Capturing Unstable Metallofullerenes
Abstract
1. Introduction
2. Unstable Fullerenes
- (1)
- Empty fullerenes. Soluble empty fullerenes have a large HOMO-LUMO gap and a closed-shell electronic structure with the singlet ground state. However, if the HOMO-LUMO gap is small, the triplet (biradical) state may be accessible or even become lower in energy than the singlet (Figure 2a). The molecule thus has an open-shell electronic structure, which is usually synonymous with enhanced reactivity. A typical example of an insoluble empty fullerene with a triplet ground state is D3h-C74 [53].
- (2)
- Monometallofullerenes with trivalent metals, MIII@C2n (Figure 2b). Since endohedral metal atoms transfer their electrons to the fullerene cage, the MIII state implies that the fullerene cage accepts three electrons and thus has a radical nature with one unpaired electron. Interestingly, some monometallofullerenes, such as MIII@C2v(9)-C82, behave as stable fullerenes and can be obtained in pure form by procedure I, while the others, such as MIII@C60 or MIII@C2v(5)-C80, are insoluble in fullerene solvents and require special procedures for extraction and isolation. MIII@Cs(6)-C82 is a good example of a dual behavior as it shows reasonable solubility and can be obtained by conventional procedure I, but tends to form dimers upon crystallization [54,55]. The boundary between the two types is blurred, indicating that the peculiarities of the π-system distribution for a given carbon cage also play an important role.
- (3)
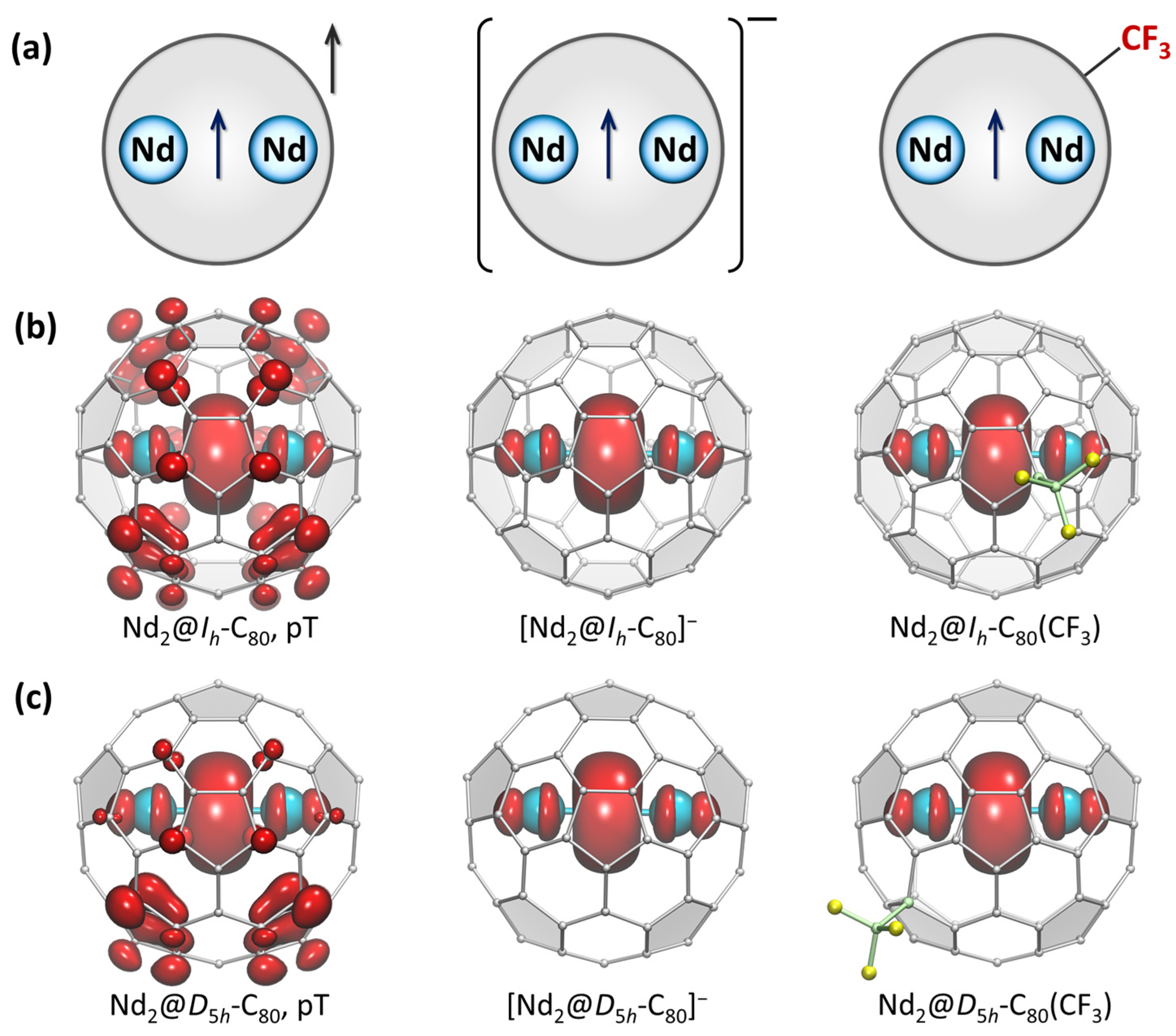
- Dimetallofullerenes of trivalent metals (Figure 2c). Their situation in some way resembles that of empty fullerenes since the transfer of six electrons from the metal dimer to the host fullerene should result in a closed-shell electronic structure. Indeed, dimetallofullerenes of early lanthanides, such as La2@C2n and Ce2@C2n, are soluble in fullerene solvents and can be purified using procedure I. The difference from empty fullerenes is that the LUMO of dimetallofullerenes is usually a metal–metal bonding orbital, and its energy depends on the metal. For heavier lanthanides, starting with Nd, the energy of the metal-based LUMO becomes so low that the triplet state, in which one unpaired electron occupies the metal-based MO and one occupies the fullerene MO, becomes more stable than the closed-shell singlet [56,57].
- (4)
- Clusterfullerenes, i.e., metallofullerenes with endohedral clusters such as M3N, M2C2, M2O, M2S, etc., usually have a closed-shell electronic structure of the carbon cage, and the vast majority of them can be obtained from the soot and separated by procedure I. It is certainly possible that some of them are unstable due to the small HOMO-LUMO gap of the carbon cage, similarly to insoluble empty fullerenes, but we are not aware of any experimentally characterized examples of clusterfullerenes that are not soluble in fullerene solvents but could be extracted and separated by other approaches. Clusterfullerenes are therefore not discussed further in this review.
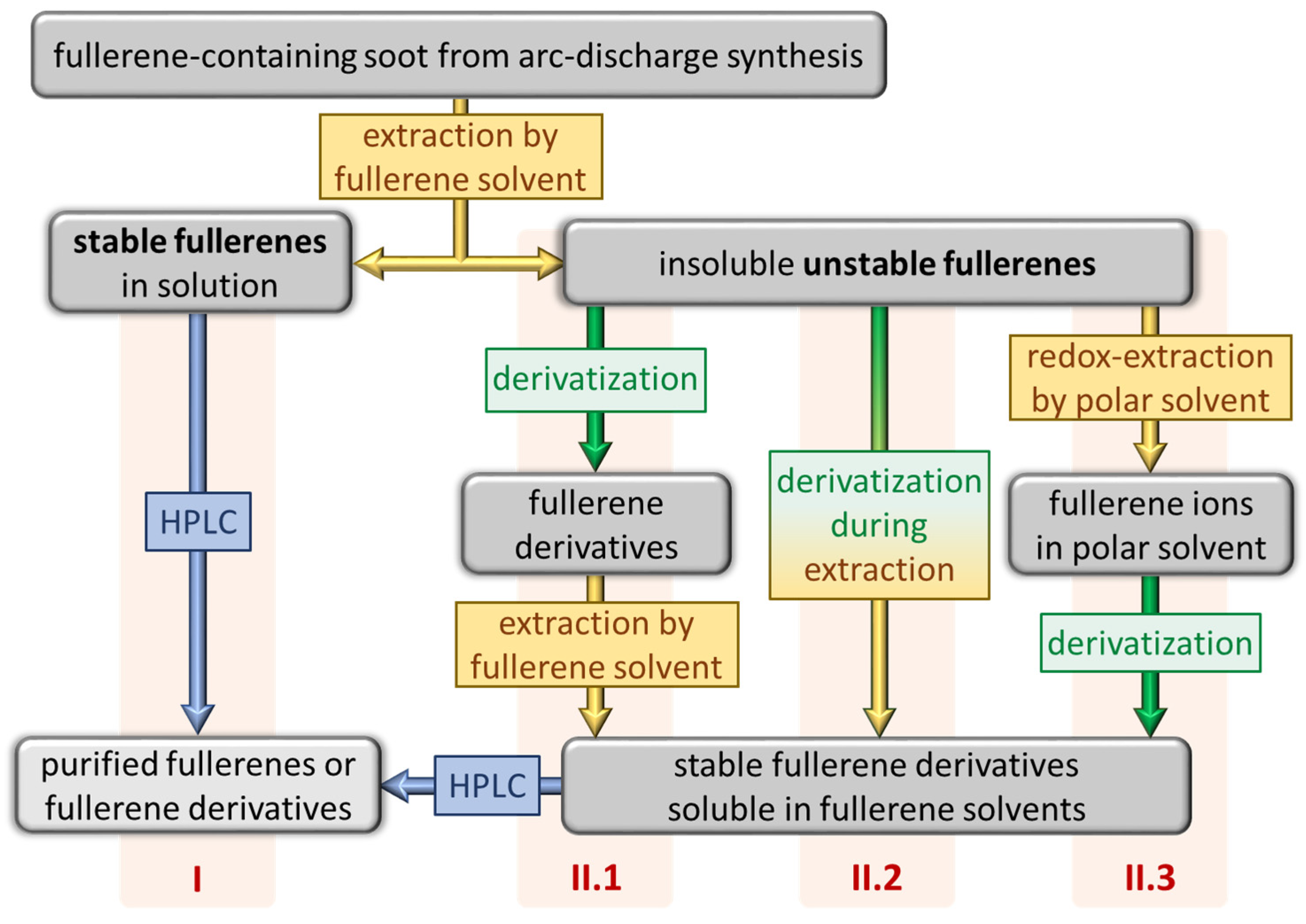
3. Redox Extraction
4. Chemical Functionalization of Unstable Fullerenes
4.1. General Remarks
- (1)
- Empty fullerenes with triplet ground state require the addition of at least two radical groups to quench the cage biradical. However, it is not guaranteed that the bisadduct C2n(R)2 will have a large HOMO-LUMO gap. Depending on the MO structure of the molecule, the bisadduct may also have a small gap, thus requiring the addition of another pair of radical groups. However, regardless of how many pairs of radical groups are ultimately required to obtain a stable derivative, it is certain that the number of groups will be even.
- (2)
- Monometallofullerenes MIII@C2n have one unpaired electron, and therefore require the addition of at least one radical group. In general, the number of groups in the stable closed-shell MIII@C2n(R)x derivative should be odd.
- (3)
- Open-shell dimetallofullerenes of trivalent metals are peculiar in that of the two unpaired valence electrons, one is localized on the fullerene cage and one on the metal dimer. The latter does not seem to be relevant for the kinetic stability, which means that only the unpaired electron of the cage should be quenched. Therefore, dimetallofullerenes require the addition of at least one and generally odd number of groups, similarly to monometallofullerenes.
4.2. Derivatization Followed by Dissolution
4.3. In Situ Derivatization during Exraction
4.4. Redox-Extraction and Derivatization of Anions
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Popov, A.A.; Yang, S.; Dunsch, L. Endohedral Fullerenes. Chem. Rev. 2013, 113, 5989–6113. [Google Scholar] [CrossRef]
- Lu, X.; Feng, L.; Akasaka, T.; Nagase, S. Current status and future developments of endohedral metallofullerenes. Chem. Soc. Rev. 2012, 41, 7723–7760. [Google Scholar] [CrossRef]
- Yang, S.; Wei, T.; Jin, F. When metal clusters meet carbon cages: Endohedral clusterfullerenes. Chem. Soc. Rev. 2017, 46, 5005–5058. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, X.; Tan, X.; Li, H.; Dai, S.; Yao, B.; Liu, X.; He, Y.; Jin, F. Recent Progress on the Functionalization of Endohedral Metallofullerenes. Inorganics 2023, 11, 346. [Google Scholar] [CrossRef]
- Li, Y.; Sun, Y.; Kopcha, W.P.; Zhang, J. Advances in Regioselective Functionalization of Endohedral Metallofullerenes. Chin. J. Chem. 2023, 41, 2025–2034. [Google Scholar] [CrossRef]
- Kopcha, W.P.; Biswas, R.; Sun, Y.; Chueng, S.-T.D.; Dorn, H.C.; Zhang, J. Water-soluble endohedral metallofullerenes: New horizons for biomedical applications. Chem. Commun. 2023, 59, 13551–13561. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Zhao, C.; Shi, Z.; Jia, C.; Guo, X. Single-Molecule Fullerenes: Current Stage and Perspective. ACS Mater. Lett. 2022, 11, 1037–1052. [Google Scholar] [CrossRef]
- Li, Y.; Kopcha, W.; Rodriguez-Fortea, A.; Zhang, J. Multicomponent Reactions Among Alkyl Isocyanides, sp reactants, and sp2 Carbon Cages. Synlett 2022, 33, 907–912. [Google Scholar]
- Li, W.; Wang, C.; Wang, T. Metallofullertube: From Tubular Endohedral Structures to Properties. ChemPhysChem 2022, 23, e202200507. [Google Scholar] [CrossRef]
- Li, M.; Zhao, R.; Dang, J.; Zhao, X. Theoretical study on the stabilities, electronic structures, and reaction and formation mechanisms of fullerenes and endohedral metallofullerenes. Coord. Chem. Rev. 2022, 471, 214762. [Google Scholar] [CrossRef]
- Shen, W.; Bao, L.; Lu, X. Endohedral Metallofullerenes: An Ideal Platform of Sub-Nano Chemistry. Chin. J. Chem. 2022, 40, 275–284. [Google Scholar] [CrossRef]
- Li, W.; Wang, C.-R.; Wang, T. Molecular structures and magnetic properties of endohedral metallofullerenes. Chem. Commun. 2021, 57, 10317–10326. [Google Scholar] [CrossRef] [PubMed]
- Akhanova, N.Y.; Shchur, D.V.; Pomytkin, A.P.; Zolotarenko, A.D.; Zolotarenko, A.D.; Gavrylyuk, N.A.; Ualkhanova, M.; Bo, W.; Ang, D. Methods for the Synthesis of Endohedral Fullerenes. J. Nanosci. Nanotechnol. 2021, 21, 2446–2459. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chang, Q.; Zhang, G.; Li, F.; Wang, X.; Yang, S.; Troyanov, S.I. Structural Studies of Giant Empty and Endohedral Fullerenes. Front. Chem. 2020, 8, 607712. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Hu, S.; Lu, X. Endohedral Metallofullerenes: New Structures and Unseen Phenomena. Chem. Eur. J. 2020, 26, 5748–5757. [Google Scholar] [CrossRef] [PubMed]
- Jalife, S.; Arcudia, J.; Pan, S.; Merino, G. Noble gas endohedral fullerenes. Chem. Sci. 2020, 11, 6642–6652. [Google Scholar] [CrossRef]
- Guan, R.; Chen, M.; Jin, F.; Yang, S. Strain Release of Fused Pentagons on Fullerene Cage via Chemical Functionalization. Angew. Chem. Int. Ed. 2020, 59, 1048–1073. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Wang, C. Functional Metallofullerene Materials and Their Applications in Nanomedicine, Magnetics, and Electronics. Small 2019, 15, e1901522. [Google Scholar] [CrossRef]
- Spree, L.; Popov, A.A. Recent advances in single molecule magnetism of dysprosium-metallofullerenes. Dalton Trans. 2019, 48, 2861–2871. [Google Scholar] [CrossRef]
- Liu, F.; Spree, L.; Krylov, D.S.; Velkos, G.; Avdoshenko, S.M.; Popov, A.A. Single-Electron Lanthanide-Lanthanide Bonds Inside Fullerenes toward Robust Redox-Active Molecular Magnets. Acc. Chem. Res. 2019, 52, 2981–2993. [Google Scholar] [CrossRef]
- Jin, P.; Li, Y.; Magagula, S.; Chen, Z. Exohedral functionalization of endohedral metallofullerenes: Interplay between inside and outside. Coord. Chem. Rev. 2019, 388, 406–439. [Google Scholar] [CrossRef]
- Feng, L.; Hao, Y.; Liu, A.; Slanina, Z. Trapping Metallic Oxide Clusters inside Fullerene Cages. Acc. Chem. Res. 2019, 52, 1802–1811. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, C.-H.; Chen, N.; Echegoyen, L. Fullerenes as Nanocontainers That Stabilize Unique Actinide Species Inside: Structures, Formation, and Reactivity. Acc. Chem. Res. 2019, 52, 1824–1833. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, X.; Chen, N. Recent Progress on Endohedral Metallic Fullerenes. Gen. Chem. 2018, 4, 180004. [Google Scholar]
- Yamada, M.; Akasaka, T.; Nagase, S. Salvaging Reactive Fullerenes from Soot by Exohedral Derivatization. Angew. Chem. Int. Ed. 2018, 57, 13394–13405. [Google Scholar] [CrossRef]
- Popov, A.A. Redox-active metal–metal bonds between lanthanides in dimetallofullerenes. Curr. Opin. Electrochem. 2018, 8, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Peng, P.; Lu, X. Bonding inside and outside Fullerene Cages. Acc. Chem. Res. 2018, 51, 810–815. [Google Scholar] [CrossRef]
- Li, T.; Dorn, H.C. Biomedical Applications of Metal-Encapsulated Fullerene Nanoparticles. Small 2017, 13, 1603152. [Google Scholar] [CrossRef] [PubMed]
- Kako, M.; Nagase, S.; Akasaka, T. Functionalization of Endohedral Metallofullerenes with Reactive Silicon and Germanium Compounds. Molecules 2017, 22, 1179. [Google Scholar] [CrossRef]
- Cerón, M.R.; Maffeis, V.; Stevenson, S.; Echegoyen, L. Endohedral Fullerenes: Synthesis, Isolation, Mono and Bis-Functionalization. Inorg. Chim. Acta 2017, 468, 16–27. [Google Scholar] [CrossRef]
- Abella, L.; Wang, Y.; Rodríguez-Fortea, A.; Chen, N.; Poblet, J.M. Current status of oxide clusterfullerenes. Inorg. Chim. Acta 2017, 468, 91–104. [Google Scholar] [CrossRef]
- Shinohara, H. Another big discovery—Metallofullerenes. Philos. Trans. R. Soc. Lond. A Math. Phys. Eng. Sci. 2016, 374, 20150325. [Google Scholar] [CrossRef]
- Romero, E.L.; Echegoyen, L. Electron spin resonance spectroscopy of empty and endohedral fullerenes. J. Phys. Org. Chem. 2016, 29, 781–792. [Google Scholar] [CrossRef]
- Zhang, Y.; Popov, A.A. Transition-Metal and Rare-Earth-Metal Redox Couples inside Carbon Cages: Fullerenes Acting as Innocent Ligands. Organometallics 2014, 33, 4537–4549. [Google Scholar] [CrossRef]
- Zhang, J.; Dorn, H.C. NMR Studies of the Dynamic Motion of Encapsulated Ions and Clusters in Fullerene Cages: A Wheel Within a Wheel. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 35–46. [Google Scholar] [CrossRef]
- Wang, T.; Wang, C. Endohedral Metallofullerenes Based on Spherical Ih-C80 Cage: Molecular Structures and Paramagnetic Properties. Acc. Chem. Res. 2014, 47, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Bao, L.; Akasaka, T.; Nagase, S. Recent progress in the chemistry of endohedral metallofullerenes. Chem. Commun. 2014, 50, 14701–14715. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.; Tang, C.; Chen, Z. Carbon Atoms Trapped in Cages: Metal Carbide Clusterfullerenes. Coord. Chem. Rev. 2014, 270–271, 89–111. [Google Scholar] [CrossRef]
- Ghiassi, K.B.; Olmstead, M.M.; Balch, A.L. Gadolinium-containing endohedral fullerenes: Structures and function as magnetic resonance imaging (MRI) agents. Dalton Trans. 2014, 43, 7346–7358. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Borras, M.; Osuna, S.; Luis, J.M.; Swart, M.; Sola, M. The role of aromaticity in determining the molecular structure and reactivity of (endohedral metallo)fullerenes. Chem. Soc. Rev. 2014, 43, 5089–5105. [Google Scholar] [CrossRef]
- Cerón, M.R.; Li, F.-F.; Echegoyen, L.A. Endohedral fullerenes: The importance of electronic, size and shape complementarity between the carbon cages and the corresponding encapsulated clusters. J. Phys. Org. Chem. 2014, 27, 258–264. [Google Scholar] [CrossRef]
- Zhang, J.; Stevenson, S.; Dorn, H.C. Trimetallic Nitride Template Endohedral Metallofullerenes: Discovery, Structural Characterization, Reactivity, and Applications. Acc. Chem. Res. 2013, 46, 1548–1557. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-P.; Lu, X.; Akasaka, T.; Nagase, S. New features in coordination chemistry: Valuable hints from X-ray analyses of endohedral metallofullerenes. Polyhedron 2013, 52, 3–9. [Google Scholar] [CrossRef]
- Lu, X.; Akasaka, T.; Nagase, S. Carbide Cluster Metallofullerenes: Structure, Properties, and Possible Origin. Acc. Chem. Res. 2013, 46, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Cong, H.; Yu, B.; Akasaka, T.; Lu, X. Endohedral metallofullerenes: An unconventional core–shell coordination union. Coord. Chem. Rev. 2013, 257, 2880–2898. [Google Scholar] [CrossRef]
- Yang, S. Synthesis, Separation, and Molecular Structures of Endohedral Fullerenes. Curr. Org. Chem. 2012, 16, 1079–1094. [Google Scholar] [CrossRef]
- Popov, A.A.; Avdoshenko, S.M.; Pendas, A.M.; Dunsch, L. Bonding between strongly repulsive metal atoms: An oxymoron made real in a confined space of endohedral metallofullerenes. Chem. Commun. 2012, 48, 8031–8050. [Google Scholar] [CrossRef]
- Akasaka, T.; Lu, X. Structural and electronic properties of endohedral metallofullerenes. Chem. Rec. 2012, 12, 256–269. [Google Scholar] [CrossRef]
- Yang, S.; Liu, F.; Chen, C.; Jiao, M.; Wei, T. Fullerenes encaging metal clusters-clusterfullerenes. Chem. Commun. 2011, 47, 11822–11839. [Google Scholar] [CrossRef]
- Rodriguez-Fortea, A.; Balch, A.L.; Poblet, J.M. Endohedral metallofullerenes: A unique host-guest association. Chem. Soc. Rev. 2011, 40, 3551–3563. [Google Scholar] [CrossRef]
- Popov, A.A.; Dunsch, L. Electrochemistry In Cavea: Endohedral Redox Reactions of Encaged Species in Fullerenes. J. Phys. Chem. Lett. 2011, 2, 786–794. [Google Scholar] [CrossRef]
- Liu, G.; Wu, Y.; Porfyrakis, K. Synthesis and Chemistry of Endohedral Fullerenes. Curr. Org. Chem. 2011, 15, 1197–1207. [Google Scholar] [CrossRef]
- Kovalenko, V.I.; Khamatgalimov, A.R. Open-shell fullerene C74: Phenalenyl-radical substructures. Chem. Phys. Lett. 2003, 377, 263–268. [Google Scholar] [CrossRef]
- Hu, S.; Liu, T.; Shen, W.; Slanina, Z.; Akasaka, T.; Xie, Y.; Uhlik, F.; Huang, W.; Lu, X. Isolation and Structural Characterization of Er@C2v(9)-C82 and Er@Cs(6)-C82: Regioselective Dimerization of a Pristine Endohedral Metallofullerene Induced by Cage Symmetry. Inorg. Chem. 2019, 58, 2177–2182. [Google Scholar] [CrossRef]
- Bao, L.; Pan, C.; Slanina, Z.; Uhlik, F.; Akasaka, T.; Lu, X. Isolation and Crystallographic Characterization of the Labile Isomer of Y@C82 Cocrystallized with Ni(OEP): Unprecedented Dimerization of Pristine Metallofullerenes. Angew. Chem. Int. Ed. 2016, 55, 9234–9238. [Google Scholar] [CrossRef]
- Yang, W.; Velkos, G.; Rosenkranz, M.; Schiemenz, S.; Liu, F.; Popov, A.A. Nd-Nd Bond in Ih and D5h Cage Isomers of Nd2@C80 Stabilized by Electrophilic CF3 Addition. Adv. Sci. 2024, 11, 2305190. [Google Scholar] [CrossRef]
- Wang, Z.; Kitaura, R.; Shinohara, H. Metal-Dependent Stability of Pristine and Functionalized Unconventional Dimetallofullerene M2@Ih-C80. J. Phys. Chem. C 2014, 118, 13953–13958. [Google Scholar] [CrossRef]
- Fu, W.; Zhang, J.; Fuhrer, T.; Champion, H.; Furukawa, K.; Kato, T.; Mahaney, J.E.; Burke, B.G.; Williams, K.A.; Walker, K.; et al. Gd2@C79N: Isolation, Characterization, and Monoadduct Formation of a Very Stable Heterofullerene with a Magnetic Spin State of S = 15/2. J. Am. Chem. Soc. 2011, 133, 9741–9750. [Google Scholar] [CrossRef]
- Zuo, T.; Xu, L.; Beavers, C.M.; Olmstead, M.M.; Fu, W.; Crawford, T.D.; Balch, A.L.; Dorn, H.C. M2@C79N (M = Y, Tb): Isolation and Characterization of Stable Endohedral Metallofullerenes Exhibiting M...M Bonding Interactions inside Aza[80]fullerene Cages. J. Am. Chem. Soc. 2008, 130, 12992–12997. [Google Scholar] [CrossRef]
- Jin, F.; Xin, J.; Guan, R.; Xie, X.-M.; Chen, M.; Zhang, Q.; Popov, A.A.; Xie, S.-Y.; Yang, S. Stabilizing Three-Center Single-Electron Metal-Metal Bond in a Fullerene Cage. Chem. Sci. 2021, 12, 6890–6895. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, P.; Li, B.; Yu, P.; Yang, L.; Ehara, M.; Jin, P.; Akasaka, T.; Lu, X. Cluster-Geometry-Associated Metal–Metal Bonding in Trimetallic Carbide Clusterfullerenes. Inorg. Chem. 2022, 61, 11277–11283. [Google Scholar] [CrossRef]
- Iiduka, Y.; Wakahara, T.; Nakahodo, T.; Tsuchiya, T.; Sakuraba, A.; Maeda, Y.; Akasaka, T.; Yoza, K.; Horn, E.; Kato, T.; et al. Structural determination of metallofuIlerene Sc3C82 revisited: A surprising finding. J. Am. Chem. Soc. 2005, 127, 12500–12501. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kurihara, H.; Suzuki, M.; Saito, M.; Slanina, Z.; Uhlik, F.; Aizawa, T.; Kato, T.; Olmstead, M.M.; Balch, A.L.; et al. Hiding and Recovering Electrons in a Dimetallic Endohedral Fullerene: Air-Stable Products from Radical Additions. J. Am. Chem. Soc. 2015, 137, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Chen, M.; Pan, C.; Yamaguchi, T.; Kato, T.; Olmstead, M.M.; Balch, A.L.; Akasaka, T.; Lu, X. Crystallographic Evidence for Direct Metal–Metal Bonding in a Stable Open-Shell La2@Ih-C80 Derivative. Angew. Chem. Int. Ed. 2016, 55, 4242–4246. [Google Scholar] [CrossRef]
- Liu, F.; Velkos, G.; Krylov, D.S.; Spree, L.; Zalibera, M.; Ray, R.; Samoylova, N.A.; Chen, C.-H.; Rosenkranz, M.; Schiemenz, S.; et al. Air-stable redox-active nanomagnets with lanthanide spins radical-bridged by a metal–metal bond. Nat. Commun. 2019, 10, 571. [Google Scholar] [CrossRef]
- Liu, F.; Krylov, D.S.; Spree, L.; Avdoshenko, S.M.; Samoylova, N.A.; Rosenkranz, M.; Kostanyan, A.; Greber, T.; Wolter, A.U.B.; Büchner, B.; et al. Single molecule magnet with an unpaired electron trapped between two lanthanide ions inside a fullerene. Nat. Commun. 2017, 8, 16098. [Google Scholar] [CrossRef]
- Zaripov, R.B.; Kandrashkin, Y.E.; Salikhov, K.M.; Büchner, B.; Liu, F.; Rosenkranz, M.; Popov, A.A.; Kataev, V. Unusually large hyperfine structure of the electron spin levels in an endohedral dimetallofullerene and its spin coherent properties. Nanoscale 2020, 12, 20513–20521. [Google Scholar] [CrossRef]
- Bubnov, V.P.; Laukhina, E.E.; Kareev, I.E.; Koltover, V.K.; Prokhorova, T.G.; Yagubskii, E.B.; Kozmin, Y.P. Endohedral Metallofullerenes: A Convenient Gram-Scale Preparation. Chem. Mater. 2002, 14, 1004–1008. [Google Scholar] [CrossRef]
- Huang, H.; Yang, S. Toward Efficient Synthesis of Endohedral Metallofullerenes by Arc Discharge of Carbon Rods Containing Encapsulated Rare Earth Carbides and Ultrasonic Soxhlet Extraction. Chem. Mater. 2000, 12, 2715–2720. [Google Scholar] [CrossRef]
- Diener, M.D.; Alford, J.M. Isolation and properties of small-bandgap fullerenes. Nature 1998, 393, 668–671. [Google Scholar] [CrossRef]
- Kubozono, Y.; Noto, T.; Ohta, T.; Maeda, H.; Kashino, S.; Emura, S.; Ukita, S.; Sogabe, T. Extractions of Ca@C60 and Sr@C60 with Aniline. Chem. Lett. 1996, 25, 453–454. [Google Scholar] [CrossRef]
- Fuchs, D.; Rietschel, H.; Michel, R.H.; Fischer, A.; Weis, P.; Kappes, M.M. Extraction and Chromatographic Elution Behavior of Endohedral Metallofullerenes: Inferences Regarding Effective Dipole Moments. J. Phys. Chem. 1996, 100, 725–729. [Google Scholar] [CrossRef]
- Ding, J.; Yang, S. Efficient N,N-Dimethylformamide Extraction of Endohedral Metallofullerenes for HPLC Purification. Chem. Mater. 1996, 8, 2824–2827. [Google Scholar] [CrossRef]
- Kubozono, Y.; Ohta, T.; Hayashibara, T.; Maeda, H.; Ishida, H.; Kashino, S.; Oshima, K.; Yamazaki, H.; Ukita, S.; Sogabe, T. Preparation and Extraction of Ca@C60. Chem. Lett. 1995, 24, 457–458. [Google Scholar] [CrossRef]
- Xiao, J.; Savina, M.R.; Martin, G.B.; Francis, A.H.; Meyerhoff, M.E. Efficient HPLC Purification of Endohedral Metallofullerenes on a Porphyrin-Silica Stationary Phase. J. Am. Chem. Soc. 1994, 116, 9341–9342. [Google Scholar] [CrossRef]
- Anderson, M.R.; Dorn, H.C.; Stevenson, S.A. Making connections between metallofullerenes and fullerenes: Electrochemical investigations. Carbon 2000, 38, 1663–1670. [Google Scholar] [CrossRef]
- Liu, B.B.; Zou, G.T.; Yang, H.B.; Yu, S.; Lu, J.S.; Liu, Z.Y.; Liu, S.Y.; Xu, W.G. Synthesis, extraction and electronic structure of Ce@C2n. J. Phys. Chem. Solids 1997, 58, 1873–1876. [Google Scholar] [CrossRef]
- Sun, D.Y.; Liu, Z.Y.; Guo, X.H.; Xu, W.G.; Liu, S.Y. High-yield extraction of endohedral rare-earth fullerenes. J. Phys. Chem. B 1997, 101, 3927–3930. [Google Scholar] [CrossRef]
- Kubozono, Y.; Maeda, H.; Takabayashi, Y.; Hiraoka, K.; Nakai, T.; Kashino, S.; Emura, S.; Ukita, S.; Sogabe, T. Extractions of Y@C60, Ba@C60, La@C60, Ce@C60, Pr@C60, Nd@C60 and Gd@C60 with aniline. J. Am. Chem. Soc. 1996, 118, 6998–6999. [Google Scholar] [CrossRef]
- Kareev, I.E.; Shulga, Y.M.; Bubnov, V.P.; Kozlovski, V.I.; Dodonov, A.F.; Martynenko, M.V.; Yagubskii, E.B. Investigation of composition of endometallofullerene extracts. Fuller. Nanotub. Carbon Nanostruct. 2004, 12, 59–63. [Google Scholar] [CrossRef]
- Kareev, I.E.; Bubnov, V.P.; Laukhina, E.E.; Dodonov, A.F.; Kozovski, V.I.; Yagubskii, E.B. Experimental evidence in support of the formation of anionic endohedral metallofullerenes during their extraction with N,N-dimethylformamide. Fuller. Nanotub. Carbon Nanostruct. 2004, 12, 65–69. [Google Scholar] [CrossRef]
- Raebiger, J.W.; Bolskar, R.D. Improved Production and Separation Processes for Gadolinium Metallofullerenes. J. Phys. Chem. C 2008, 112, 6605–6612. [Google Scholar] [CrossRef]
- Bolskar, R.D.; Alford, J.M. Chemical oxidation of endohedral metallofullerenes: Identification and separation of distinct classes. Chem. Commun. 2003, 11, 1292–1293. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, S.; Nishibori, E.; Sawa, H.; Sugimoto, K.; Takata, M.; Miyata, Y.; Kitaura, R.; Shinohara, H.; Okada, H.; Sakai, T.; et al. A layered ionic crystal of polar Li@C60 superatoms. Nat. Chem. 2010, 2, 678–683. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Komuro, T.; Sakai, T.; Matsuo, Y.; Ono, Y.; Omote, K.; Yokoo, K.; Kawachi, K.; Kasama, Y.; Ono, S.; et al. Preparation of endohedral fullerene containing lithium (Li@C60) and isolation as pure hexafluorophosphate salt ([Li+@C60][PF6−]). RSC Adv. 2012, 2, 10624–10631. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Wakahara, T.; Shirakura, S.; Maeda, Y.; Akasaka, T.; Kobayashi, K.; Nagase, S.; Kato, T.; Kadish, K.M. Reduction of endohedral metallofullerenes: A convenient method for isolation. Chem. Mat. 2004, 16, 4343–4346. [Google Scholar] [CrossRef]
- Laukhina, E.E.; Bubnov, V.P.; Estrin, Y.I.; Golod, Y.A.; Khodorkovskii, M.A.; Koltover, V.K.; Yagubskii, E.B. Novel proficient method for isolation of endometallofullerenes from fullerene-containing soots by two-step o-xylene-N,N-dimethylformamide extraction. J. Mater. Chem. 1998, 8, 893–895. [Google Scholar] [CrossRef]
- Yamamoto, K.; Funasaka, H.; Takahashi, T.; Akasaka, T. Isolation of an ESR-Active Metallofullerene of La@C82. J. Phys. Chem. 1994, 98, 2008–2011. [Google Scholar] [CrossRef]
- Solodovnikov, S.P.; Tumanskii, B.L.; Bashilov, V.V.; Lebedkin, S.F.; Sokolov, V.I. Spectral study of reactions of La@C82 and Y@C82 with amino-containing solvents. Russ. Chem. Bull. 2001, 50, 2242–2244. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Wakahara, T.; Lian, Y.F.; Maeda, Y.; Akasaka, T.; Kato, T.; Mizorogi, N.; Nagase, S. Selective extraction and purification of endohedral metallofullerene from carbon soot. J. Phys. Chem. B 2006, 110, 22517–22520. [Google Scholar] [CrossRef]
- Tagmatarchis, N.; Shinohara, H. Production, Separation, Isolation, and Spectroscopic Study of Dysprosium Endohedral Metallofullerenes. Chem. Mater. 2000, 12, 3222–3226. [Google Scholar] [CrossRef]
- Sun, B.; Gu, Z. Solvent-dependent Anion Studies on Enrichment of Metallofullerene. Chem. Lett. 2002, 31, 1164–1165. [Google Scholar] [CrossRef]
- Lu, X.; Li, H.; Sun, B.; Shi, Z.; Gu, Z. Selective reduction and extraction of Gd@C82 and Gd2@C80 from soot and the chemical reaction of their anions. Carbon 2005, 43, 1546–1549. [Google Scholar] [CrossRef]
- Shustova, N.B.; Kuvychko, I.V.; Bolskar, R.D.; Seppelt, K.; Strauss, S.H.; Popov, A.A.; Boltalina, O.V. Trifluoromethyl derivatives of insoluble small-HOMO-LUMO-gap hollow higher fullerenes. NMR and DFT structure elucidation of C2-(C74-D3h)(CF3)12, Cs-(C76-Td(2))(CF3)12, C2-(C78-D3h(5))(CF3)12, Cs-(C80-C2v(5))(CF3)12, and C2-(C82-C2(5))(CF3)12. J. Am. Chem. Soc. 2006, 128, 15793–15798. [Google Scholar] [CrossRef]
- Shustova, N.B.; Newell, B.S.; Miller, S.M.; Anderson, O.P.; Bolskar, R.D.; Seppelt, K.; Popov, A.A.; Boltalina, O.V.; Strauss, S.H. Discovering and verifying elusive fullerene cage isomers: Structures of C2-p11-(C74-D3h)(CF3)12 and C2-p11-(C78-D3h(5))(CF3)12. Angew. Chem. Int. Ed. 2007, 46, 4111–4114. [Google Scholar] [CrossRef]
- Wang, Z.; Nakanishi, Y.; Noda, S.; Niwa, H.; Zhang, J.; Kitaura, R.; Shinohara, H. Missing Small-Bandgap Metallofullerenes: Their Isolation and Electronic Properties. Angew. Chem. Int. Ed. 2013, 52, 11770–11774. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, A.; Nishino, M.; Niwa, H.; Ishino, K.; Wang, Z.; Omachi, H.; Furukawa, K.; Yamaguchi, T.; Kato, T.; Bandow, S.; et al. Crystalline functionalized endohedral C60 metallofullerides. Nat. Commun. 2018, 9, 3073. [Google Scholar] [CrossRef]
- Nakagawa, A.; Aoyagi, S.; Omachi, H.; Ishino, K.; Nishino, M.; Rio, J.; Ewels, C.; Shinohara, H. Isolation and structure determination of missing fullerenes Gd@C74(CF3)n through in situ trifluoromethylation. R. Soc. Open Sci. 2018, 5, 181015. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Aoyagi, S.; Omachi, H.; Kitaura, R.; Shinohara, H. Isolation and Structure Determination of a Missing Endohedral Fullerene La@C70 through In Situ Trifluoromethylation. Angew. Chem. Int. Ed. 2016, 55, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.Y.; Gao, F.; Lu, X.; Huang, R.B.; Wang, C.R.; Zhang, X.; Liu, M.L.; Deng, S.L.; Zheng, L.S. Capturing the labile fullerene[50] as C50Cl10. Science 2004, 304, 699. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.-Z.; Chen, R.-T.; Liao, Z.-J.; Li, J.; Zhu, F.; Lu, X.; Xie, S.-Y.; Li, J.; Huang, R.-B.; Zheng, L.-S. Carbon arc production of heptagon-containing fullerene[68]. Nat. Commun. 2011, 2, 420. [Google Scholar] [CrossRef]
- Zhong, Y.-Y.; Chen, Z.-C.; Du, P.; Cui, C.-H.; Tian, H.-R.; Shi, X.-M.; Deng, S.-L.; Gao, F.; Zhang, Q.; Gao, C.-L.; et al. Double Negatively Curved C70 Growth through a Heptagon-Involving Pathway. Angew. Chem. Int. Ed. 2019, 58, 14095–14099. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.R.; Shi, Z.Q.; Wan, L.J.; Lu, X.; Dunsch, L.; Shu, C.Y.; Tang, Y.L.; Shinohara, H. C64H4: Production, isolation, and structural characterizations of a stable unconventional fulleride. J. Am. Chem. Soc. 2006, 128, 6605–6610. [Google Scholar] [CrossRef]
- Tian, H.-R.; Chen, M.-M.; Wang, K.; Chen, Z.-C.; Fu, C.-Y.; Zhang, Q.; Li, S.-H.; Deng, S.-L.; Yao, Y.-R.; Xie, S.-Y.; et al. An Unconventional Hydrofullerene C66H4 with Symmetric Heptagons Retrieved in Low-Pressure Combustion. J. Am. Chem. Soc. 2019, 141, 6651–6657. [Google Scholar] [CrossRef]
- Xie, F.-F.; Chen, Z.-C.; Zhang, M.; Xie, X.-M.; Chen, L.-F.; Tian, H.-R.; Deng, S.-L.; Xie, S.-Y.; Zheng, L.-S. Capturing nonclassical C70 with double heptagons in low-pressure combustion. Chem. Commun. 2022, 58, 9814–9817. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Vicente, A.; Mulet-Gas, M.; Dunk, P.W.; Poblet, J.M.; Rodríguez-Fortea, A. Probing the formation of halogenated endohedral metallofullerenes: Predictions confirmed by experiments. Carbon 2018, 129, 750–757. [Google Scholar] [CrossRef]
- Maeda, Y.; Tsuchiya, T.; Kikuchi, T.; Nikawa, H.; Yang, T.; Zhao, X.; Slanina, Z.; Suzuki, M.; Yamada, M.; Lian, Y.; et al. Effective Derivatization and Extraction of Insoluble Missing Lanthanum Metallofullerenes La@C2n (n=36–38) with Iodobenzene. Carbon 2016, 98, 67–73. [Google Scholar] [CrossRef]
- Wakahara, T.; Nikawa, H.; Kikuchi, T.; Nakahodo, T.; Rahman, G.M.A.; Tsuchiya, T.; Maeda, Y.; Akasaka, T.; Yoza, K.; Horn, E.; et al. La@C72 having a non-IPR carbon cage. J. Am. Chem. Soc. 2006, 128, 14228–14229. [Google Scholar] [CrossRef]
- Nikawa, H.; Kikuchi, T.; Wakahara, T.; Nakahodo, T.; Tsuchiya, T.; Rahman, G.M.A.; Akasaka, T.; Maeda, Y.; Yoza, K.; Horn, E.; et al. Missing metallofullerene La@C74. J. Am. Chem. Soc. 2005, 127, 9684–9685. [Google Scholar] [CrossRef]
- Lu, X.; Nikawa, H.; Kikuchi, T.; Mizorogi, N.; Slanina, Z.; Tsuchiya, T.; Nagase, S.; Akasaka, T. Radical Derivatives of Insoluble La@C74: X-ray Structures, Metal Positions, and Isomerization. Angew. Chem. Int. Ed. 2011, 50, 6356–6359. [Google Scholar] [CrossRef]
- Nikawa, H.; Yamada, T.; Cao, B.P.; Mizorogi, N.; Slanina, Z.; Tsuchiya, T.; Akasaka, T.; Yoza, K.; Nagase, S. Missing Metallofullerene with C80 Cage. J. Am. Chem. Soc. 2009, 131, 10950–10954. [Google Scholar] [CrossRef]
- Akasaka, T.; Lu, X.; Kuga, H.; Nikawa, H.; Mizorogi, N.; Slanina, Z.; Tsuchiya, T.; Yoza, K.; Nagase, S. Dichlorophenyl Derivatives of La@C3v(7)-C82: Endohedral Metal Induced Localization of Pyramidalization and Spin on a Triple-Hexagon Junction. Angew. Chem. Int. Ed. 2010, 49, 9715–9719. [Google Scholar] [CrossRef]
- Kareev, I.E.; Lebedkin, S.F.; Bubnov, V.P.; Yagubskii, E.B.; Ioffe, I.N.; Khavrel, P.A.; Kuvychko, I.V.; Strauss, S.H.; Boltalina, O.V. Trifluoromethylated endohedral metallofullerenes: Synthesis and characterization of Y@C82(CF3)5. Angew. Chem. Int. Ed. 2005, 44, 1846–1849. [Google Scholar] [CrossRef]
- Maeda, Y.; Akita, S.; Suzuki, M.; Yamada, M.; Akasaka, T.; Kobayashi, K.; Nagase, S. Controlling the reactivity of La@C82 by reduction: Reaction of the La@C82 anion with alkyl halide with high regioselectivity. Beilstein J. Org. Chem. 2023, 19, 1858–1866. [Google Scholar] [CrossRef]
- Wang, Y.; Velkos, G.; Israel, N.J.; Rosenkranz, M.; Büchner, B.; Liu, F.; Popov, A.A. Electrophilic Trifluoromethylation of Dimetallofullerene Anions en Route to Air-Stable Single-Molecule Magnets with High Blocking Temperature of Magnetization. J. Am. Chem. Soc. 2021, 143, 18139–18149. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Xin, J.; Yao, Y.; Liang, Z.; Qiu, Y.; Chen, M.; Yang, S. Capturing the Long-Sought Dy@C2v(5)-C80 via Benzyl Radical Stabilization. Nanomaterials 2022, 12, 3291. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yao, Y.-R.; Hu, Y.; Yang, L.; Yang, S.; Zhang, Y.; Zhang, Q.; Peng, P.; Jin, P.; Li, F.-F. Reactivity of Open-Shell Metallofullerene Anions: Synthesis, Crystal Structures, and Electrochemical Properties of Benzylated Gd@C2v-C82. Inorganics 2023, 11, 349. [Google Scholar] [CrossRef]
- Yang, S.; Ioffe, I.N.; Troyanov, S.I. Chlorination-Promoted Skeletal Transformations of Fullerenes. Acc. Chem. Res. 2019, 52, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Lebedeva, M.A.; Chamberlain, T.W.; Khlobystov, A.N. Harnessing the Synergistic and Complementary Properties of Fullerene and Transition-Metal Compounds for Nanomaterial Applications. Chem. Rev. 2015, 115, 11301–11351. [Google Scholar] [CrossRef]
- Boltalina, O.V.; Popov, A.A.; Kuvychko, I.V.; Shustova, N.B.; Strauss, S.H. Perfluoroalkylfullerenes. Chem. Rev. 2015, 115, 1051–1105. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Akasaka, T.; Nagase, S. Carbene Additions to Fullerenes. Chem. Rev. 2013, 113, 7209–7264. [Google Scholar] [CrossRef] [PubMed]
- Tzirakis, M.D.; Orfanopoulos, M. Radical Reactions of Fullerenes: From Synthetic Organic Chemistry to Materials Science and Biology. Chem. Rev. 2013, 113, 5262–5321. [Google Scholar] [CrossRef]
- Lu, X.; Akasaka, T.; Nagase, S. Chemistry of endohedral metallofullerenes: The role of metals. Chem. Commun. 2011, 47, 5942–5957. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Akasaka, T.; Nagase, S. Endohedral Metal Atoms in Pristine and Functionalized Fullerene Cages. Acc. Chem. Res. 2010, 43, 92–102. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Popov, A.A. Capturing Unstable Metallofullerenes. Inorganics 2024, 12, 48. https://doi.org/10.3390/inorganics12020048
Liu F, Popov AA. Capturing Unstable Metallofullerenes. Inorganics. 2024; 12(2):48. https://doi.org/10.3390/inorganics12020048
Chicago/Turabian StyleLiu, Fupin, and Alexey A. Popov. 2024. "Capturing Unstable Metallofullerenes" Inorganics 12, no. 2: 48. https://doi.org/10.3390/inorganics12020048
APA StyleLiu, F., & Popov, A. A. (2024). Capturing Unstable Metallofullerenes. Inorganics, 12(2), 48. https://doi.org/10.3390/inorganics12020048