Abstract
The current research is about the synthesis of pure nickel sulfide, a series of Te (0, 0.5, 1, 1.5, 2, and 3 wt.%)-doped NiS (Te@NiS) nanoparticles (NPs), and a series of S-g-C3N4 (10, 30, 50, 70, and 80 wt.%)/Te@NiS nanocomposites (NCs), fabricated through a hydrothermal route. XRD and FTIR spectroscopic techniques demonstrated the successful synthesis of NPs and NCs. SEM-EDX images confirmed the flakelike structure and elemental constituents of the fabricated materials. Tauc plots were drawn, to calculate the band gaps of the synthesized samples. Te doping resulted in a significant reduction in the band gap of the NiS NPs. The photocatalytic efficiency of the NPs and NCs was investigated against MB, under sunlight. The results obtained for the photocatalytic activity, showed that 1%Te@NiS nanoparticles have an excellent dye degradation capacity in sunlight. This was made even better by making a series of SGCN/1% Te@NiS nanocomposites with different amounts of S-g-C3N4. When compared to NiS, Te@NiS, SGCN, and 70%SGCN/1%Te@NiS, the 70%SGCN/1%Te@NiS NCs have excellent antifungal ability. The higher impact of SGCN/Te@NiS, may be due to its enhanced ability to disperse and interact with the membranes and intracellular proteins of fungi. The 70%SGCN/1%Te@NiS NCs showed excellent antibacterial and photocatalytic efficiency. Thus, the 70%SGCN/1%Te@NiS NCs might prove fruitful in antibacterial and photocatalytic applications.
1. Introduction
The textile industry is an important contributor to the global economy. It accounts for a large part of global exports and creates many jobs. Still, it has also been regarded as a significant contributor to global pollution [1,2,3,4]. Most of the damage that the textile industry does to the environment, is caused by the discharge of untreated effluent into waterways, which accounts for about 80% of all emissions from the industry [5,6,7]. The effluent discharged by the textile industry, is a combination of metals, dyes, and other pollutants [8]. Textile dyes are our biggest concern, because they are mutagenic, can cause cancer, and stay in the environment for a long time. They also affect whole food chains and bioaccumulate, which means that species higher up the food chain have higher levels of contamination than their prey [5,9,10,11]. In this regard, azo-type textile dyes deserve special attention, because during the dyeing process, approximately 15% to 50% of them are discharged into water that emerging countries regularly utilize for irrigation [12]. This water has negative impacts on the soil and living beings, and enters the food chain. Therefore, in order to preserve the sustainability of the ecosystem for future generations, remediation measures must be implemented [13,14,15].
Regarding solving problems that arise from textile effluent, semiconductor-based photocatalysts, such as metal oxides (e.g., TiO2 [16,17], ZnO [18,19], Bi2WO6 [20], CuO [21,22], WO3 [23], and Fe2O3 [24]), metal sulfides (e.g., ZnS [25,26], CuS [27,28], CdS [29,30], Ag2S [31], Bi2S3 [32], and NiS [33]), and chitosan coated zinc oxide (CS-ZnO) nanocomposites [34], have attracted wide attention because of their ability to degrade organic pollutants by utilizing solar energy [35,36,37], and for the production of hydrogen [38]. Among these photocatalysts, metal-sulfide-based semiconductors are the most well-known for degrading dyes with cost-effective, eco-friendly, and long-lasting treatment methods [39,40]. Among the metal sulfide photocatalysts, NiS plays an ideal role in the selection of p-type semiconductors, that match with the energy levels of TiO2 [41]. Furthermore, its binary Ni–S systems have more earth abundant resources, possessing multiple phases, such as Ni3S2, Ni9S8, Ni7S6, NiS2, Ni3S4, NiS, etc. [42]. NiS2 nanostructures, including nanoparticles, hollow microspheres, nanowires, and nanosheets, have been hailed as potential semiconducting materials [43] for catalytic applications because of their stability, affordability, and nontoxic nature [42,44,45]. However, despite having optoelectronic properties [46], the efficiency of photodegradation remains low when using metal sulfides, due to the fast electron–hole recombination [47]. Similarly, photocorrosion and low quantum efficiency also affect metal sulfides. So, overcoming these issues before applying metal sulfides as photocatalysts, is necessary [48]. In this regard, enormous efforts have been undertaken, such as modifications to the structure of the nanoparticles, or doping.
Doping of high-surface-area particles or materials is required for increasing the photocatalytic performance and charge transfer characteristics of the catalyst [49]. Ceramic oxide nanodoping (Al2O3, TiO2, SiO2, etc.) can improve the dielectric characteristics of composites, while maintaining their high thermal durability [50]. Electrocatalytic H2 generation can be improved by doping with a non-noble metal catalyst. This helps to solve the issue of low efficiency related to the production of either metal electrocatalytic H2 or non-noble metal H2. Previously, metal/nonmetal doping has been used to improve the morphology and characteristics of nanodoped NiS. Cobalt doping on nickel sulfide improves its efficiency to evolve electrocatalytic hydrogen [51].
Properly engineered heterojunctions/nanocomposites are the newest trend in nanostructured material development. They are characterized as a mixture of two or more phases with distinct structures and compositions, one of which is in the nanoscale regime [52]. NiS is a p-type semiconductor, whereas g-C3N4 is typically thought of as an n-type semiconductor. Both of these semiconductors’ p-n heterostructures can aid in the promotion of charge transfer and separation [41,53]. The p-n heterostructures of both, can help to promote the charge transfer/separation [54,55]. Previous studies have also shown that the synergistic interactions between g-C3N4 and the other parts of the heterostructures, inhibit the recombination of photogenerated charge carriers and give the photocatalysts some unique properties [56]. A photocatalyst consisting of NiS/g-C3N4/SrTiO3 (NS/CN/STO) was prepared using a hydrothermal method. The combination of the CN/STO heterojunction and NiS cocatalyst, resulted in enhanced photocatalytic hydrogen evolution activity in the NS/CN/STO system [57]. A p-n heterostructured NiS/BiVO4 (NiS/BVO) photocatalyst was designed, by pairing NiS (p-type) nanoparticles with nanostructured BVO (n-type), and used to photodegrade an organic anion dye, in Li et al. The light harvesting and the separation and transfer of electrons were improved. A molar ratio of 0.7 for NiS/BVO, gave the highest photocatalytic capability, of 95.6%, within 90 min of irradiation, and a degradation rate of 1.7970 h−1 was achieved, which was almost 16 and 33 times greater than pure BVO and NiS, respectively [58]. Vignesh et al. developed the magnetically separable g-C3N4/α-Fe2O3 nanocomposite, for the photocatalytic degradation of mixed RhB and MB pollutants. They observed that the NC exhibited better photocatalytic efficiency than g-C3N4 and α-Fe2O3 [59].
We first describe the hydrothermal synthesis process of sulfur-doped graphitic carbon nitride composites with Te@NiS nanoparticles (SGCN/Te@NiS), and then study their photocatalytic and antibacterial activity. In the current work, initially, NiS NPs were doped with a series of weight percentages of Te (0.5, 1, 1.5, and 3%) to improve their photocatalytic efficiency. In the photocatalytic MB degradation experiment, 1%Te@NiS NPs exhibited the best catalytic activity. Next, 1%Te@NiS NPs were homogenized with S-g-C3N4. The weight percentages of S-g-C3N4, in a series of S-g-C3N4/Te@NiS nanocomposites, were 10, 30, 50, 70, and 80 wt.%. The photocatalytic and antibacterial activity of the S-g-C3N4/Te@NiS composite was found to be better than that of Te@NiS and S-g-C3N4. Te doping in the NiS matrix and simultaneous coupling with S-g-C3N4, were primarily responsible for this improvement. A logical photocatalytic mechanism has also been postulated for the synthesized composite’s enhanced photocatalytic and antibacterial activities under sunlight irradiation.
2. Experimental
2.1. Chemicals
The chemical reagents required for the synthesis of NiS, SGCN, Te@NiS nanoparticles, and the SGCN/Te@NiS nanocomposite, and for photocatalytic activity, were thiourea (CH4N2S), nickel nitrate hexahydrate (Ni (NO3)2.6H2O), sodium dodecyl sulfate (SDS) (C12H25O4S.Na), methylene blue (MB) (C16H18C1N3S), ethanol (for washing only) (C2H5OH), and tellurium (Te). Nutrient broth and nutrient agar were used in the antibacterial study. All chemicals/reagents were used as purchased.
2.2. Synthesis of NiS and Te@NiS Nanoparticles
The NiS nanoparticles were assembled using a modified version of a previously reported hydrothermal technique. The typical synthesis experiment involved preparing two solutions: solution “A” was prepared by dissolving 0.75 g of thiourea into 10 mL of distilled water, under magnetic stirring. Nickel nitrate hexahydrate (1.63 g) and 1 g of sodium dodecyl sulfide, were added to 15 mL of distilled water, under constant stirring. This solution was marked as solution “B.” Then, solutions A and B were mixed in one beaker, and constantly stirred for about 20 min. Next, this solution was transferred into a 25 mL Teflon-lined, sealed stainless autoclave. The autoclave was kept in an oven at 80 °C for about 8 h. Greyish-black precipitates were obtained and ground into powder. Following filtering and rinsing with water/ethanol, the residues were dried in an oven at 70 °C, for two hours. A series of weight percentages of Te (0.5, 1, 1.5, and 3%) was doped into NiS nanoparticles, employing the same process, except for varying the tellurium concentration.
2.3. Synthesis of SGCN
To fabricate SGCN nanosheets by simple means of calcination, 10 g of thiourea was placed in a covered alumina crucible and calcined in a muffle furnace. The temperature was raised progressively until it reached 550 °C, and then that temperature was maintained for about 4 h. The resulting yellowish product was cooled and then ground into a fine powder [60].
2.4. Synthesis of SGCN/Te@NiS Nanocomposites
The Te@NiS NPs were modified by integrating 0.11, 0.34, 0.63, 0.84, and 0.93 g of SGCN, to prepare 10, 30, 50, 70, and 80 wt.% nanocomposites. To synthesize 70 wt.% SGCN/Te@NiS composites, 1.22 g of nickel nitrate hexahydrate and 1 g of sodium dodecyl sulfide were dissolved in 15 mL of distilled water, with constant stirring at room temperature (solution A). Solution “B” was prepared by dissolving 0.75 g of thiourea, 0.014 g of tellurium powder, and 0.84 g of SGCN. After that, the two solutions were put into one beaker and constantly stirred for about 20 min. Then, that solution was poured into a 25 mL Teflon-lined autoclave and placed in an oven at 200 °C, for about 8 h. The greyish-black residue obtained, was dried at 80 °C for 2 h. Later on, the composite was collected and stored for characterization. Other nanocomposites were prepared in a similar fashion. Figure 1 depicts a schematic diagram for the fabrication of the nanocomposites.
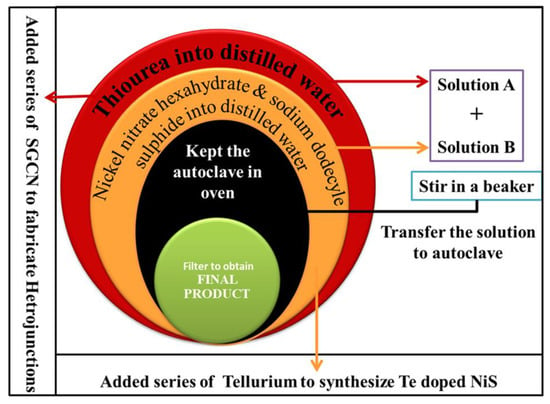
Figure 1.
Schematic diagram for the synthesis of nanoparticles.
2.5. Photocatalytic Study
A volume of 100 mL of MB aqueous solution was mixed with 0.2 g of each material. in a typical photocatalytic experiment. The mixture was left to stir in the dark for around 30 min, to reach the adsorption–desorption equilibrium. After that, the suspension was placed in an open environment, with sunlight, and the degradation of the MB dye was checked by taking 5 mL aliquots at regular intervals (30 min). After centrifuging these aliquots, a UV-visible spectrophotometer was used to record the samples’ absorption spectra. In order to determine the rate constant for degradation, k, from the first-order plot, it was hypothesized that the rate of degradation would follow pseudo-first-order kinetics (Equation (1)).
where A0 is the initial absorbance, A is the absorbance after a time (t), and k is the pseudo-first-order rate constant.
The percentage degradation efficiency of the polluted dyes was studied using Equation (2):
2.6. Band Gap Calculations for Photocatalysts
The band gap was calculated by using UV-visible spectroscopy, to measure the absorbance of samples, and then converting the absorbance data into a Tauc plot. The Tauc plot was drawn with energy on the x-axis and (αhv)2 on the y-axis. The calculation involves the following equation:
where, hv is easily calculated by
and α is calculated by the Beer-Lambert equation
2.7. Antimicrobial Study
Antimicrobial activities were carried out with the best photocatalytic activity NPs and NCs. The Antimicrobial activity determination was carried out using the agar-well diffusion method. The process that was followed is described below:
- The cultured bacteria and fungi were used to prepare inoculum. Two flasks were taken, having 25 mL water each with 0.32 g of nutrient broth. The flasks were autoclaved for about 15 min and labeled with the name of bacterial strains, i.e., Bacillus subtilis (B. subtilis) and Escherichia coli (E. coli) and fungi strains i.e. Monilinia laxa (M. laxa) and Fusarium oxysporum (F. oxysporum). Two drops of bacterial strain were added into the respective flasks and placed in a shaker for 24 h. The fungal strains were incubated for 48 h.
- Then, the photocatalyst solutions (mg/L) were prepared, by using the relation: M1V1 = M2V2
- The medium was prepared by adding 1 g agar and 2 g broth to 100 mL water, in a flask. The solution was heated until it boiled. The sample was poured into Petri dishes and was allowed to cool down and solidify.
- After cooling, holes were punched in the gel by hole puncture, according to the number of mg/L solutions prepared (blank, 100 ppm, 200 ppm, 300 ppm, 400 ppm, 500 ppm). The prepared mg/L solutions were added into the respective holes. The Petri dishes were covered and incubated for about 24 h at 37 °C and at 28 °C for 48 h for fungal strains.
3. Results and Discussion
3.1. Fourier-Transform Infrared Spectroscopy (FTIR)
FTIR spectroscopy was employed to analyze the functional groups present in the samples. The FTIR spectrum of NiS (Figure 2a), revealed a stretching vibration of –OH at approximately 3261.7 cm−1 and at around 3116.9 cm−1 for 1%Te@NiS, showing the presence of water. The occurrence of strong adsorption peaks for Te@NiS in a lower frequency range, at 973 and 609 cm−1, is an indication of the successful incorporation of tellurium into the NiS NPs (Figure 2b). The stretching modes of the C–H group have been attributed to the bands appearing at approximately 2949.6 and 2919.02 cm−1. A distinct absorption band at 973 cm−1 was identified as a specific peak for the Ni–S bond. The development of a metal–sulfur coordination bond was confirmed by the lower C=S shift stretching frequency at 609.9 cm−1. These peaks showed a slightly greater sharpness, and wavenumbers that are almost identical to the absorption bands of NiS. The FTIR spectrum of SGCN (Figure 2c), showed a sharp and prominent absorption band at 808 cm−1, that is assigned to the stretching mode of the triazine group. The presence of C–N heterocycles and their vibrational stretching, is indicated by the absorption bands detected in the range of 1228–1616 cm–1. A broad band at 3111 cm−1, is probably attributable to O–H bond stretching and uncondensed amine groups. These observed peaks agree with earlier reported research [61]. Specific peaks at 2923.2, 1631.2, and 1315.7 cm−1 in the composite spectrum, correspond to Te@NiS peaks, while peaks at 1242.3, 1452.2, and 1542.2 cm−1, correspond to SGCN’s absorption bands. This demonstrates that the SGCN/Te@NiS nanocomposite was successfully synthesized (Figure 2d).
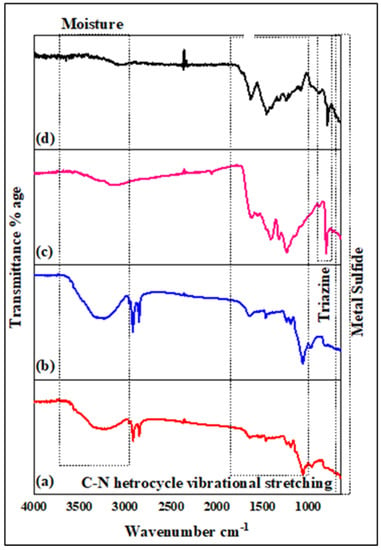
Figure 2.
FTIR spectra of synthesized samples (a) NiS, (b) Te@NiS, (c) SGCN, and (d) SGCN/Te@NiS.
3.2. X-ray Diffraction Pattern
X-ray diffractograms were used to distinguish the phase, and lattice parameters of the synthesized photocatalysts (NiS, Te@NiS, SGCN, and SGCN/Te@NiS). The XRD pattern of nickel sulfide showed peaks at 2θ° = 30.70°, 32.62°, 36.07°, 38.36°, 40.94°, 49.38°, 50.21°, 52.73°, 56.67°, 57.89°, 60.08°, 71.09°, and 73.08°, which correspond to the 101, 300, 021, 220, 211, 131, 410, 401, 321, 330, 312, and 431 crystalline planes of NiS (Figure 3a). The most intense peak was found at angle 2θ = 46.22°. These outcomes were consistent with earlier work [62]. The XRD patterns of Te@NiS and SGCN/Te@NiS, show a minor shift towards the lower angles, demonstrating the effective doping of Te into NiS and the coupling of SGCN with Te@NiS photocatalysts, respectively (Figure 3b). These findings show that nickel has been adequately replaced by tellurium in the NiS lattice. Figure 3c shows the XRD pattern for SGCN has two distinct peaks, at 13.52° and 27.2°, having facets of crystals (100) and (002), respectively. Figure 3c shows the XRD pattern for SGCN, with a distinct peak at 13.52°, having the facet of crystal (100). In the X-ray diffractogram of SGCN@Te/NiS, the distinct peaks obtained at 2θ = 11.42°, 28.28° are due to SGCN, while other peaks observed (31.73°, 34.22°, 37.83°, 38.59°, 40.93°, 50.33°, 51.75°, 53.98°, 56.47°, 57.49°, 60.13°, 70.79°, and 72.77°), correspond to the crystalline planes of Te@NiS.
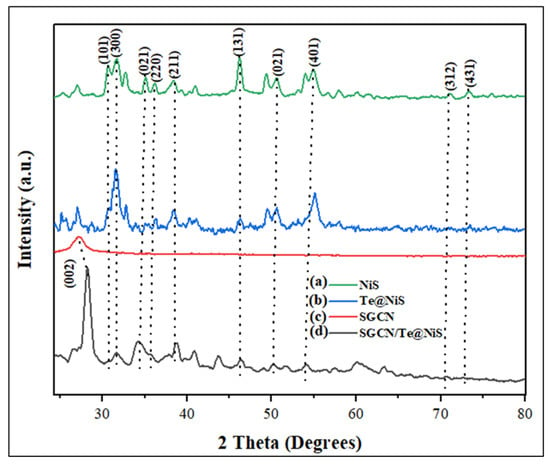
Figure 3.
XRD pattern of (a) NiS, (b) Te@NiS, (c) SGCN, and (d) SGCN/Te@NiS.
3.3. SEM
Figure 4 displays SEM images showing the morphological structure of NiS, Te@NiS NPs, and SGCN/Te@NiS NCs. Figure 4a,b show SEM images of NiS at two different magnifications. In these images, NiS showed a two dimensional flakelike structure. In Figure 4c,d, the agglomerated Te@NiS nanoparticles can be seen in various sizes. Two distinct magnifications of SGCN/Te@SGCN are shown in Figure 4e,f. The images reveal that the agglomerated flakes found in the Te@NiS NPs, are aligned on the surface of the SGCN sheets. The EDX spectrum of SGCN/Te@NiS (Figure 4g), revealed the existence of carbon (C), nitrogen (N), nickel (Ni), oxygen (O), tellurium (Te), and sulfur (S). The percentage composition of the components found in the SGCN/Te@NiS composite was in the order Ni > C > O > S > Te. This demonstrates that C and O have greater content ratios, whereas Te has the lowest concentration. The detected elements in the EDX spectrum, confirm the successful synthesis of SGCN/Te@NiS.
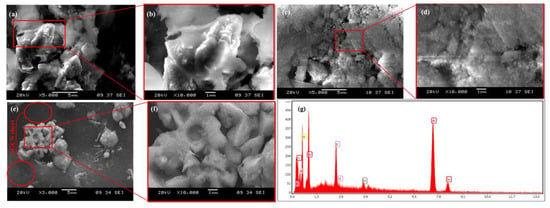
Figure 4.
SEM images of (a,b) NiS, (c,d) Te@NiS, and (e,f) SGCN/Te@NiS. (g) EDX spectrum of SGCN/Te@NiS composite.
3.4. Band Gap
The band gap is the key property for determining the photocatalytic performance of the synthesized samples. The calculated band gap for NiS is about 3.70 eV [63] (Figure 5a), which decreases to 3.23 eV on doping with Te (Figure 5b), confirming the massive reduction in e−/h+ pair recombination rate, resulting in an increase in the photodegradation efficiency of MB. The calculated band gap for SGCN is 2.67 eV (Figure 5c). This energy bandgap of SGCN is similar to that previously reported [64]. A further decrease in the band gap (to 2.60 eV) was observed, by the hybridization of SGCN with Te@NiS (Figure 5d). It was deduced from the results, that the photogenerated e− can easily jump from the impure state to the conduction band, or from the valence band to the impure state. In other words, the photodegradation efficiency of NiS NPs is enhanced by the formation of composites.
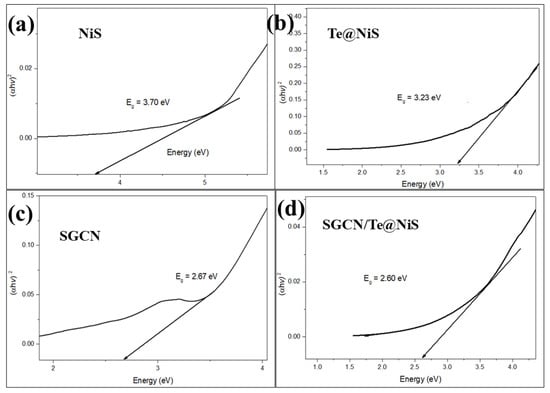
Figure 5.
Tauc plots for band gap calculations (a) NiS, (b) Te@NiS, (c) SGCN, and (d) SGCN/Te@NiS.
3.5. XPS
XPS was used to analyze the surface chemistry of SGCN/Te@NiS, as well as the interaction between SGCN and Te@NiS. The binding energy at 283.3 eV might be attributed to the C–C coordination of the surface adventitious carbon, while that at 284.8 eV corresponds to sp2-bonded carbon, according to the high-resolution spectrum of C 1s (Figure 6a) (C–NH2). This shift was attributed to the interactions between SGCN and Te@NiS, since the binding energies of C 1s for SGCN/Te@NiS NCs, were somewhat lower than the published values for SGCN [65,66]. The graphitic N, pyrrolic N, and pyridine N could each be identified by one of three different peaks, at 402.5, 399.1, and 397.6 eV, in the SGCN N 1s peak. When compared to the previously published value for SGCN (398.5 eV), the binding energy of pyridine N in the SGCN/Te@NiS sample was reduced by 0.18 eV, thus demonstrating the interfacial contact between SGCN and Te@NiS (Figure 6b) [67,68]. Four characteristic peaks, of Te 3d5/2 (573.21 eV), Te 3d3/2 (583.21 eV), and TiOx (576.71 and 587.04 eV), were assigned to the metallic Te and tellurium ions, Te4+, in the Te 3d XPS spectrum (Figure 6c), which fully corresponds with the literature. The Ni 2p3/2 and Ni 2p1/2 can be ascribed to the peaks that occurred at 853.34 eV and 870.74 eV, respectively, in the Ni 2p spectrum of SGCN/Te@NiS (Figure 6d). Furthermore, the signal’s satellite peaks, occurring at 862.41 and 880.24 eV, are similar to those noted elsewhere in the literature. Additionally, as shown in Figure S1, the deconvoluted XPS spectrum of the S 2p was performed for SGCN/Te@NiS, to analyze the valence state of the S. In SGCN/Te@NiS NCs, sulfur is generally associated with two distinct peaks, at 159.93 and 160.13 eV.
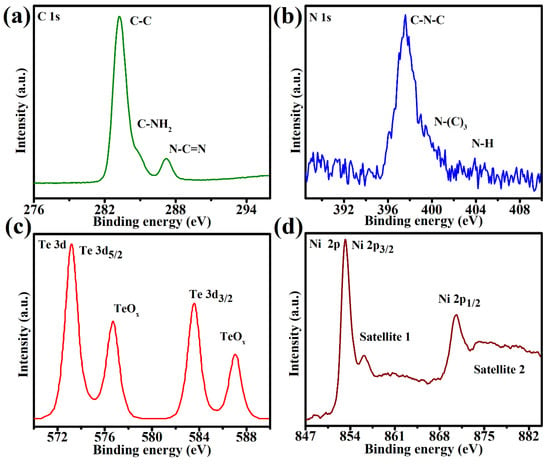
Figure 6.
High-resolution XPS spectra of 70%SGCN/1%Te@NiS NCs: (a) C 1s, (b) N 1s, (c) Te 3d, and (d) Ni 2p.
3.6. Photocatalytic Activity Measurements
Using a UV-visible spectrophotometer, the photodegradation of methylene blue by pure NiS, Te@NiS nanoparticles, and SGCN/Te@NiS nanocomposites was observed under sunlight. The organic dye, methylene blue, was degraded by the synthesized nanocatalysts, resulting in the formation of the inorganic species H2O and CO2. UV-visible spectrophotometer results are shown in Figure 7a,b. The rate of degradation of methylene blue was found to be wavelength dependent. A high rate of degradation was observed in the visible region, at ~664 nm, and a low rate was found in the UV region, at ~292 nm (Figure 7c). The absorption strength reduced over time, which aided in increasing the photodegradation of methylene blue (Figure 7c). In the series of doped photocatalysts, the 1%Te@NiS nanoparticles were found to show excellent degradation of MB dye, as compared to other Te@NiS nanoparticles (Figure 7a,b), due to the synergistic photocatalytic effect of both Te metal and NiS. The decrease in the bandgap due to Te doping, helps to increase the charge separation and degradation rate of the MB dye. Thus, the degradation rate of MB increased for Te@NiS NPs, as compared to NiS NPs, and 1%Te@NiS exhibited the best photocatalytic activity. Next, 1%Te@NiS nanoparticles were integrated with SGCN (10, 30, 50, 70, and 80 wt.%), to develop more effective nanocomposites, and their photocatalytic activity against MB was examined.
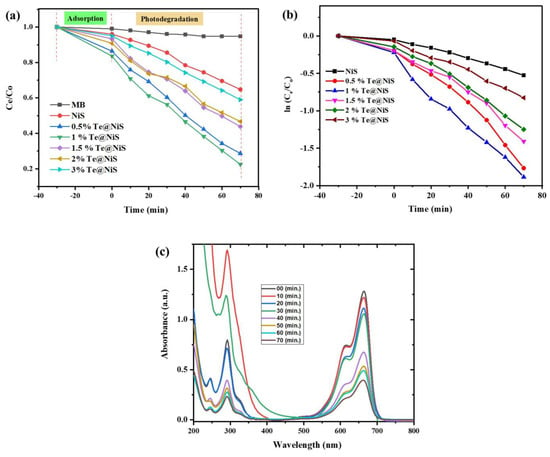
Figure 7.
Degradation efficiency (a). Kinetic study (b). Effect of time on degradation of MB by Te@NiS NPs (c).
From the photocatalytic activity of the prepared series of nanocomposites, it was observed that, as the proportion of SGCN increased, so did the degrading tendency of MB. The fabricated NCs were mixed with dye and placed in the dark under stirring, to check the dye adsorption ability of the NCs (Figure 8). Then, the sample and dye mixtures were exposed to the sunlight, to verify their photocatalytic efficiencies against the model pollutant. In the composite photocatalyst series, 70%SGCN/Te@NiS deteriorated MB faster (97%) than the other composites (Figure 8a).
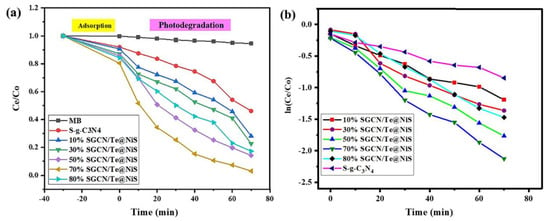
Figure 8.
Degradation efficiency (a). Kinetic study of photocatalytic degradation of MB by SGCN/Te@NiS NCs (b).
All of the synthetic photocatalysts were found to adhere to first-order kinetics (Figure 7b and Figure 8b). The following sequence of kinetic rates was determined from the pseudo-first-order reaction: SGCN/Te@NiS > Te@NiS > NiS. The 70% SGCN is naturally the best concentration for the SGCN/Te@NiS NC, to achieve its highest photocatalytic activity. Additionally, a rise in SGCN concentration (< 70%) may result in e–h pair combination centers, which gradually reduce photocatalytic efficiency. The fact that the kinetic rate of SGCN/Te@NiS was higher than that of Te@NiS and NiS, suggests that SGCN/Te@NiS has outstanding photocatalytic activity against MB. The photocatalytic activity of the 7% SGCN/Te@NiS NC was found to be better than that of some recent reported composites, because SGCN and Te@NiS might successfully develop good heterojunctions [55,56,57,58,59,60,61] (Table 1). Te atoms may also aid in the transport and separation of e- and h+ in the composite.

Table 1.
Photocatalytic efficiency of the SGCN/Te@NiS NCs and some previously reported work.
The EPR spectra of 70%SGCN/1%Te@NiS NCs were studied, to further corroborate the validation of the functional species ·O2− and ·OH in the photocatalysis event (Figure 9a,b). Although the signals are not visible in the dark, the obvious ESR signals are associated with the DMPO−•OH and DMPO−•O2− adducts under sunlight, indicating that both •OH and •O2− are formed during the photocatalysis reaction. The EPR results not only confirm the development of the 70%SGCN/1%Te@NiS NCs, but also show how effective the self-assembled approach, built using SGCN/Te@NiS, is for pollutant degradation.
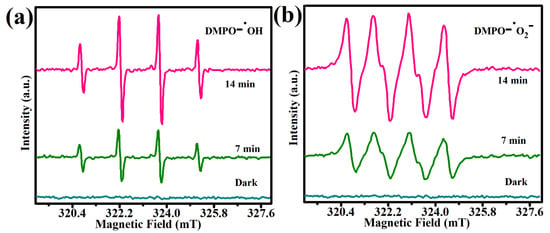
Figure 9.
70%SGCN/1%Te@NiS NCs ESR spectra. For DMPO−•OH (a), this was performed in an aqueous suspension, and for DMPO−•O2− (b) this was performed in a methanol suspension.
3.7. Antimicrobial Activity
The antimicrobial activity was explored by using various concentrations of NiS, Te-doped NiS nanoparticles, and composites (100, 200, 300, 400, and 500 mg/L solutions), against two bacteria (B.subtilis and E. coli) and two fungi (M. laxa and F. oxysporum) strains. The corresponding bactericidal results were noted by measuring the zone of inhibition (ZOI) and are given in Table 2. It was noted that the SGCN/Te@NiS with a concentration of 500 mg/L showed excellent antibacterial behavior and produces a 19 ± 0.8 mm ZOI against Bacillus subtilis, and 18 ± 0.8 mm ZOI against E. coli (Table 2). The ordered bactericidal effect of the samples against bacteria was found to be NiS < Te@NiS < SGCN/Te@NiS. According to the proposed photocatalytic and bactericidal mechanism, the synthesized nanocatalysts generate e–/h+ pairs and reactive oxygen species (ROS) under light irradiation (Figure 10) [76,77]. The maximal zone of inhibition values of 3Te-ZnO@40SCN NCs, for M. laxa and F. oxysporum, are 39.2 mm and 36.5 mm, respectively, according to the antifungal potential data (Table 2). The resulting reactive species cause the degradation of the dye and the damage to the cellular membrane, which leads to the death of the microbial cell [78]. In the SGCN/Te@NiS composite, SGCN acts as an electron acceptor, which helps with charge transfer and separates charge carriers, to slow down the rate of recombination. The prolonged lifetime of the e–/h+ pair, produces more radicals, which enhances the bactericidal activity of the SGCN/Te@NiS composite. Thus, the improved antibacterial behavior of composites refers to the synergistic effect of the Te@NiS and SGCN substrates. As a result, SGCN/Te@NiS NCs could be used in future pollution remediation and antimicrobial applications.

Table 2.
NiS, Te@NiS, SGCN, and SGCN/Te@NiS NCs have zones of inhibition for their antifungal potential when employed in the agar-well diffusion method.
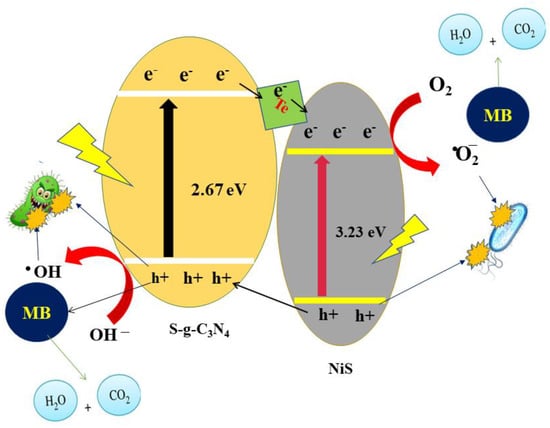
Figure 10.
Schematic antimicrobial and catalytic dye degradation mechanisms under sunlight of the SGCN/Te@NiS NCs.
4. Conclusions
In this study, a series of Te-doped nickel sulfides were made, by adding tellurium as an impurity (wt.%), and a series of composites were made, by integrating different concentrations (wt.%) of SGCN into 1%Te@NiS. The successful syntheses of NiS, Te@NiS, and SGCN/1Te@NiS were examined by various characterization techniques, where XRD results confirmed crystallinity, SEM checked the structural morphologies, EDX verified the elemental composition, and FTIR spectra validated the presence of functional groups. The antimicrobial and photocatalytic efficiency of the synthesized samples was checked against bacteria, fungi and MB, respectively. The photocatalytic activity and antimicrobial results showed that the 70%SGCN/1%Te@NiS nanocomposite is an efficient photocatalyst. Further, the good photocatalytic efficiency of the NCs was validated by applying a kinetic study. The Te doping and coupling of SGCN with NiS material, played a vital role in improving the efficiency of the SGCN/Te@NiS NCs. Consequently, the SGCN/Te@NiS NC is a promising material, with prospective uses in water disinfection and purification via photocatalytic decomposition of contaminants.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics11040156/s1, Figure S1: High-resolution S 2p XPS spectrum of 70%SGCN/1%Te@NiS NCs.
Author Contributions
Conceptualization, writing-original draft preparation, M.R. and M.J.; validation, performed major experimental works, supervision, S.I.; methodology, software, acquisition of data, A.A.; Q.M.; data curation, design of study, K.A., A.B. and M.A.Q.; reviewed original manuscript and critical revision, N.S.A. and H.A.I.; writing reviewing and editing, software, acquisition of funding, M.M.A.-A. and E.B.E. All authors have read and agreed to the published version of the manuscript.
Funding
The authors express their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for funding this work through research group program under grant number RGP.2/273/44. This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R7), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors express their appreciation to the Deanship of Scientific Research at King Khalid University, Saudi Arabia, for funding this work through research group program under grant number RGP.2/273/44. This research was funded by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R7), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aldalbahi, A.; El-Naggar, M.E.; El-Newehy, M.H.; Rahaman, M.; Hatshan, M.R.; Khattab, T.A. Effects of technical textiles and synthetic nanofibers on environmental pollution. Polymers 2021, 13, 155. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Khan, M. Pakistan textile industry facing new challenges. Res. J. Int. Stud. 2010, 14, 21–29. [Google Scholar]
- Memon, J.A.; Aziz, A.; Qayyum, M. The rise and fall of Pakistan’s textile industry: An analytical view. Eur. J. Bus. Manag. 2020, 12, 136–142. [Google Scholar]
- Singh, S.; Khajuria, R. Penicillium enzymes for the textile industry. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 201–215. [Google Scholar]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Mansoor, S.; Shahid, S.; Ashiq, K.; Alwadai, N.; Javed, M.; Iqbal, S.; Fatima, U.; Zaman, S.; Nazim Sarwar, M.; Alshammari, F.H.; et al. Controlled growth of nanocomposite thin layer based on Zn-Doped MgO nanoparticles through Sol-Gel technique for biosensor applications. Inorg. Chem. Commun. 2022, 142, 109702. [Google Scholar] [CrossRef]
- Iqbal, S.; Javed, M.; Qamar, M.A.; Bahadur, A.; Fayyaz, M.; Akbar, A.; Alsaab, H.O.; Awwad, N.S.; Ibrahium, H.A.J.C. Synthesis of Cu-ZnO/Polyacrylic Acid Hydrogel as Visible-Light-Driven Photocatalyst for Organic Pollutant Degradation. Chem. Sel. 2022, 7, e202103694. [Google Scholar] [CrossRef]
- Yaseen, D.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Singh, Z.; Chadha, P. Textile industry and occupational cancer. J. Occup. Med. Toxicol. 2016, 11, 39. [Google Scholar] [CrossRef]
- Erkurt, H.A. Biodegradation of Azo Dyes; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Newman, M. The Science of Pollution (Cuarta). 2015. Available online: https://bit.ly/33OMYAp (accessed on 12 December 2022).
- Rehman, K.; Shahzad, T.; Sahar, A.; Hussain, S.; Mahmood, F.; Siddique, M.H.; Siddique, M.A.; Rashid, M.I. Effect of Reactive Black 5 azo dye on soil processes related to C and N cycling. PeerJ 2018, 6, e4802. [Google Scholar] [CrossRef]
- Sathiyavimal, S.; Vasantharaj, S.; Bharathi, D.; Saravanan, M.; Manikandan, E.; Kumar, S.S.; Pugazhendhi, A. Biogenesis of copper oxide nanoparticles (CuONPs) using Sida acuta and their incorporation over cotton fabrics to prevent the pathogenicity of Gram negative and Gram positive bacteria. J. Photochem. Photobiol. B Biol. 2018, 188, 126–134. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Shariq, M.; Fadhali, M.M.; Madkhali, O.; Ali, S.K.; Syed, I.S.; Awaji, M.Y.; Shakir Khan, M. Accelerated Decoloration of Organic Dyes from Wastewater Using Ternary Metal/g-C3N4/ZnO Nanocomposites: An Investigation of Impact of g-C3N4 Concentration and Ni and Mn Doping. Catalysts 2022, 12, 1388. [Google Scholar] [CrossRef]
- Afzal, M.I.; Shahid, S.; Mansoor, S.; Javed, M.; Iqbal, S.; Hakami, O.; Yousef, E.S.; Al-Fawzan, F.F.; Elkaeed, E.B.; Pashameah, R.A.; et al. Fabrication of a Ternary Nanocomposite g-C3N4/Cu@CdS with Superior Charge Separation for Removal of Organic Pollutants and Bacterial Disinfection from Wastewater under Sunlight Illumination. Toxics 2022, 10, 657. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Park, S.-J. TiO2 photocatalyst for water treatment applications. J. Ind. Eng. Chem. 2013, 19, 1761–1769. [Google Scholar] [CrossRef]
- Kiwaan, H.; Atwee, T.; Azab, E.; El-Bindary, A. Photocatalytic degradation of organic dyes in the presence of nanostructured titanium dioxide. J. Mol. Struct. 2020, 1200, 127115. [Google Scholar] [CrossRef]
- Dodoo-Arhin, D.; Asiedu, T.; Agyei-Tuffour, B.; Nyankson, E.; Obada, D.; Mwabora, J. Photocatalytic degradation of Rhodamine dyes using zinc oxide nanoparticles. Mater. Today Proc. 2021, 38, 809–815. [Google Scholar] [CrossRef]
- Rajendrachari, S.; Taslimi, P.; Karaoglanli, A.C.; Uzun, O.; Alp, E.; Jayaprakash, G.K. Photocatalytic degradation of Rhodamine B (RhB) dye in waste water and enzymatic inhibition study using cauliflower shaped ZnO nanoparticles synthesized by a novel One-pot green synthesis method. Arab. J. Chem. 2021, 14, 103180. [Google Scholar] [CrossRef]
- Lai, M.T.L.; Lai, C.W.; Lee, K.M.; Chook, S.W.; Yang, T.C.K.; Chong, S.H.; Juan, J.C. Facile one-pot solvothermal method to synthesize solar active Bi2WO6 for photocatalytic degradation of organic dye. J. Alloys Compd. 2019, 801, 502–510. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, V.; Kim, K.-H.; Rawat, M. Biogenic synthesis of copper oxide nanoparticles using plant extract and its prodigious potential for photocatalytic degradation of dyes. Environ. Res. 2019, 177, 108569. [Google Scholar] [CrossRef]
- Muthuvel, A.; Jothibas, M.; Manoharan, C. Synthesis of copper oxide nanoparticles by chemical and biogenic methods: Photocatalytic degradation and in vitro antioxidant activity. Nanotechnol. Environ. Eng. 2020, 5, 14. [Google Scholar] [CrossRef]
- Verma, M.; Singh, K.P.; Kumar, A. Reactive magnetron sputtering based synthesis of WO3 nanoparticles and their use for the photocatalytic degradation of dyes. Solid State Sci. 2020, 99, 105847. [Google Scholar] [CrossRef]
- Abdelrahman, E.A.; Hegazey, R.; Kotp, Y.H.; Alharbi, A. Facile synthesis of Fe2O3 nanoparticles from Egyptian insecticide cans for efficient photocatalytic degradation of methylene blue and crystal violet dyes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 222, 117195. [Google Scholar] [CrossRef]
- Aziz, A.; Ali, N.; Khan, A.; Bilal, M.; Malik, S.; Ali, N.; Khan, H. Chitosan-zinc sulfide nanoparticles, characterization and their photocatalytic degradation efficiency for azo dyes. Int. J. Biol. Macromol. 2020, 153, 502–512. [Google Scholar] [CrossRef]
- Ahadi, M.; Saber Tehrani, M.; Aberoomand Azar, P.; Waqif Husain, S. Novel preparation of sensitized ZnS nanoparticles and its use in photocatalytic degradation of tetracycline. Int. J. Environ. Sci. Technol. 2016, 13, 2797–2804. [Google Scholar] [CrossRef]
- Ayodhya, D.; Venkatesham, M.; Santoshi Kumari, A.; Reddy, G.B.; Ramakrishna, D.; Veerabhadram, G. Photocatalytic degradation of dye pollutants under solar, visible and UV lights using green synthesised CuS nanoparticles. J. Exp. Nanosci. 2016, 11, 418–432. [Google Scholar] [CrossRef]
- Borthakur, P.; Boruah, P.K.; Darabdhara, G.; Sengupta, P.; Das, M.R.; Boronin, A.I.; Kibis, L.S.; Kozlova, M.N.; Fedorov, V.E. Microwave assisted synthesis of CuS-reduced graphene oxide nanocomposite with efficient photocatalytic activity towards azo dye degradation. J. Environ. Chem. Eng. 2016, 4, 4600–4611. [Google Scholar] [CrossRef]
- Kumar, S.; Ojha, A.K. In-situ synthesis of reduced graphene oxide decorated with highly dispersed ferromagnetic CdS nanoparticles for enhanced photocatalytic activity under UV irradiation. Mater. Chem. Phys. 2016, 171, 126–136. [Google Scholar] [CrossRef]
- Kakodkar, S.B. Synthesis, characterisation and photocatalytic activity of cadmium sulphide nanoparticles. Chem. Sci. Trans 2016, 5, 75–78. [Google Scholar]
- Ayodhya, D.; Veerabhadram, G. Green synthesis, characterization, photocatalytic, fluorescence and antimicrobial activities of Cochlospermum gossypium capped Ag2S nanoparticles. J. Photochem. Photobiol. B Biol. 2016, 157, 57–69. [Google Scholar] [CrossRef]
- Sharma, S.; Khare, N. Hierarchical Bi2S3 nanoflowers: A novel photocatalyst for enhanced photocatalytic degradation of binary mixture of Rhodamine B and Methylene blue dyes and degradation of mixture of p-nitrophenol and p-chlorophenol. Adv. Powder Technol. 2018, 29, 3336–3347. [Google Scholar] [CrossRef]
- Guo, H.; Ke, Y.; Wang, D.; Lin, K.; Shen, R.; Chen, J.; Weng, W. Efficient adsorption and photocatalytic degradation of Congo red onto hydrothermally synthesized NiS nanoparticles. J. Nanoparticle Res. 2013, 15, 1475. [Google Scholar] [CrossRef]
- Bharathi, D.; Ranjithkumar, R.; Chandarshekar, B.; Bhuvaneshwari, V. Preparation of chitosan coated zinc oxide nanocomposite for enhanced antibacterial and photocatalytic activity: As a bionanocomposite. Int. J. Biol. Macromol. 2019, 129, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Oshida, Y. Bioscience and Bioengineering of Titanium Materials; Elsevier: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Chen, X.; Zhang, H.; Ci, C.; Sun, W.; Wang, Y. Few-layered boronic ester based covalent organic frameworks/carbon nanotube composites for high-performance K-organic batteries. ACS Nano 2019, 13, 3600–3607. [Google Scholar] [CrossRef] [PubMed]
- Qamar, M.A.; Javed, M.; Shahid, S.; Iqbal, S.; Abubshait, S.A.; Abubshait, H.A.; Ramay, S.M.; Mahmood, A.; Ghaithan, H.M. Designing of highly active g-C3N4/Co@ ZnO ternary nanocomposites for the disinfection of pathogens and degradation of the organic pollutants from wastewater under visible light. J. Nanopart. Res. 2021, 9, 105534. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent advances and applications of semiconductor photocatalytic technology. Appl. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. A review on recent advances in photodegradation of dyes using doped and heterojunction based semiconductor metal sulfide nanostructures for environmental protection. Mater. Today Energy 2018, 9, 83–113. [Google Scholar]
- Iqbal, S. Spatial charge separation and transfer in L-cysteine capped NiCoP/CdS nano-heterojunction activated with intimate covalent bonding for high-quantum-yield photocatalytic hydrogen evolution. Appl. Catal. B Environ. 2020, 274, 119097. [Google Scholar] [CrossRef]
- Alenizia, M.; Alserourya, F.; Kumarc, R.; Aslamd, M.; Barakatc, M. Removal of trichlorophenol from wastewater using NiS/RGO/TiO. Composites 2020, 24, 26. [Google Scholar]
- Gomaa, M.M.; Sayed, M.H.; Abdel-Wahed, M.S.; Boshta, M. A facile chemical synthesis of nanoflake NiS 2 layers and their photocatalytic activity. RSC Adv. 2022, 12, 10401–10408. [Google Scholar] [CrossRef]
- Guo, Y.; Guo, D.; Ye, F.; Wang, K.; Shi, Z. Synthesis of lawn-like NiS2 nanowires on carbon fiber paper as bifunctional electrode for water splitting. Int. J. Hydrogen Energy 2017, 42, 17038–17048. [Google Scholar] [CrossRef]
- Xie, Y.; Zhou, Y.; Gao, C.; Liu, L.; Zhang, Y.; Chen, Y.; Shao, Y. Construction of AgBr/BiOBr S-scheme heterojunction using ion exchange strategy for high-efficiency reduction of CO2 to CO under visible light. Sep. Purif. Technol. 2022, 303, 122288. [Google Scholar] [CrossRef]
- Sher, M.; Javed, M.; Shahid, S.; Hakami, O.; Qamar, M.A.; Iqbal, S.; AL-Anazy, M.M.; Baghdadi, H.B. Designing of highly active g-C3N4/Sn doped ZnO heterostructure as a photocatalyst for the disinfection and degradation of the organic pollutants under visible light irradiation. J. Photochem. Photobiol. A Chem. 2021, 418, 113393. [Google Scholar] [CrossRef]
- Shinde, D.V.; Patil, S.A.; Cho, K.; Ahn, D.Y.; Shrestha, N.K.; Mane, R.S.; Lee, J.K.; Han, S.H. Revisiting Metal Sulfide Semiconductors: A Solution-Based General Protocol for Thin Film Formation, Hall Effect Measurement, and Application Prospects. Adv. Funct. Mater. 2015, 25, 5739–5747. [Google Scholar] [CrossRef]
- Isac, L.; Cazan, C.; Enesca, A.; Andronic, L. Copper sulfide based heterojunctions as photocatalysts for dyes photodegradation. Front. Chem. 2019, 7, 694. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ou, X.; Xiang, Q.; Carabineiro, S.A.; Fan, J.; Lv, K. Research progress in metal sulfides for photocatalysis: From activity to stability. Chemosphere 2022, 135085. [Google Scholar] [CrossRef] [PubMed]
- Santhosh, C.; Malathi, A.; Daneshvar, E.; Kollu, P.; Bhatnagar, A. Photocatalytic degradation of toxic aquatic pollutants by novel magnetic 3D-TiO2@ HPGA nanocomposite. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef]
- Yang, Y.; He, J.; Wu, G.; Hu, J. “Thermal Stabilization Effect” of Al2O3 nano-dopants improves the high-temperature dielectric performance of polyimide. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef]
- Peng, Z.; Lou, S.; Gao, Y.; Kong, L.; Yan, S.; Wang, K.; Song, H. Effect of Co doping on electrocatalytic performance of Co-NiS2/CoS2 heterostructures. Nanomaterials 2021, 11, 1245. [Google Scholar] [CrossRef]
- Tomke, P.D.; Rathod, V.K. Nanoengineering Tools in Beverage Industry. In Nanoengineering in the Beverage Industry; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–69. [Google Scholar]
- Jing, J.; Chen, Z.; Feng, C.; Sun, M.; Hou, J. Transforming g-C3N4 from amphoteric to n-type semiconductor: The important role of p/n type on photoelectrochemical cathodic protection. J. Alloys Compd. 2021, 851, 156820. [Google Scholar] [CrossRef]
- Mao, M.; Xu, J.; Li, L.; Zhao, S.; Li, X. The pn heterojunction constructed by NiMnO3 nanoparticles and Ni3S4 to promote charge separation and efficient catalytic hydrogen evolution. Int. J. Hydrogen Energy 2021, 46, 23190–23204. [Google Scholar] [CrossRef]
- Matsuura, K.; Mizumoto, N.; Kobayashi, K.; Nozaki, T.; Fujita, T.; Yashiro, T.; Fuchikawa, T.; Mitaka, Y.; Vargo, E.L. A genomic imprinting model of termite caste determination: Not genetic but epigenetic inheritance influences offspring caste fate. Am. Nat. 2018, 191, 677–690. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C 3 N 4 -Based Heterostructured Photocatalysts. Adv. Energy Mater. 2017, 8, 1701503. [Google Scholar] [CrossRef]
- Luo, X.-L.; He, G.-L.; Fang, Y.-P.; Xu, Y.-H. Nickel sulfide/graphitic carbon nitride/strontium titanate (NiS/g-C3N4/SrTiO3) composites with significantly enhanced photocatalytic hydrogen production activity. J. Colloid Interface Sci. 2018, 518, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, X.; Wang, X.-T.; Jian, L.-J.; Abdallah, N.I.M.; Dong, X.-F.; Wang, C.-W. Pn Heterostructured design of decahedral NiS/BiVO4 with efficient charge separation for enhanced photodegradation of organic dyes. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125565. [Google Scholar] [CrossRef]
- Vignesh, S.; Kim, H. Interfacial coupling effects in α-Fe2O3/g-C3N4 composite magnetically separable heterojunction with upgraded Z-scheme photocatalytic performance of mixed organic pollutant degradation. J. Phys. Chem. Solids 2022, 169, 110869. [Google Scholar] [CrossRef]
- Vinoth, S.; Rajaitha, P.M.; Venkadesh, A.; Devi, K.S.; Radhakrishnan, S.; Pandikumar, A. Nickel sulfide-incorporated sulfur-doped graphitic carbon nitride nanohybrid interface for non-enzymatic electrochemical sensing of glucose. Nanoscale Adv. 2020, 2, 4242–4250. [Google Scholar] [CrossRef]
- Javed, M.; Abid, M.; Hussain, S.; Shahwar, D.; Arshad, S.; Ahmad, N.; Arif, M.; Khan, H.; Nadeem, S.; Raza, H. Synthesis, characterization and photocatalytic applications of s-doped graphitic carbon nitride nanocomposites with nickel doped zinc oxide nanoparticles. Dig. J. Nanomater. Biostruct. 2020, 15, 1097–1105. [Google Scholar] [CrossRef]
- Mi, L.; Chen, Y.; Wei, W.; Chen, W.; Hou, H.; Zheng, Z. Large-scale urchin-like micro/nano-structured NiS: Controlled synthesis, cation exchange and lithium-ion battery applications. RSC Adv. 2013, 3, 17431–17439. [Google Scholar] [CrossRef]
- Pitchaiya, S.; Natarajan, M.; Santhanam, A.; Ramakrishnan, V.M.; Asokan, V.; Palanichamy, P.; Rangasamy, B.; Sundaram, S.; Velauthapillai, D. Nickel sulphide-carbon composite hole transporting material for (CH3NH3PbI3) planar heterojunction perovskite solar cell. Mater. Lett. 2018, 221, 283–288. [Google Scholar] [CrossRef]
- Wang, K.; Li, Q.; Liu, B.; Cheng, B.; Ho, W.; Yu, J. Sulfur-doped g-C3N4 with enhanced photocatalytic CO2-reduction performance. Appl. Catal. B Environ. 2015, 176–177, 44–52. [Google Scholar] [CrossRef]
- Abubshait, S.A.; Iqbal, S.; Abubshait, H.A.; AlObaid, A.A.; Al-Muhimeed, T.I.; Abd-Rabboh, H.S.; Bahadur, A.; Li, W.J.C.; Physicochemical, S.A.; Aspects, E. Effective heterointerface combination of 1D/2D Co-NiS/Sg-C3N4 heterojunction for boosting spatial charge separation with enhanced photocatalytic degradation of organic pollutants and disinfection of pathogens. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127390. [Google Scholar] [CrossRef]
- Iqbal, S.; Bahadur, A.; Javed, M.; Liu, G.; Al-Muhimeed, T.I.; AlObaid, A.A.; Ahmad, Z.; Feng, K.; Xiao, D.J. Synergetic intimate interface contacts of 2D/1D Sg-C3N4/Co-NiS heterojunction with spatial charge separation for boosting photodegradation of MB and inactivation of pathogens under visible light irradiation. J. Alloys Compd. 2022, 892, 162012. [Google Scholar] [CrossRef]
- Bahadur, A.; Iqbal, S.; Alsaab, H.O.; Awwad, N.S.; Ibrahium, H.A. Designing a novel visible-light-driven heterostructure Ni–ZnO/SgC 3 N 4 photocatalyst for coloured pollutant degradation. RSC Adv. 2021, 11, 36518–36527. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, S.; Amjad, A.; Javed, M.; Alfakeer, M.; Mushtaq, M.; Rabea, S.; Elkaeed, E.B.; Pashameah, R.A.; Alzahrani, E.; Farouk, A.-E. Boosted Spatial Charge Carrier Separation of Binary ZnFe2O4/Sg-C3N4 Heterojunction for Visible-light-driven Photocatalytic activity and Antimicrobial Performance. Front. Chem. 2022, 894. [Google Scholar] [CrossRef]
- Ye, Z.; Kong, L.; Chen, F.; Chen, Z.; Lin, Y.; Liu, C. A comparative study of photocatalytic activity of ZnS photocatalyst for degradation of various dyes. Optik 2018, 164, 345–354. [Google Scholar] [CrossRef]
- Kokilavani, S.; Syed, A.; Kumar, B.H.; Elgorban, A.M.; Bahkali, A.H.; Ahmed, B.; Das, A.; Khan, S.S. Facile synthesis of MgS/Ag2MoO4 nanohybrid heterojunction: Outstanding visible light harvesting for boosted photocatalytic degradation of MB and its anti-microbial applications. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127097. [Google Scholar] [CrossRef]
- Lin, X.P.; Huang, F.Q.; Wang, W.D.; Zhang, K.L. A novel photocatalyst BiSbO4 for degradation of methylene blue. Appl. Catal. A Gen. 2006, 307, 257–262. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, L.; Chang, C.; Fu, Y.; Chu, X. Preparation of magnetic composite photocatalyst Bi2WO6/CoFe2O4by two-step hydrothermal method and itsphotocatalytic degradation of bisphenol A. Catal. Commun. 2013, 37, 92–95. [Google Scholar] [CrossRef]
- Zhang, P.; Munawar, T.; Soltane, R.; Javed, M.; Liu, G.; Iqbal, S.; Qamar, M.A.; Dera, A.A.; Alrbyawi, H.; Alfakeer, M. Fabrication of Cr-ZnFe2O4/Sg-C3N4 Heterojunction Enriched Charge Separation for Sunlight Responsive Photocatalytic Performance and Antibacterial Study. Molecules 2022, 27, 6330. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Lam, S.-M.; Sin, J.-C.; Zeng, H.; Mohamed, A.R. Magnetically recoverable Pd-loaded BiFeO3 microcomposite with enhanced visible light photocatalytic performance for pollutant, bacterial and fungal elimination. Sep. Purif. Technol. 2020, 236, 116195. [Google Scholar] [CrossRef]
- Javed, M.; Iqbal, S.; Qamar, M.A.; Shariq, M.; Ahmed, I.A.; BaQais, A.; Alzahrani, H.; Ali, S.K.; Masmali, N.; Althagafi, T.M. Fabrication of Effective Co-SnO2/SGCN Photocatalysts for the Removal of Organic Pollutants and Pathogen Inactivation. Crystals 2023, 13, 163. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Bahadur, A.; AL-Anazy, M.M.; Laref, A.; Li, D. Designing of highly active g-C3N4/Ni-ZnO photocatalyst nanocomposite for the disinfection and degradation of the organic dye under sunlight radiations. Colloids Surf. A Physicochem. Eng. Asp. 2021, 614, 126176. [Google Scholar] [CrossRef]
- Elavarasan, N.; Vignesh, S.; Srinivasan, M.; Venkatesh, G.; Palanisamy, G.; Ramasamy, P.; Palanivel, B.; Al-Enizi, A.M.; Ubaidullah, M.; Reddy, V.R.M. Synergistic S-Scheme mechanism insights of g-C3N4 and rGO combined ZnO-Ag heterostructure nanocomposite for efficient photocatalytic and anticancer activities. J. Alloys Compd. 2022, 906, 164255. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S.; Sher, M.; Akbar, M.B. Highly efficient g-C3N4/Cr-ZnO nanocomposites with superior photocatalytic and antibacterial activity. J. Photochem. Photobiol. A Chem. 2020, 401, 112776. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).