Dithiodipropionate and Fumarate Ni, Cu, and Zn Mixed Ligand Complexes
Abstract
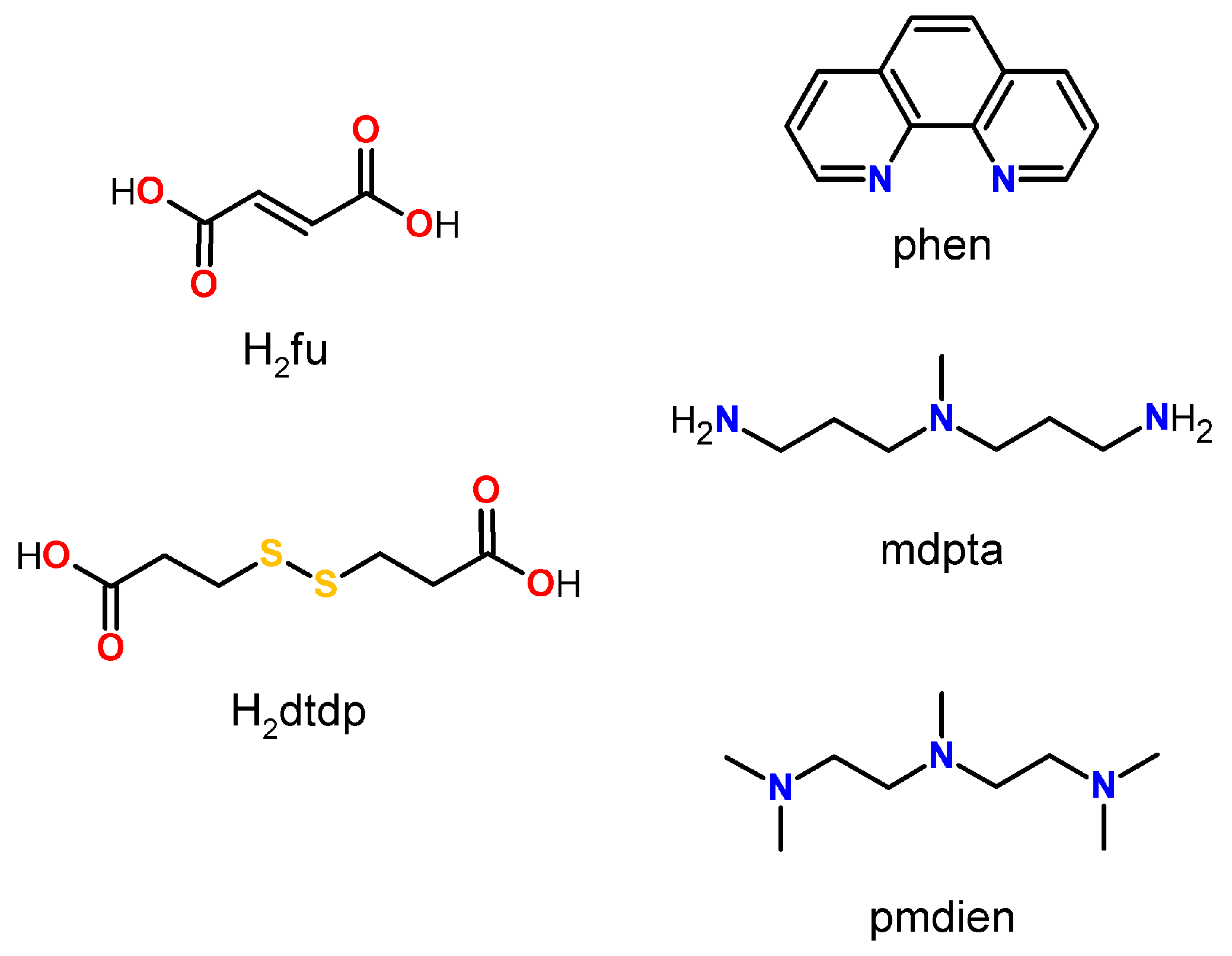
1. Introduction
2. Results and Discussion
2.1. Characterization of Complexes
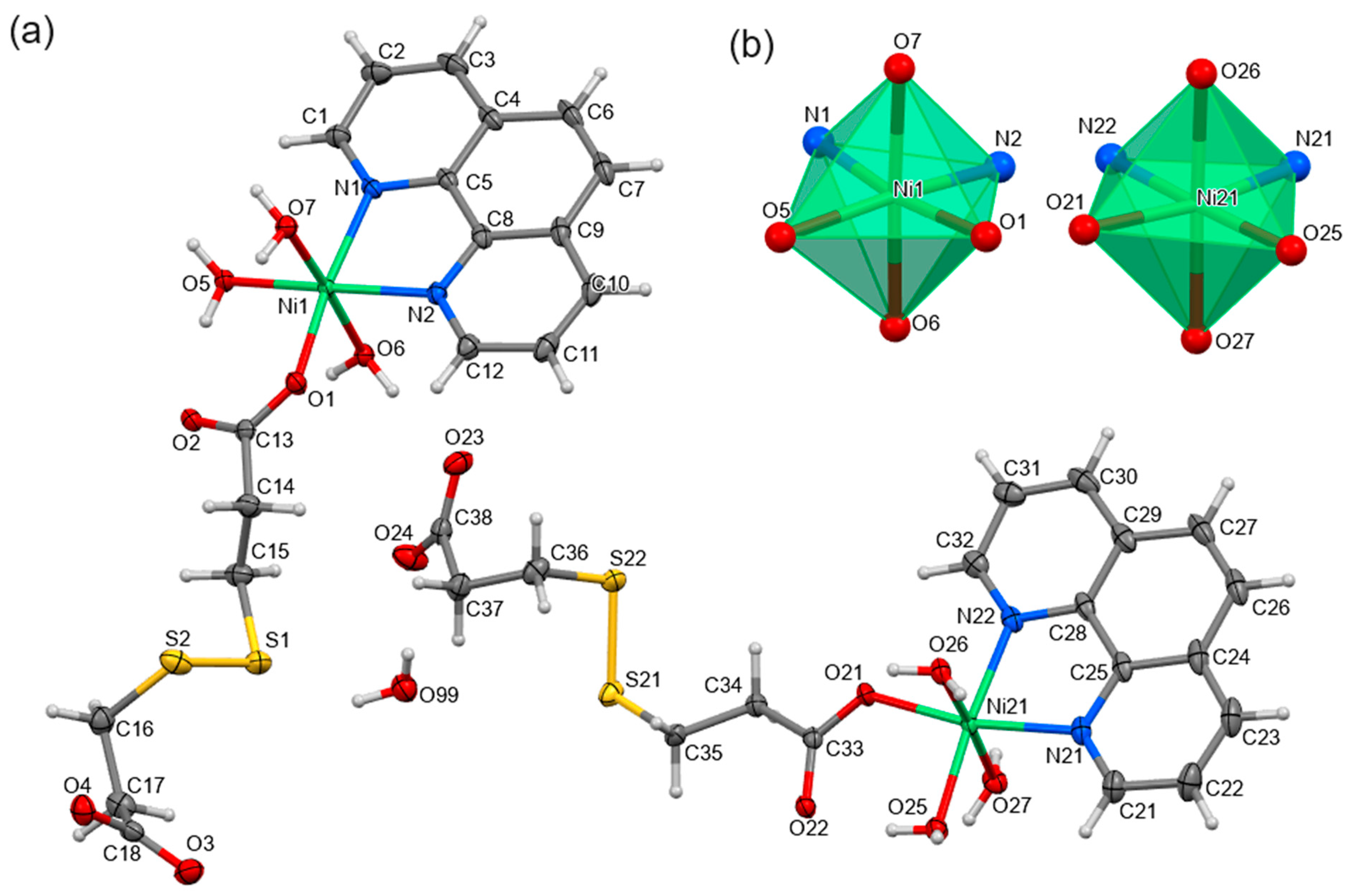
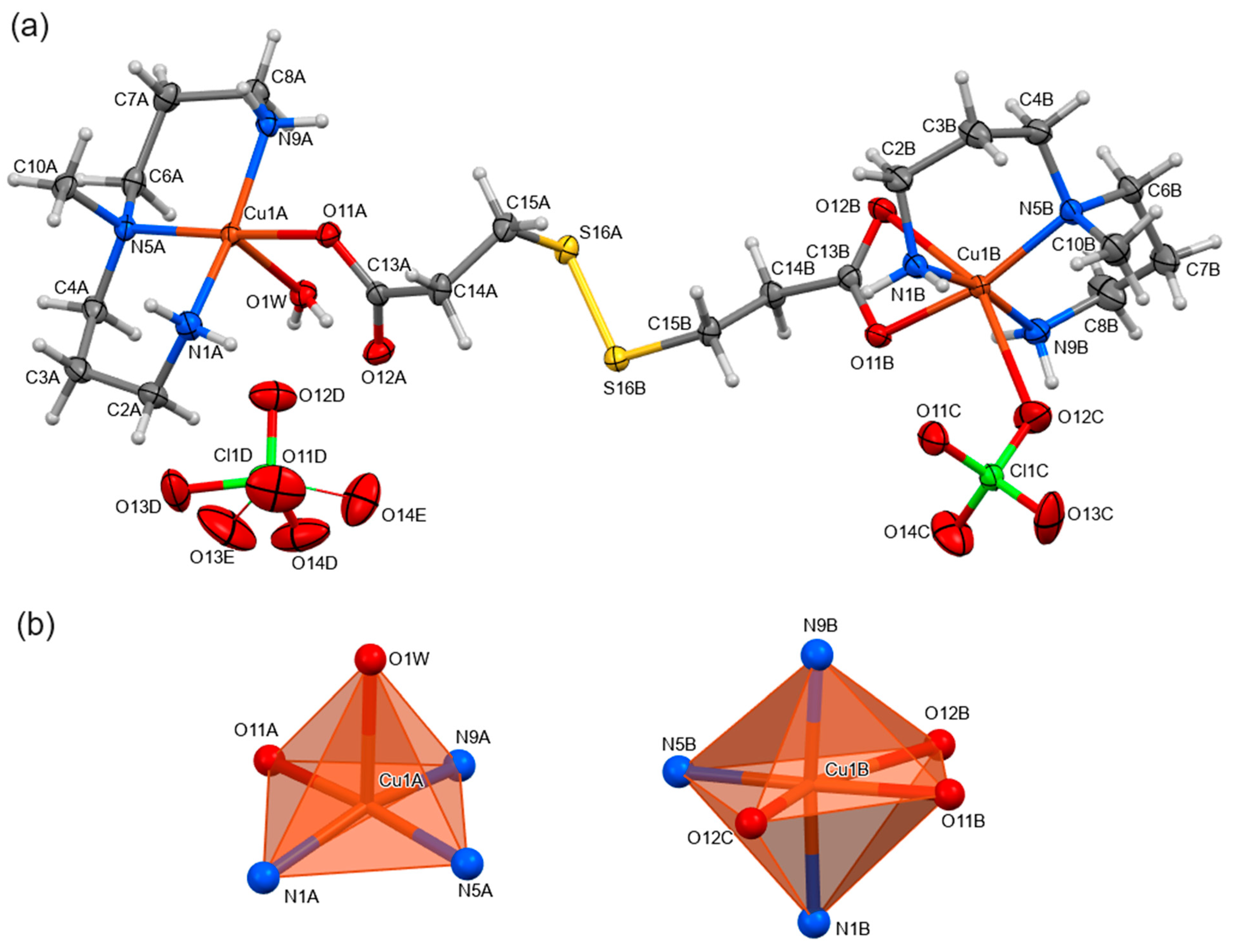
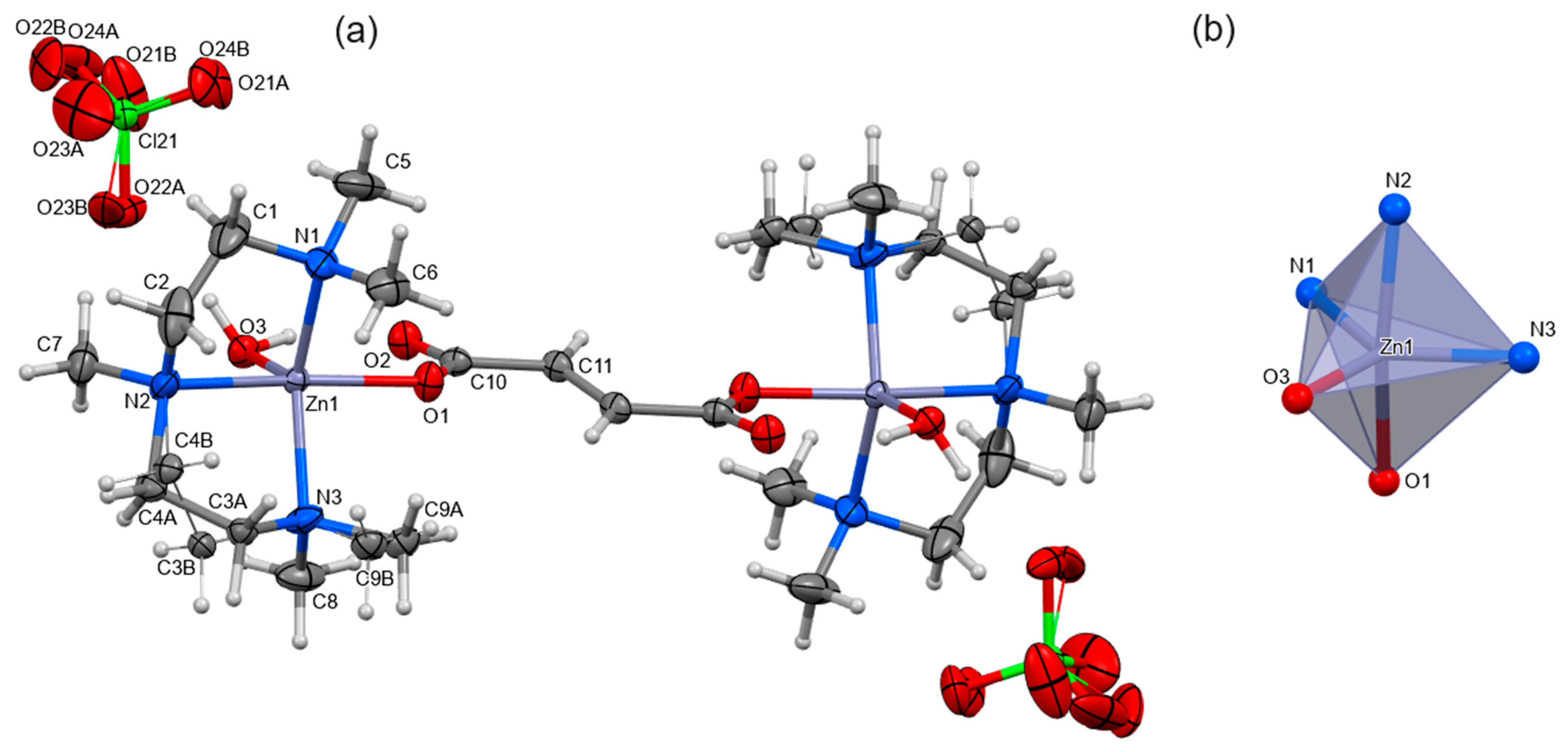
2.2. Structural Analysis
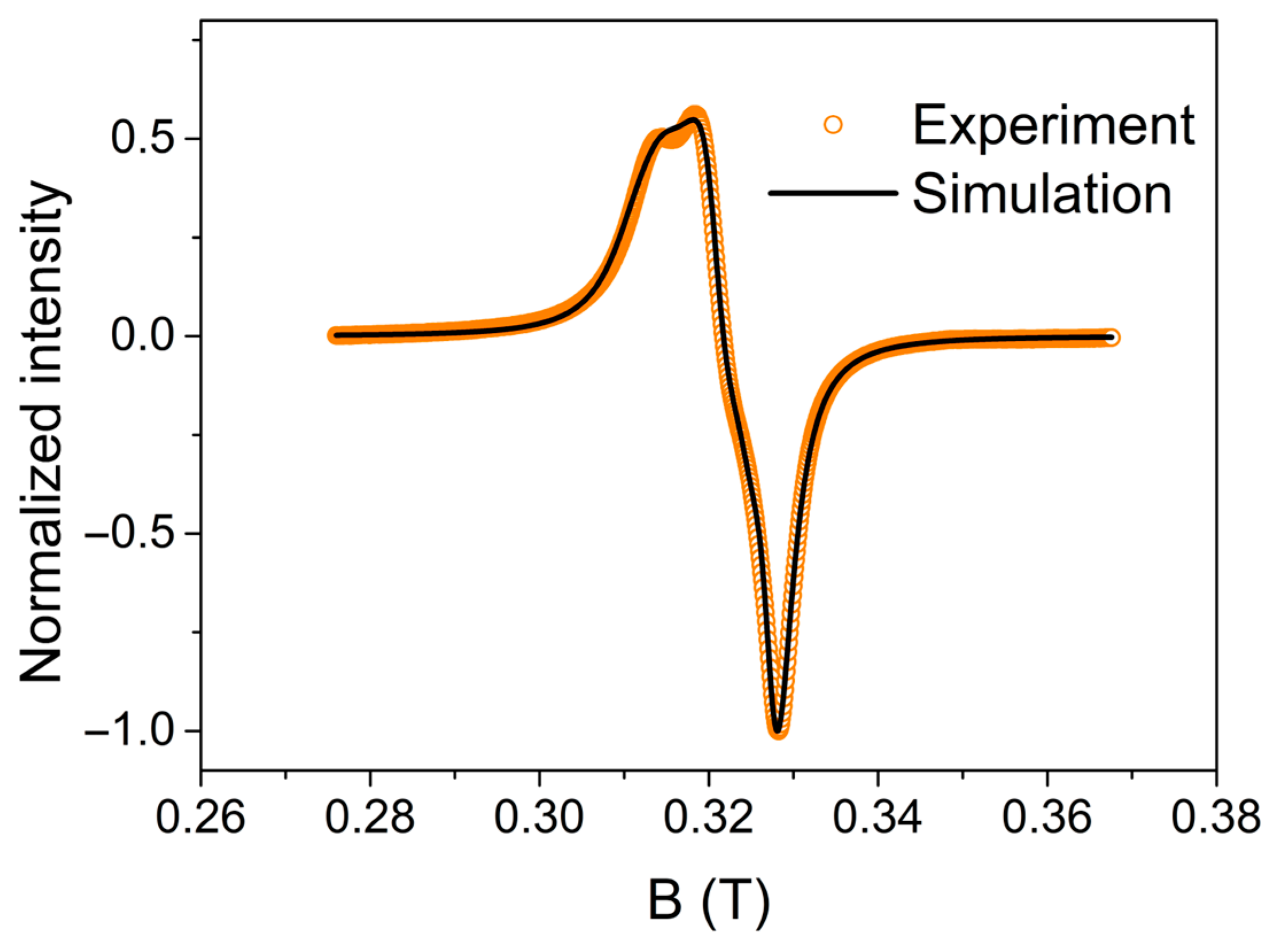
2.3. EPR Study of [(ClO4)(mdpta)Cu(μ-dtdp)Cu(mdpta)(H2O)](ClO4) (2)
2.4. Antimicrobial Activity of the Complexes
3. Materials and Methods
3.1. Chemicals and Methods
3.2. Complex Preparation
3.2.1. Ni(dtdp)(H2O)
3.2.2. [Ni(dtdp)(phen)(H2O)3]∙0.5H2O (1)
3.2.3. [(ClO4)(mdpta)Cu(μ-dtdp)Cu(mdpta)(H2O)](ClO4) (2)
3.2.4. [{Zn(pmdien)(H2O)}2(μ-fu)](ClO4)2 (3)
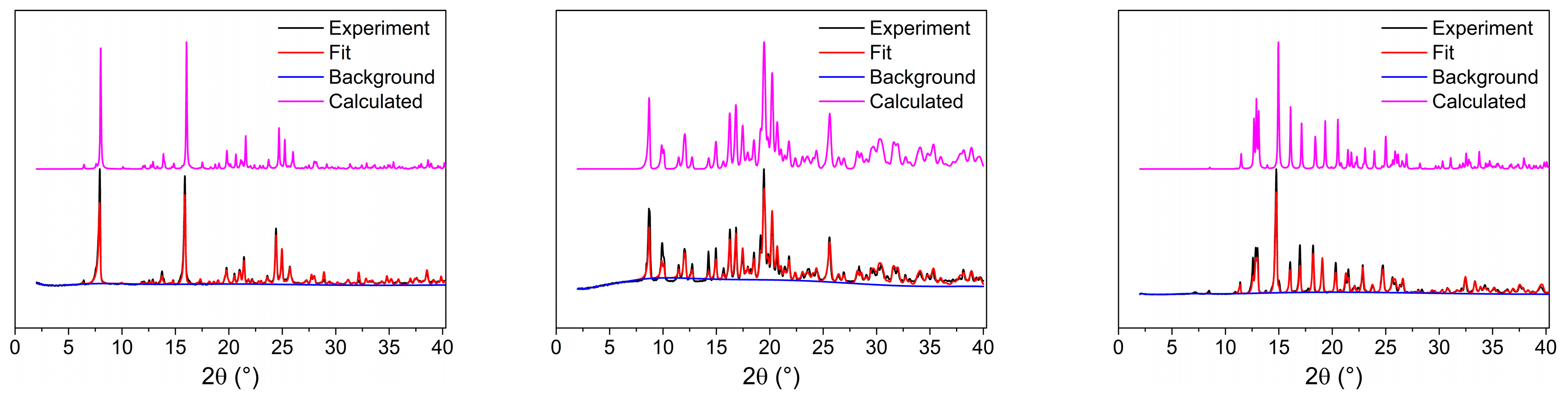
3.3. Determination of Crystal Structures
3.4. Determination of Antibacterial Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Li, S.; Song, J.; Ni, J.C.; Wang, Z.N.; Gao, X.; Shi, Z.; Bai, F.Y.; Xing, Y.H. Photoelectric properties and potential nitro derivatives sensing by a highly luminescent of Zn(II) and Cd(II) metal-organic frameworks assembled by the flexible hexapodal ligand, 1,3,5-triazine-2,4,6-triamine hexaacetic acid. RSC Adv. 2016, 6, 36000–36010. [Google Scholar] [CrossRef]
- Swiatkowski, M.; Kruszynski, R. Structurally diverse coordination compounds of zinc as effective precursors of zinc oxide nanoparticles with various morphologies. Appl. Organomet. Chem. 2019, 33, 18. [Google Scholar] [CrossRef]
- Svorec, J.; Polakovicova, P.; Moncol, J.; Kuchtanin, V.; Breza, M.; Soralova, S.; Padelkova, Z.; Mrozinski, J.; Lis, T.; Segla, P. Structural, magnetic and quantum-chemical study of dinuclear copper(II) thiophenecarboxylate and furancarboxylate complexes. Polyhedron 2014, 81, 216–226. [Google Scholar] [CrossRef]
- Kuchtanin, V.; Moncol, J.; Mrozinski, J.; Kalinska, B.; Padelková, Z.; Svorec, J.; Segl’a, P.; Melník, M. Study of copper(II) thiophenecarboxylate complexes with N-methylnicotinamide. Polyhedron 2013, 50, 546–555. [Google Scholar] [CrossRef]
- Shintoyo, S.; Murakami, K.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Watanabe, M.; Tsuchimoto, M.; Mrozinski, J.; et al. Crystal Field Splitting of the Ground State of Terbium(III) and Dysprosium(III) Complexes with a Triimidazolyl Tripod Ligand and an Acetate Determined by Magnetic Analysis and Luminescence. Inorg. Chem. 2014, 53, 10359–10369. [Google Scholar] [CrossRef]
- Ashraf, J.; Riaz, M.A. Biological potential of copper complexes: A review. Turk. J. Chem. 2022, 46, 595–623. [Google Scholar] [CrossRef]
- Molinaro, C.; Martoriati, A.; Pelinski, L.; Cailliau, K. Copper Complexes as Anticancer Agents Targeting Topoisomerases I and II. Cancers 2020, 12, 2863. [Google Scholar] [CrossRef]
- Switlicka-Olszewska, A.; Machura, B.; Mrozinski, J.; Kalinska, B.; Kruszynski, R.; Penkala, M. Effect of N-donor ancillary ligands on structural and magnetic properties of oxalate copper(II) complexes. New J. Chem. 2014, 38, 1611–1626. [Google Scholar] [CrossRef]
- Switlicka-Olszewska, A.; Machura, B.; Mrozinski, J. Synthesis, magnetic behavior and structural characterization of novel one-dimensional copper(II) coordination polymer based on azide and oxalate bridges. Inorg. Chem. Commun. 2014, 43, 86–89. [Google Scholar] [CrossRef]
- Esfahani, M.H.; Behzad, M. Crystal structure and antibacterial properties of a new dinuclear copper complex based on an unsymmetrical NN′O type Schiff base ligand. J. Coord. Chem. 2020, 73, 154–163. [Google Scholar] [CrossRef]
- Kopel, P.; Kamenícek, J.; Petrícek, V.; Kurecka, A.; Kalinska, B.; Mrozinski, J. Syntheses and study on nickel and copper complexes with 1,3,5-benzenetricarboxylic acid.: Crystal and molecular structure of Cu3(mdpta)3(btc) (ClO4)3·4H2O. Polyhedron 2007, 26, 535–542. [Google Scholar] [CrossRef]
- Loubalová, I.; Kopel, P. Coordination Compounds of Cu, Zn, and Ni with Dicarboxylic Acids and N Donor Ligands, and Their Biological Activity: A Review. Molecules 2023, 28, 1445. [Google Scholar] [CrossRef] [PubMed]
- Mautner, F.A.; Vicente, R.; Louka, F.R.Y.; Massoud, S.S. Dinuclear fumarato- and terephthalato-bridged copper(II) complexes: Structural characterization and magnetic properties. Inorg. Chim. Acta 2008, 361, 1339–1348. [Google Scholar] [CrossRef]
- Alarcon-Payer, C.; Pivetta, T.; Choquesillo-Lazarte, D.; Gonzalez-Perez, J.M.; Crisponi, G.; Castineiras, A.; Niclos-Gutierrez, J. Thiodiacetato-copper(II) chelates with or without N-heterocyclic donor ligands: Molecular and/or crystal structures of Cu(tda) (n), Cu(tda)(Him)(2)(H2O) and Cu(tda)(5Mphen) center dot 2H(2)O (Him = imidazole, 5Mphen=5-methyl-1,10-phenanthroline). Inorg. Chim. Acta 2005, 358, 1918–1926. [Google Scholar] [CrossRef]
- Bonomo, R.P.; Rizzarelli, E.; Brescianipahor, N.; Nardin, G. Properties and X-ray crystal-structures of copper(ii) mixed complexes with thiodiacetate and 2,2′-bipyridyl or 2,2′-6′,2′-terpyridyl. J. Chem. Soc.-Dalton Trans. 1982, 681–685. [Google Scholar] [CrossRef]
- Neuman, N.I.; Perec, M.; Gonzalez, P.J.; Passeggi, M.C.G.; Rizzi, A.C.; Brondino, C.D. Single Crystal EPR Study of the Dinuclear Cu(II) Complex Cu(tda)(phen) (2)center dot H(2)tda (tda = Thiodiacetate, phen = Phenanthroline): Influence of Weak Interdimeric Magnetic Interactions. J. Phys. Chem. A 2010, 114, 13069–13075. [Google Scholar] [CrossRef]
- Kopel, P.; Travnicek, Z.; Marek, J.; Korabik, M.; Mrozinski, J. Syntheses and properties of binuclear copper(II) mixed-ligand complexes involving thiodiglycolic acid. The crystal structures of (phen)(2)Cu(mu-tdga)Cu(phen) (NO3)(2)center dot 5H(2)O and (H2O)(pmdien)Cu(mu-tdga)Cu(pmdien)(H2O) (ClO4)(2). Polyhedron 2003, 22, 411–418. [Google Scholar] [CrossRef]
- Khullar, S.; Mandal, S.K. Effect of Spacer Atoms in the Dicarboxylate Linkers on the Formation of Coordination Architectures-Molecular Rectangles vs 1D Coordination Polymers: Synthesis, Crystal Structures, Vapor/Gas Adsorption Studies, and Magnetic Properties. Cryst. Growth Des. 2014, 14, 6433–6444. [Google Scholar] [CrossRef]
- Baggio, R.; Perec, M.; Garland, M.T. Aqua(2,2′-bipyriayl-N,N′)(thiodiacetato-O,O′,S)zinc(II) tetrahydrate. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 1996, 52, 2457–2460. [Google Scholar] [CrossRef]
- Arici, M.; Yesilel, O.Z.; Sahin, O.; Tas, M. Dinuclear and polynuclear copper(II) complexes with 3,31-thiodipropionate and unprecedented coordination mode. Polyhedron 2014, 71, 62–68. [Google Scholar] [CrossRef]
- Kopel, P.; Travnicek, Z.; Marek, J.; Mrozinski, J. Syntheses and study on nickel(II) complexes with thiodiglycolic acid and nitrogen-donor ligands. X-ray structures of Ni(bpy)(tdga)(H2O)center dot 4H(2)O and (en)Ni(mu-tdga)(2)NI(en) center dot 4H(2)O (tdgaH(2)= thiodiglycolic acid). Polyhedron 2004, 23, 1573–1578. [Google Scholar] [CrossRef]
- Loubalová, I.; Zahradníková, E.; Masaryk, L.; Nemec, I.; Hochvaldová, L.; Panáček, A.; Kvítek, L.; Večeřová, R.; Swiatkowski, M.; Kopel, P. Antibacterial study on nickel and copper dicarboxylate complexes. Inorg. Chim. Acta 2023, 545, 121273. [Google Scholar] [CrossRef]
- Lazarou, K.N.; Terzis, A.; Perlepes, S.P.; Raptopoulou, C.P. Synthetic, structural and spectroscopic studies of complexes derived from the copper(II) perchlorate/fumaric acid/N,N′-chelates tertiary reaction systems. Polyhedron 2010, 29, 46–53. [Google Scholar] [CrossRef]
- Dalai, S.; Mukherjee, P.S.; Rogez, G.; Mallah, T.; Drew, M.G.B.; Chaudhuri, N.R. Synthesis, crystal structures, and magnetic properties of two new 1D copper(II) coordination polymers containing fumarate(-2) and chelating N,N′-donor as ligands. Eur. J. Inorg. Chem. 2002, 2002, 3292–3297. [Google Scholar] [CrossRef]
- Burrows, A.D.; Harrington, R.W.; Mahon, M.F.; Price, C.E. The influence of hydrogen bonding on the structure of zinc co-ordination polymers. J. Chem. Soc.-Dalton Trans. 2000, 3845–3854. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Lin, J.L.; Chen, B.Y. New catenary coordination polymers using fumarato ligand as bridging spacer: Crystal structures of Mn(phen)(2)(H2O)(2) L center dot 4H(2)O, Mn(phen)(H2O)(2)L and Zn(phen)L center dot H2L with-H2L fumaric acid. J. Mol. Struct. 2003, 646, 151–159. [Google Scholar] [CrossRef]
- Konar, S.; Zangrando, E.; Drew, M.G.B.; Ribas, J.; Chaudhuri, N.R. Synthesis, structural analysis, and magnetic behaviour of three fumarate bridged coordination polymers: Five-fold interpenetrated diamond-like net of Ni-II, sheets of Ni-II and Co-II. Dalton Trans. 2004, 260–266. [Google Scholar] [CrossRef]
- Ohmura, T.; Mori, W.; Hasegawa, M.; Takei, T.; Ikeda, T.; Hasegawa, E. Crystal structures and magnetic and gas-occlusion properties of microporous materials containing infinite chains of mononuclear metal (Cu(II), Zn(II), and Ni(II)) dicarboxylates unit. Bull. Chem. Soc. Jpn. 2003, 76, 1387–1395. [Google Scholar] [CrossRef]
- Abbaszadeh, A.; Safari, N.; Amani, V.; Notash, B.; Raei, F.; Eftekhar, F. Mononuclear and Dinuclear Copper(II) Complexes Containing N, O and S Donor Ligands: Synthesis, Characterization, Crystal Structure Determination and Antimicrobial Activity of Cu(phen)(tda) center dot 2H(2)O and (phen)(2)Cu(mu-tda)Cu(phen) (ClO4)(2 center dot)1.5H(2)O. Iran J. Chem. Chem. Eng.-Int. Engl. Ed. 2014, 33, 1–13. [Google Scholar]
- Buchtelova, H.; Skubalova, Z.; Strmiska, V.; Michalek, P.; Kociova, S.; Smerkova, K.; Kruszynski, R.; Bienko, A.; Kaj, M.; Lewinska, A.; et al. Synthesis and structural characterization of antimicrobial binuclear copper (II) coordination compounds bridged by hydroxy- and/or thiodipropionic acid. J. Inorg. Biochem. 2019, 191, 8–20. [Google Scholar] [CrossRef]
- Lahiri, D.; Bhowmick, T.; Pathak, S.; Shameema, O.; Patra, A.K.; Ramakumar, S.; Chakravarty, A.R. Anaerobic Photocleavage of DNA in Red Light by Dicopper(II) Complexes of 3,3′-Dithiodipropionic Acid. Inorg. Chem. 2009, 48, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Figuerola, A.; Bertolasi, V.; Manna, S.C. DNA/protein binding and magnetic properties of a 1D Cu(II) complex containing fumarate and tridentate Schiff base ligands. Polyhedron 2016, 119, 460–470. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE. Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; Universitat de Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Casanova, D.; Cirera, J.; Llunell, M.; Alemany, P.; Avnir, D.; Alvarez, S. Minimal Distortion Pathways in Polyhedral Rearrangements. J. Am. Chem. Soc. 2004, 126, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Etter, M.C.; MacDonald, J.C. Graph-Set Analysis of Hydrogen-Bond Patterns in Organic Crystals. Acta Crystallogr. Sect. B 1990, 46, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Taut, T.; Kleeberg, R.; Bergmann, J. The new seifert Rietveld program and its application to quantitative phase analysis. In Proceedings of the XVII Conference on Applied Crystallography, Wisla, Poland, 31 August–4 September 1997; pp. 87–92. [Google Scholar]
- Garribba, E.; Micera, G. The determination of the geometry of Cu(II) complexes—An EPR spectroscopy experiment. J. Chem. Educ. 2006, 83, 1229–1232. [Google Scholar] [CrossRef]
- Hathaway, B.J.; Billing, D.E. Electronic properties and stereochemistry of mono-nuclear complexes of copper(ii) ion. Coord. Chem. Rev. 1970, 5, 143–207. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A graphical user interface for the Rietveld refinement program BGMN. J. Appl. Crystallogr. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Chilton, N.F.; Anderson, R.P.; Turner, L.D.; Soncini, A.; Murray, K.S. PHI: A powerful new program for the analysis of anisotropic monomeric and exchange-coupled polynuclear d- and f-block complexes. J. Comput. Chem. 2013, 34, 1164–1175. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C-Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
| MIC [g/L] | ||||
|---|---|---|---|---|
| Complex | E. coli | P. aeruginosa | E. faecalis | S. aureus |
| 1 | 25 | 25 | 6.25 | 12.5 |
| 2 | 6.25 | 12.5 | 3.13 | 0.78 |
| 3 | 6.25 | >25 | 6.25 | 12.5 |
| Compound (CCDC Number). | 1 (2369177) | 2 (2314973) | 3 (2369178) |
|---|---|---|---|
| Empirical formula | C36H46N4Ni2O15S4 | C20H48Cl2Cu2N6O13S2 | C11H26ClN3O7Zn |
| Formula weight | 1020.43 | 842.74 | 826.33 |
| Temperature (K) | 100.3 (5) | 120 (2) | 100.0 (1) |
| Crystal system | orthorhombic | monoclinic | monoclinic |
| Space group | Pbca | Cc | P21/c |
| a (Å) | 22.0565 (1) | 15.1199 (5) | 8.1853 (1) |
| b (Å) | 14.4105 (1) | 11.2259 (4) | 15.4006 (2) |
| c (Å) | 27.4138 (1) | 20.9787 (6) | 14.2517 (2) |
| α (°) | 90 | 90 | 90 |
| β (°) | 90 | 105.828 (1) | 101.713 (2) |
| γ (°) | 90 | 90 | 90 |
| Volume (Å3) | 8713.34 (8) | 3425.80 (19) | 1759.14 (4) |
| Z | 8 | 4 | 2 |
| ρcalc (g/cm3) | 1.556 | 1.634 | 1.560 |
| μ (mm−1) | 3.481 | 1.586 | 1.584 |
| F(000) | 4240 | 1752 | 864 |
| Crystal size (mm3) | 0.469 × 0.081 × 0.051 | 0.166 × 0.116 × 0.108 | 0.427 × 0.229 × 0.207 |
| Radiation | Cu Kα (λ = 1.54184) | Mo Kα (λ = 0.71073) | Mo Kα (λ = 0.71073) |
| θ range for data collect. (°) | 3.224 to 78.895 | 2.018 to 27.482 | 2.645 to 31.581 |
| Index ranges | −28 ≤ h ≤ 27, | −19 ≤ h ≤ 17, | −10 ≤ h ≤ 11, |
| −18 ≤ k ≤ 16, | −14 ≤ k ≤ 14, | −22 ≤ k ≤ 20, | |
| −34 ≤ l ≤ 33 | −27 ≤ l ≤ 27 | −19 ≤ l ≤ 20 | |
| Reflections collected/independent | 201405/9260 | 28941/7369 | 59565/5458 |
| Rint | 0.0734 | 0.0305 | 0.0305 |
| Data/restraints/parameters | 9260/21/592 | 7369/26/427 | 5458/14/280 |
| Goodness-of-fit on F2 | 1.100 | 1.056 | 1.071 |
| Final R indexes [I > 2s(I)] | R1 = 0.0408, | R1 = 0.0174, | R1 = 0.0297, |
| wR2 = 0.1077 | wR2 = 0.0426 | wR2 = 0.0764 | |
| R indexes (all data) | R1 = 0.0426, | R1 = 0.0179, | R1 = 0.0366, |
| wR2 = 0.1088 | wR2 = 0.0429 | wR2 = 0.0809 | |
| Largest diff. peak and hole (e·Å−3) | 0.905/−0.725 | 0.319/−0.259 | 1.179/−0.440 |
| Flack parameter | - | 0.020 (3) | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loubalová, I.; Kotrle, K.; Antal, P.; Hochvaldová, L.; Panáček, A.; Císařová, I.; Świątkowski, M.; Kopel, P. Dithiodipropionate and Fumarate Ni, Cu, and Zn Mixed Ligand Complexes. Inorganics 2024, 12, 260. https://doi.org/10.3390/inorganics12100260
Loubalová I, Kotrle K, Antal P, Hochvaldová L, Panáček A, Císařová I, Świątkowski M, Kopel P. Dithiodipropionate and Fumarate Ni, Cu, and Zn Mixed Ligand Complexes. Inorganics. 2024; 12(10):260. https://doi.org/10.3390/inorganics12100260
Chicago/Turabian StyleLoubalová, Ivana, Kamil Kotrle, Peter Antal, Lucie Hochvaldová, Aleš Panáček, Ivana Císařová, Marcin Świątkowski, and Pavel Kopel. 2024. "Dithiodipropionate and Fumarate Ni, Cu, and Zn Mixed Ligand Complexes" Inorganics 12, no. 10: 260. https://doi.org/10.3390/inorganics12100260
APA StyleLoubalová, I., Kotrle, K., Antal, P., Hochvaldová, L., Panáček, A., Císařová, I., Świątkowski, M., & Kopel, P. (2024). Dithiodipropionate and Fumarate Ni, Cu, and Zn Mixed Ligand Complexes. Inorganics, 12(10), 260. https://doi.org/10.3390/inorganics12100260







