Synthesis and Characterization of Green Zinc-Metal-Pillared Bentonite Mediated Curcumin Extract (Zn@CN/BE) as an Enhanced Antioxidant and Anti-Diabetes Agent
Abstract
1. Introduction
2. Results and Discussion
2.1. Characterization of the Synthetic Structure
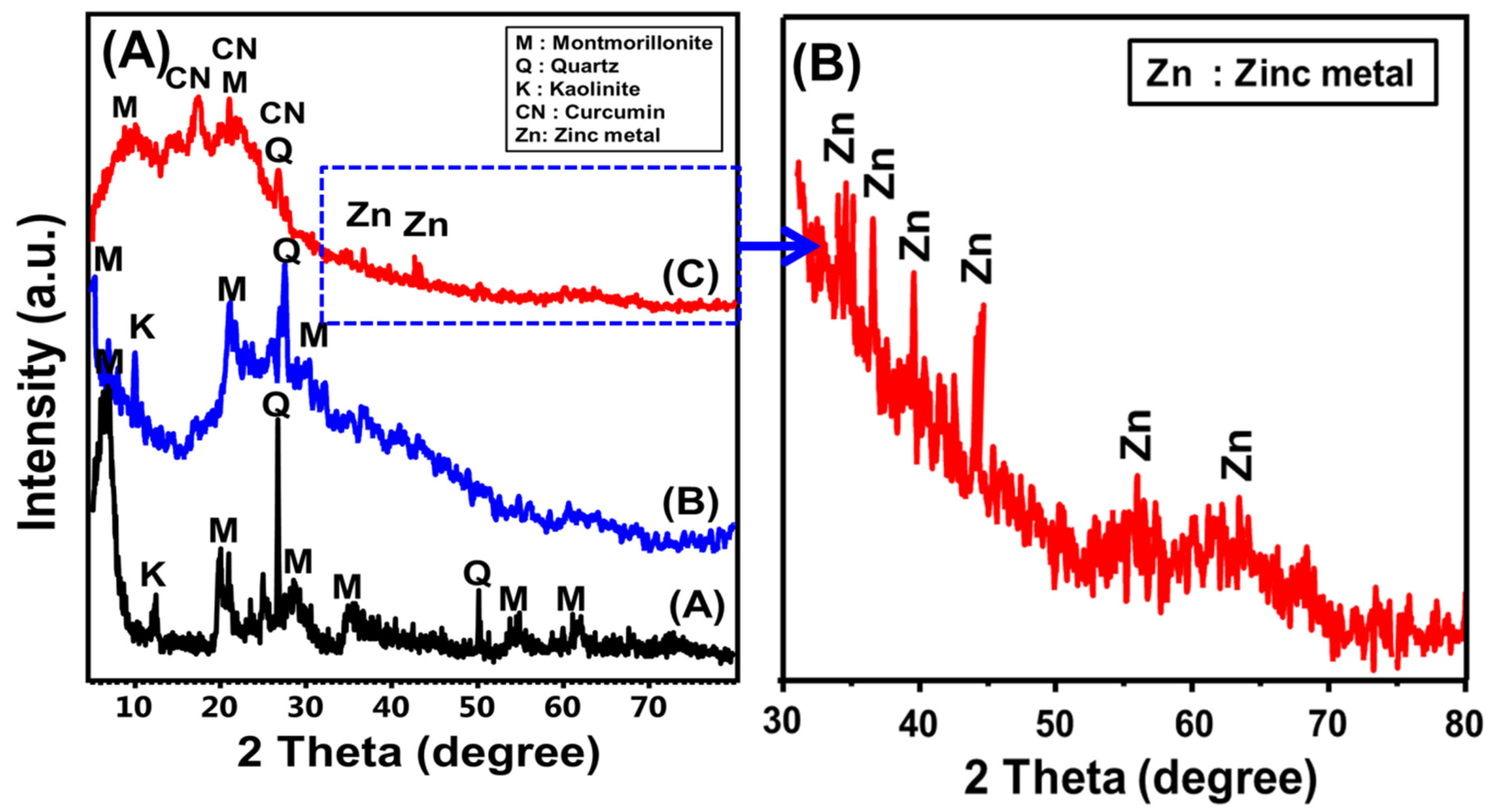
2.1.1. Structural Properties
2.1.2. Morphological and Textural Properties
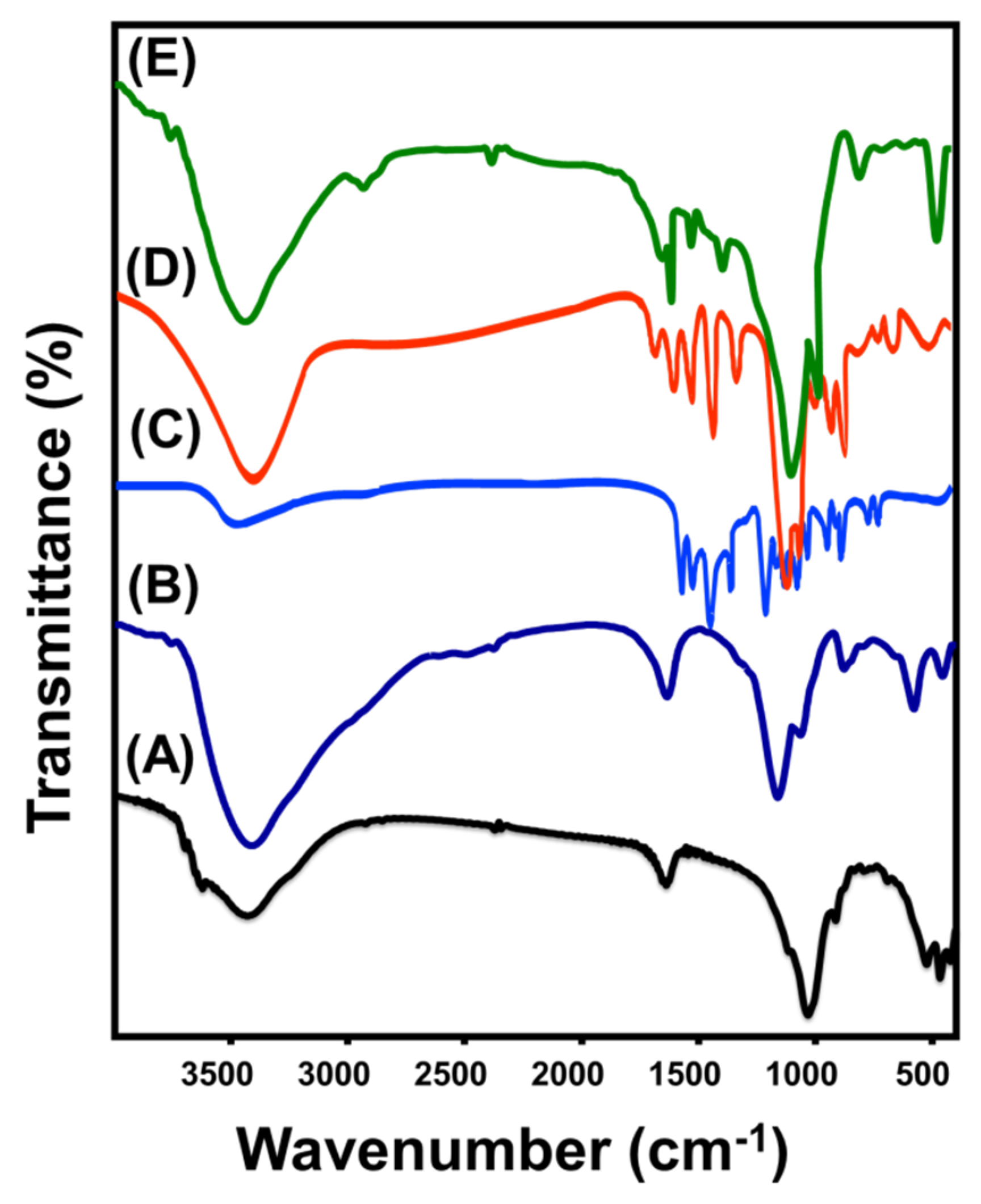
2.1.3. Chemical Properties
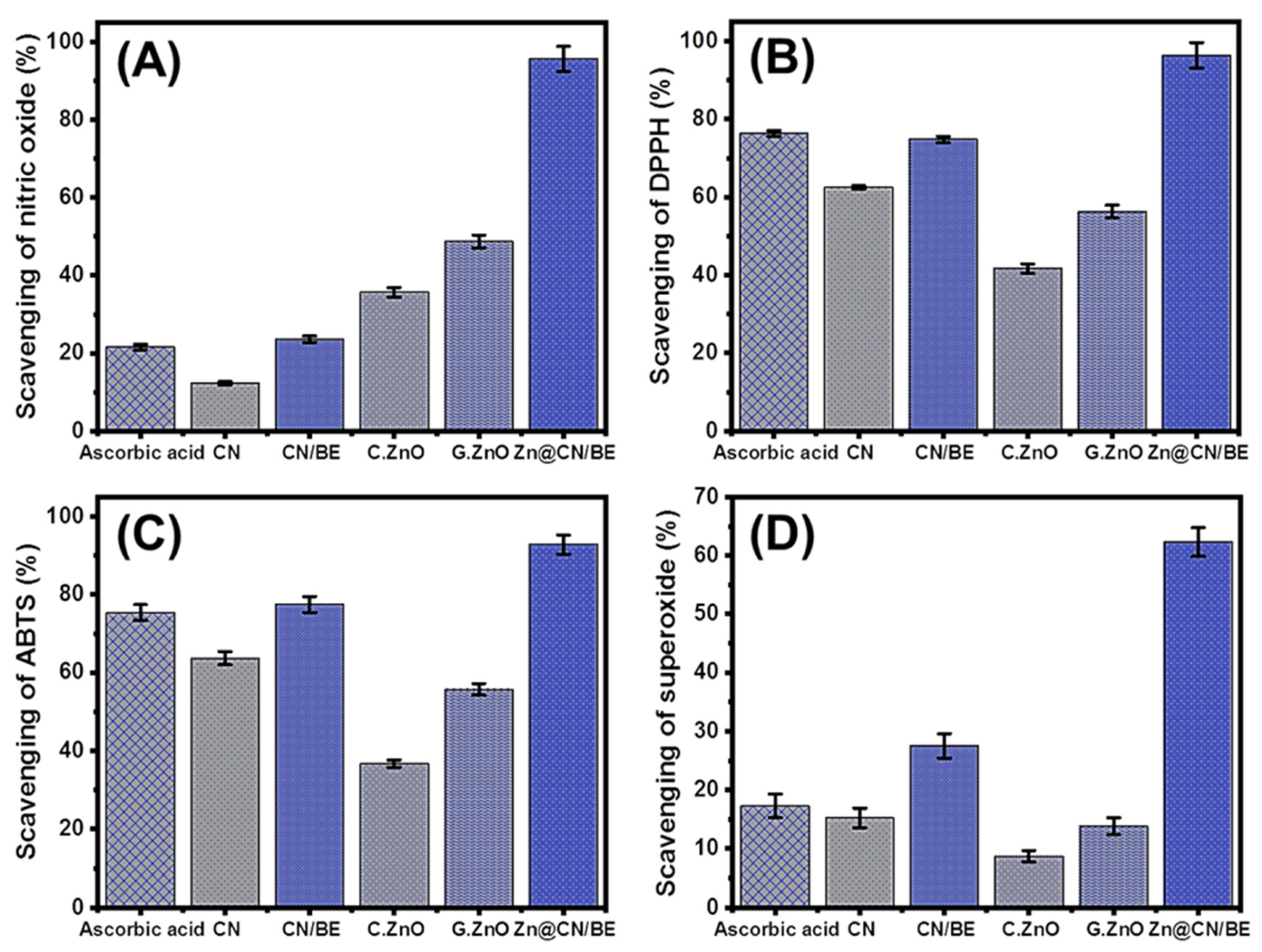
2.2. Antioxidant Properties
2.2.1. Scavenging of Nitric Oxide
2.2.2. Scavenging of DPPH Radical
2.2.3. Scavenging of ABTS Radical
2.2.4. Scavenging of Superoxide Radical
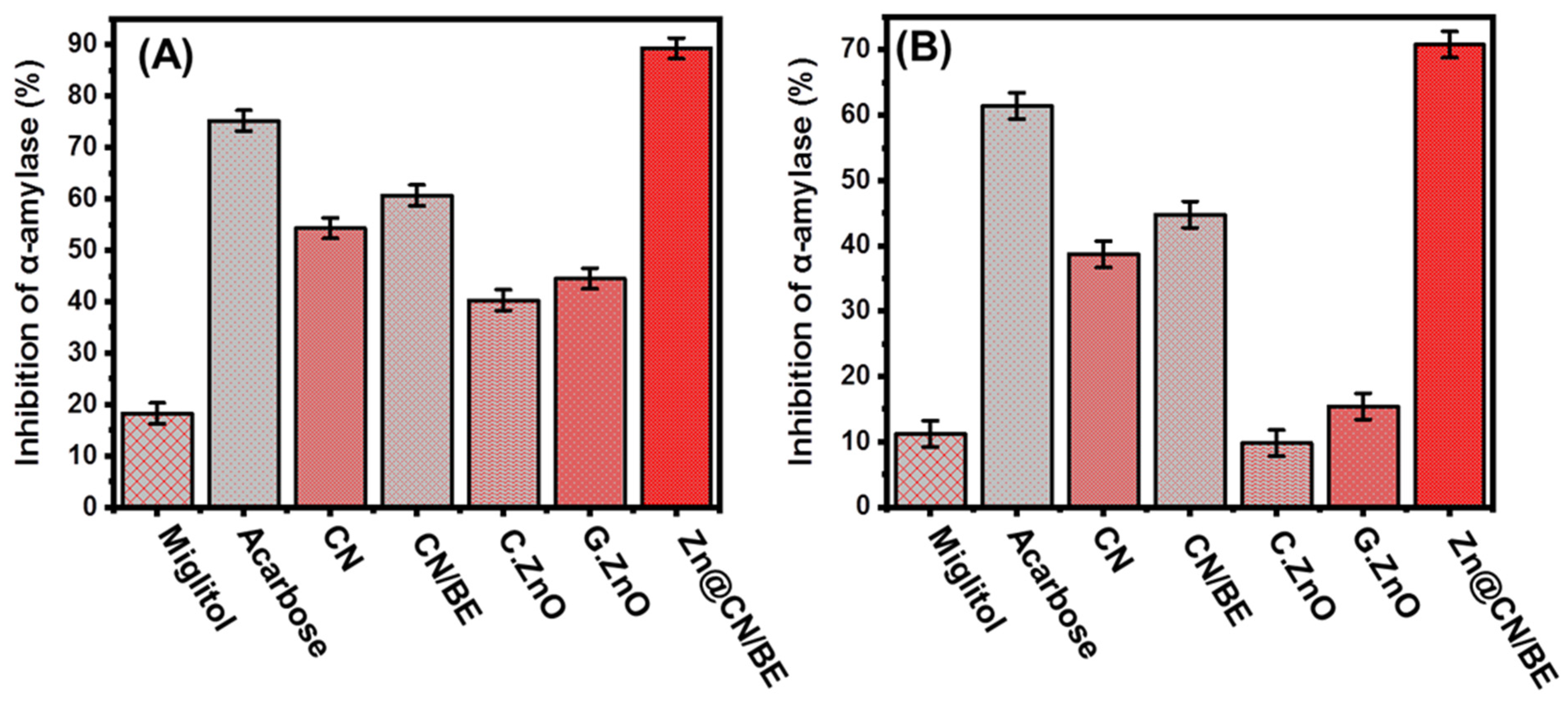
2.3. Antidiabetic Properties
2.3.1. Porcine Pancreatic α-Amylase Inhibition Assay
2.3.2. Murine Pancreatic α-Amylase Inhibition
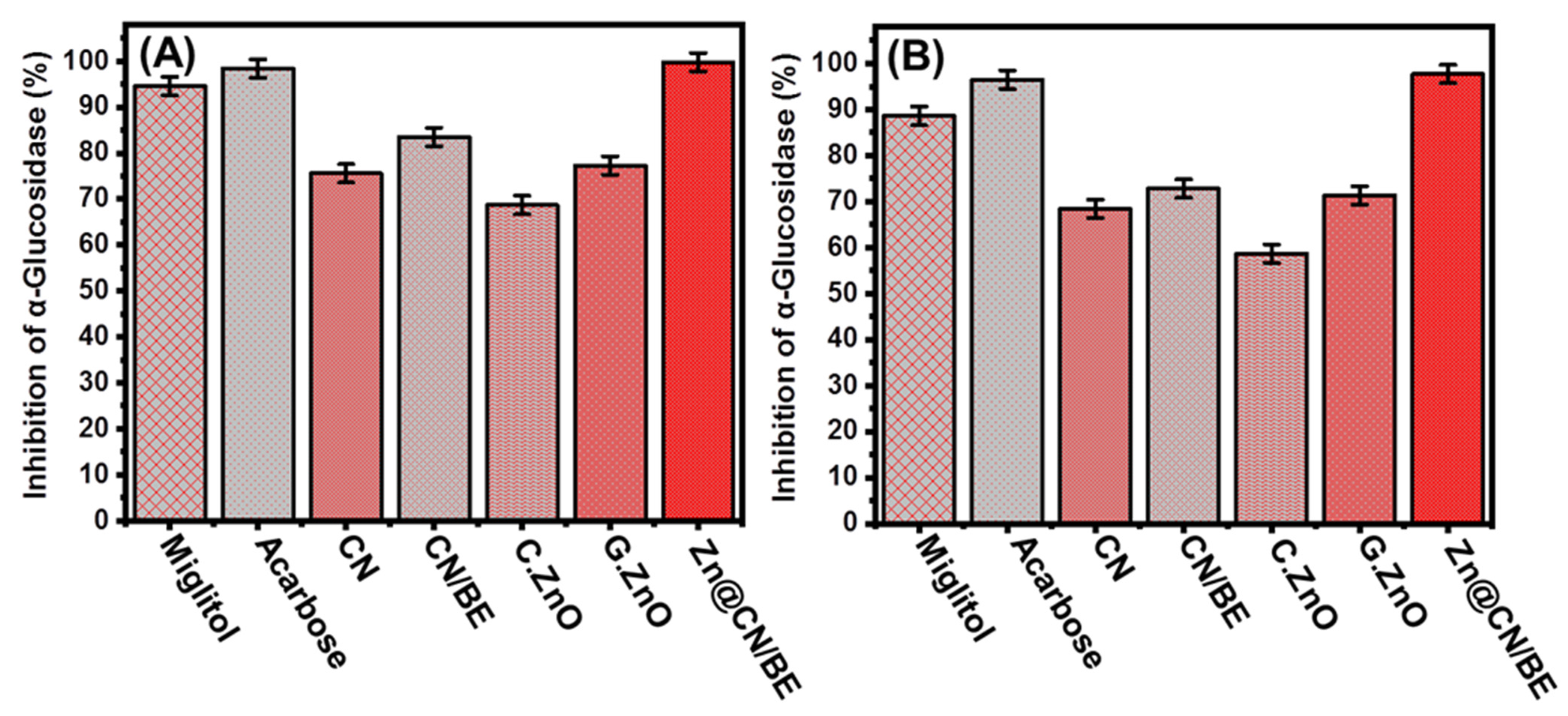
2.3.3. Pancreatic α-Glucosidase Inhibition
2.3.4. Murine Intestinal α-Glucosidase Inhibition
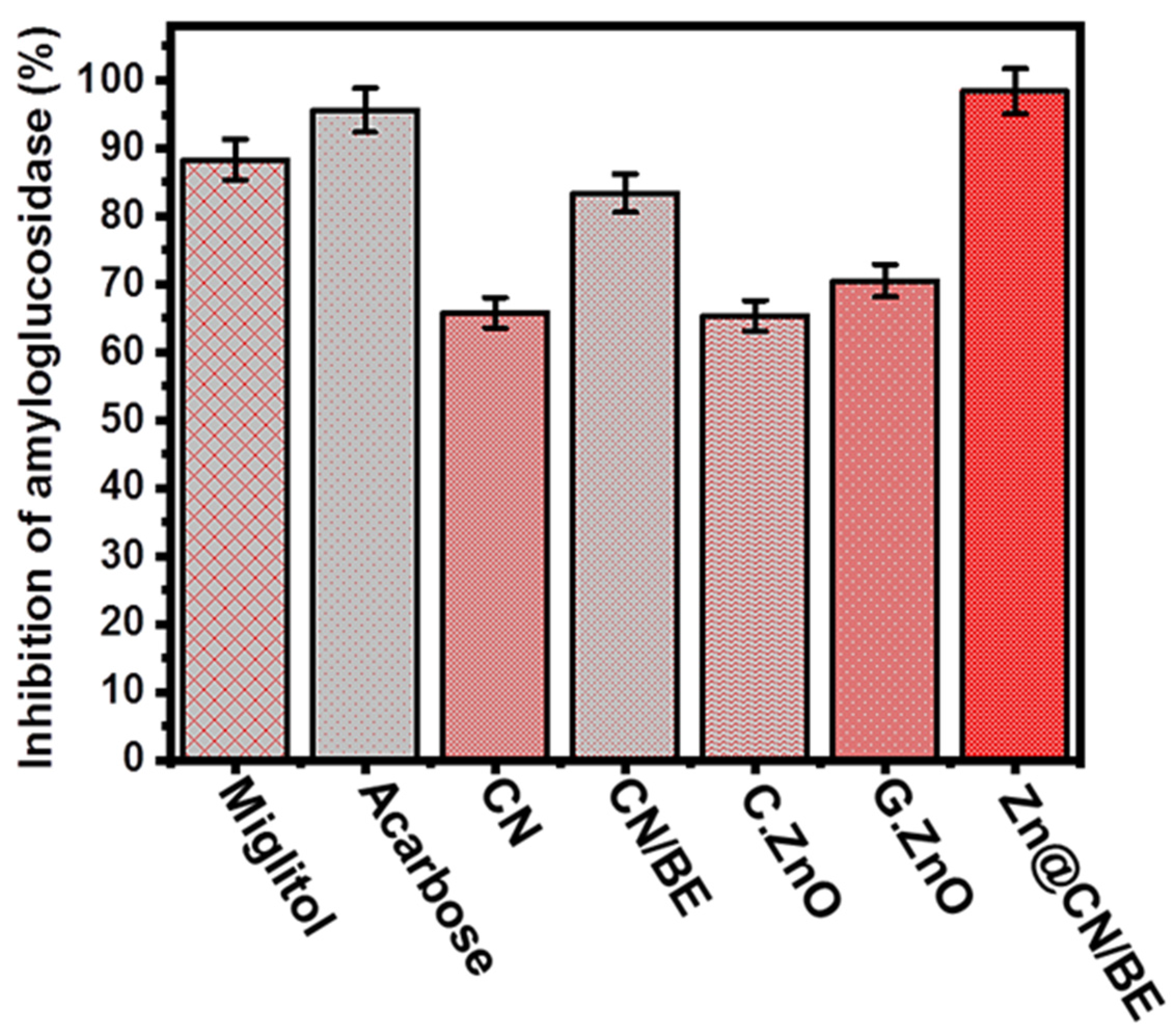
2.3.5. Amyloglucosidase Inhibition
3. Experimental Work
3.1. Materials and Chemicals
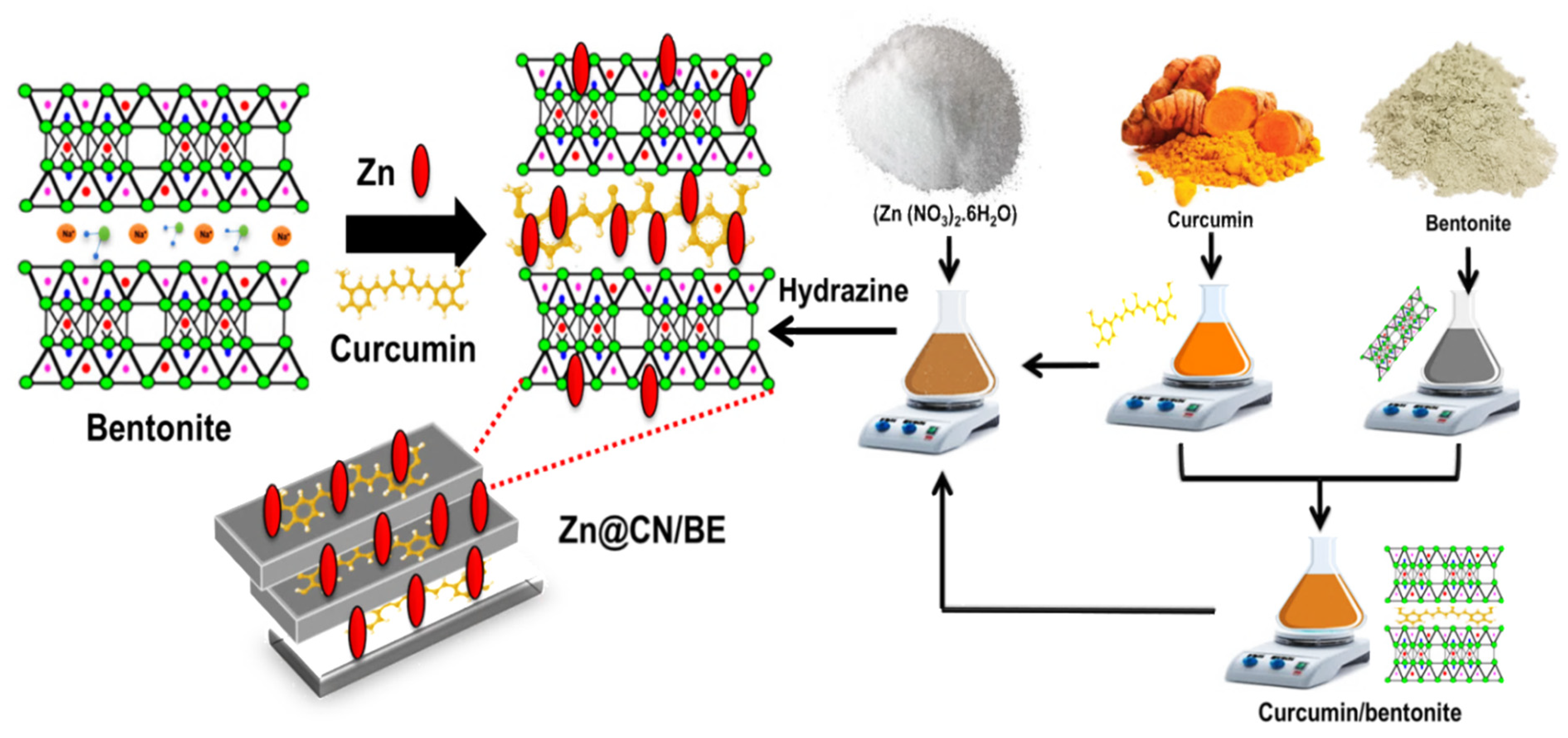
3.2. Synthesis of Green Zinc-Pillared Bentonite Mediated Curcumin Extract (Zn@CN/BE)
3.2.1. Acid Activation of Bentonite
3.2.2. Synthesis of Curcumin-Intercalated Bentonite (CN/BE)
3.2.3. Synthesis of Zinc-Pillared Bentonite Mediated Curcumin Extract (Zn@CN/BE)
3.3. Characterization Techniques
3.4. Antioxidant Studies
3.4.1. Nitric Oxide Radical Scavenging Assay
3.4.2. DPPH Radical Scavenging Assay
3.4.3. ABTS Radical Scavenging Assay
3.4.4. Superoxide Radical Scavenging Assay
3.5. Antidiabetic Studies
3.5.1. Porcine Pancreatic Amylase Inhibition Assay
3.5.2. Crude Murine Pancreatic Amylase Inhibition Assay
3.5.3. α-Glucosidase Inhibition Assay
3.5.4. Crude Murine Intestinal α-Glucosidase Inhibition Assay
3.5.5. Amyloglucosidase Inhibition Assay
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arvanag, F.M.; Bayrami, A.; Habibi-Yangjeh, A.; Pouran, S.R. A comprehensive study on antidiabetic and antibacterial activities of ZnO nanoparticles biosynthesized using Silybum marianum L seed extract. Mater. Sci. Eng. C 2018, 97, 397–405. [Google Scholar] [CrossRef]
- Behl, T.; Kaur, I.; Sehgal, A.; Sharma, E.; Kumar, A.; Grover, M.; Bungau, S. Unfolding Nrf2 in diabetes mellitus. Mol. Biol. Rep. 2021, 48, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Billacura, M.P.; Lavilla, C.J.; Cripps, M.J.; Hanna, K.; Sale, C.; Turner, M.D. β-alanine scavenging of free radicals protects mitochondrial function and enhances both insulin secretion and glucose uptake in cells under metabolic stress. Adv. Redox Res. 2022, 6, 100050. [Google Scholar] [CrossRef]
- Hsu, W.-H.; Chang, H.-M.; Lee, Y.-L.; Prasannan, A.; Hu, C.-C.; Wang, J.-S.; Lai, J.-Y.; Yang, J.M.; Jebaranjitham, N.; Tsai, H.-C. Biodegradable polymer-nanoclay composites as intestinal sleeve implants installed in digestive tract for obesity and type 2 diabetes treatment. Mater. Sci. Eng. C 2020, 110, 110676. [Google Scholar] [CrossRef]
- Sagandira, C.R.; Khasipo, A.Z.; Sagandira, M.B.; Watts, P. An overview of the synthetic routes to essential oral anti-diabetes drugs. Tetrahedron 2021, 96, 132378. [Google Scholar] [CrossRef]
- Robkhob, P.; Ghosh, S.; Bellare, J.; Jamdade, D.; Tang, I.-M.; Thongmee, S. Effect of silver doping on antidiabetic and antioxidant potential of ZnO nanorods. J. Trace Elements Med. Biol. 2020, 58, 126448. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Hajra, D. Study of glucose uptake enhancing potential of fenugreek (Trigonella foenum graecum) leaves extract on 3T3 L1 cells line and evaluation of its antioxidant potential. Pharmacogn. Res. 2018, 10, 347. [Google Scholar] [CrossRef]
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Dedvisitsakul, P.; Watla-Iad, K. Antioxidant activity and antidiabetic activities of Northern Thai indigenous edible plant extracts and their phytochemical constituents. Heliyon 2022, 8, e10740. [Google Scholar] [CrossRef]
- Kim, M.-S.; Jung, Y.S.; Jang, D.; Cho, C.H.; Lee, S.-H.; Han, N.S.; Kim, D.-O. Antioxidant capacity of 12 major soybean isoflavones and their bioavailability under simulated digestion and in human intestinal Caco-2 cells. Food Chem. 2022, 374, 131493. [Google Scholar] [CrossRef]
- Asmat, U.; Abad, K.; Ismail, K. Diabetes mellitus and oxidative stress—A concise review. Saudi Pharm. J. 2016, 24, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Ben Ahmed, Z.; Hefied, F.; Yousfi, M.; Demeyer, K.; Heyden, Y.V. Study of the antioxidant activity of Pistacia atlantica Desf. Gall extracts and evaluation of the responsible compounds. Biochem. Syst. Ecol. 2022, 100, 104358. [Google Scholar] [CrossRef]
- Yilmazer-Musa, M.; Griffith, A.M.; Michels, A.J.; Schneider, E.; Frei, B. Grape Seed and Tea Extracts and Catechin 3-Gallates Are Potent Inhibitors of α-Amylase and α-Glucosidase Activity. J. Agric. Food Chem. 2012, 60, 8924–8929. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.R.; Sharif, S.; Shaheen, F.; Khalid, M.; Iqbal, Y.; Faisal, A.; Aziz, M.H.; Atif, M.; Ahmad, S.; Fakhar-e-Alam, M.; et al. Green synthesis of RGO-ZnO mediated Ocimum basilicum leaves extract nanocomposite for antioxidant, antibacterial, antidiabetic and photocatalytic activity. J. Saudi Chem. Soc. 2022, 26, 101438. [Google Scholar] [CrossRef]
- Singh, T.A.; Sharma, A.; Tejwan, N.; Ghosh, N.; Das, J.; Sil, P.C. A state of the art review on the synthesis, antibacterial, antioxidant, antidiabetic and tissue regeneration activities of zinc oxide nanoparticles. Adv. Colloid Interface Sci. 2021, 295, 102495. [Google Scholar] [CrossRef]
- Madan, H.R.; Sharma, S.C.; Suresh, D.; Vidya, Y.S.; Nagabhushana, H.; Rajanaik, H.; Anantharaju, K.S.; Prashantha, S.C.; Maiya, P.S. Facile green fabrication of nanostructure ZnO plates, bullets, flower, prismatic tip, closed pine cone: Their antibacterial, antioxidant, photoluminescent and photocatalytic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 152, 404–416. [Google Scholar] [CrossRef]
- Ağagündüz, D.; Yılmaz, B.; Koçak, T.; Başar, H.B.A.; Rocha, J.M.; Özoğul, F. Novel Candidate Microorganisms for Fermentation Technology: From Potential Benefits to Safety Issues. Foods 2022, 11, 3074. [Google Scholar] [CrossRef]
- Velsankar, K.; Venkatesan, A.; Muthumari, P.; Suganya, S.; Mohandoss, S.; Sudhahar, S. Green inspired synthesis of ZnO nanoparticles and its characterizations with biofilm, antioxidant, anti-inflammatory, and anti-diabetic activities. J. Mol. Struct. 2022, 1255, 132420. [Google Scholar]
- Ansari, A.; Ali, A.; Khan, N.; Umar, M.S.; Owais, M. Shamsuzzaman Synthesis of steroidal dihydropyrazole derivatives using green ZnO NPs and evaluation of their anticancer and antioxidant activity. Steroids 2022, 188, 109113. [Google Scholar] [CrossRef]
- Noohpisheh, Z.; Amiri, H.; Farhadi, S.; Mohammadi-Gholami, A. Green synthesis of Ag-ZnO nanocomposites using Trigonella foenum-graecum leaf extract and their antibacterial, antifungal, antioxidant and photocatalytic properties. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118595. [Google Scholar] [CrossRef]
- Sharma, A.; Nagraik, R.; Sharma, S.; Sharma, G.; Pandey, S.; Azizov, S.; Chauhan, P.K.; Kumar, D. Green synthesis of ZnO nanoparticles using Ficus palmata: Antioxidant, antibacterial and antidiabetic studies. Results Chem. 2022, 4, 100509. [Google Scholar] [CrossRef]
- Yusof, N.A.A.; Zain, N.M.; Pauzi, N. Synthesis of ZnO nanoparticles with chitosan as stabilizing agent and their antibacterial properties against Gram-positive and Gram-negative bacteria. Int. J. Biol. Macromol. 2019, 124, 1132–1136. [Google Scholar] [CrossRef]
- Shaaban, S.; Adam, M.S.S.; El-Metwaly, N.M. Novel organoselenium-based N-mealanilic acid and its zinc (II) chelate: Catalytic, anticancer, antimicrobial, antioxidant, and computational assessments. J. Mol. Liq. 2022, 363, 119907. [Google Scholar] [CrossRef]
- Meer, B.; Andleeb, A.; Iqbal, J.; Ashraf, H.; Meer, K.; Ali, J.S.; Drouet, S.; Anjum, S.; Mehmood, A.; Khan, T.; et al. Bio-Assisted Synthesis and Characterization of Zinc Oxide Nanoparticles from Lepidium Sativum and Their Potent Antioxidant, Antibacterial and Anticancer Activities. Biomolecules 2022, 12, 855. [Google Scholar] [CrossRef] [PubMed]
- Hasanzadeh, A.; Gholipour, B.; Rostamnia, S.; Eftekhari, A.; Tanomand, A.; Khaksar, S.; Khalilov, R. Biosynthesis of AgNPs onto the urea-based periodic mesoporous organosilica (AgxNPs/Ur-PMO) for antibacterial and cell viability assay. J. Colloid Interface Sci. 2021, 585, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Baran, A.; Keskin, C.; Baran, M.F.; Huseynova, I.; Khalilov, R.; Eftekhari, A.; Irtegun-Kandemir, S.; Kavak, D.E. Ecofriendly Synthesis of Silver Nanoparticles Using Ananas comosus Fruit Peels: Anticancer and Antimicrobial Activities. Bioinorg. Chem. Appl. 2021, 2021, 2058149. [Google Scholar] [CrossRef]
- Prasad, S.; Lall, R. Zinc-curcumin based complexes in health and diseases: An approach in chemopreventive and therapeutic improvement. J. Trace Elements Med. Biol. 2022, 73, 127023. [Google Scholar] [CrossRef]
- Deng, J.; Wang, J.; Hu, H.; Hong, J.; Yang, L.; Zhou, H.; Xu, D. Application of mesoporous calcium silicate nanoparticles as a potential SD carrier to improve the solubility of curcumin. J. Dispers. Sci. Technol. 2022, 1–9. [Google Scholar] [CrossRef]
- Kour, P.; Afzal, S.; Gani, A.; Zargar, M.I.; Tak, U.N.; Rashid, S.; Dar, A.A. Effect of nanoemulsion-loaded hybrid biopolymeric hydrogel beads on the release kinetics, antioxidant potential and antibacterial activity of encapsulated curcumin. Food Chem. 2022, 376, 131925. [Google Scholar] [CrossRef]
- Yixuan, L.; Qaria, M.A.; Sivasamy, S.; Jianzhong, S.; Daochen, Z. Curcumin production and bioavailability: A comprehensive review of curcumin extraction, synthesis, biotransformation and delivery systems. Ind. Crop. Prod. 2021, 172, 114050. [Google Scholar] [CrossRef]
- Khansili, N.; Krishna, P.M. Curcumin functionalized TiO2 modified bentonite clay nanostructure for colorimetric Aflatoxin B1 detection in peanut and corn. Sens. Bio Sens. Res. 2022, 35, 100480. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Preparation of carbohydrate-based functional composite films incorporated with curcumin. Food Hydrocoll. 2020, 98, 105302. [Google Scholar] [CrossRef]
- Bernardo, A.; Plumitallo, C.; De Nuccio, C.; Visentin, S.; Minghetti, L. Curcumin promotes oligodendrocyte differentiation and their protection against TNF-α through the activation of the nuclear receptor PPAR-γ. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Abukhadra, M.R.; Adlii, A.; El-Sherbeeny, A.M.; Soliman, A.T.A.; Elgawad, A.E.E.A. Promoting the decontamination of different types of water pollutants (Cd2+, safranin dye, and phosphate) using a novel structure of exfoliated bentonite admixed with cellulose nanofiber. J. Environ. Manag. 2020, 273, 111130. [Google Scholar] [CrossRef]
- Wasim, M.; Shi, F.; Liu, J.; Zhang, H.; Zhu, K.; Tian, Z. Synthesis and characterization of curcumin/MMT-clay-treated bacterial cellulose as an antistatic and ultraviolet-resistive bioscaffold. J. Polym. Res. 2022, 29, 423. [Google Scholar] [CrossRef]
- Mohammadhosseini, S.; Al-Musawi, T.J.; Romero Parra, R.M.; Qutob, M.; Gatea, M.A.; Ganji, F.; Balarak, D. UV and Visible Light Induced Photodegradation of Reactive Red 198 Dye and Textile Factory Wastewater on Fe2O3/Bentonite/TiO2 Nanocomposite. Minerals 2022, 12, 1417. [Google Scholar] [CrossRef]
- Tong, L.; Liang, T.; Tian, Y.; Zhang, Q.; Pan, Y. Research progress on treatment of mine wastewater by bentonite composite. Arab. J. Geosci. 2022, 15, 681. [Google Scholar] [CrossRef]
- Koksal, E.; Afsin, B.; Tabak, A.; Caglar, B. Butylamine-resadiye bentonite composite characterization. Spectrosc. Lett. 2020, 53, 745–750. [Google Scholar] [CrossRef]
- Darmawan, M.A.; Muhammad, B.Z.; Harahap, A.F.P.; Ramadhan, M.Y.A.; Sahlan, M.; Haryuni; Supriyadi, T.; Abd-Aziz, S.; Gozan, M. Reduction of the acidity and peroxide numbers of tengkawang butter (Shorea stenoptera) using thermal and acid activated bentonites. Heliyon 2020, 6, e05742. [Google Scholar] [CrossRef]
- Nagahashi, E.; Ogata, F.; Saenjum, C.; Nakamura, T.; Kawasaki, N. Preparation and Characterization of Acid-Activated Bentonite with Binary Acid Solution and Its Use in Decreasing Electrical Conductivity of Tap Water. Minerals 2021, 11, 815. [Google Scholar] [CrossRef]
- Chauhan, M.; Saini, V.K.; Suthar, S. Ti-pillared montmorillonite clay for adsorptive removal of amoxicillin, imipramine, diclofenac-sodium, and paracetamol from water. J. Hazard. Mater. 2020, 399, 122832. [Google Scholar] [CrossRef] [PubMed]
- Dardir, F.M.; Mohamed, A.S.; Abukhadra, M.R.; Ahmed, E.A.; Soliman, M.F. Cosmetic and pharmaceutical qualifications of Egyptian bentonite and its suitability as drug carrier for Praziquantel drug. Eur. J. Pharm. Sci. 2018, 115, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Abdel Salam, M.; Mokhtar, M.; Albukhari, S.M.; Baamer, D.F.; Palmisano, L.; Jaremko, M.; Abukhadra, M.R. Synthesis and Characterization of Green ZnO@ polynaniline/Bentonite Tripartite Structure (G. Zn@ PN/BE) as Adsorbent for As (V) Ions: Integration, Steric, and Energetic Properties. Polymers 2022, 14, 2329. [Google Scholar] [CrossRef] [PubMed]
- Khatun, B.; Banik, N.; Hussain, A.; Ramteke, A.; Maji, T. Genipin crosslinked curcumin loaded chitosan/montmorillonite K-10 (MMT) nanoparticles for controlled drug delivery applications. J. Microencapsul. 2018, 35, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, E.; Andreescu, D.; Andreescu, S. Artificial Nanoparticle Antioxidants. In Oxidative Stress: Diagnostics, Prevention, and Therapy; American Chemical Society: Washington, DC, USA, 2011; pp. 235–253. [Google Scholar] [CrossRef]
- Parul, R.; Kundu, S.K.; Saha, P. In vitro nitric oxide scavenging activity of methanol extracts of three Bangladeshi medicinal plants. Pharma Innov. 2013, 1, 83. [Google Scholar]
- Song, Y.; Yang, F.; Ma, M.; Kang, Y.; Hui, A.; Quan, Z.; Wang, A. Green synthesized Se–ZnO/attapulgite nanocomposites using Aloe vera leaf extract: Characterization, antibacterial and antioxidant activities. LWT 2022, 165, 113762. [Google Scholar] [CrossRef]
- Saad, A.M.; Abukhadra, M.R.; Ahmed, S.A.-K.; Elzanaty, A.M.; Mady, A.H.; Betiha, M.A.; Shim, J.-J.; Rabie, A.M. Photocatalytic degradation of malachite green dye using chitosan supported ZnO and Ce–ZnO nano-flowers under visible light. J. Environ. Manag. 2020, 258, 110043. [Google Scholar] [CrossRef]
- Wang, Z.-J.; Xu, J.-J.; Ji, F.-Y.; Luo, S.-Z.; Li, X.-J.; Mu, D.-D.; Jiang, S.-T.; Zheng, Z. Fabrication and characterization of soy β-conglycinin-dextran-polyphenol nanocomplexes: Improvement on the antioxidant activity and sustained-release property of curcumin. Food Chem. 2022, 395, 133562. [Google Scholar] [CrossRef]
- Ayodhya, D.; Veerabhadram, G. Facile thermal fabrication of CuO nanoparticles from Cu (II)-Schiff base complexes and its catalytic reduction of 4-nitrophenol, antioxidant, and antimicrobial studies. Chem. Data Collect. 2019, 23, 100259. [Google Scholar] [CrossRef]
- Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour. Technol. 2017, 3, 506–515. [Google Scholar] [CrossRef]
- Ramachandiran, D.; Elangovan, M.; Rajesh, K. Structural, optical, biological and photocatalytic activities of platinum nanoparticles using salixtetrasperma leaf extract via hydrothermal and ultrasonic methods. Optik 2021, 244, 167494. [Google Scholar] [CrossRef]
- Hosny, M.; Fawzy, M.; El-Badry, Y.A.; Hussein, E.E.; Eltaweil, A.S. Plant-assisted synthesis of gold nanoparticles for photocatalytic, anticancer, and antioxidant applications. J. Saudi Chem. Soc. 2022, 26, 101419. [Google Scholar] [CrossRef]
- Adersh, A.; Kulkarni, A.R.; Ghosh, S.; More, P.; Chopade, B.A.; Gandhi, M.N. Surface defect rich ZnO quantum dots as antioxidant inhibitingα-amylase and α-glucosidase: A potential anti-diabetic nanomedicine. J. Mater. Chem. B 2015, 3, 4597–4606. [Google Scholar]
- Liu, Y.; Ying, D.; Cai, Y.; Le, X. Improved antioxidant activity and physicochemical properties of curcumin by adding ovalbumin and its structural characterization. Food Hydrocoll. 2017, 72, 304–311. [Google Scholar] [CrossRef]
- Oun, A.A.; Bae, A.Y.; Shin, G.H.; Park, M.-K.; Kim, J.T. Comparative study of oregano essential oil encapsulated in halloysite nanotubes and diatomaceous earth as antimicrobial and antioxidant composites. Appl. Clay Sci. 2022, 224, 106522. [Google Scholar] [CrossRef]
- Mohammadian, M.; Salami, M.; Moghadam, M.; Amirsalehi, A.; Emam-Djomeh, Z. Mung bean protein as a promising biopolymeric vehicle for loading of curcumin: Structural characterization, antioxidant properties, and in vitro release kinetics. J. Drug Deliv. Sci. Technol. 2021, 61, 102148. [Google Scholar] [CrossRef]
- Lin, D.; Xiao, L.; Qin, W.; Loy, D.A.; Wu, Z.; Chen, H.; Zhang, Q. Preparation, characterization and antioxidant properties of curcumin encapsulated chitosan/lignosulfonate micelles. Carbohydr. Polym. 2022, 281, 119080. [Google Scholar] [CrossRef]
- Hamasaki, T.; Kashiwagi, T.; Imada, T.; Nakamichi, N.; Aramaki, S.; Toh, K.; Morisawa, S.; Shimakoshi, H.; Hisaeda, Y.; Shirahata, S. Kinetic Analysis of Superoxide Anion Radical-Scavenging and Hydroxyl Radical-Scavenging Activities of Platinum Nanoparticles. Langmuir 2008, 24, 7354–7364. [Google Scholar] [CrossRef]
- Xie, J.; Wang, N.; Dong, X.; Wang, C.; Du, Z.; Mei, L.; Yong, Y.; Huang, C.; Li, Y.; Gu, Z.; et al. Graphdiyne Nanoparticles with High Free Radical Scavenging Activity for Radiation Protection. ACS Appl. Mater. Interfaces 2018, 11, 2579–2590. [Google Scholar] [CrossRef]
- Shu, G.; Xu, D.; Xie, S.; Chang, L.-J.; Liu, X.; Yang, J.; Li, Y.; Wang, X. The antioxidant, antibacterial, and infected wound healing effects of ZnO quantum dots-chitosan biocomposite. Appl. Surf. Sci. 2023, 611, 155727. [Google Scholar] [CrossRef]
- Selvan, D.S.A.; Kumar, R.S.; Murugesan, S.; Shobana, S.; Rahiman, A.K. Antidiabetic activity of phytosynthesized Ag/CuO nanocomposites using Murraya koenigii and Zingiber officinale extracts. J. Drug Deliv. Sci. Technol. 2022, 67, 102838. [Google Scholar] [CrossRef]
- Haritha, V.; Gowri, S.; Janarthanan, B.; Faiyazuddin; Karthikeyan, C.; Sharmila, S. Biogenic synthesis of nickel oxide nanoparticles using Averrhoa bilimbi and investigation of its antibacterial, antidiabetic and cytotoxic properties. Inorg. Chem. Commun. 2022, 144, 109930. [Google Scholar] [CrossRef]
- Sabeena, G.; Rajaduraipandian, S.; Manju, T.; Alhadlaq, H.A.; Mohan, R.; Annadurai, G.; Ahamed, M. In vitro antidiabetic and anti-inflammatory effects of Fe-doped CuO-rice husk silica (Fe-CuO-SiO2) nanocomposites and their enhanced innate immunity in zebrafish. J. King Saud Univ. Sci. 2022, 34, 102121. [Google Scholar] [CrossRef]
- Vinotha, V.; Iswarya, A.; Thaya, R.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Khaled, J.M.; Al-Anbr, M.N.; Vaseeharan, B. Synthesis of ZnO nanoparticles using insulin-rich leaf extract: Anti-diabetic, antibiofilm and anti-oxidant properties. J. Photochem. Photobiol. B Biol. 2019, 197, 111541. [Google Scholar] [CrossRef]
- Rehana, D.; Mahendiran, D.; Kumar, R.S.; Rahiman, A.K. In vitro antioxidant and antidiabetic activities of zinc oxide nanoparticles synthesized using different plant extracts. Bioprocess Biosyst. Eng. 2017, 40, 943–957. [Google Scholar] [CrossRef]
- Alkaladi, A.; Abdelazim, A.; Afifi, M. Antidiabetic Activity of Zinc Oxide and Silver Nanoparticles on Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 2014, 15, 2015–2023. [Google Scholar] [CrossRef]
- Dhobale, S.; Thite, T.; Laware, S.L.; Rode, C.V.; Koppikar, S.J.; Ghanekar, R.-K.; Kale, S.N. Zinc oxide nanoparticles as novel alpha-amylase inhibitors. J. Appl. Phys. 2008, 104, 094907. [Google Scholar] [CrossRef]
- Kitture, R.; Ghosh, S.; More, P.A.; Date, K.; Gaware, S.; Datar, S.; Chopade, B.A.; Kale, S.N. Curcumin-Loaded, Self-Assembled Aloevera Template for Superior Antioxidant Activity and Trans-Membrane Drug Release. J. Nanosci. Nanotechnol. 2015, 15, 4039–4045. [Google Scholar] [CrossRef]
- Dappula, S.S.; Kandrakonda, Y.R.; Shaik, J.B.; Mothukuru, S.L.; Lebaka, V.R.; Mannarapu, M.; Amooru, G.D. Biosynthesis of zinc oxide nanoparticles using aqueous extract of Andrographis alata: Characterization, optimization and assessment of their antibacterial, antioxidant, antidiabetic and anti-Alzheimer’s properties. J. Mol. Struct. 2023, 1273, 134264. [Google Scholar] [CrossRef]
- Sanap, S.P.; Ghosh, S.; Jabgunde, A.M.; Pinjari, R.V.; Gejji, S.P.; Singh, S.; Chopade, B.A.; Dhavale, D.D. Synthesis, computational study and glycosidase inhibitory activity of polyhydroxylated conidine alkaloids—A bicyclic iminosugar. Org. Biomol. Chem. 2010, 8, 3307–3315. [Google Scholar] [CrossRef] [PubMed]
- Lawande, P.P.; Sontakke, V.A.; Kumbhar, N.M.; Bhagwat, T.R.; Ghosh, S.; Shinde, V.S. Polyhydroxylated azetidine iminosugars: Synthesis, glycosidase inhibitory activity and molecular docking studies. Bioorganic Med. Chem. Lett. 2017, 27, 5291–5295. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bellucci, S.; Rudayni, H.A.; Shemy, M.H.; Aladwani, M.; Alneghery, L.M.; Allam, A.A.; Abukhadra, M.R. Synthesis and Characterization of Green Zinc-Metal-Pillared Bentonite Mediated Curcumin Extract (Zn@CN/BE) as an Enhanced Antioxidant and Anti-Diabetes Agent. Inorganics 2023, 11, 154. https://doi.org/10.3390/inorganics11040154
Bellucci S, Rudayni HA, Shemy MH, Aladwani M, Alneghery LM, Allam AA, Abukhadra MR. Synthesis and Characterization of Green Zinc-Metal-Pillared Bentonite Mediated Curcumin Extract (Zn@CN/BE) as an Enhanced Antioxidant and Anti-Diabetes Agent. Inorganics. 2023; 11(4):154. https://doi.org/10.3390/inorganics11040154
Chicago/Turabian StyleBellucci, Stefano, Hassan Ahmed Rudayni, Marwa H. Shemy, Malak Aladwani, Lina M. Alneghery, Ahmed A. Allam, and Mostafa R. Abukhadra. 2023. "Synthesis and Characterization of Green Zinc-Metal-Pillared Bentonite Mediated Curcumin Extract (Zn@CN/BE) as an Enhanced Antioxidant and Anti-Diabetes Agent" Inorganics 11, no. 4: 154. https://doi.org/10.3390/inorganics11040154
APA StyleBellucci, S., Rudayni, H. A., Shemy, M. H., Aladwani, M., Alneghery, L. M., Allam, A. A., & Abukhadra, M. R. (2023). Synthesis and Characterization of Green Zinc-Metal-Pillared Bentonite Mediated Curcumin Extract (Zn@CN/BE) as an Enhanced Antioxidant and Anti-Diabetes Agent. Inorganics, 11(4), 154. https://doi.org/10.3390/inorganics11040154







