PEPPSI-Type Pd(II)—NHC Complexes on the Base of p-tert-Butylthiacalix[4]arene: Synthesis and Catalytic Activities
Abstract
1. Introduction
2. Results and Discussion
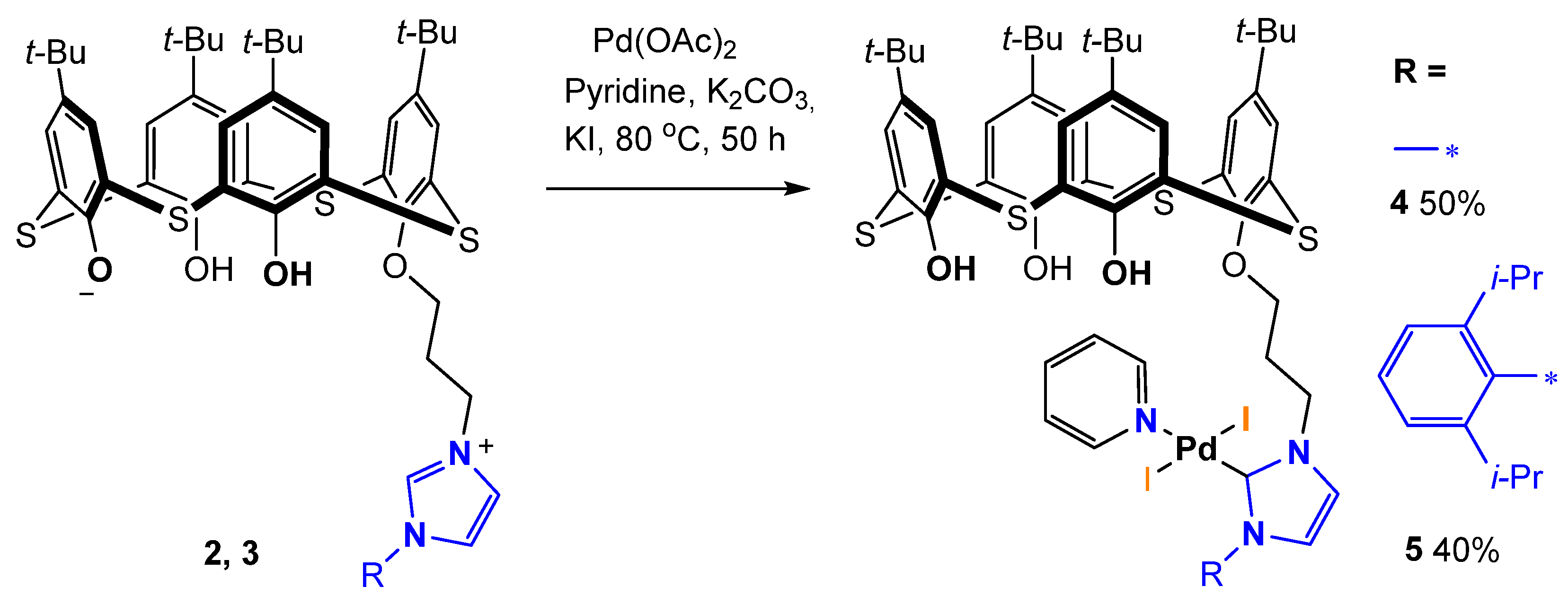
2.1. Synthesis
2.2. Catalytic Activities
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xie, Q.; Li, J.; Wen, X.; Huang, Y.; Hu, Y.; Huang, Q.; Xu, G.; Xie, Y.; Zhou, H. Carbohydrate-substituted N-heterocyclic carbenes Palladium complexes: High efficiency catalysts for aqueous Suzuki–Miyaura reaction. Carbohydr. Res. 2022, 512, 108516. [Google Scholar] [CrossRef]
- Farhang, M.; Akbarzadeh, A.R.; Rabbani, M.; Ghadiri, A.M. A retrospective-prospective review of Suzuki–Miyaura reaction: From cross-coupling reaction to pharmaceutical industry applications. Polyhedron 2022, 227, 116124. [Google Scholar] [CrossRef]
- Al Nasr, I.; Touj, N.; Koko, W.; Khan, T.; Özdemir, I.; Yaşar, S.; Hamdi, N. Biological activities of nhc–pd(Ii) complexes based on benzimidazolylidene n-heterocyclic carbene (nhc) ligands bearing aryl substituents. Catalysts 2020, 10, 1190. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Chang, S.Y.; Ejaz, M.; Samy, M.M.; Mousa, A.O.; Kuo, S.W. Design and Synthesis of Bisulfone-Linked Two-Dimensional Conjugated Microporous Polymers for CO2 Adsorption and Energy Storage. Molecules 2023, 28, 3234. [Google Scholar] [CrossRef] [PubMed]
- Koy, M.; Bellotti, P.; Das, M.; Glorius, F. N-Heterocyclic carbenes as tunable ligands for catalytic metal surfaces. Nat. Catal. 2021, 4, 352–363. [Google Scholar] [CrossRef]
- Valente, C.; Çalimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The development of bulky palladium NHC complexes for the most-challenging cross-coupling reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef]
- Smith, C.A.; Narouz, M.R.; Lummis, P.A.; Singh, I.; Nazemi, A.; Li, C.H.; Crudden, C.M. N-Heterocyclic Carbenes in Materials Chemistry. Chem. Rev. 2019, 119, 4986–5056. [Google Scholar] [CrossRef]
- Fortman, G.C.; Nolan, S.P. N-Heterocyclic carbene (NHC) ligands and palladium in homogeneous cross-coupling catalysis: A perfect union. Chem. Soc. Rev. 2011, 40, 5151–5169. [Google Scholar] [CrossRef]
- Johansson Seechurn, C.C.C.; Kitching, M.O.; Colacot, T.J.; Snieckus, V. Palladium-catalyzed cross-coupling: A historical contextual perspective to the 2010 nobel prize. Angew. Chem. Int. Ed. 2012, 51, 5062–5085. [Google Scholar] [CrossRef] [PubMed]
- Vasu, G.R.P.; Venkata, K.R.M.; Kakarla, R.R.; Ranganath, K.V.S.; Aminabhavi, T.M. Recent advances in sustainable N-heterocyclic carbene-Pd(II)-pyridine (PEPPSI) catalysts: A review. Environ. Res. 2023, 225, 115515. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, G.; Guob, D.; Wang, J. Recent developments on NHC-driven dual catalytic approaches. Org. Chem. Front. 2022, 9, 5016–5040. [Google Scholar] [CrossRef]
- Boztepe, C.; Künkül, A.; Gürbüz, N. Hydrogel supported vinylimidazole based PEPPSI-Pd-NHC catalysts: The catalytic activities in Heck and Suzuki-Miyaura coupling reactions. J. Mol. Struct. 2020, 1209, 127948. [Google Scholar] [CrossRef]
- Kaloğlu, N.; Özdemir, İ. PEPPSI-Pd-NHC catalyzed Suzuki-Miyaura cross-coupling reactions in aqueous media. Tetrahedron 2019, 75, 2306–2313. [Google Scholar] [CrossRef]
- Eremin, D.B.; Denisova, E.A.; Kostyukovich, A.Y.; Martens, J.; Berden, G.; Oomens, J.; Khrustalev, V.N.; Chernyshev, V.M.; Ananikov, V.P. Ionic Pd/NHC Catalytic System Enables Recoverable Homogeneous Catalysis: Mechanistic Study and Application in the Mizoroki–Heck Reaction. Chem. Eur. J. 2019, 25, 16564–16572. [Google Scholar] [CrossRef]
- Korukçu, M.Ç.; Can, S. Pd–PEPPSI type complexes bearing unsymmetrical NHC ligand with phenyl-substituted backbone: Highly efficient catalysts for Heck–Mizoroki and Suzuki–Miyaura cross-coupling reactions Meliha. Appl. Organomet. Chem. 2023, 37, 7057. [Google Scholar] [CrossRef]
- Lima, C.G.S.; Pauli, F.P.; Madriaga, V.G.; Amaral, A.A.P.; Graciano, I.A.; Meira, V.L.; Forezi, L.d.S.M.; Ferreira, V.F.; Lima, T.d.M.; de Carvalho da Silva, F. Supramolecular Catalysts for Organic Synthesis: Preparation and Applications of Cyclodextrins and Calixarenes in C−C Cross-Coupling Reactions. Eur. J. Org. Chem. 2022, 2022, e202200904. [Google Scholar] [CrossRef]
- Snelders, D.J.M.; Van Koten, G.; Klein Gebbink, R.J.M. Hexacationic Dendriphos ligands in the Pd-catalyzed Suzuki-Miyaura cross-coupling reaction: Scope and mechanistic studies. J. Am. Chem. Soc. 2009, 131, 11407–11416. [Google Scholar] [CrossRef]
- Jover, J.; Fey, N.; Purdie, M.; Lloyd-Jones, G.C.; Harvey, J.N. A computational study of phosphine ligand effects in Suzuki-Miyaura coupling. J. Mol. Catal. A Chem. 2010, 324, 39–47. [Google Scholar] [CrossRef]
- Yang, J.; Liu, J.; Wang, Y.; Wang, J. Synthesis, structure and catalysis/applications of N-heterocyclic carbene based on macrocycles. J. Incl. Phenom. Macrocycl. Chem. 2018, 90, 15–37. [Google Scholar] [CrossRef]
- Almallah, H.; Nos, M.; Ayzac, V.; Brenner, E.; Matt, D.; Gourlaouen, C.; Jahjah, M.; Hijazi, A. Complexes featuring N-heterocyclic carbenes with bowl-shaped wingtips. Comptes Rendus Chim. 2019, 22, 299–309. [Google Scholar] [CrossRef]
- Şahin, N.; Semeril, D.; Brenner, E.; Matt, D.; Kaya, C.; Toupet, L. Palladium-catalysed Suzuki-Miyaura cross-coupling with imidazolylidene ligands substituted by crowded resorcinarenyl and calixarenyl units. Turk. J. Chem. 2015, 39, 1171–1179. [Google Scholar] [CrossRef]
- Kaloğlu, M.; Sémeril, D.; Brenner, E.; Matt, D.; Özdemir, I.; Toupet, L. The Influence of Imidazolylidene Ligands with Bulky Resorcinarenyl Substituents on Catalysts for Suzuki-Miyaura Coupling. Eur. J. Inorg. Chem. 2016, 2016, 1115–1120. [Google Scholar] [CrossRef]
- Brenner, E.; Matt, D.; Henrion, M.; Teci, M.; Toupet, L. Calix[4]arenes with one and two N-linked imidazolium units as precursors of N-heterocyclic carbene complexes. Coordination chemistry and use in Suzuki-Miyaura cross-coupling. Dalton Trans. 2011, 40, 9889–9898. [Google Scholar] [CrossRef] [PubMed]
- Burilov, V.A.; Gafiatullin, M.B.K.; Mironova, D.A.; Sultanova, E.D.; Evtugyn, V.G.; Osin, Y.N.; Islamov, D.R.; Usachev, K.S.; Solovieva, S.E.; Antipin, I.S. Amphiphilic PdII-NHC Complexes on 1,3-Alternate p-tert-Butylthiacalix[4]arene Platform: Synthesis and Catalytic Activities in Coupling and Hydrogenation Reactions. Eur. J. Org. Chem. 2020, 2020, 2180–2189. [Google Scholar] [CrossRef]
- Gafiatullin, B.K.; Paskevich, I.V.; Burilov, V.A.; Solovieva, S.E.; Antipin, I.S. One-pot Synthesis of Mono-substituted Quaternized p-tert-Butylthiacalix[4]arenes. Macroheterocycles 2022, 15, 53–58. [Google Scholar] [CrossRef]
- Lamouchi, M.; Jeanneau, E.; Coulm, J.; Brioude, A.; Desroches, C. Synthesis and properties of mono-O-[(N-(aminoalkyl)aminocarbonyl)-methoxy] thiacalix[4]arenes and novel mono-O-bridged bisthiacalix[4]arene. Comptes Rendus Chim. 2013, 16, 1073–1078. [Google Scholar] [CrossRef]
- Mague, J.T.; Akkurt, M.; Mohamed, S.K.; Omran, O.A.; Albayati, M.R. N, N, N-Triethylethanaminium 5,11,17,23-tetra- tert -butyl-25-[(ethoxycarbonyl)methoxy]-26,28-dihydroxy-27-oxido-2,8,14,20-tetrathiacalix[4]arene: A molecular salt. IUCrData 2016, 1, x161465. [Google Scholar] [CrossRef]
- Zhao, J.L.; Tomiyasu, H.; Ni, X.L.; Zeng, X.; Elsegood, M.R.J.; Redshaw, C.; Rahman, S.; Georghiou, P.E.; Yamato, T. Synthesis and evaluation of a novel ionophore based on a thiacalix[4]arene derivative bearing imidazole units. New J. Chem. 2014, 38, 6041–6049. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air-and Moisture-Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef]
- Serdaroğlu, G.; Şahin, N.; Üstün, E.; Tahir, M.N.; Arıcı, C.; Gürbüz, N.; Özdemir, İ. PEPPSI type complexes: Synthesis, x-ray structures, spectral studies, molecular docking and theoretical investigations. Polyhedron 2021, 204, 115281. [Google Scholar] [CrossRef]
- Chernenko, A.Y.; Astakhov, A.V.; Kutyrev, V.V.; Gordeev, E.G.; Burykina, J.V.; Minyaev, M.E.; Khrustalev, V.N.; Chernyshev, V.M.; Ananikov, V.P. Stabilization of the Pd–NHC framework with 1,2,4-triazol-5-ylidene ligands toward decomposition in alkaline media. Inorg. Chem. Front. 2021, 8, 3382–3401. [Google Scholar] [CrossRef]
- Miyaura, N.; Yamada, K.; Suzuki, A. A new stereospecific cross-coupling by the palladium-catalyzed reaction of 1-alkenylboranes with 1-alkenyl or 1-alkynyl halides. Tetrahedron Lett. 1979, 20, 3437–3440. [Google Scholar] [CrossRef]
- Joshi, C.; Macharia, J.M.; Izzo, J.A.; Wambua, V.; Kim, S.; Hirschi, J.S.; Vetticatt, M.J. Isotope Effects Reveal the Catalytic Mechanism of the Archetypical Suzuki-Miyaura Reaction. ACS Catal. 2022, 12, 2959–2966. [Google Scholar] [CrossRef]
- Lennox, A.J.J.; Lloyd-Jones, G.C. Selection of boron reagents for Suzuki-Miyaura coupling. Chem. Soc. Rev. 2014, 43, 412–443. [Google Scholar] [CrossRef] [PubMed]
- Kaushal, J.; Singh, S.; Oswal, P.; Arora, A.; Nautiyal, D.; Kumar, A. Suzuki−Miyaura coupling and O−arylation reactions catalysed by palladium(II) complexes of bulky ligands bearing naphthalene core, Schiff base functionality and biarylphosphine moiety. J. Mol. Struct. 2022, 1253, 132099. [Google Scholar] [CrossRef]
- Valente, C.; Belowich, M.E.; Hadei, N.; Organ, M.G. Pd-PEPPSI complexes and the Negishi reaction. Eur. J. Org. Chem. 2010, 2010, 4343–4354. [Google Scholar] [CrossRef]
- Lima, C.F.R.A.C.; Rodrigues, A.S.M.C.; Silva, V.L.M.; Silva, A.M.S.; Santos, L.M.N.B.F. Role of the base and control of selectivity in the suzuki-miyaura cross-coupling reaction. ChemCatChem 2014, 6, 1291–1302. [Google Scholar] [CrossRef]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lub, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef]
- Burilov, V.A.; Nugmanov, R.I.; Ibragimova, R.R.; Solovieva, S.E.; Antipin, I.S. “Click chemistry” in the synthesis of new amphiphilic 1,3-alternate thiacalixarenes. Mendeleev Commun. 2015, 25, 177–179. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Zhao, J.; Zhao, Y.; Li, L.; Zhang, H. A Modified Procedure for the Synthesis of 1-Arylimidazoles. Synthesis 2003, 2003, 2661–2666. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT-Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; Van De Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]

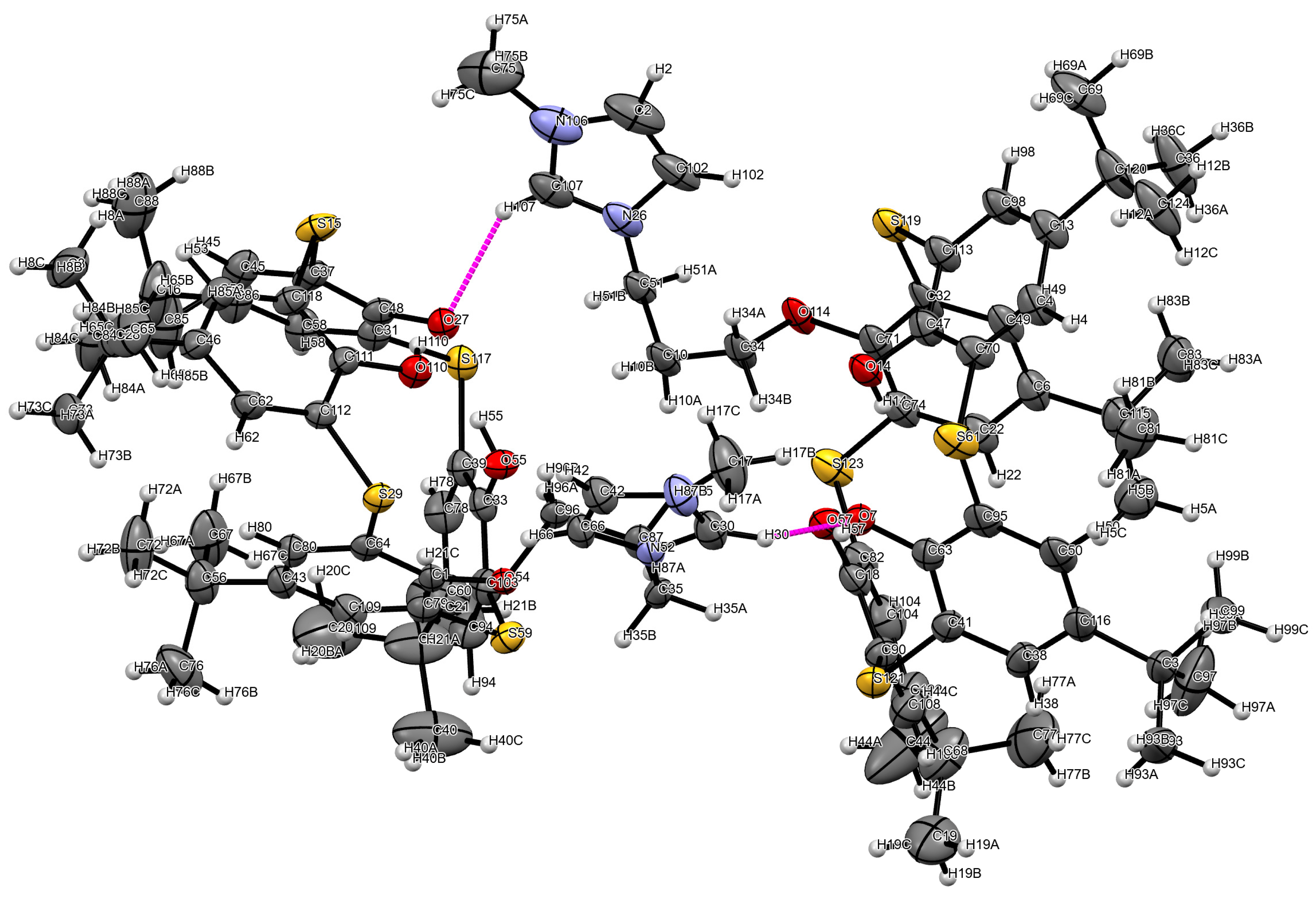
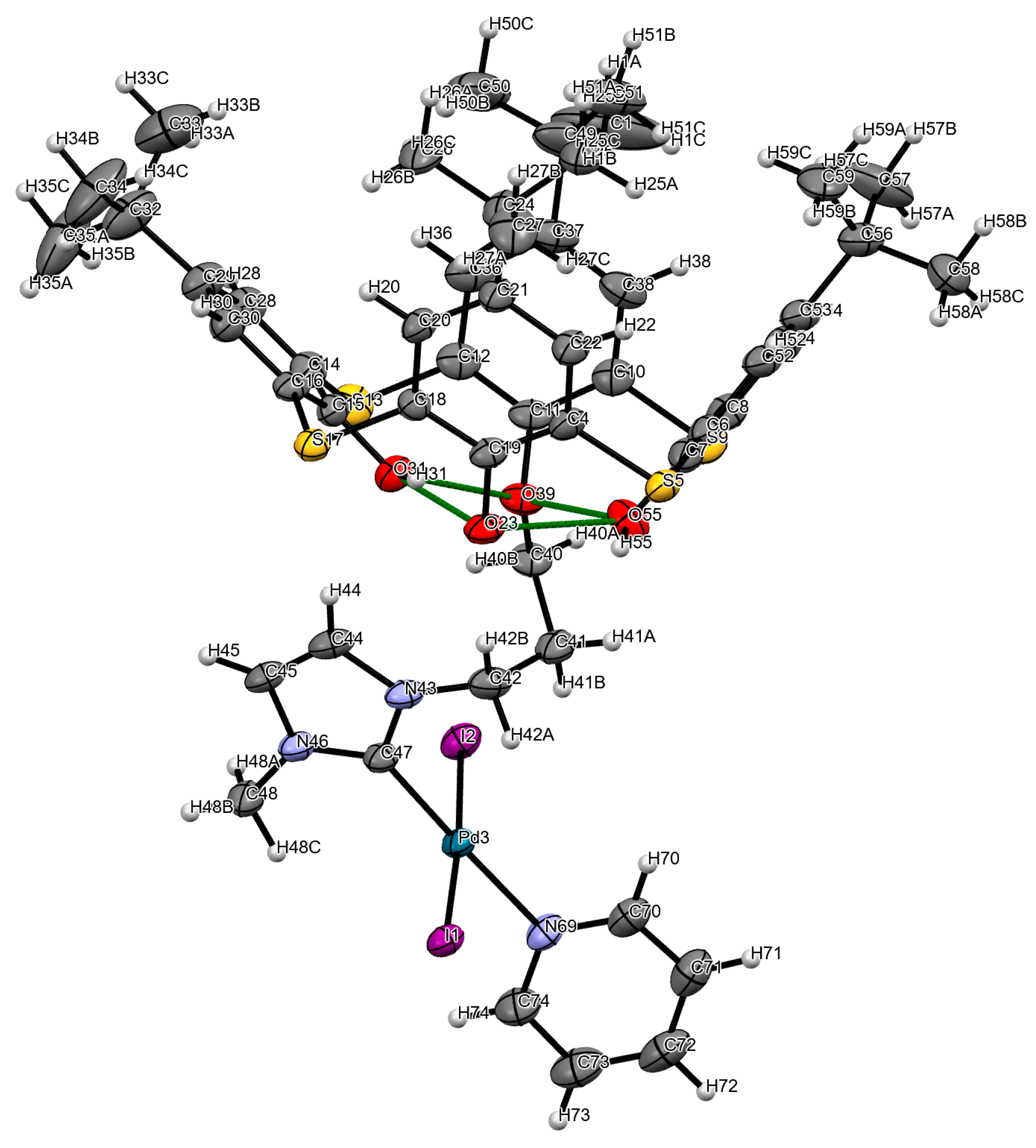
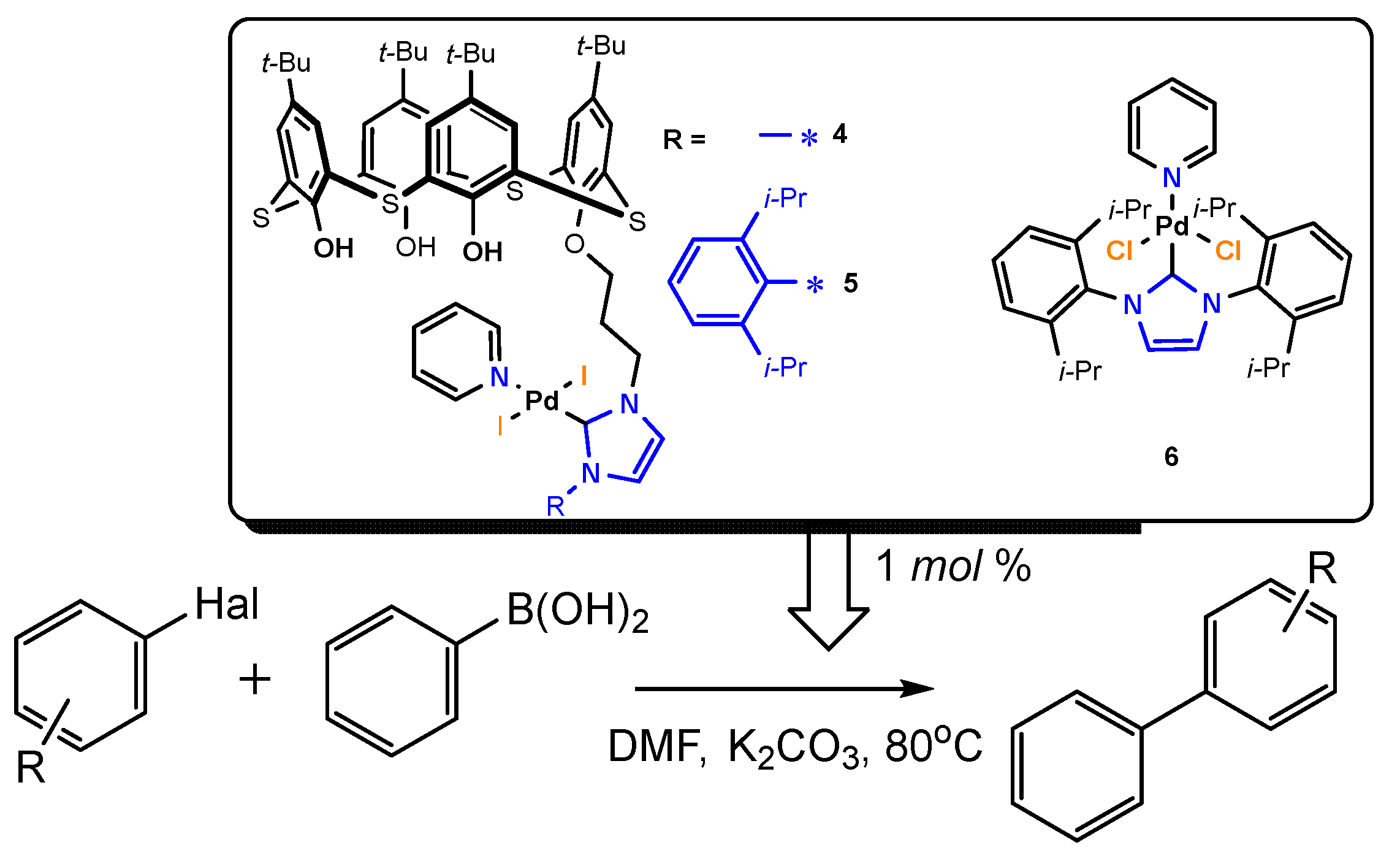
| Entry | Complex | Hal | R | Conversion, % | Isolated yield, % | Selectivity, % |
|---|---|---|---|---|---|---|
| 1 | 6 | -I | p-OCH3 | 66 | 53 | 85 |
| 2 | 6 * | 99 | 71 | |||
| 3 | 6 ** | 99 | 65 | |||
| 4 | 6 *** | 99 | 69 | |||
| 5 | 5 | 75 | 61 | 91 | ||
| 6 | 5 * | 99 | 82 | |||
| 7 | 5 ** | 99 | 85 | |||
| 8 | 5 *** | 99 | 84 | |||
| 9 | 4 | 27 | 14 | 96 | ||
| 10 | 4 * | 99 | 91 | |||
| 11 | 4 ** | 99 | 88 | |||
| 12 | 4 *** | 99 | 78 | |||
| 13 | 6 | -I | p-NO2 | 99 | 65 | 99 |
| 14 | 6 * | 99 | 72 | |||
| 15 | 5 | 93 | 35 | 99 | ||
| 16 | 5 * | 99 | 95 | |||
| 17 | 4 | 75 | 35 | 99 | ||
| 18 | 4 * | 95 | 80 | |||
| 19 | 6 | -Br | p-OCH3 | 70 | 48 | 71 |
| 20 | 6 * | 94 | 56 | 71 | ||
| 21 | 5 | 54 | 37 | 75 | ||
| 22 | 5 * | 62 | 42 | 76 | ||
| 23 | 4 | 6 | 3 | 99 | ||
| 24 | 4 * | 18 | 12 | 99 | ||
| 25 | 5 * | -Br | p-CH3 | 56 | 71 | |
| 26 | 5 * | -Mesityl | 23 | 30 | ||
| 27 | 5 * | p-COCH3 | 99 | 98 |
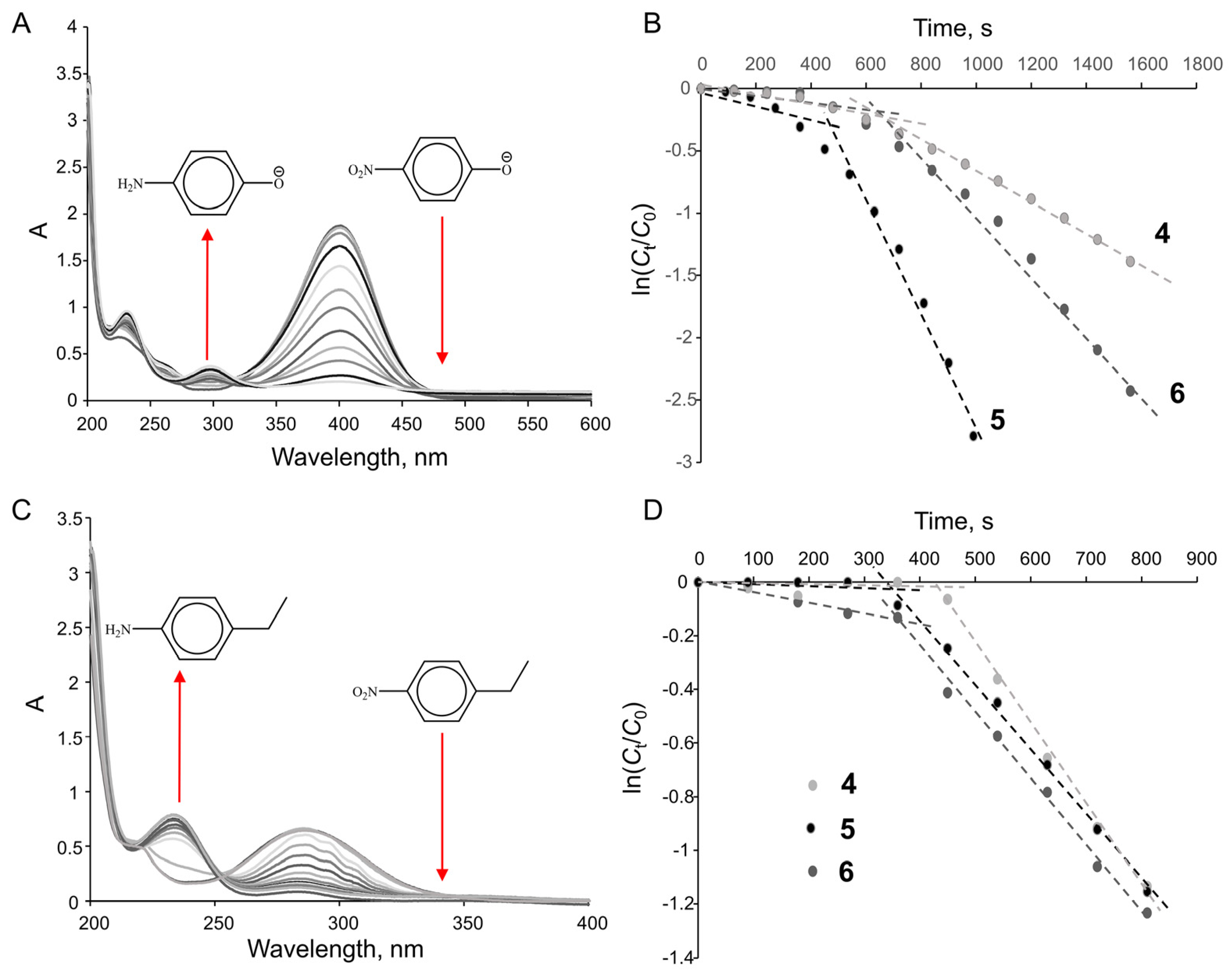
| System | p-Nitrophenol | p-Ethylnitrobenzene | ||
|---|---|---|---|---|
| Apparent Rate Constant, k, s−1 | Specific Catalytic Activity, Ka, × 105 mol1s−1 | Apparent Rate Constant, k, s−1 | Specific Catalytic Activity, Ka, × 105 mol1s−1 | |
| 6 | 2.1 × 10−3 | 4.2 | 2.2 × 10−3 | 4.4 |
| 5 | 4.2 × 10−3 | 8.4 | 2.4 × 10−3 | 4.8 |
| 4 | 1.2 × 10−3 | 2.4 | 2.7 × 10−3 | 5.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gafiatullin, B.; Akchurina, A.; Fedoseeva, A.; Sultanova, E.; Islamov, D.; Usachev, K.; Burilov, V.; Solovieva, S.; Antipin, I. PEPPSI-Type Pd(II)—NHC Complexes on the Base of p-tert-Butylthiacalix[4]arene: Synthesis and Catalytic Activities. Inorganics 2023, 11, 326. https://doi.org/10.3390/inorganics11080326
Gafiatullin B, Akchurina A, Fedoseeva A, Sultanova E, Islamov D, Usachev K, Burilov V, Solovieva S, Antipin I. PEPPSI-Type Pd(II)—NHC Complexes on the Base of p-tert-Butylthiacalix[4]arene: Synthesis and Catalytic Activities. Inorganics. 2023; 11(8):326. https://doi.org/10.3390/inorganics11080326
Chicago/Turabian StyleGafiatullin, Bulat, Aigul Akchurina, Angelina Fedoseeva, Elza Sultanova, Daut Islamov, Konstantin Usachev, Vladimir Burilov, Svetlana Solovieva, and Igor Antipin. 2023. "PEPPSI-Type Pd(II)—NHC Complexes on the Base of p-tert-Butylthiacalix[4]arene: Synthesis and Catalytic Activities" Inorganics 11, no. 8: 326. https://doi.org/10.3390/inorganics11080326
APA StyleGafiatullin, B., Akchurina, A., Fedoseeva, A., Sultanova, E., Islamov, D., Usachev, K., Burilov, V., Solovieva, S., & Antipin, I. (2023). PEPPSI-Type Pd(II)—NHC Complexes on the Base of p-tert-Butylthiacalix[4]arene: Synthesis and Catalytic Activities. Inorganics, 11(8), 326. https://doi.org/10.3390/inorganics11080326