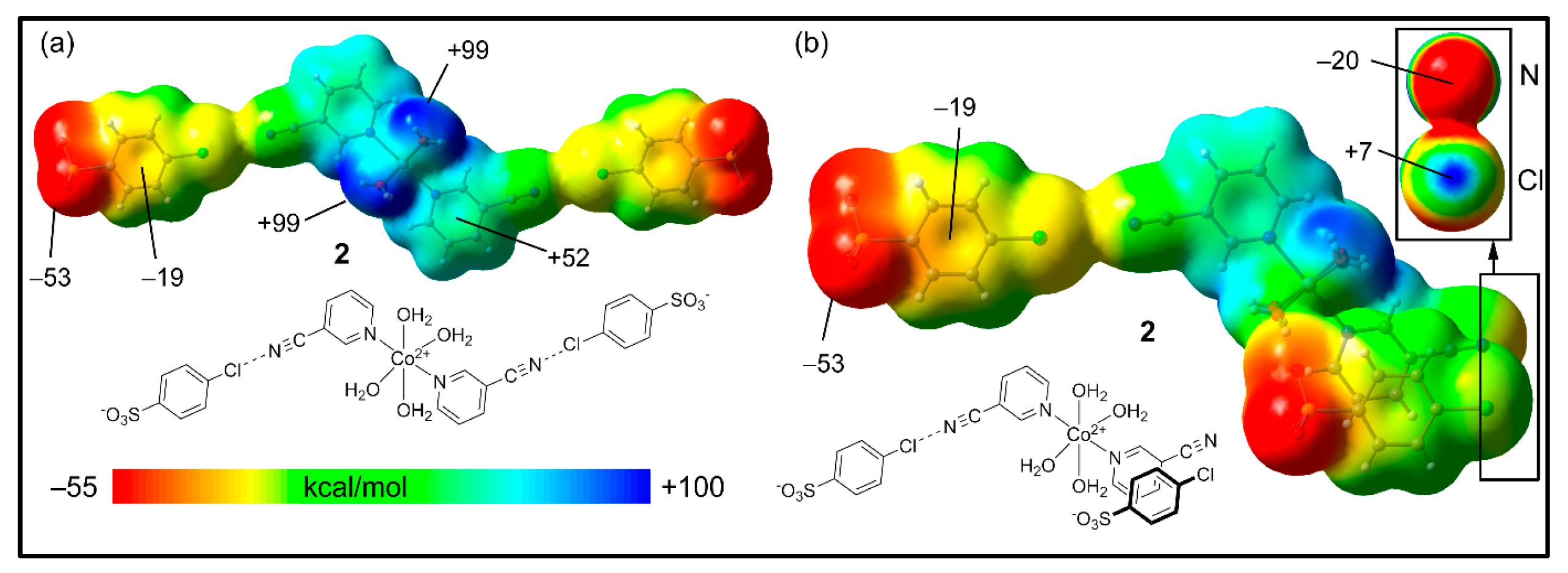
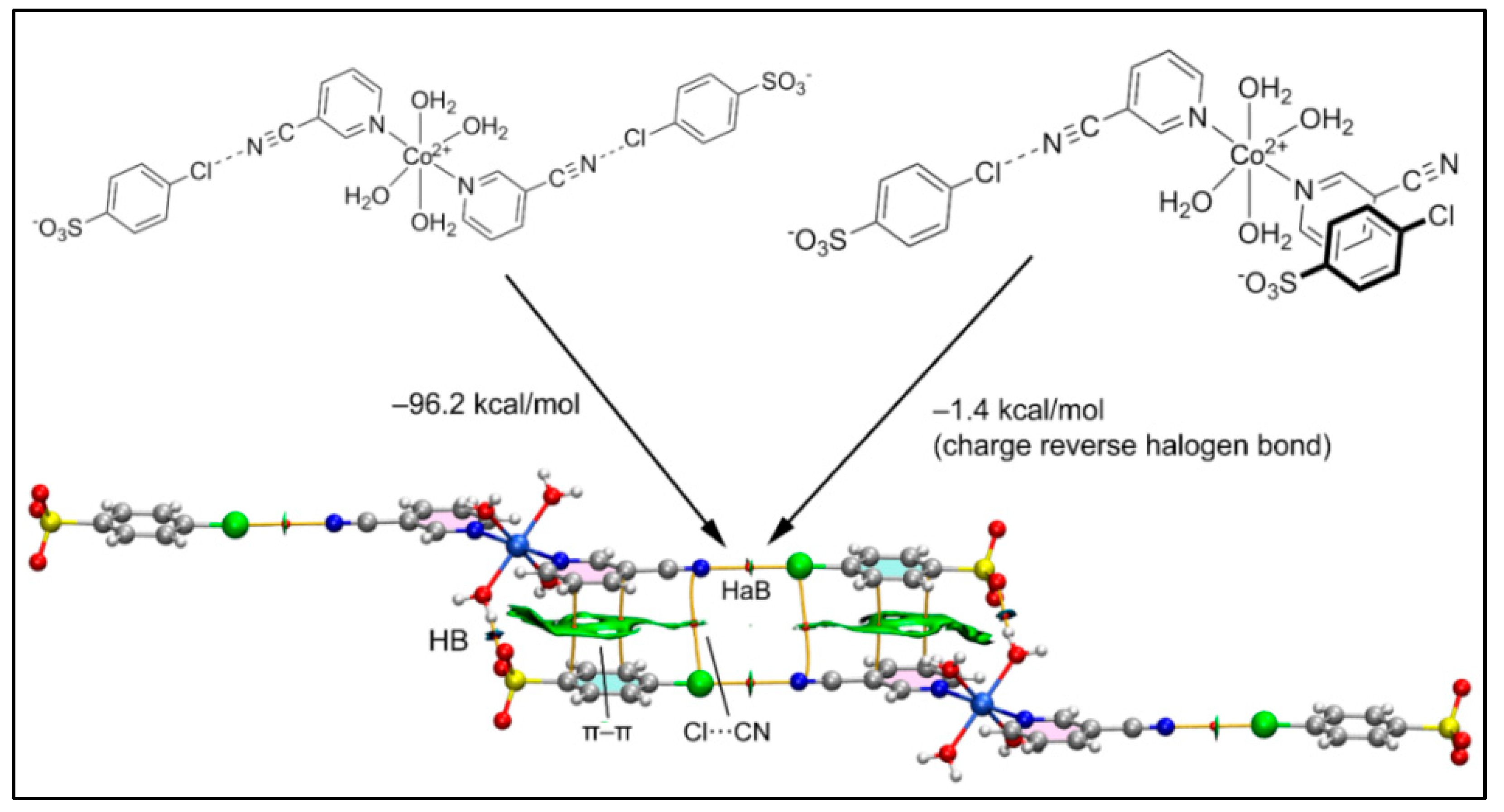
2.5. Theoretical Study
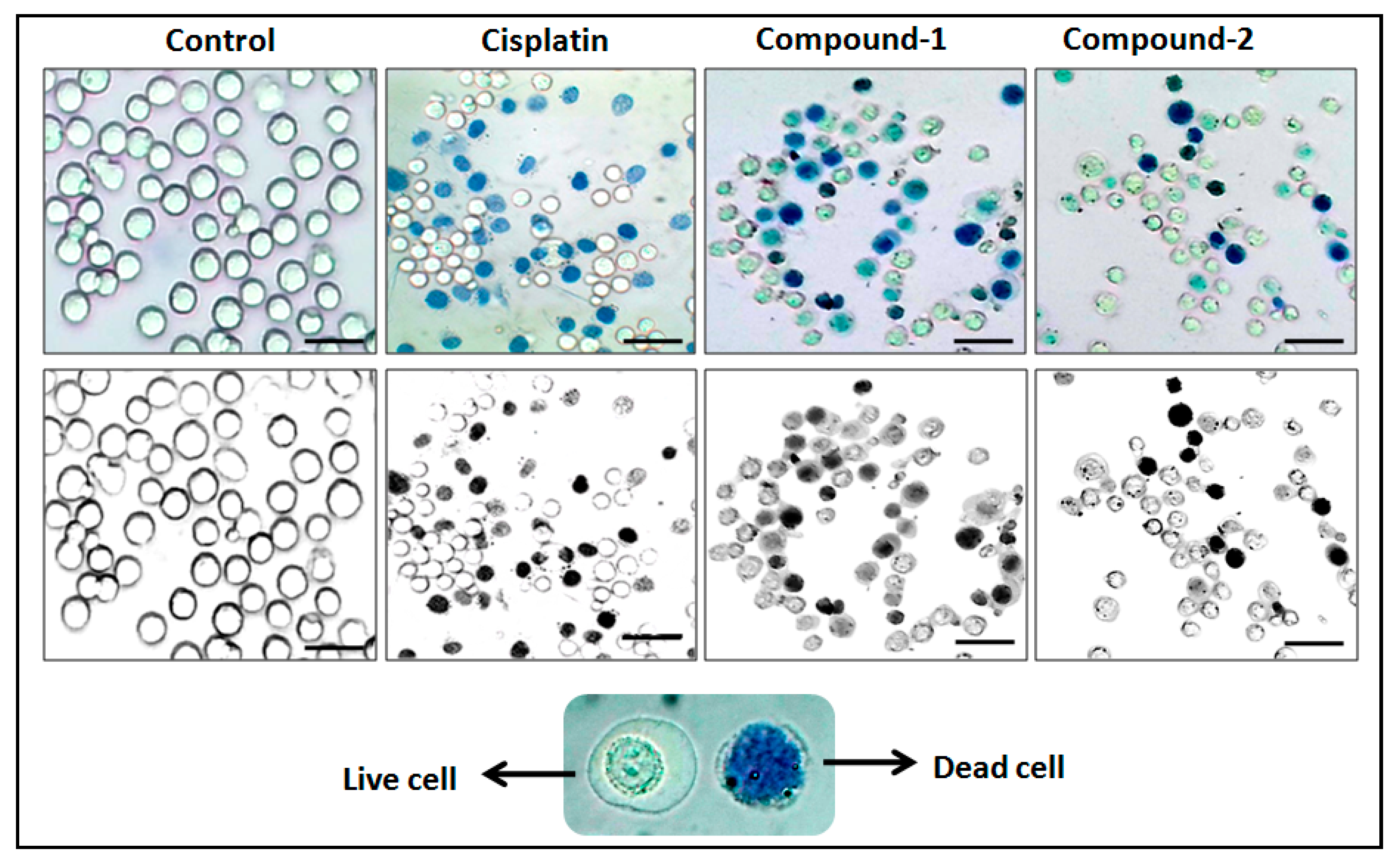
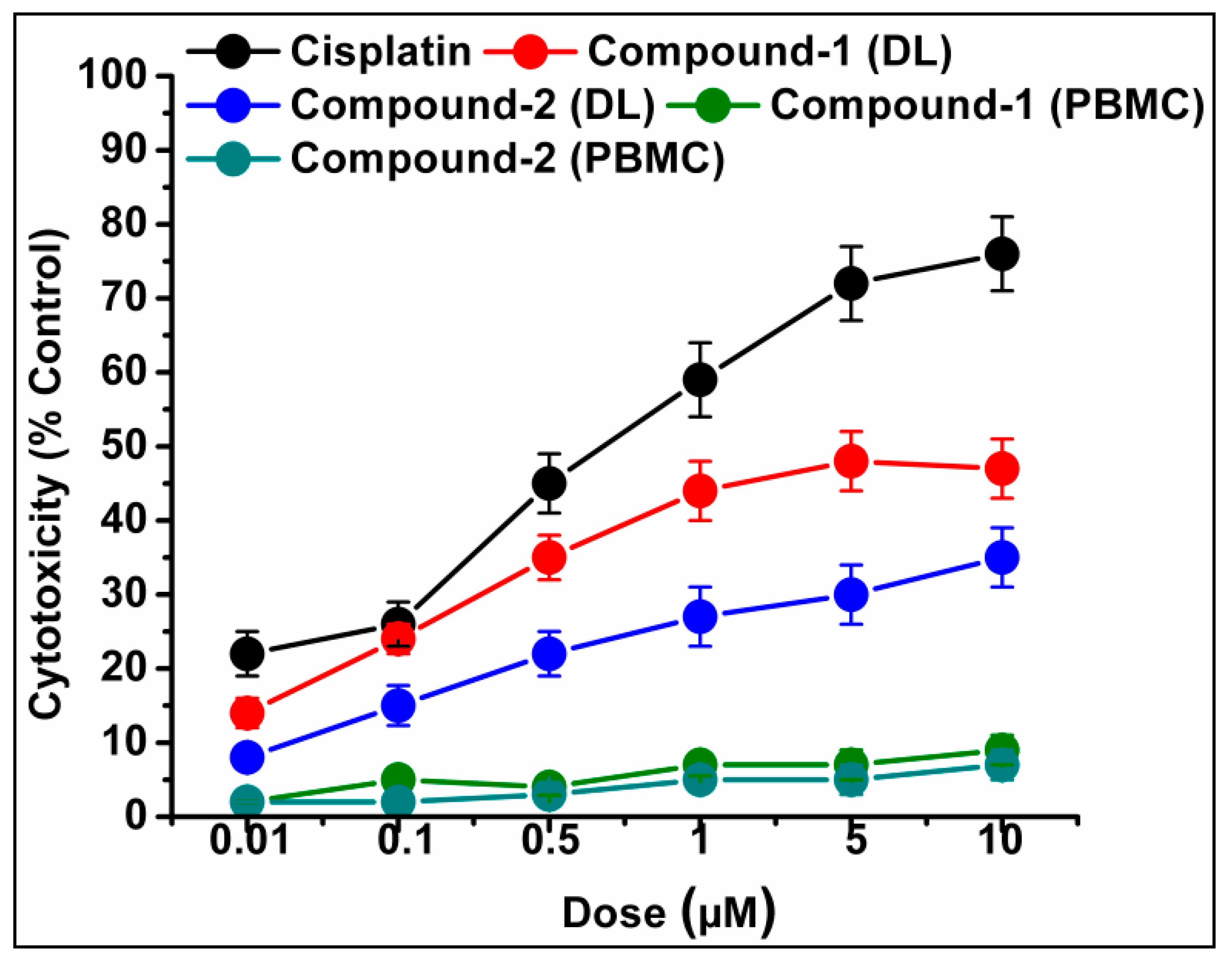
2.6. Cytotoxicity Assay Using Trypan Blue Method
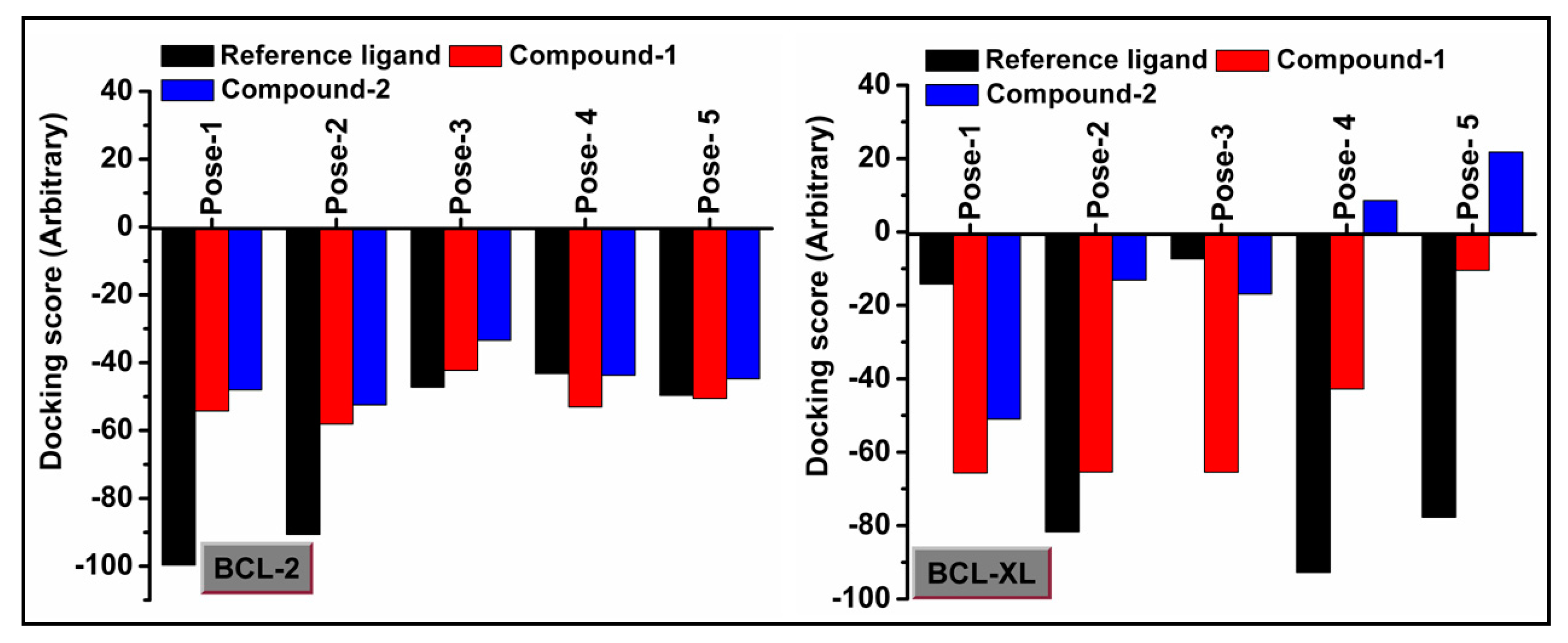
2.7. Molecular Docking Simulation
3. Materials and Methods
3.1. Synthesis
3.1.1. Synthesis of [Ni(3-CNpy)2(H2O)4]ADS·2.75H2O (1)
3.1.2. Synthesis of [Co(3-CNpy)2(H2O)4] (4-ClbzSO3)2 (2)
3.2. Crystallographic Data Collection and Refinement
3.3. Computational Methods
3.4. Cell Line and Drug Preparation
3.5. Cytotoxicity Study Using Trypan Blue Exclusion Method
3.6. Molecular Docking Simulation
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Barszcz, B.; Masternak, J.; Kowalik, M. Structural Insights into Coordination Polymers Based on 6s2 Pb(II) and Bi(III) Centres Connected via Heteroaromatic Carboxylate Linkers and Their Potential Applications. Coord. Chem. Rev. 2021, 443, 213935–213948. [Google Scholar] [CrossRef]
- Yue, Q.; Wang, Y.-Y.; Hu, X.-L.; Guo, W.-X.; Gao, E.-Q. Eight Coordination Compounds Assembled from Unexplored Semi-Rigid Ether-Based Unsymmetrical Tetracarboxylate and Various Dipyridyl Ligands: Structural Variation, Magnetic and Photoluminescence Properties. CrystEngComm 2019, 21, 6719–6732. [Google Scholar] [CrossRef]
- Rathnayake, H. Introductory Chapter:Self-Assembly of Molecules into Supramolecular Structures. InSelf-Assembly of Materials and Their Applications; IntechOpen: Rijeka, Croatia, 2023. [Google Scholar]
- Uchida, K.; Mita, K.; Yamamoto, S.; Tanaka, K. Conformational Relaxation of Ethylene-Propylene-Diene Terpolymer at a Solid Interface. Polym. J. 2023, 55, 683–690. [Google Scholar] [CrossRef]
- Li, X.-Z.; Tian, C.-B.; Sun, Q.-F. Coordination-Directed Self-Assembly of Functional Polynuclear Lanthanide Supramolecular Architectures. Chem. Rev. 2022, 122, 6374–6458. [Google Scholar] [CrossRef] [PubMed]
- Beheshti, A.; Fard, E.S.M.; Kubicki, M.; Mayer, P.; Abrahams, C.T.; Razatofighi, S.E. Design, Synthesis and Characterization of Copper-Based Coordination Compounds with Bidentate (N,N and N,O) Ligands: Reversible Uptake of Iodine, Dye Adsorption and Assessment of Their Antibacterial Properties. CrystEngComm 2019, 21, 251–262. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural Variation in Copper(I) Complexes with Pyridylmethylamide Ligands: Structural Analysis with a New Four-Coordinate Geometry Index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, J.; Chen, M.; Tang, C.; Zhao, H.; Dong, Q.; Yu, W.-D.; Jiang, Z.; Chen, B.; Li, X.; et al. Construction of a π-Stacked Supramolecular Framework Using a Triphenylene-Cored Metallo-Organic Cage. Inorg. Chem. Front. 2023, 10, 621–629. [Google Scholar] [CrossRef]
- Dutta, D.; Islam, S.M.N.; Saha, U.; Chetry, S.; Guha, A.K.; Bhattacharyya, M.K. Structural topology of weak non-covalent interactions in a layered supramolecular coordination solid of zinc involving 3-aminopyridine and benzoate: Experimental and theoretical studies. J. Chem. Crystallogr. 2018, 48, 156–163. [Google Scholar] [CrossRef]
- Liu, H.-Y.; Bo, L.; Yang, J.; Liu, Y.-Y.; Ma, J.-F.; Wu, H. Two Novel Inorganic–Organic Hybrid Materials Constructed from Two Kinds of Octamolybdate Clusters and Flexible Tetradentate Ligands. Dalton Trans. 2011, 40, 9782–9788. [Google Scholar] [CrossRef] [PubMed]
- Aakeröy, C.B.; Champness, N.R.; Janiak, C. Recent Advances in Crystal Engineering. CrystEngComm 2010, 12, 22–43. [Google Scholar] [CrossRef]
- Mirzaei, M.; Eshtiagh-Hosseini, H.; Bolouri, Z.; Rahmati, Z.; Esmaeilzadeh, A.; Hassanpoor, A.; Bauza, A.; Ballester, P.; Barceló-Oliver, M.; Mague, J.T.; et al. Rationalization of Noncovalent Interactions within Six New MII/8-Aminoquinoline Supramolecular Complexes (MII = Mn, Cu, and Cd): A Combined Experimental and Theoretical DFT Study. Cryst. Growth Des. 2015, 15, 1351–1361. [Google Scholar] [CrossRef]
- Schießl, J.; Schulmeister, J.; Doppiu, A.; Wörner, E.; Rudolph, M.; Karch, R.; Hashmi, A.S.K. An Industrial Perspective on Counter Anions in Gold Catalysis: On Alternative Counter Anions. Adv. Synth. Catal. 2018, 360, 3949–3959. [Google Scholar] [CrossRef]
- Khavasi, H.R.; Mohammad Sadegh, B.M. Temperature-Dependent Supramolecular Motif in Coordination Compounds. Inorg. Chem. 2010, 49, 5356–5358. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.-S.; Park, S.H.; Kim, K.M.; Jang, H.G. Solvent-Dependent Structures of Co(NO3)2 with 1,2-Bis(4-pyridyl)ethylene. Interconversion of Molecular Ladders versus Mononuclear Complexes. Inorg. Chem. 1998, 37, 5781–5785. [Google Scholar] [CrossRef]
- Das, A.; Sharma, P.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Ahmed, R.S.; Hussain, S.; Bhattacharyya, M.K. Supramolecular Assemblies Involving Biologically Relevant Antiparallel π-Stacking and Unconventional Solvent Driven Structural Topology in Maleato and Fumarato Bridged Zn(II) Coordination Polymers: Antiproliferative Evaluation and Theoretical Studies. New J. Chem. 2021, 45, 13040–13055. [Google Scholar] [CrossRef]
- Aitipamula, S.; Banerjee, R.; Bansal, A.K.; Biradha, K.; Cheney, M.L.; Choudhury, A.R.; Desiraju, G.R.; Dikundwar, A.G.; Dubey, R.; Duggirala, N.; et al. Polymorphs, Salts, and Cocrystals: What’s in a Name? Cryst. Growth Des. 2012, 12, 2147–2152. [Google Scholar] [CrossRef]
- Chettri, A.; Subba, A.; Singh, G.P.; Bag, P.P. Pharmaceutical Co-Crystals: A Green Way to Enhance Drug Stability and Solubility for Improved Therapeutic Efficacy. J. Pharm. Pharmacol. 2023, 76, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Barman, D.; Annadhasan, M.; Bidkar, A.P.; Rajamalli, P.; Barman, D.; Ghosh, S.S.; Chandrasekar, R.; Iyer, P.K. Highly Efficient Color-Tunable Organic Co-Crystals Unveiling Polymorphism, Isomerism, Delayed Fluorescence for Optical Waveguides and Cell-Imaging. Nat. Commun. 2023, 14, 6648–6664. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Wang, Y.; Xue, J.; Liu, J.; Qin, J.; Hong, Z.; Du, Y. Solid Phase Drug-Drug Pharmaceutical Co-Crystal Formed Between Pyrazinamide and Diflunisal: Structural Characterization Based on Terahertz/Raman Spectroscopy Combining with DFT Calculation. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2020, 234, 118265–118272. [Google Scholar] [CrossRef]
- Abidi, S.S.A.; Garg, U.; Azim, Y.; Alam, M.; Gupta, A.K.; Pradeep, C.P.; Azum, N.; Asiri, A.M. Spectroscopic, Structural, DFT and Molecular Docking Studies on Novel Cocrystal Salt Hydrate of Chromotropic Acid and Its Antibiofilm Activity. Arab J. Sci. Eng. 2021, 46, 353–364. [Google Scholar] [CrossRef]
- Hammud, H.H.; McManus, G.J.; Zaworotko, M.J.; Tabesh, R.N.; Ibrahim, H.I.M.; Ayub, K.; Ludwig, R. The Co-Crystal of Copper(II) Phenanthroline Chloride Complex Hydrate with p-Aminobenzoic Acid: Structure, Cytotoxicity, Thermal Analysis, and DFT Calculation. Monatsh. Chem. 2021, 152, 323–336. [Google Scholar] [CrossRef]
- Hegde, T.A.; Vinitha, G. Chloridocobaltate(II) Metal–Organic Cocrystal Delivering Intermolecular-Charge Transfer-Enhanced Passive Optical Limiting: A Comprehensive Study on Structure–Property Relation. Eur. Phys. J. D 2021, 75, 214. [Google Scholar] [CrossRef]
- Hegde, T.A.; Dutta, A.; Sabari Girisun, T.C.; Vinitha, G. A Novel Organic-Inorganic Ionic Cocrystal—Piperazine-1,4-Diium Tetrachloridocuprate(II) Dihydrate Delivering Efficient Optical Limiting. Chem. Phys. Lett. 2021, 781, 138971–138980. [Google Scholar] [CrossRef]
- Cao, F.-Y.; Huang, M.-P.; Gao, H.-L.; Zhao, X.-L.; Han, J.; Chen, X.-D. Lanthanide Complexes of Anthraquinone-1,8-Disulfonate: Syntheses, Structures and Catalytic Studies. Inorg. Chem. Commun. 2021, 130, 108682–108687. [Google Scholar] [CrossRef]
- Shimizu, G.K.H.; Vaidhyanathan, R.; Taylor, J.M. Phosphonate and Sulfonate Metal Organic Frameworks. Chem. Soc. Rev. 2009, 38, 1430–1449. [Google Scholar] [CrossRef] [PubMed]
- Platero-Prats, A.E.; Iglesias, M.; Snejko, N.; Monge, Á.; Gutiérrez-Puebla, E. From Coordinatively Weak Ability of Constituents to Very Stable Alkaline-Earth Sulfonate Metal−Organic Frameworks. Cryst. Growth Des. 2011, 11, 1750–1758. [Google Scholar] [CrossRef]
- D’Vries, R.F.; Snejko, N.; Gutiérrez-Puebla, E.; Iglesias, M.; Monge, M. Angeles. Supramolecular Structures via Hydrogen Bonds and π-Stacking Interactions in Novel Anthraquinonedisulfonates of Zinc, Nickel, Cobalt, Copper and Manganese. Inorganica Chim. Acta 2012, 382, 119–126. [Google Scholar] [CrossRef]
- Lian, Z.-X.; Li, H.-H. Bis(ethylenediamine-κ2N,N′)copper(II) 9,10-Dioxoanthracene-2,6-Disulfonate. Acta Cryst. 2007, 63, 853–855. [Google Scholar]
- Cao, Y.-Y. Aqua (9,10-Dioxoanthracene-1,5-Disulfonato-κO1)bis (1,10-Phenanthroline-κ2N,N′)nickel(II). Acta Cryst. 2010, 66, 1188. [Google Scholar]
- Jia, J.; Feng, W.; Zhao, H.-K.; Yang, E.-C. catena-Poly[[[bis (1,10-phenanthroline-κ2N,N′)manganese(II)]-μ-9,10-dioxoanthracene-1,5-disulfonato-κ2O1:O5] tetrahydrate]. Acta Cryst. 2009, 65, 695–696. [Google Scholar]
- Li, Y.; Guo, F.; Chen, T.; Zhang, L.; Wang, Z.; Su, Q.; Feng, L. Design, Synthesis, Molecular Docking, and Biological Evaluation of New Emodin Anthraquinone Derivatives as Potential Antitumor Substances. Chem. Biodivers. 2020, 17, 2000328–2000359. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, Y.-B.; Kurtán, T.; Liu, M.-X.; Tang, H.; Zhuang, C.-L.; Zhang, W. Anthraquinone Derivatives from a Coral Associated Fungus Stemphylium lycopersici. Nat. Prod. Res. 2020, 34, 2116–2123. [Google Scholar] [CrossRef]
- Tian, W.; Wang, C.; Li, D.; Hou, H. Novel Anthraquinone Compounds as Anticancer Agents and Their Potential Mechanism. Future Med. Chem. 2020, 12, 627–644. [Google Scholar] [CrossRef] [PubMed]
- Partl, G.J.; Nussbaumer, F.; Schuh, W.; Kopacka, H.; Wurst, K.; Peringer, P. Crystal Structures of Two PCN Pincer Iridium Complexes and One PCP Pincer Carbodiphosphorane Iridium Intermediate: Substitution of One Phosphine Moiety of a Carbodiphosphorane by an Organic Azide. Acta Cryst. E 2019, 75, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Biswas, K.; Ghosh, P.; Basu, B. New 1,2-Dithioether Based 2D Copper(I) Coordination Polymer: From Synthesis to Catalytic Application in A3-Coupling Reaction. J. Coord. Chem. 2019, 72, 1810–1819. [Google Scholar] [CrossRef]
- Li, F.F.; Ma, J.F.; Song, S.Y.; Yang, J.; Jia, H.Q.; Hu, N.H. Syntheses, structures, and characterizations of four new silver (I) sulfonate coordination polymers with neutral ligands. Cryst. Growth Des. 2006, 6, 209–215. [Google Scholar] [CrossRef]
- Zhang, J.-P.; Lin, Y.-Y.; Huang, X.-C.; Chen, X.-M. From One- to Three-Dimensional Architectures: Supramolecular Isomerism of Copper(I) 3,5-Di(4-pyridyl)-1,2,4-triazolate Involving in Situ Ligand Synthesis. Cryst. Growth Des. 2006, 6, 519–523. [Google Scholar] [CrossRef]
- Du, M.; Zhao, X.-J.; Batten, S.R.; Ribas, J. From 1-D Coordination Polymers to 3-D Hydrogen-Bonding Networks: Crystal Engineering and Magnetism of CuII−dca−Cyanopyridine Supramolecular Systems (dca = Dicyanamide, N(CN)2−). Cryst. Growth Des. 2005, 5, 901–909. [Google Scholar] [CrossRef]
- Dinolfo, P.H.; Benkstein, K.D.; Stern, C.L.; Hupp, J.T. C- and Z-Shaped Coordination Compounds. Synthesis, Structure, and Spectroelectrochemistry of cis- and trans-[Re(CO)3(L)]2-2,2‘-bisbenzimidizolate with L = 4-Phenylpyridine, 2,4‘-Bipyridine, or Pyridine. Inorg. Chem. 2005, 44, 8707–8714. [Google Scholar] [CrossRef] [PubMed]
- Noro, S.; Kitagawa, S.; Nakamura, T.; Wada, T. Synthesis and Crystallographic Characterization of Low-Dimensional and Porous Coordination Compounds Capable of Supramolecular Aromatic Interaction Using the 4,4‘-Azobis(pyridine) Ligand. Inorg. Chem. 2005, 44, 3960–3971. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Zimmerman, S.C.; Nakashima, S. A Highly Stable Quadruply Hydrogen-Bonded Heterocomplex Useful for Supramolecular Polymer Blends. J. Am. Chem. Soc. 2005, 127, 6520–6521. [Google Scholar] [CrossRef] [PubMed]
- Sijbesma, R.P.; Beijer, F.H.; Brunsveld, L.; Folmer, B.J.B.; Hirschberg, J.H.K.K.; Lange, R.F.M.; Lowe, J.K.L.; Meijer, E.W. Reversible Polymers Formed from Self-Complementary Monomers Using Quadruple Hydrogen Bonding. Science 1997, 278, 1601–1604. [Google Scholar] [CrossRef] [PubMed]
- Hobza, P.; Zahradník, R.; Müller-Dethlefs, K. The World of Non-Covalent Interactions: 2006. Collect. Czech. Chem. Commun. 2006, 71, 443–531. [Google Scholar] [CrossRef]
- Niemann, T.; Stange, P.; Strate, A.; Ludwig, R. Like-likes-Like: Cooperative Hydrogen Bonding Overcomes Coulomb Repulsion in Cationic Clusters with Net Charges up to Q=+6e. ChemPhysChem 2018, 19, 1691–1695. [Google Scholar] [CrossRef] [PubMed]
- Mudsainiyan, R.K.; Pandey, S.K. A Combined Theoretical Calculation and Hirshfeld Surface Analysis of Cooperative Non-covalent Interactions in the Crystal Packing in [Cu(L1)2(EDA)]. Z. Anorg. Allg. Chem. 2017, 643, 1245–1252. [Google Scholar] [CrossRef]
- Sikorski, A.; Trzybiński, D. Networks of Intermolecular Interactions Involving Nitro Groups in the Crystals of Three Polymorphs of 9-Aminoacridinium 2,4-Dinitrobenzoate⋅2,4-Dinitrobenzoic Acid. J. Mol. Struct. 2013, 1049, 90–98. [Google Scholar] [CrossRef]
- Ojala, C.R.; Ojala, W.H.; Britton, D. Solid-State Intermolecular Contacts Involving the Nitrile Group in p-Cyano-N-(p-cyanobenzylidene)aniline and 4,4′-(azinodimethylidyne)bis-benzonitrile. J. Chem. Crystallogr. 2011, 41, 464–469. [Google Scholar] [CrossRef]
- Dutta, D.; Nashre-ul-Islam, S.M.; Saha, U.; Frontera, A.; Bhattacharyya, M.K. Cu(II) and Co(II) Coordination Solids Involving Unconventional Parallel Nitrile(π)–Nitrile(π) and Energetically Significant Cooperative Hydrogen Bonding Interactions: Experimental and Theoretical Studies. J. Mol. Struct. 2019, 1195, 733–743. [Google Scholar] [CrossRef]
- Lee, S.; Mallik, A.B.; Fredrickson, D.C. Dipolar−Dipolar Interactions and the Crystal Packing of Nitriles, Ketones, Aldehdyes, and C(sp2)−F Groups. Cryst. Growth Des. 2004, 4, 279–290. [Google Scholar] [CrossRef]
- Wood, P.A.; Borwick, S.J.; Watkin, D.J.; Motherwell, W.D.S.; Allen, F.H. Dipolar C≡N⋯C≡N Interactions in Organic Crystal Structures: Database Analysis and Calculation of Interaction Energies. Acta Cryst. B 2008, 64, 393–396. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Kopylovich, M.N.; Sabbatini, A.; Drew, M.G.B.; Martins, L.M.D.R.S.; Pettinari, C.; Pombeiro, A.J.L. Cooperative Metal–Ligand Assisted E/Z Isomerization and Cyano Activation at CuII and CoII Complexes of Arylhydrazones of Active Methylene Nitriles. Inorg. Chem. 2014, 53, 9946–9958. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, N.; Onami, T. Magnesium-Induced Copper-Catalyzed Synthesis of Unsymmetrical Diaryl Chalcogenide Compounds from Aryl Iodide via Cleavage of the Se−Se or S−S Bond. J. Org. Chem. 2004, 69, 915–920. [Google Scholar] [CrossRef] [PubMed]
- Wolstenholme, D.J.; Dobson, J.L.; McGrady, G.S. Homopolar Dihydrogen Bonding in Main Group Hydrides: Discovery, Consequences, and Applications. Dalton Trans. 2015, 44, 9718–9731. [Google Scholar] [CrossRef] [PubMed]
- Bertani, R.; Sgarbossa, P.; Venzo, A.; Lelj, F.; Amati, M.; Resnati, G.; Pilati, T.; Metrangolo, P.; Terraneo, G. Halogen Bonding in Metal–Organic–Supramolecular Networks. Coord. Chem. Rev. 2010, 254, 677–695. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.; Murray, J.S. An Overview of Halogen Bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Murray, J.S.; Fanfrlík, J.; Řezáč, J.; Solá, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen Bond Tunability I: The Effects of Aromatic Fluorine Substitution on the Strengths of Halogen-Bonding Interactions Involving Chlorine, Bromine, and Iodine. J. Mol. Model. 2011, 17, 3309–3318. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Ranjani, A.; Gayathri, L.; Saha, S.; Pasan, J.; Dhanasekaran, D.; Akbarsha, M.A.; Maji, M.; Biswas, B. Recognition ofSelf-Assembled Water-Nitrate Cluster in a Co(III)-2,2′-Bipyridine Host: Synthesis, X-Ray Structure, DNA Cleavage, Molecular Docking and Anticancer Activity. J. Chem. Sci. 2016, 128, 1755–1764. [Google Scholar] [CrossRef]
- Saha, U.; Dutta, D.; Nath, H.; Franconetti, A.; Frontera, A.; Bhattacharyya, M.K. Supramolecular Association in Cu(II) Coordination Complexes Involving Energetically Significant NO⋯NO π–Hole Interaction and Cooperative π-Stacked Ternary Assembly: Experimental and Theoretical Studies. Inorganica Chim. Acta 2019, 488, 159–169. [Google Scholar] [CrossRef]
- Siu, C.-K.; Balaj, O.P.; Bondybey, V.E.; Beyer, M.K. Reactions of Large Water Cluster Anions with Hydrogen Chloride: Formation of Atomic Hydrogen and Phase Separation in the Gas Phase. J. Am. Chem. Soc. 2007, 129, 3238–3246. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Ni, J.; Zheng, F.; Cui, Y.; Wang, Q.; Ng, S.W.; Zhu, W. Tetra- and Binuclear Complexes of Hydroxy-Rich Ligands: Supramolecular Structures, Stabilization of Unusual Water Clusters, and Magnetic Properties. Cryst. Growth Des. 2009, 9, 118–126. [Google Scholar] [CrossRef]
- Bai, S.-Q.; Quek, G.Y.H.; Koh, L.L.; Hor, T.S.A. Crystallographic Analysis of Different Water—Halide Cluster Blends in Cationic [(SNS)PdII] Pincer Complexes. CrystEngComm 2010, 12, 226–233. [Google Scholar] [CrossRef]
- Hu, T.; Zhao, X.; Hu, X.; Xu, Y.; Sun, D.; Sun, D. Two Novel Isomeric Organic Anion-Water Aggregations: 1D Tape and 3D 2-Fold Interpenetrated Diamond Network. RSC Adv. 2011, 1, 1682–1686. [Google Scholar] [CrossRef]
- Safin, D.A.; Szell, P.M.; Keller, A.; Korobkov, I.; Bryce, D.L.; Murugesu, M. Interaction of 2,4,6-tris(2-pyrimidyl)-1,3,5-triazine (TPymT) with CoX2 (X= Cl, Br) in water: Trapping of new self-assembled water–chloride/bromide clusters in a [Co (bpca)2]+ host (bpca= bis(2-pyrimidylcarbonyl)amidate anion). New J. Chem. 2015, 39, 7147–7152. [Google Scholar] [CrossRef]
- Nath, H.; Dutta, D.; Sharma, P.; Frontera, A.; Verma, A.K.; Barceló-Oliver, M.; Devi, M.; Bhattacharyya, M.K. Adipato Bridged Novel Hexanuclear Cu(II) and Polymeric Co(II) Coordination Compounds Involving Cooperative Supramolecular Assemblies and Encapsulated Guest Water Clusters in a Square Grid Host: Antiproliferative Evaluation and Theoretical Studies. Dalton Trans. 2020, 49, 9863–9881. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.-G.; Wu, S.-H.; Pan, Z.-H.; Xiao, Z.-J.; Dai, J.-C. Formation of Different Polymeric Water Clusters via Organic Anionic Templates: More Carboxylate Groups Used, More Water Molecules Gathered. Inorg. Chem. Commun. 2014, 39, 34–38. [Google Scholar] [CrossRef]
- Sharma, P.; Nath, H.; Frontera, A.; Barcelo-Oliver, A.K.; Verma, S.; Hussain, M.K.; Bhattacharyya, M.K. Biologically Relevant Unusual Cooperative Assemblies and Fascinating Infinite Crown-Like Supramolecular Nitrate–Water Hosts Involving Guest Complex Cations in Bipyridine and Phenanthroline-Based Cu(II) Coordination Compounds: Antiproliferative Evaluation and Theoretical Studies. New J. Chem. 2021, 45, 8269–8282. [Google Scholar]
- Alamri, M.A.; Al-Jahdali, M.; Al-Radadi, N.S.; Hussien, M.A. Characterization, Theoretical Investigation, and Biological Applications of Mn(II), Co(II), Ni(II), Cu(II), and Zn(II) Complexes of a Triazene Ligand Containing a Benzothiazole Ring. Appl. Organometal. Chem. 2022, 36, e6466. [Google Scholar] [CrossRef]
- Hosseini-Yazdi, S.A.; Samadzadeh-Aghdam, P.; Mirzaahmadi, A.; Khandar, A.A.; Mahmoudi, G.; Ruck, M.; Doert, T.; Balula, S.S.; Cunha-Silva, L. Synthesis, Crystal Structures, Spectroscopic and Electrochemical Studies on Cu(II) and Ni(II) Complexes with Compartmental Nitrogen–Oxygen Mixed Donor Ligands. Polyhedron 2014, 80, 41–46. [Google Scholar] [CrossRef]
- Das, B.K.; Bora, S.J.; Bhattacharyya, M.K.; Barman, R.K. Inverse Bilayer Structure of Mononuclear CoII and NiII Complexes of the Type M(H2O)3(SO4)(4-CNpy)2. Acta Cryst. B 2009, 65, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Nashre-ul-Islam, S.M.; Dutta, D.; Frontera, A.; Bhattacharyya, M.K. Supramolecular Association Involving Nitrile–Nitrile Interactions in Polymeric Mn(II) Coordination Complexes: A Combined Experimental and Theoretical Study. Inorg. Chim. Acta 2019, 487, 424–432. [Google Scholar] [CrossRef]
- Janiak, C. A Critical Account on π–π Stacking in Metal Complexes with Aromatic Nitrogen-Containing Ligands. J. Chem. Soc., Dalton Trans. 2000, 21, 3885–3896. [Google Scholar] [CrossRef]
- Wei, Y.; Yu, Y.; Sa, R.; Li, Q.; Wu, K. Two Cobalt(II) Coordination Polymers [Co2(H2O)4(Hbidc)2]n and [Co(Hbidc)]n (Hbidc = 1H-Benzimidazole-5,6-Dicarboxylate): Syntheses, Crystal Structures, and Magnetic Properties. CrystEngComm 2009, 11, 1054–1060. [Google Scholar] [CrossRef]
- Dutta, D.; Sharma, P.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Baishya, T.; Bhattacharyya, M.K. Supramolecular Assemblies Involving Unconventional Non-Covalent Contacts in Pyrazole-Based Coordination Compounds of Co(II) and Cu(II) Pyridinedicarboxylates: Antiproliferative Evaluation and Theoretical Studies. Polyhedron 2022, 224, 116025–116037. [Google Scholar] [CrossRef]
- Sharma, P.; Sarma, P.; Frontera, A.; Hussain, S.; Verma, A.K.; Bhattacharyya, M.K. Energetically Significant Anti-Parallel π-Stacking and Unconventional Anion-π Interactions in Phenanthroline Based Ni(II) and Cu(II) Coordination Compounds: Antiproliferative Evaluation and Theoretical Studies. Inorganica Chim. Acta 2021, 516, 120082–120093. [Google Scholar] [CrossRef]
- Diehr, S.; Wöhlert, S.; Boeckmann, J.; Näther, C. Tetrakis(3-cyanopyridine-κN1)bis(thiocyanato-κN)cobalt(II) 1,4-Dioxane Disolvate. Acta Cryst. E 2011, 67, m1898. [Google Scholar] [CrossRef] [PubMed]
- Esrafili, M.D.; Ahmadi, B. A Theoretical Investigation on the Nature of Cl⋯N and Br⋯N Halogen Bonds in FArX⋯NCY Complexes (X=Cl, Br and Y=H, F, Cl, Br, OH, NH2, CH3 and CN). Comput. Theor. Chem. 2012, 997, 77–82. [Google Scholar] [CrossRef]
- Bilewicz, E.; Rybarczyk-Pirek, A.J.; Dubis, A.T.; Grabowski, S.J. Halogen Bonding in Crystal Structure of 1-Methylpyrrol-2-yl Trichloromethyl Ketone. J. Mol. Struct. 2007, 829, 208–211. [Google Scholar] [CrossRef]
- Orhan, O.; Çolak, A.T.; Emen, F.M.; Kismali, G.; Meral, O.; Sel, T.; Çilgi, G.K.; Taş, M. Syntheses of Crystal Structures and In Vitro Cytotoxic Activities of New Copper(II) Complexes of Pyridine-2,6-Dicarboxylate. J. Coord. Chem. 2015, 68, 4003–4016. [Google Scholar] [CrossRef]
- Manna, S.C.; Mistri, S.; Jana, A.D. A Rare Supramolecular Assembly Involving Ion Pairs of Coordination Complexes with a Host–Guest Relationship: Synthesis, Crystal Structure, Photoluminescence and Thermal Study. CrystEngComm 2012, 14, 7415–7422. [Google Scholar] [CrossRef]
- Sharma, P.; Gogoi, A.; Verma, A.K.; Frontera, A.; Bhattacharyya, M.K. Charge-Assisted Hydrogen Bond and Nitrile⋯Nitrile Interaction Directed Supramolecular Associations in Cu(II) and Mn(II) Coordination Complexes: Anticancer, Hematotoxicity and Theoretical Studies. New J. Chem. 2020, 44, 5473–5488. [Google Scholar] [CrossRef]
- Figgis, B.N.; Hitchman, M.A. Ligand Field Theory and Its Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 1999. [Google Scholar]
- Heine, M.; Fink, L.; Schmidt, M.U. 4-Cyanopyridine Complexes [MX2(4-CNpy)x]n (with X = Cl, Br and x = 1, 2): Crystal Structures, Thermal Properties and a Comparison with [MX2(3-CNpy)x]n Complexes. CrystEngComm 2020, 22, 2067–2082. [Google Scholar] [CrossRef]
- Hou, X.-Y.; Wang, X.; Fu, F.; Wang, J.-J.; Tang, L.; Wu, Y.-P. Silver(I) Coordination Polymer Based on Anthraquinone-2,6-Disulfonate: Synthesis, Crystal Structure, and Luminescent Properties. J. Coord. Chem. 2012, 65, 2935–2944. [Google Scholar] [CrossRef]
- Fu, M.; Wu, X.-Y.; Jia, J.; Zhao, X.-J.; Yang, E.-C. Four Anthraquinone-1,5-Disulfonate-Based Metal Complexes Incorporating N-Heterocyclic Coligands: Synthesis, Crystal Structures, and Fluorescence. J. Coord. Chem. 2011, 64, 1770–1781. [Google Scholar] [CrossRef]
- Mautner, F.A.; Scherzer, M.; Berger, C.; Fischer, R.C.; Vicente, R.; Massoud, S.S. Synthesis and Characterization of Five New Thiocyanato- and Cyanato-Metal(II) Complexes with 4-Azidopyridine as Co-Ligand. Polyhedron 2015, 85, 20–26. [Google Scholar] [CrossRef]
- Chetry, S.; Sharma, P.; Frontera, A.; Saha, U.; Verma, A.K.; Sarma, B.; Kalita, P.J.; Bhattacharyya, M.K. Biologically Relevant and Energetically Significant Cooperative Ternary (π–π)2/(π–π)1/(π–π)2 Assemblies and Fascinating Discrete (H2O)21 Clusters in Isostructural 2,5-Pyridine Dicarboxylato Co(II) and Zn(II) Phenanthroline Compounds: Antiproliferative Evaluation and Theoretical Studies. New J. Chem. 2021, 45, 3699–3715. [Google Scholar]
- Batool, S.S.; Gilani, S.R.; Tahir, M.N.; Harrison, W.T.A.Z. Syntheses and Structures of Monomeric and Dimeric Ternary Complexes of Copper(II) with 2,2′-Bipyridyl and Carboxylate Ligands. Anorg. Allg. Chem. 2016, 642, 1364–1368. [Google Scholar] [CrossRef]
- Gogoi, A.; Islam, S.M.N.; Frontera, A.; Bhattacharyya, M.K. Supramolecular association in Cu(II) and Co(II) coordination complexes of 3,5-dimethylpyrazole: Experimental and theoretical studies. Inorg. Chim. Acta 2019, 484, 133–141. [Google Scholar] [CrossRef]
- Basumatary, D.; Lal, R.A.; Kumar, A. Synthesis, and characterization of low- and high-spin manganese(II) complexes of polyfunctional adipoyldihydrazone: Effect of coordination of N-donor ligands on stereo-redox chemistry. J. Mol. Struct. 2015, 1092, 122–129. [Google Scholar] [CrossRef]
- Bruijnincx, P.C.; Sadler, P.J. New Trends for Metal Complexes with Anticancer Activity. Curr. Opin. Chem. Biol. 2008, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, S.; Annaraj, J.; Athappan, P.R. Spectral and Redox Studies on Mixed Ligand Complexes of Cobalt(III) Phenanthroline/Bipyridyl and Benzoylhydrazones, Their DNA Binding and Antimicrobial Activity. J. Inorg. Biochem. 2005, 99, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P.; Saini, A.; Singh, S.; Singh, A.; Venugoplalan, P.; Ferretti, V. Second Sphere Coordination Complexes: Synthesis, Characterization, Single Crystal Structure and Packing Analyses of trans-Cu(en)2(H2O)22 where L1=p-Toluenesulphonate, L2=5-Bromo-2-Methoxybenzenesulphonate. J. Mol. Struct. 2010, 969, 155–162. [Google Scholar] [CrossRef]
- Ma, Y.; Mu, B.; Huang, R.-D. Syntheses, Structures and Properties of a Series of Nickel(II) Complexes Derived from Amino-5-Mercapto-1,3,4-Thiadiazole. Transit. Met. Chem. 2018, 43, 103–113. [Google Scholar] [CrossRef]
- Mandal, T.; Dey, A.; Pathak, S.; Islam, M.M.; Konar, S.; Castro, J.O.; Seth, S.K.; Ray, P.P.; Frontera, A.; Mukhopadhyay, S. Structures, Photoresponse Properties and DNA Binding Abilities of 4-(4-Pyridinyl)-2-Pyridone Salts. RSC Adv. 2019, 9, 9663–9677. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Baishya, T.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Das, J.; Bhattacharyya, M.K. Structural Topologies Involving Energetically Significant Antiparallel π-Stacking and Unconventional N(nitrile)⋯π(fumarate) Contacts in Dinuclear Zn(II) and Polymeric Mn(II) Compounds: Antiproliferative Evaluation and Theoretical Studies. New J. Chem. 2022, 46, 5296–5311. [Google Scholar] [CrossRef]
- Manikandan, M.; Chen, T.; Sun, Z.; Zhang, S.; Luo, J. Conformational Polymorphism on Styryl Quinolinium Salts of 2-[(E)-2-(4-Hydroxy-3-Methoxystyrl)-1-Methyl]-Quinolinium 4-Chlorobenzenesulfonate (HMQ-CBS): Potential Nonlinear Optical Crystals for Terahertz Application. Inorg. Chem. Commun. 2015, 61, 165–168. [Google Scholar] [CrossRef]
- Yang, W.; Li, W.; Yu, B.; Liu, C.; Wang, H. Fluorescence Charge-Assisted Hydrogen-Bonded Organic Frameworks Assembled from Tetraphenylethene Amidinium Cation. Inorg. Chem. Commun. 2022, 139, 109396–109415. [Google Scholar] [CrossRef]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Tennant, J.R. Evaluation of the Trypan Blue Technique for Determination of Cell Viability. Transplantation 1964, 2, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Dijt, F.J.; Fichtinger-Schepman, A.M.J.; Berends, F.; Reedijk, J. Formation and Repair of Cisplatin-Induced Adducts to DNA in Cultured Normal and Repair-Deficient Human Fibroblasts. Cancer Res. 1988, 48, 6058–6062. [Google Scholar] [PubMed]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef] [PubMed]
- Lv, D.; Pal, P.; Liu, X.; Jia, Y.; Thummuri, D.; Zhang, P.; Hu, W.; Pei, J.; Zhang, Q.; Zhou, S.; et al. Development of a BCL-xL and BCL-2 Dual Degrader with Improved Anti-Leukemic Activity. Nat. Commun. 2021, 12, 6896. [Google Scholar] [CrossRef]
- APEX3, SAINT, SADABS and XP; Bruker AXS Inc.: Madison, WI, USA, 2015.
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Farrugia, L.J. WinGX and ORTEP for Windows: An Update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Shao, D.; Zhou, Y.; Pi, Q.; Shen, F.-X.; Yang, S.-R.; Zhang, S.-L.; Wang, X.-Y. Two-Dimensional Frameworks Formed by Pentagonal Bipyramidal Cobalt(II) Ions and Hexacyanometallates: Antiferromagnetic Ordering, Metamagnetism and Slow Magnetic Relaxation. Dalton Trans. 2017, 46, 9088–9096. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Brandenburg, K. Diamond 3.1f; Crystal Impact GbR: Bonn, Germany, 2008. [Google Scholar]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate ab initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104–154122. [Google Scholar] [CrossRef]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic Structure Calculations on Workstation Computers: The Program System TURBOMOLE. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Contreras-García, J.; Johnson, E.R.; Keinan, S.; Chaudret, R.; Piquemal, J.-P.; Beratan, D.N.; Yang, W. NCIPLOT: A Program for Plotting Noncovalent Interaction Regions. J. Chem. Theory Comput. 2011, 7, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Bader, R.F.W. A Quantum Theory of Molecular Structure and Its Applications. Chem. Rev. 2002, 102, 731–738. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graphics 1996, 14, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Klein, G. Comparative studies of mouse tumors with respect to their capacity for growth as ‘ascitic tumor’ and their average nucleic acid content. Exp Cell Res 1951, 2, 518–524. [Google Scholar] [CrossRef]
- Koiri, R.K.; Trigun, S.K.; Mishra, L.; Pandey, K.; Dixit, D. Regression of Dalton’s lymphoma in vivo via decline in lactate dehydrogenase and induction of apoptosis by a ruthenium(II)-complex containing 4-carboxy N-ethylbenzamide as ligand. Invest New Drugs 2009, 27, 503–516. [Google Scholar] [CrossRef]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.; Christensen, M.H. MolDock: A New Technique for High-Accuracy Molecular Docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Chetry, S.; Sharma, P.; Frontera, A.; Dutta, D.; Verma, A.K.; Bhattacharyya, M.K. Unconventional Formation of a 1D-Chain of H-Bonded Water Molecules in Bipyridine-Based Supramolecular Hexameric Hosts of Isostructural Coordination Compounds of Co(II) and Zn(II): Antiproliferative Evaluation and Theoretical Studies. Polyhedron 2020, 191, 114809–114824. [Google Scholar] [CrossRef]
- Bhattacharyya, M.K.; Saha, U.; Dutta, D.; Das, A.; Verma, A.K.; Frontera, A. Solvent-Driven Structural Topology Involving Energetically Significant Intra- and Intermolecular Chelate Ring Contacts and Anticancer Activities of Cu(II) Phenanthroline Complexes Involving Benzoates: Experimental and Theoretical Studies. RSC Adv. 2019, 9, 16339–16356. [Google Scholar] [CrossRef] [PubMed]
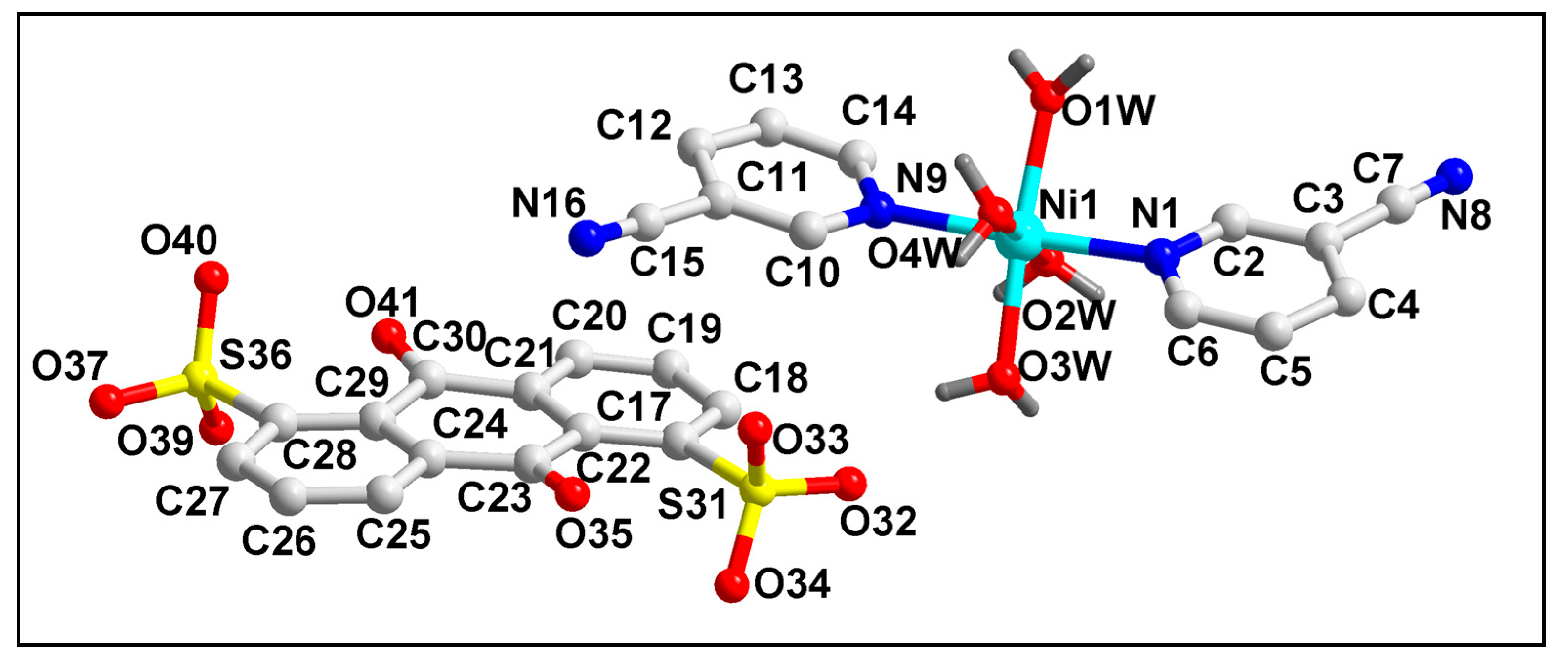


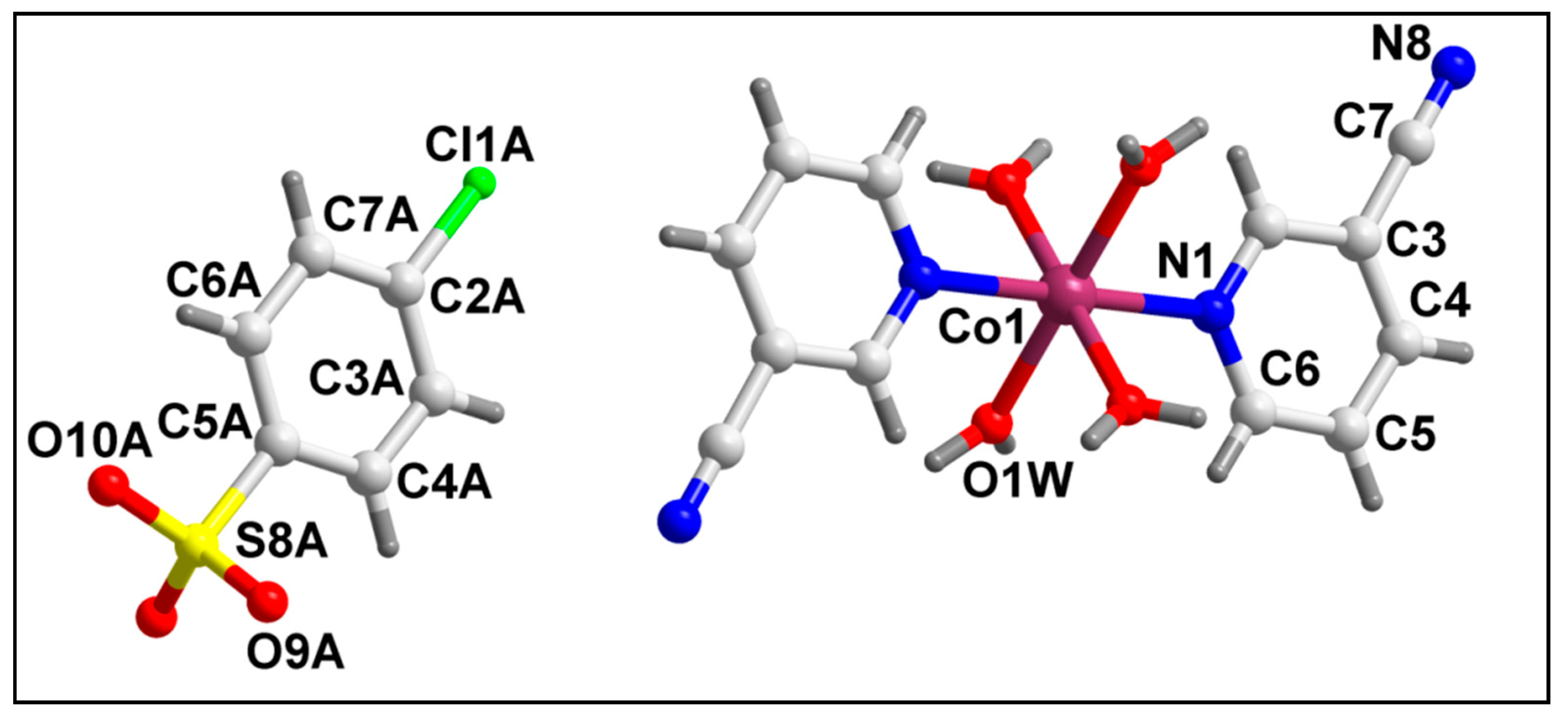
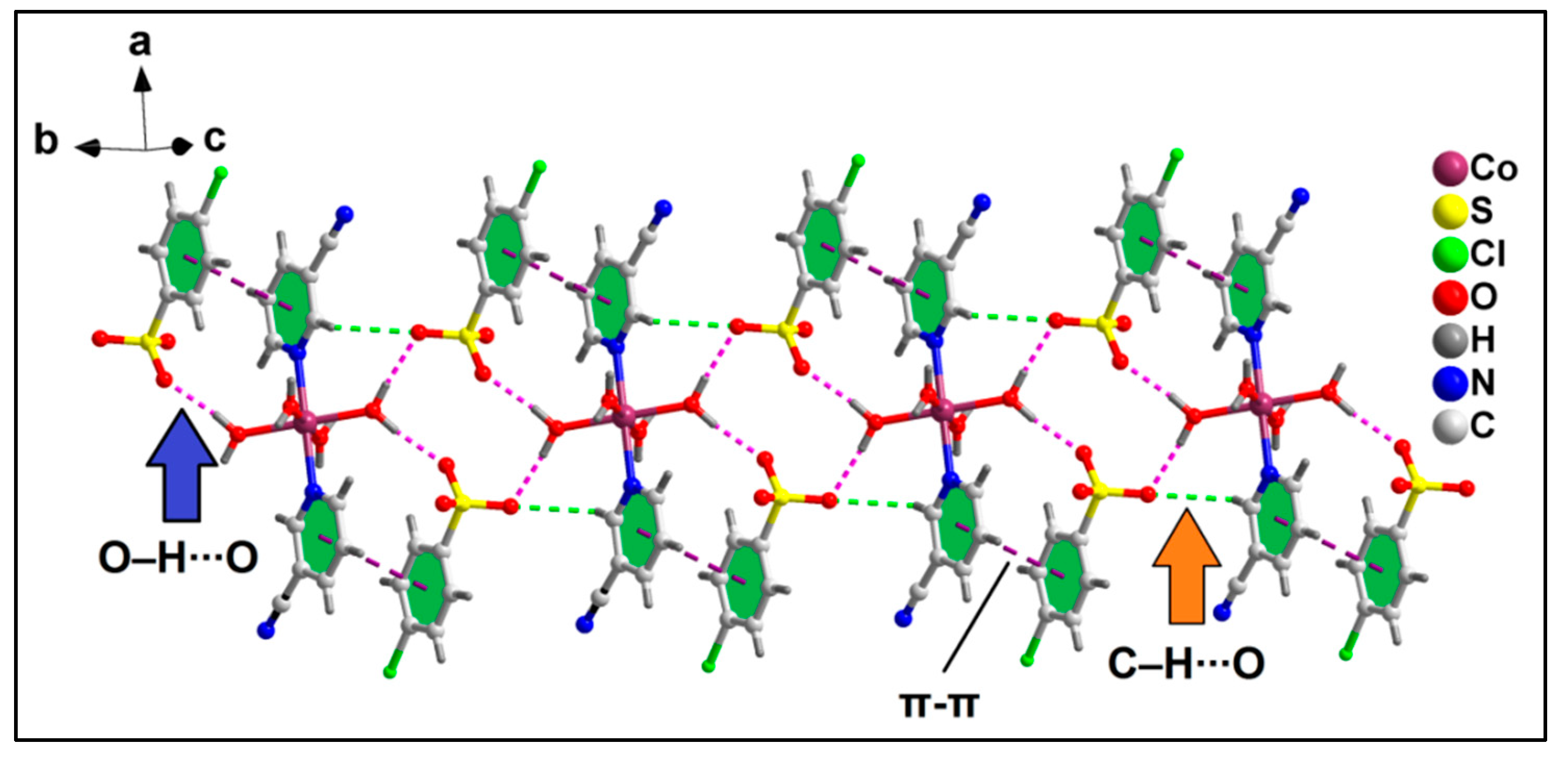
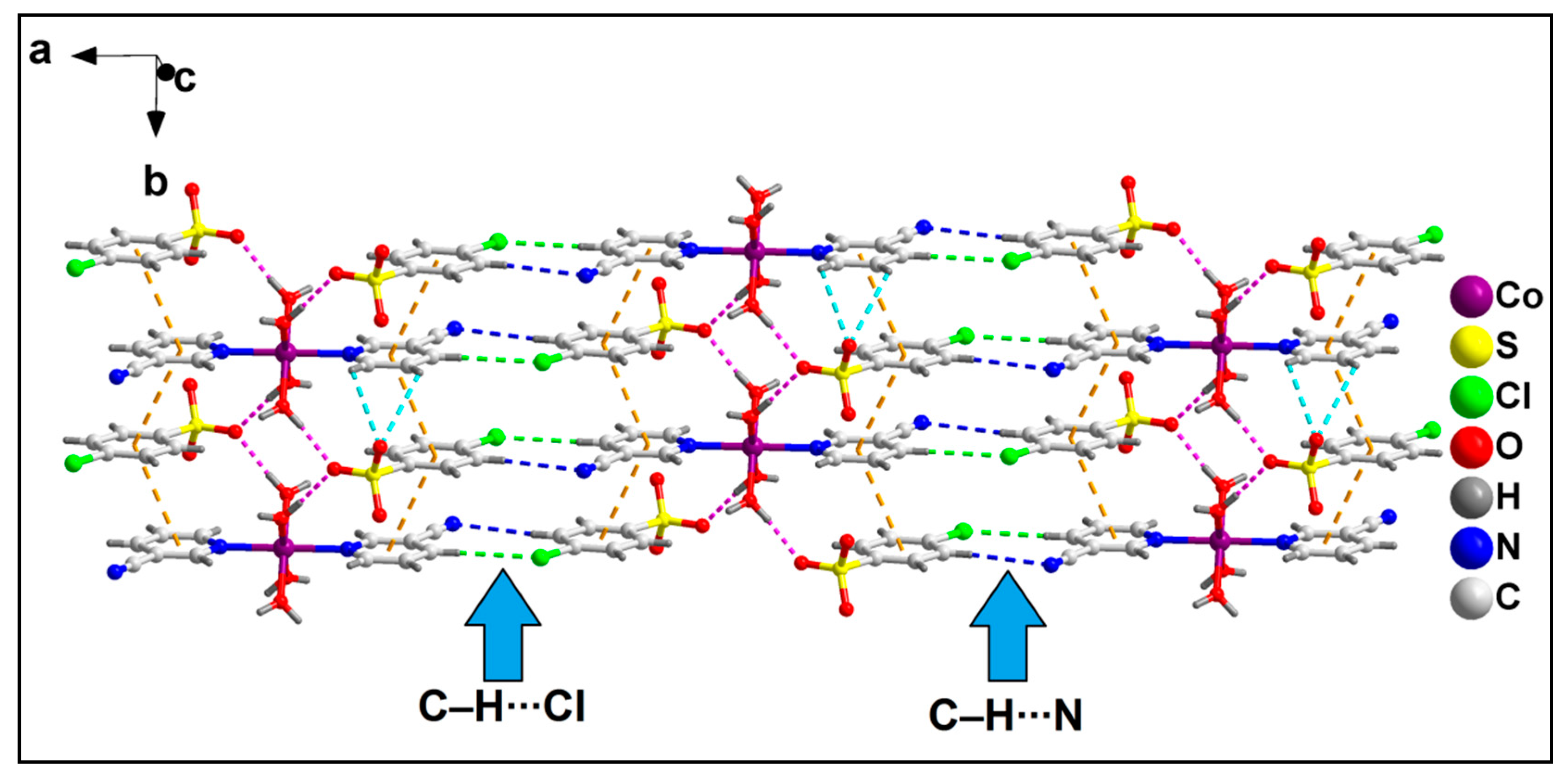




| D–H⋯A | d(D–H) | d(D⋯A) | d(H⋯A) | <(DHA) |
|---|---|---|---|---|
| 1 | ||||
| C5–H5⋯O35#1 | 0.95 | 2.37 | 3.141(3) | 137.9 |
| C13–H13⋯O40#2 | 0.95 | 2.45 | 3.144(3) | 130.0 |
| O1W–H1WA⋯O5W#3 | 0.87 | 1.81 | 2.592(4) | 148.9 |
| O1W–H1WB⋯O32#4 | 0.87 | 1.84 | 2.705(3) | 175.6 |
| O2W–H2WA⋯O8W | 0.87 | 1.90 | 2.769(11) | 174.5 |
| O2W–H2WA⋯O9W | 0.87 | 1.91 | 2.743(10) | 159.4 |
| O2W–H2WB⋯O40#5 | 0.87 | 1.83 | 2.700(3) | 174.4 |
| O3W–H3WA⋯O32 | 0.87 | 1.91 | 2.694(3) | 149.6 |
| O3W–H3WB⋯O37#5 | 0.87 | 1.93 | 2.799(3) | 171.8 |
| O4W–H4WA⋯O6W | 0.87 | 1.90 | 2.751(10) | 166.4 |
| O4W–H4WA⋯O7W | 0.87 | 1.90 | 2.733(11) | 160.8 |
| O4W–H4WB⋯O37#6 | 0.87 | 1.81 | 2.675(3) | 172.4 |
| C27–H27⋯O5W#7 | 0.95 | 2.40 | 3.185(5) | 140.0 |
| O6W–H6WA⋯O33 | 0.87 | 1.81 | 2.669(8) | 167.1 |
| O8W–H8WA⋯O34#8 | 0.87 | 2.00 | 2.857(12) | 170.0 |
| O8W–H8WB⋯O4W#9 | 0.87 | 2.21 | 3.073(12) | 173.8 |
| O9W–H9WA⋯O34# | 0.87 | 1.95 | 2.776(9) | 157.1 |
| O9W–H9WB⋯O4W#9 | 0.87 | 2.26 | 3.009(11) | 144.9 |
| O5W–H5WA⋯O6W | 0.87 | 1.59 | 2.41(2) | 155.5 |
| O5W–H5WB⋯O39#6 | 0.87 | 1.96 | 2.780(4) | 156.9 |
| O7W–H7WA⋯O7W#1 | 0.87 | 1.35 | 2.19(4) | 162.1 |
| O7WH7WB⋯O33 | 0.87 | 1.92 | 2.765(11) | 164.8 |
| 2 | ||||
| C3A–H3A∙∙∙N8 | 0.93 | 3.950 | 2.99 | 168.1 |
| C4–H4∙∙∙Cl1A | 0.93 | 3.943 | 2.86 | 165.2 |
| C5–H5∙∙∙N8 | 0.93 | 3.460 | 2.64 | 146.4 |
| C7A–H7A∙∙∙O9A | 0.93 | 3.644 | 2.92 | 135.1 |
| O1W–H1WA∙∙∙O10A | 0.87 | 2.757(2) | 1.90 | 170.6 |
| O1W–H1WB∙∙∙O9A#10 | 0.87 | 2.770(2) | 1.90 | 176.0 |
| C2–H2∙∙∙O9A | 0.93 | 3.531 | 2.69 | 147.2 |
| Parameters | 1 | 2 |
|---|---|---|
| Formula | C26H27.5N4NiO14.75S2 | C24H24N4O10S2Cl2Co |
| Formula weight | 754.85 | 722.42 |
| Temp, [K] | 100.00 | 294.15 |
| Crystal system | Monoclinic | Monoclinic |
| Space group | P21/c | C2/m |
| a, [Å] | 14.3963(1) | 31.6924(9) |
| b, [Å] | 14.1160(1) | 6.9038(2) |
| c, [Å] | 14.8840(1) | 6.8614(2) |
| α, [°] | 90 | 90 |
| β, [°] | 98.551(2) | 95.4620(1) |
| γ, [°] | 90 | 90 |
| V, [Å3] | 2991.1(4) | 1494.44(7) |
| Z | 4 | 2 |
| Absorption coefficient (mm−1) | 2.973 | 7.993 |
| F(0 0 0) | 1558 | 738 |
| D (calcd), [Mg/m3] | 1.676 | 1.605 |
| Index ranges | −17 ≤ h ≤ 17, −16 ≤ k ≤ 17, −17 ≤ l ≤ 17 | −38 ≤ h ≤ 38, −8 ≤ k ≤ 8, −8 ≤ l ≤ 8 |
| Crystal size, [mm3] | 0.15 × 0.09 × 0.05 | 0.18 × 0.12 × 0.06 |
| θ range, [°] | 4.34 to 68.521 | 2.801 to 68.189 |
| Independent Reflections | 5408 | 1494 |
| Reflections collected | 30,922 | 9510 |
| Refinement method | Full-matrix leastsquares on F2 | Full-matrix leastsquares on F2 |
| Data/restraints/parameters | 5408/0/473 | 1494/0/128 |
| Goodness-of-fit on F2 | 1.130 | 1.085 |
| Final R indices [I >2σ(I)] | R1 = 0.0445, wR2 = 0.1074 | R1 = 0.0311, wR2 = 0.0822 |
| R indices (all data) | R1 = 0.0473, wR2 = 0.1088 | R1 = 0.0313, wR2 = 0.0827 |
| Largest hole and peak [e·Å−3] | −0.44 and 0.42 | −0.44 and 0.41 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banik, S.; Baishya, T.; Gomila, R.M.; Frontera, A.; Barcelo-Oliver, M.; Verma, A.K.; Das, J.; Bhattacharyya, M.K. ‘Charge Reverse’ Halogen Bonding Contacts in Metal-Organic Multi-Component Compounds: Antiproliferative Evaluation and Theoretical Studies. Inorganics 2024, 12, 111. https://doi.org/10.3390/inorganics12040111
Banik S, Baishya T, Gomila RM, Frontera A, Barcelo-Oliver M, Verma AK, Das J, Bhattacharyya MK. ‘Charge Reverse’ Halogen Bonding Contacts in Metal-Organic Multi-Component Compounds: Antiproliferative Evaluation and Theoretical Studies. Inorganics. 2024; 12(4):111. https://doi.org/10.3390/inorganics12040111
Chicago/Turabian StyleBanik, Subham, Trishnajyoti Baishya, Rosa M. Gomila, Antonio Frontera, Miquel Barcelo-Oliver, Akalesh K. Verma, Jumi Das, and Manjit K. Bhattacharyya. 2024. "‘Charge Reverse’ Halogen Bonding Contacts in Metal-Organic Multi-Component Compounds: Antiproliferative Evaluation and Theoretical Studies" Inorganics 12, no. 4: 111. https://doi.org/10.3390/inorganics12040111
APA StyleBanik, S., Baishya, T., Gomila, R. M., Frontera, A., Barcelo-Oliver, M., Verma, A. K., Das, J., & Bhattacharyya, M. K. (2024). ‘Charge Reverse’ Halogen Bonding Contacts in Metal-Organic Multi-Component Compounds: Antiproliferative Evaluation and Theoretical Studies. Inorganics, 12(4), 111. https://doi.org/10.3390/inorganics12040111










