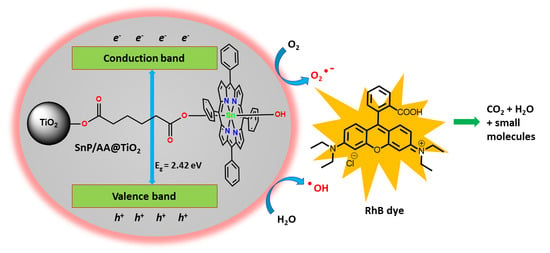
Sn(IV)porphyrin-Anchored TiO2 Nanoparticles via Axial-Ligand Coordination for Enhancement of Visible Light-Activated Photocatalytic Degradation
Abstract
1. Introduction
2. Results and Discussion
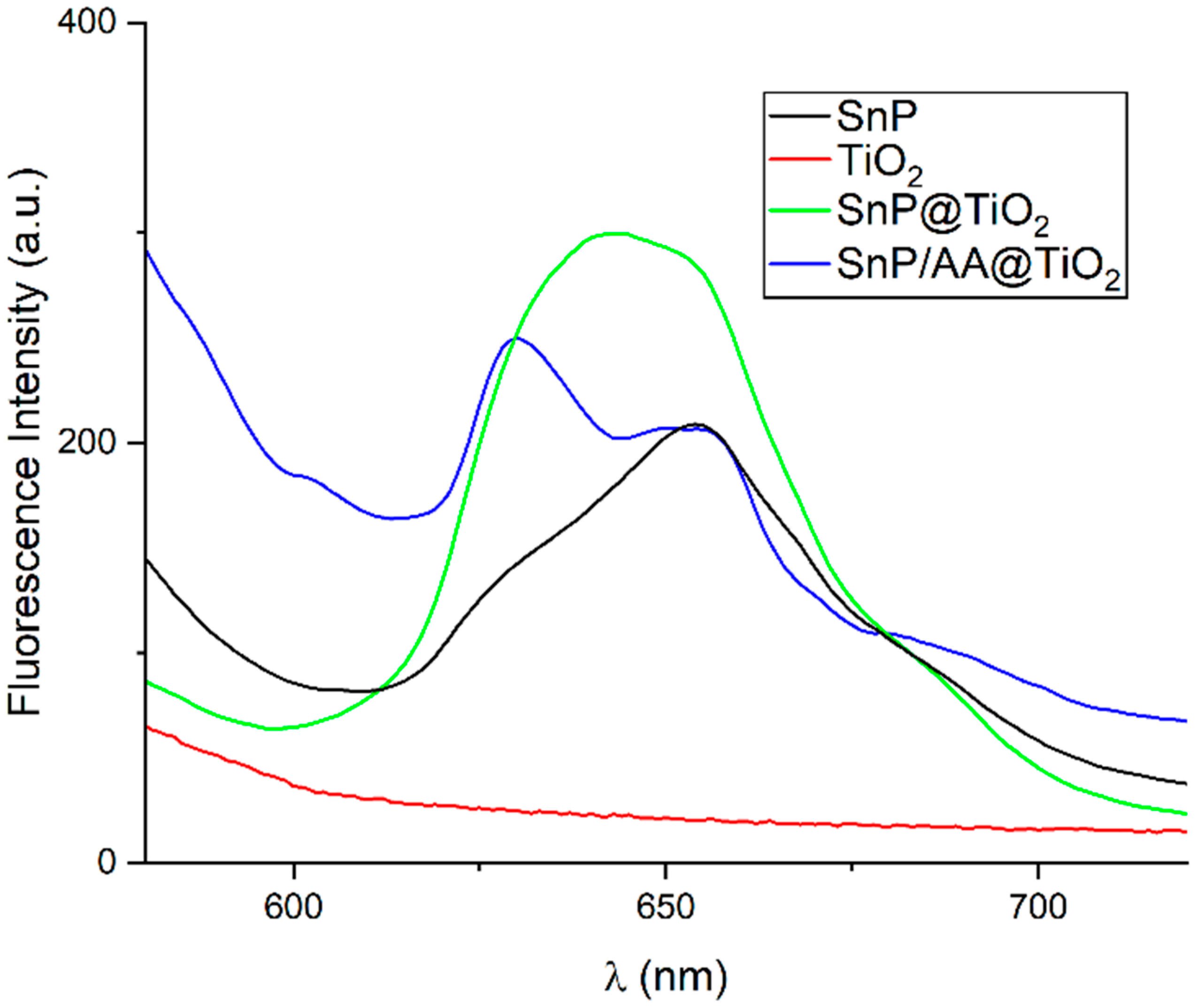
2.1. Fabrication and Characterization of Photocatalysts
2.2. Photocatalytic Degradation of Rhodamine B (RhB)
3. Materials and Methods
3.1. Synthesis of SnP/AA@TiO2
3.2. Synthesis of SnP@TiO2
3.3. Photocatalytic Degradation Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, Z.; Walker, G.W.; Muir, D.C.G.; Nagatani-Yoshida, K. Toward a Global Understanding of Chemical Pollution: A First Comprehensive Analysis of National and Regional Chemical Inventories. Environ. Sci. Technol. 2020, 54, 2575–2584. [Google Scholar] [CrossRef]
- Naidu, R.; Biswas, B.; Willett, I.R.; Cribb, J.; Kumar Singh, B.; Paul Nathanail, C.; Coulon, F.; Semple, K.T.; Jones, K.C.; Barclay, A.; et al. Chemical pollution: A growing peril and potential catastrophic risk to humanity. Environ. Int. 2021, 156, 106616. [Google Scholar] [CrossRef]
- Ren, G.; Han, H.; Wang, Y.; Liu, S.; Zhao, J.; Meng, X.; Li, Z. Recent Advances of Photocatalytic Application in Water Treatment: A Review. Nanomaterials 2021, 11, 1804. [Google Scholar]
- Parvulescu, V.I.; Epron, F.; Garcia, H.; Granger, P. Recent Progress and Prospects in Catalytic Water Treatment. Chem. Rev. 2022, 122, 2981–3121. [Google Scholar] [CrossRef]
- Rao, C.; Zhou, L.; Pan, Y.; Lu, C.; Qin, X.; Sakiyama, H.; Muddassir, M.; Liu, J. The Extra-Large Calixarene-Based MOFs-Derived Hierarchical Composites for Photocatalysis of Dye: Facile Syntheses and Contribution of Carbon Species. J. Alloys Compd. 2022, 897, 163178. [Google Scholar] [CrossRef]
- Hollender, J.; Zimmermann, S.G.; Koepke, S.; Krauss, M.; McArdell, C.S.; Ort, C.; Singer, H.; von Gunten, U.; Siegrist, H. Elimination of Organic Micropollutants in a Municipal Wastewater Treatment Plant Upgraded with a Full-Scale Post-Ozonation Followed by Sand Filtration. Environ. Sci. Technol. 2009, 43, 7862–7869. [Google Scholar]
- Xu, Q.; Huang, Q.-S.; Luo, T.-Y.; Wu, R.-L.; Wei, W.; Ni, B.-J. Coagulation removal and photocatalytic degradation of microplastics in urban waters. Chem. Eng. J. 2021, 416, 129123. [Google Scholar]
- Dong, X.; Li, Y.; Li, D.; Liao, D.; Qin, T.; Prakash, O.; Kumar, A.; Liu, J. A new 3D 8-connected Cd(II) MOF as a potentphotocatalyst for oxytetracycline antibiotic degradation. CrystEngComm 2022, 24, 6933. [Google Scholar] [CrossRef]
- Saratale, R.G.; Saratale, G.D.; Chang, J.S.; Govindwar, S.P. Bacterial decolorization and degradation of azo dyes: A review. J. Taiwan Inst. Chem. Eng. 2011, 42, 138–157. [Google Scholar] [CrossRef]
- Łuba, M.; Mikołajczyk, T.; Pierożyński, B.; Smoczyński, L.; Wojtacha, P.; Kuczyński, M. Electrochemical Degradation of Industrial Dyes in Wastewater through the Dissolution of Aluminum Sacrificial Anode of Cu/Al Macro-Corrosion Galvanic Cell. Molecules 2020, 25, 4108. [Google Scholar]
- Deng, Y.; Zhao, R. Advanced Oxidation Processes (AOPs) in Wastewater Treatment. Curr. Pollut. Rep. 2015, 1, 167–176. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J.C.G.E. Advanced Oxidation Processes Coupled with Nanomaterials for Water Treatment. Nanomaterials 2021, 11, 2045. [Google Scholar] [CrossRef]
- Palma, D.; Deganello, F.; Liotta, L.F.; La Parola, V.; Bianco Prevot, A.; Malandrino, M.; Laurenti, E.; Boffa, V.; Magnacca, G. Main Issues in the Synthesis and Testing of Thermocatalytic Ce-Doped SrFeO3 Perovskites for Wastewater Pollutant Removal. Inorganics 2023, 11, 85. [Google Scholar] [CrossRef]
- Franco, P.; Sacco, O.; De Marco, I.; Vaiano, V. Zinc Oxide Nanoparticles Obtained by Supercritical Anti solvent Precipitation for the Photocatalytic Degradation of Crystal Violet Dye. Catalysts 2019, 9, 346. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability. Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Guang, H.; Yang, J.; Duan, X.; Farnood, R.; Yang, C.; Yang, J.; Liu, W.; Liu, Q. Recent developments and challenges in zeolite-based composite photocatalysts for environmental applications. Chem. Eng. J. 2021, 417, 129209. [Google Scholar]
- Jin, J.C.; Wang, J.; Guo, J.; Yan, M.H.; Wang, J.; Srivastava, D.; Kumar, A.; Sakiyama, H.; Muddassir, M.; Pan, Y. A 3D rare cubane-like tetramer Cu(II)-based MOF with 4-fold dia topology as an efficient photocatalyst for dye degradation. Colloids Surf. A 2023, 656, 130475. [Google Scholar] [CrossRef]
- Subhiksha, V.; Kokilavani, S.; Khan, S.S. Recent advances in degradation of organic pollutant in aqueous solutions using bismuth based photocatalysts: A review. Chemosphere 2022, 290, 133228. [Google Scholar] [CrossRef]
- Heng, Z.W.; Chong, W.C.; Pang, Y.L.; Koo, C.H. An Overview of the Recent Advances of Carbon Quantum Dots/metal Oxides in the Application of Heterogeneous Photocatalysis in Photodegradation of Pollutants towards Visible-Light and Solar Energy Exploitation. J. Environ. Chem. Eng. 2021, 9, 105199. [Google Scholar] [CrossRef]
- Yao, S.; Yuan, X.; Jiang, L.; Xiong, T.; Zhang, J. Recent Progress on Fullerene-Based Materials: Synthesis, Properties, Modifications, and Photocatalytic Applications. Materials 2020, 13, 2924. [Google Scholar] [CrossRef]
- Sandhu, I.S.; Chitkara, M.; Rana, S.; Dhillon, G.; Taneja, A.; Kumar, S. Photocatalytic performances of stand-alone graphene oxide (GO) and reduced graphene oxide (rGO) nanostructures. Opt Quant Electron 2020, 52, 359. [Google Scholar] [CrossRef]
- La, D.D.; Ngo, H.H.; Nguyen, D.D.; Tran, N.T.; Vo, H.T.; Nguyen, X.H.; Chang, S.W.; Chung, W.J.; Nguyen, M.D. Advances and prospects of porphyrin-based nanomaterials via self-assembly for photocatalytic applications in environmental treatment. Coord. Chem. Rev. 2022, 463, 214543. [Google Scholar]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-Organized Porphyrinic Materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar] [CrossRef]
- Hasobe, T. Porphyrin-Based Supramolecular Nanoarchitectures for Solar Energy Conversion. J. Phys. Chem. Lett. 2013, 4, 1771–1780. [Google Scholar] [CrossRef]
- Drain, C.M.; Lehn, J.M. Self-assembly of Square Multiporphyrin Arrays by Metal Ion Coordination. J. Chem. Soc. Chem. Commun. 1994, 19, 2313–2315. [Google Scholar] [CrossRef]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and functionalization of porphyrins through organometallic methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef]
- Mota, H.P.; Quadrado, R.F.N.; Iglesias, B.A.; Fajardo, A.R. Enhanced photocatalytic degradation of organic pollutants mediated by Zn(II)-porphyrin/poly(acrylic acid) hybrid microparticles. Appl. Catal. B Environ. 2020, 277, 119208. [Google Scholar] [CrossRef]
- Liu, S.; Xia, S.; Wang, J.; Ren, X.; Chen, S.; Zhong, Y.; Bai, F. Synthesis of the ZnTPyP/WO3 nanorod-on-nanorod heterojunction direct Z-scheme with spatial charge separation ability for enhanced photocatalytic hydrogen generation. Nanoscale 2023, 15, 2871–2881. [Google Scholar] [CrossRef]
- Nadeem, S.; Mutalib, I.A.; Shahrun, M.S. Synthesis of Metalloporphyrin Encapsulated Zeolite A for Photocatalytic Orange II Degradation. Procedia Eng. 2016, 148, 1282–1288. [Google Scholar] [CrossRef]
- Min, K.S.; Kumar, R.S.; Lee, J.H.; Kim, K.S.; Lee, S.G.; Son, Y.A. Synthesis of new TiO2/porphyrin-based composites and photocatalytic studies on methylene blue degradation. Dyes Pigments 2019, 160, 37–47. [Google Scholar] [CrossRef]
- La, D.D.; Hangarge, R.V.; Bhosale, S.V.; Ninh, H.D.; Jones, L.A.; Bhosale, S.V. Arginine-Mediated Self-Assembly of Porphyrin on Graphene: A Photocatalyst for Degradation of Dyes. Appl. Sci. 2017, 7, 643. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, A.; Zhao, W.; Li, C.; Chen, X.; Wang, Y.; Zhu, W.; Zhong, Q. Influence of Metal-Porphyrins on the Photocatalysis of Graphitic Carbon Nitride. Dyes Pigm. 2018, 153, 241–247. [Google Scholar] [CrossRef]
- Vo, H.T.; Nguyen, A.T.; Tran, C.V.; Nguyen, S.X.; Tung, N.T.; Pham, D.T.; Nguyen, D.D.; La, D.D. Self-Assembly of Porphyrin Nanofibers on ZnO Nanoparticles for the Enhanced Photocatalytic Performance for Organic Dye Degradation. ACS Omega 2021, 6, 23203–23210. [Google Scholar] [CrossRef]
- Kim, W.; Tachikawa, T.; Majima, T.; Li, C.; Kim, H.-J.; Choi, W. Tin-porphyrin sensitized TiO2 for the production of H2 under visible light. Energy Environ. Sci. 2010, 3, 1789–1795. [Google Scholar] [CrossRef]
- Duan, M.Y.; Li, J.; Mele, G.; Wang, C.; Lu, X.F.; Vasapollo, G.; Zhang, F.X. Photocatalytic Activity of Novel Tin Porphyrin/TiO2 Based Composites. J. Phys. Chem. C 2010, 114, 7857–7862. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kim, H.; Mergu, N.; Son, Y.-A. A photocatalytic comparison study between tin complex and carboxylic acid derivatives of porphyrin/TiO2 composites. Res. Chem. Intermed. 2020, 46, 313–328. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Li, X.; Kang, S.-Z.; Mu, J. Visible Light Photocatalytic Activity of Porphyrin Tin(IV) Sensitized TiO2 Nanoparticles for the Degradation of 4-Nitrophenol and Methyl Orange. J. Dispersion Sci. Technol. 2011, 32, 943–947. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Supramolecular Porphyrin Nanostructures Based on Coordination-Driven Self-Assembly and Their Visible Light Catalytic Degradation of Methylene Blue Dye. Nanomaterials 2020, 10, 2314. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Self-Assembled Nanomaterials Based on Complementary Sn(IV) and Zn(II)-Porphyrins, and Their Photocatalytic Degradation for Rhodamine B Dye. Molecules 2021, 26, 3598. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, H.-J. Sn(IV)-Porphyrin-Based Nanostructures Featuring Pd(II)-Mediated Supramolecular Arrays and Their Photocatalytic Degradation of Acid Orange 7 Dye. Int. J. Mol. Sci. 2022, 23, 13702. [Google Scholar] [CrossRef]
- Shee, N.K.; Kim, H.-J. Supramolecular squares of Sn(IV)porphyrins with Re(I)-corners for the fabrication of self-assembled nanostructures performing photocatalytic degradation of Eriochrome Black T dye. Inorg. Chem. Front. 2023, 10, 174–183. [Google Scholar] [CrossRef]
- Shee, N.K.; Park, B.-H.; Kim, H.-J. Hybrid Composite of Sn(IV)-Porphyrin and Mesoporous Structure for Enhanced Visible Light Photocatalytic Degradation of Organic Dyes. Molecules 2023, 28, 1886. [Google Scholar] [CrossRef]
- Fallon, G.D.; Langford, S.J.; Lee, M.A.-P.; Lygris, E. Self-assembling Mixed Porphyrin Trimers—The Use of Diaxial Sn(IV) Porphyrin Phenolates as an Organising Precept. Inorg. Chem. Commun. 2002, 5, 715–718. [Google Scholar] [CrossRef]
- Hawley, J.C.; Bampos, N.; Sanders, J.K.M. Synthesis and Characterization of Carboxylate Complexes of SnIV Porphyrin Monomers and Oligomers. Chem. Eur. J. 2003, 9, 5211–5222. [Google Scholar]
- Arnold, D.P.; Blok, J. The coordination chemistry of tin porphyrin complexes. Coord. Chem. Rev. 2004, 248, 299–319. [Google Scholar] [CrossRef]
- Ou, Z.; E, W.; Zhu, W.; Thordarson, P.; Sintic, P.J.; Crossley, M.J.; Kadish, K.M. Effect of Axial Ligands and Macrocyclic Structure on Redox Potentials and Electron-Transfer Mechanisms of Sn(IV) Porphyrins. Inorg. Chem. 2007, 46, 10840–10849. [Google Scholar] [CrossRef]
- Shetti, V.S.; Ravikanth, M. Sn(IV) Porphyrin based axial-bonding type porphyrin triads containing heteroporphyrins as axial ligands. Inorg. Chem. 2010, 49, 2692–2700. [Google Scholar] [CrossRef]
- Sharma, R.; Ghosh, A.; Wolfram, B.; Bröring, M.; Ravikanth, M. Synthesis and Characterization of Hexa-Coordinated Sn(IV) Complexes of Meso-Aryl Dipyrrins. Dalton Trans. 2013, 42, 5627–5630. [Google Scholar] [CrossRef]
- Pareek, Y.; Lakshmi, V.; Ravikanth, M. Axially bonded pentads constructed on the Sn(IV) porphyrin scaffold. Dalton Trans. 2014, 43, 6870–6879. [Google Scholar] [CrossRef]
- Natali, M.; Amati, A.; Demitri, N.; Iengo, E. Formation of a Long-Lived Radical Pair State in a Sn(IV) Porphyrin-di-(L-tyrosinato) Conjugate Driven by Proton-Coupled Electron-Transfer. Chem. Commun. 2018, 54, 6148–6152. [Google Scholar] [CrossRef]
- Ravikumar, M.; Raghav, D.; Rathinasamy, K.; Kathiravan, A.; Mothi, E.M. DNA Targeting Long-Chain Alkoxy Appended Tin(IV) Porphyrin Scaffolds: Photophysical and Antimicrobial PDT Investigations. ACS Appl. Bio Mater. 2018, 1, 1705–1716. [Google Scholar] [CrossRef] [PubMed]
- Shee, N.K.; Kim, M.K.; Kim, H.-J. Fluorescent chemosensing for aromatic compounds by supramolecular complex composed of tin(IV) porphyrin, viologen, and cucurbit[8]uril. Chem. Commun. 2019, 55, 10575–10578. [Google Scholar] [CrossRef]
- Chatterjee, S.; Tyagi, A.K.; Ayyub, P. Efficient Photocatalytic Degradation of Rhodamine B Dye by Aligned Arrays of Self Assembled Hydrogen Titanate Nanotubes. J. Nanomater. 2014, 2014, 328618. [Google Scholar] [CrossRef]
- Sundararajan, M.; Sailaja, V.; Kennedy, L.J.; Vijaya, J.J. Photocatalytic degradation of Rhodamine B under visible light using nanostructured zinc doped cobalt ferrite: Kinetics and mechanism. Ceram. Int. 2017, 43, 540–548. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Li, C.; Yu, J.; Jiang, X.; Zheng, Y.; Hu, W.; Jiao, F. Enhanced photocatalytic degradation of rhodamine B, methylene blue and 4-nitrophenol under visible light irradiation using TiO2/MgZnAl layered double hydroxide. J. Mater. Sci. Mater. Electron. 2018, 29, 7002–7014. [Google Scholar] [CrossRef]
- Luna-Flores, A.; Morales, M.A.; Agustín-Serrano, R.; Portillo, R.; Luna-López, J.A.; Pérez-Sánchez, G.F.; Luz, A.D.H.-d.l.; Tepale, N. Improvement of the Photocatalytic Activity of ZnO/Burkeite Heterostructure Prepared by Combustion Method. Catalysts 2019, 9, 817. [Google Scholar] [CrossRef]
- Pontes do Nascimento, N.M.; Machado de Lima, B.R.; Zamian, J.R.; Ferreira da Costa, C.E.; Adriano Santos do Nascimento, L.; Luque, R.; Filho, G.N.d.R. Synthesis of Mesoporous Zn1–xMxAl2O4 Substituted by Co2+ and Ni2+ Ions and Application in the Photodegradation of Rhodamine B. Materials 2020, 13, 2150. [Google Scholar] [CrossRef]
- Huang, J.; Nie, G.; Ding, Y. Metal-Free Enhanced Photocatalytic Activation of Dioxygen by g-C3N4 Doped with Abundant Oxygen-Containing Functional Groups for Selective N-Deethylation of Rhodamine B. Catalysts 2020, 10, 6. [Google Scholar] [CrossRef]
- Suvaitha, P.; Selvam, S.; Ganesan, D.; Rajangam, V.; Raji, A. Green Synthesis of SnO2 Nanoparticles for Catalytic Degradation of Rhodamine B. Iran. J. Sci. Technol. Trans. A Sci. 2020, 44, 661–676. [Google Scholar]
- Zhou, C.; Liu, Z.; Fang, L.; Guo, Y.; Feng, Y.; Yang, M. Kinetic and Mechanistic Study of Rhodamine B Degradation by H2O2 and Cu/Al2O3/g-C3N4 Composite. Catalysts 2020, 10, 317. [Google Scholar] [CrossRef]
- Ahmad, M.; Rehman, W.; Khan, M.M.; Qureshi, M.T.; Gul, A.; Haq, S.; Ullah, R.; Rab, A.; Menaa, F. Phytogenic fabrication of ZnO and gold decorated ZnO nanoparticles for photocatalytic degradation of Rhodamine B. J. Environ. Chem. Eng. 2021, 9, 104725. [Google Scholar] [CrossRef]
- Alorku, K.; Manoj, M.; Yanjuan, C.; Zhou, H.; Yuan, A. Nanomixture of 0-D ternary metal oxides (TiO2–SnO2–Al2O3) cooperating with 1-D hydroxyapatite (HAp) nanorods for RhB removal from synthetic wastewater and hydrogen evolution via water splitting. Chemosphere 2021, 273, 128575. [Google Scholar] [CrossRef]
- Amakali, T.; Živković, A.; Warwick, M.E.A.; Jones, D.R.; Dunnill, C.W.; Daniel, L.S.; Uahengo, V.; Mitchell, C.E.; Dzade, N.Y.; de Leeuw, N.H. Photocatalytic degradation of rhodamine B dye and hydrogen evolution by hydrothermally synthesized NaBH4-spiked ZnS nanostructures. Front. Chem. 2022, 10, 835832. [Google Scholar] [CrossRef]
- Rojas-García, E.; García-Martínez, D.C.; López-Medina, R.; Rubio-Marcos, F.; Castañeda-Ramírez, A.A.; Maubert-Franco, A.M. Photocatalytic Degradation of Dyes Using Titania Nanoparticles Supported in Metal-Organic Materials Based on Iron. Molecules 2022, 27, 7078. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Song, J.; Chung, J.S.; Hur, S.H.; Choi, W.M. ZnO/Boron Nitride Quantum Dots Nanocomposites for the Enhanced Photocatalytic Degradation of Methylene Blue and Methyl Orange. Molecules 2022, 27, 6833. [Google Scholar] [CrossRef] [PubMed]
- Benhebal, H.; Wolfs, C.; Kadi, S.; Tilkin, R.G.; Allouche, B.; Belabid, R.; Collard, V.; Felten, A.; Louette, P.; Lambert, S.D.; et al. Visible Light Sensitive SnO2/ZnCo2O4 Material for the Photocatalytic Removal of Organic Pollutants in Water. Inorganics 2019, 7, 77. [Google Scholar] [CrossRef]
- Xu, J.; Gao, Q.; Wang, Z.; Zhu, Y. An all-organic 0D/2D supramolecular porphyrin/g-C3N4 heterojunction assembled via π-π interaction for efficient visible photocatalytic oxidation. Appl. Catal. B 2021, 291, 120059. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, H.; Peng, Y.; Li, P.; Chen, S.; Yang, B.; Zhang, J. Photocatalytic Performance and Degradation Pathway of Rhodamine B with TS-1/C3N4 Composite under Visible Light. Nanomaterials 2020, 10, 756. [Google Scholar] [CrossRef]
- Alharthi, F.A.; Ababtain, A.S.; Aldubeikl, H.K.; Alanazi, H.S.; Hasan, I. Deep Eutectic Solvent-Mediated Synthesis of Ni3V2O8/N-Doped RGO for Visible-Light-Driven H2 Evolution and Simultaneous Degradation of Dyes. Inorganics 2023, 11, 67. [Google Scholar] [CrossRef]






| Photocatalyst | Rate Constant (min−1) | Reference |
|---|---|---|
| ZnP-SnP-ZnP triad nanostructure | 0.0100 | [41] |
| H2Ti3O7 nanotube | 0.0020 | [55] |
| TiO2 (P-25) | 0.0010 | [55] |
| Co0.6Zn0.4Fe2O4 | 0.0150 | [56] |
| TiO2 | 0.0015 | [57] |
| TiO2/MgZnAl | 0.0050 | [57] |
| ZnO | 0.0090 | [58] |
| ZnO/Burkeite | 0.0280 | [58] |
| Ni0.5Zn0.5Al2O4 | 0.0050 | [59] |
| g-C3N4 | 0.0032 | [60] |
| O-g-C3N4 | 0.0790 | [60] |
| SnO2-Acalypha Indica | 0.0062 | [61] |
| H2O2@Cu/Al2O3/g-C3N4 | 0.0820 | [62] |
| Au/ZnO | 0.0300 | [63] |
| TiO2–SnO2–Al2O3 | 0.0610 | [64] |
| ZnS-NaBH4 | 0.0123 | [65] |
| SnP | 0.0016 | this study |
| TiO2 | 0.0060 | this study |
| SnP@TiO2 | 0.0078 | this study |
| SnP/AA@TiO2 | 0.0366 | this study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shee, N.K.; Kim, H.-J. Sn(IV)porphyrin-Anchored TiO2 Nanoparticles via Axial-Ligand Coordination for Enhancement of Visible Light-Activated Photocatalytic Degradation. Inorganics 2023, 11, 336. https://doi.org/10.3390/inorganics11080336
Shee NK, Kim H-J. Sn(IV)porphyrin-Anchored TiO2 Nanoparticles via Axial-Ligand Coordination for Enhancement of Visible Light-Activated Photocatalytic Degradation. Inorganics. 2023; 11(8):336. https://doi.org/10.3390/inorganics11080336
Chicago/Turabian StyleShee, Nirmal Kumar, and Hee-Joon Kim. 2023. "Sn(IV)porphyrin-Anchored TiO2 Nanoparticles via Axial-Ligand Coordination for Enhancement of Visible Light-Activated Photocatalytic Degradation" Inorganics 11, no. 8: 336. https://doi.org/10.3390/inorganics11080336
APA StyleShee, N. K., & Kim, H.-J. (2023). Sn(IV)porphyrin-Anchored TiO2 Nanoparticles via Axial-Ligand Coordination for Enhancement of Visible Light-Activated Photocatalytic Degradation. Inorganics, 11(8), 336. https://doi.org/10.3390/inorganics11080336