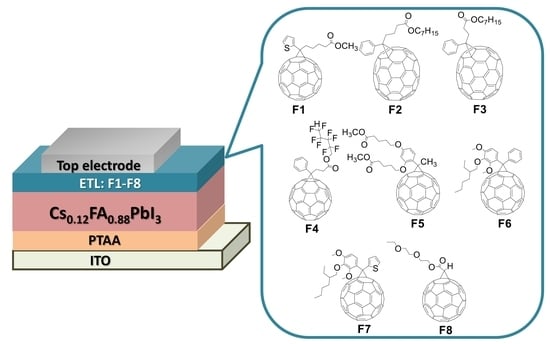
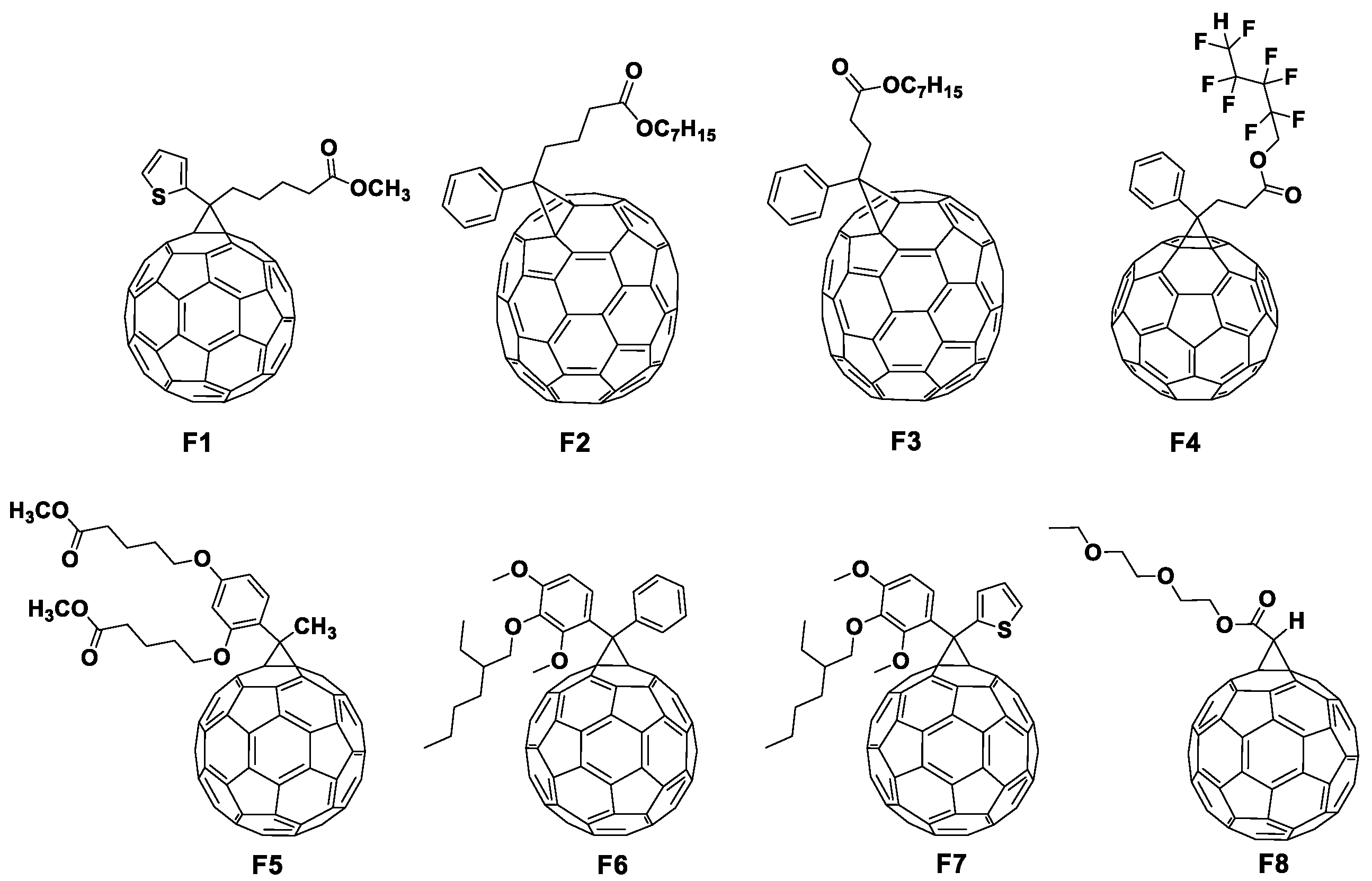
Rational Design of Fullerene Derivatives for Improved Stability of p-i-n Perovskite Solar Cells
Abstract
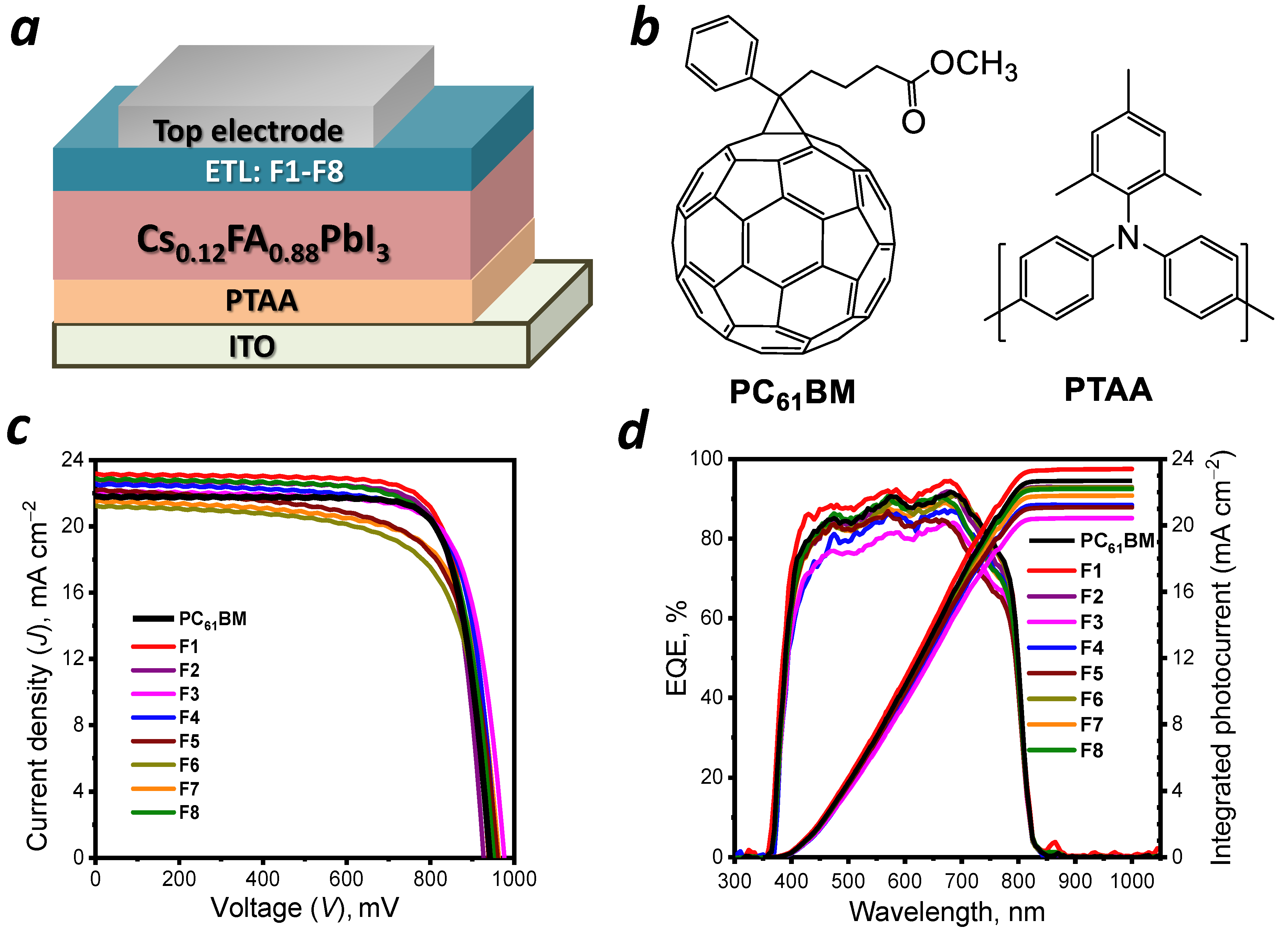
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General
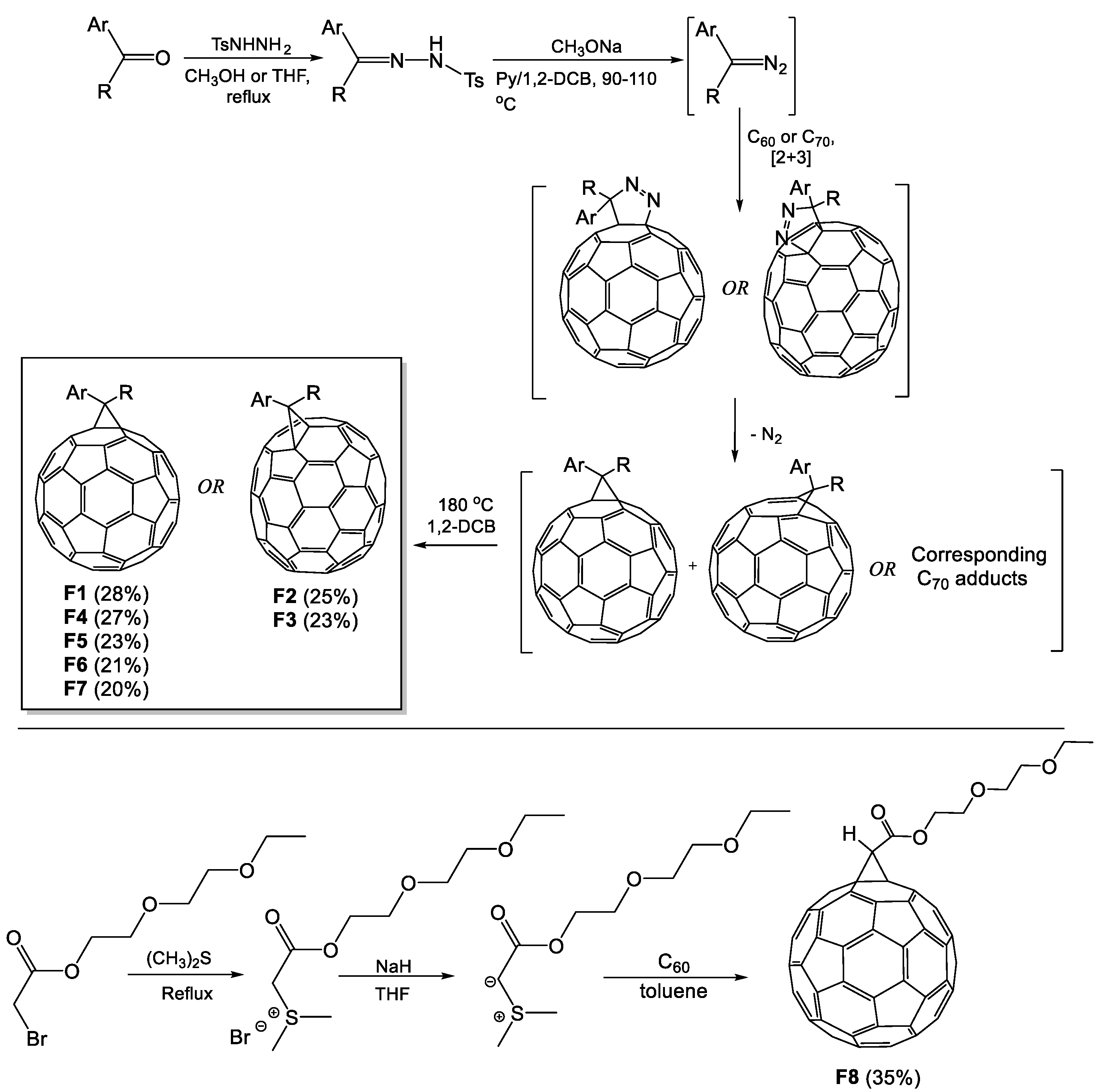
4.2. Synthesis of F1–F7
4.3. Synthesis of F8
4.4. PSCs Fabrication and Characterization
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Green, M.A.; Dunlop, E.D.; Hohl-Ebinger, J.; Yoshita, M.; Kopidakis, N.; Bothe, K.; Hinken, D.; Rauer, M.; Hao, X. Solar Cell Efficiency Tables (Version 60). Prog. Photovolt. 2022, 30, 687–701. [Google Scholar] [CrossRef]
- Sahare, S.; Pham, H.D.; Angmo, D.; Ghoderao, P.; MacLeod, J.; Khan, S.B.; Lee, S.; Singh, S.P.; Sonar, P. Emerging Perovskite Solar Cell Technology: Remedial Actions for the Foremost Challenges. Adv. Energy Mater. 2021, 11, 2101085. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.-W.; Jung, H.S.; Shin, H.; Park, N.-G. High-Efficiency Perovskite Solar Cells. Chem. Rev. 2020, 120, 7867–7918. [Google Scholar] [CrossRef]
- Schulz, P.; Cahen, D.; Kahn, A. Halide Perovskites: Is It All about the Interfaces? Chem. Rev. 2019, 119, 3349–3417. [Google Scholar] [CrossRef] [PubMed]
- Akbulatov, A.F.; Frolova, L.A.; Griffin, M.P.; Gearba, I.R.; Dolocan, A.; Vanden Bout, D.A.; Tsarev, S.; Katz, E.A.; Shestakov, A.F.; Stevenson, K.J.; et al. Effect of Electron-Transport Material on Light-Induced Degradation of Inverted Planar Junction Perovskite Solar Cells. Adv. Energy Mater. 2017, 7, 1700476. [Google Scholar] [CrossRef]
- Wu, S.; Chen, R.; Zhang, S.; Babu, B.H.; Yue, Y.; Zhu, H.; Yang, Z.; Chen, C.; Chen, W.; Huang, Y.; et al. A Chemically Inert Bismuth Interlayer Enhances Long-Term Stability of Inverted Perovskite Solar Cells. Nat. Commun. 2019, 10, 1161. [Google Scholar] [CrossRef]
- Chen, R.; Zhang, W.; Guan, X.; Raza, H.; Zhang, S.; Zhang, Y.; Troshin, P.A.; Kuklin, S.A.; Liu, Z.; Chen, W. Rear Electrode Materials for Perovskite Solar Cells. Adv. Funct. Mater. 2022, 32, 2200651. [Google Scholar] [CrossRef]
- Elnaggar, M.; Boldyreva, A.G.; Elshobaki, M.; Tsarev, S.A.; Fedotov, Y.S.; Yamilova, O.R.; Bredikhin, S.I.; Stevenson, K.J.; Aldoshin, S.M.; Troshin, P.A. Decoupling Contributions of Charge-Transport Interlayers to Light-Induced Degradation of P-i-n Perovskite Solar Cells. Sol. RRL 2020, 4, 2000191. [Google Scholar] [CrossRef]
- Liu, K.; Tian, C.; Liang, Y.; Luo, Y.; Xie, L.; Wei, Z. Progress toward Understanding the Fullerene-Related Chemical Interactions in Perovskite Solar Cells. Nano Res. 2022, 15, 7139–7153. [Google Scholar] [CrossRef]
- Zahran, R.; Hawash, Z. Fullerene-Based Inverted Perovskite Solar Cell: A Key to Achieve Promising, Stable, and Efficient Photovoltaics. Adv. Mater. Inter. 2022, 9, 2201438. [Google Scholar] [CrossRef]
- Deng, L.-L.; Xie, S.-Y.; Gao, F. Fullerene-Based Materials for Photovoltaic Applications: Toward Efficient, Hysteresis-Free, and Stable Perovskite Solar Cells. Adv. Electron. Mater. 2018, 4, 1700435. [Google Scholar] [CrossRef]
- Foo, S.; Thambidurai, M.; Senthil Kumar, P.; Yuvakkumar, R.; Huang, Y.; Dang, C. Recent Review on Electron Transport Layers in Perovskite Solar Cells. Int. J. Energy Res. 2022, 46, 21441–21451. [Google Scholar] [CrossRef]
- Kim, T.; Lim, J.; Song, S. Recent Progress and Challenges of Electron Transport Layers in Organic–Inorganic Perovskite Solar Cells. Energies 2020, 13, 5572. [Google Scholar] [CrossRef]
- Zhang, K.; Yu, H.; Liu, X.; Dong, Q.; Wang, Z.; Wang, Y.; Chen, N.; Zhou, Y.; Song, B. Fullerenes and Derivatives as Electron Transport Materials in Perovskite Solar Cells. Sci. China Chem. 2017, 60, 144–150. [Google Scholar] [CrossRef]
- Yao, Y.; Cheng, C.; Zhang, C.; Hu, H.; Wang, K.; De Wolf, S. Organic Hole-Transport Layers for Efficient, Stable, and Scalable Inverted Perovskite Solar Cells. Adv. Mater. 2022, 34, 2203794. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, Z.; Li, Z. Recent Advances in Developing High-Performance Organic Hole Transporting Materials for Inverted Perovskite Solar Cells. Front. Optoelectron. 2022, 15, 46. [Google Scholar] [CrossRef]
- Ahmad, T.; Wilk, B.; Radicchi, E.; Fuentes Pineda, R.; Spinelli, P.; Herterich, J.; Castriotta, L.A.; Dasgupta, S.; Mosconi, E.; De Angelis, F.; et al. New Fullerene Derivative as an N-Type Material for Highly Efficient, Flexible Perovskite Solar Cells of a P-i-n Configuration. Adv. Funct. Mater. 2020, 30, 2004357. [Google Scholar] [CrossRef]
- Tian, C.; Betancourt-Solis, G.; Nan, Z.; Liu, K.; Lin, K.; Lu, J.; Xie, L.; Echegoyen, L.; Wei, Z. Efficient and Stable Inverted Perovskite Solar Cells Enabled by Inhibition of Self-Aggregation of Fullerene Electron-Transporting Compounds. Sci. Bull. 2021, 66, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Delgado, O.; Chandrasekhar, P.S.; Cano-Sampaio, N.; Simon, Z.C.; Puente-Santiago, A.R.; Liu, F.; Castro, E.; Echegoyen, L. The Role of Fullerene Derivatives in Perovskite Solar Cells: Electron Transporting or Electron Extraction Layers? J. Mater. Chem. C 2021, 9, 10759–10767. [Google Scholar] [CrossRef]
- Elnaggar, M.; Elshobaki, M.; Mumyatov, A.; Luchkin, S.Y.; Dremova, N.N.; Stevenson, K.J.; Troshin, P.A. Molecular Engineering of the Fullerene-Based Electron Transport Layer Materials for Improving Ambient Stability of Perovskite Solar Cells. Sol. RRL 2019, 3, 1900223. [Google Scholar] [CrossRef]
- Chen, R.; Wang, W.; Bu, T.; Ku, Z.; Zhong, J.; Peng, Y.; Xiao, S.; You, W.; Huang, F.; Cheng, Y.; et al. Low-Cost Fullerene Derivative as an Efficient Electron Transport Layer for Planar Perovskite Solar Cells. Acta Phys.-Chim. Sinica 2019, 35, 401–407. [Google Scholar] [CrossRef]
- Liu, F.; Xing, Z.; Ren, Y.; Huang, R.-J.; Xu, P.-Y.; Xie, F.-F.; Li, S.-H.; Zhong, X. Tailoring Functional Terminals on Solution-Processable Fullerene Electron Transporting Materials for High Performance Perovskite Solar Cells. Nanomaterials 2022, 12, 1046. [Google Scholar] [CrossRef] [PubMed]
- Pascual, J.; Collavini, S.; Völker, S.F.; Phung, N.; Palacios-Lidon, E.; Irusta, L.; Grande, H.-J.; Abate, A.; Tena-Zaera, R.; Delgado, J.L. Unravelling Fullerene–Perovskite Interactions Introduces Advanced Blend Films for Performance-Improved Solar Cells. Sust. Energy Fuels 2019, 3, 2779–2787. [Google Scholar] [CrossRef]
- Shao, G.; Niu, C.; Liu, H.-W.; Yang, H.; Chen, J.-S.; Yao, Y.-R.; Yang, S.; Wang, G.-W. [60]Fullerene-Fused Cyclopentanes: Mechanosynthesis and Photovoltaic Application. Org. Lett. 2023, 25, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Przypis, L.; Ahmad, T.; Misztal, K.; Honisz, D.; Radicchi, E.; Mosconi, E.; Domagala, W.; De Angelis, F.; Wojciechowski, K. Designing New Indene-Fullerene Derivatives as Electron-Transporting Materials for Flexible Perovskite Solar Cells. J. Phys. Chem. C 2021, 125, 27344–27353. [Google Scholar] [CrossRef]
- Younes, E.M.; Gurung, A.; Bahrami, B.; El-Maghraby, E.M.; Qiao, Q. Enhancing Efficiency and Stability of Inverted Structure Perovskite Solar Cells with Fullerene C60 Doped PC61BM Electron Transport Layer. Carbon 2021, 180, 226–236. [Google Scholar] [CrossRef]
- Li, S.-H.; Xing, Z.; Wu, B.-S.; Chen, Z.-C.; Yao, Y.-R.; Tian, H.-R.; Li, M.-F.; Yun, D.-Q.; Deng, L.-L.; Xie, S.-Y.; et al. Hybrid Fullerene-Based Electron Transport Layers Improving the Thermal Stability of Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 20733–20740. [Google Scholar] [CrossRef]
- Liu, Z.; Siekmann, J.; Klingebiel, B.; Rau, U.; Kirchartz, T. Interface Optimization via Fullerene Blends Enables Open-Circuit Voltages of 1.35 V in CH3NH3Pb(I0.8Br0.2)3 Solar Cells. Adv. Energy Mater. 2021, 11, 2003386. [Google Scholar] [CrossRef]
- Saranin, D.; Pescetelli, S.; Pazniak, A.; Rossi, D.; Liedl, A.; Yakusheva, A.; Luchnikov, L.; Podgorny, D.; Gostischev, P.; Didenko, S.; et al. Transition Metal Carbides (MXenes) for Efficient NiO-Based Inverted Perovskite Solar Cells. Nano Energy 2021, 82, 105771. [Google Scholar] [CrossRef]
- Luo, Z.; Wu, F.; Zhang, T.; Zeng, X.; Xiao, Y.; Liu, T.; Zhong, C.; Lu, X.; Zhu, L.; Yang, S.; et al. Designing a Perylene Diimide/Fullerene Hybrid as Effective Electron Transporting Material in Inverted Perovskite Solar Cells with Enhanced Efficiency and Stability. Angew. Chem. Int. Ed. 2019, 58, 8520–8525. [Google Scholar] [CrossRef]
- Cheng, Z.; Fang, Y.; Wang, A.; Ma, T.; Liu, F.; Gao, S.; Yan, S.; Di, Y.; Qin, T. Highly Soluble Dendritic Fullerene Derivatives as Electron Transport Material for Perovskite Solar Cells. J. Cent. South Univ. 2021, 28, 3714–3727. [Google Scholar] [CrossRef]
- Huang, H.-H.; Tsai, H.; Raja, R.; Lin, S.-L.; Ghosh, D.; Hou, C.-H.; Shyue, J.-J.; Tretiak, S.; Chen, W.; Lin, K.-F.; et al. Robust Unencapsulated Perovskite Solar Cells Protected by a Fluorinated Fullerene Electron Transporting Layer. ACS Energy Lett. 2021, 6, 3376–3385. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, L.; Ding, L.; Yang, S. Using Fluorinated and Crosslinkable Fullerene Derivatives to Improve the Stability of Perovskite Solar Cells. J. Semicond. 2021, 42, 120201. [Google Scholar] [CrossRef]
- Xing, Z.; Li, S.-H.; Xie, F.-F.; Xu, P.-Y.; Deng, L.-L.; Zhong, X.; Xie, S.-Y. Mixed Fullerene Electron Transport Layers with Fluorocarbon Chains Assembling on the Surface: A Moisture-Resistant Coverage for Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2020, 12, 35081–35087. [Google Scholar] [CrossRef] [PubMed]
- Troshin, P.A.; Khakina, E.A.; Egginger, M.; Goryachev, A.E.; Troyanov, S.I.; Fuchsbauer, A.; Peregudov, A.S.; Lyubovskaya, R.N.; Razumov, V.F.; Sariciftci, N.S. Self-Assembly of Thiophene- and Furan-Appended Methanofullerenes with Poly(3-Hexylthiophene) in Organic Solar Cells. ChemSusChem 2010, 3, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Mumyatov, A.V.; Goryachev, A.E.; Prudnov, F.A.; Mukhacheva, O.A.; Sagdullina, D.K.; Chernyak, A.V.; Troyanov, S.I.; Troshin, P.A. Monocyclopropanated Fullerene Derivatives with Decreased Electron Affinity as Promising Electron Acceptor Materials for Organic Solar Cells. Synth. Met. 2020, 270, 116565. [Google Scholar] [CrossRef]
- Mumyatov, A.V.; Troshin, P.A. A Review on Fullerene Derivatives with Reduced Electron Affinity as Acceptor Materials for Organic Solar Cells. Energies 2023, 16, 1924. [Google Scholar] [CrossRef]
- Hummelen, J.C.; Knight, B.W.; LePeq, F.; Wudl, F.; Yao, J.; Wilkins, C.L. Preparation and Characterization of Fulleroid and Methanofullerene Derivatives. J. Org. Chem. 1995, 60, 532–538. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, J.; Schuster, D.I.; Wilson, S.R. A Superior Synthesis of [6,6]-Methanofullerenes: The Reaction of Sulfonium Ylides with C60. Tetrahedron Lett. 1995, 36, 6843–6846. [Google Scholar] [CrossRef]
- Wienk, M.M.; Kroon, J.M.; Verhees, W.J.H.; Knol, J.; Hummelen, J.C.; van Hal, P.A.; Janssen, R.A.J. Efficient Methano[70]Fullerene/MDMO-PPV Bulk Heterojunction Photovoltaic Cells. Angew. Chem. Int. Ed. 2003, 42, 3371–3375. [Google Scholar] [CrossRef]
- Troshin, P.A.; Peregudov, A.S.; Peregudova, S.M.; Lyubovskaya, R.N. Highly Regio- and Stereoselective [2+3] Cycloadditions of Azomethine Ylides to [70]Fullerene. Eur. J. Org. Chem. 2007, 2007, 5861–5866. [Google Scholar] [CrossRef]
- Powell, W.H.; Cozzi, F.; Moss, G.P.; Thilgen, C.; Hwu, R.J.-R.; Yerin, A. Nomenclature for the C60-Ih and C70-D5h(6) Fullerenes (IUPAC Recommendations 2002). Pure Appl. Chem. 2002, 74, 629–695. [Google Scholar] [CrossRef]
- Gharibzadeh, S.; Fassl, P.; Hossain, I.M.; Rohrbeck, P.; Frericks, M.; Schmidt, M.; Duong, T.; Khan, M.R.; Abzieher, T.; Nejand, B.A.; et al. Two Birds with One Stone: Dual Grain-Boundary and Interface Passivation Enables >22% Efficient Inverted Methylammonium-Free Perovskite Solar Cells. Energy Environ. Sci. 2021, 14, 5875–5893. [Google Scholar] [CrossRef]
- Domanski, K.; Alharbi, E.A.; Hagfeldt, A.; Grätzel, M.; Tress, W. Systematic Investigation of the Impact of Operation Conditions on the Degradation Behaviour of Perovskite Solar Cells. Nat. Energy 2018, 3, 61–67. [Google Scholar] [CrossRef]
- Emelianov, N.A.; Ozerova, V.V.; Zhidkov, I.S.; Korchagin, D.V.; Shilov, G.V.; Litvinov, A.L.; Kurmaev, E.Z.; Frolova, L.A.; Aldoshin, S.M.; Troshin, P.A. Nanoscale Visualization of Photodegradation Dynamics of MAPbI3 Perovskite Films. J. Phys. Chem. Lett. 2022, 13, 2744–2749. [Google Scholar] [CrossRef] [PubMed]
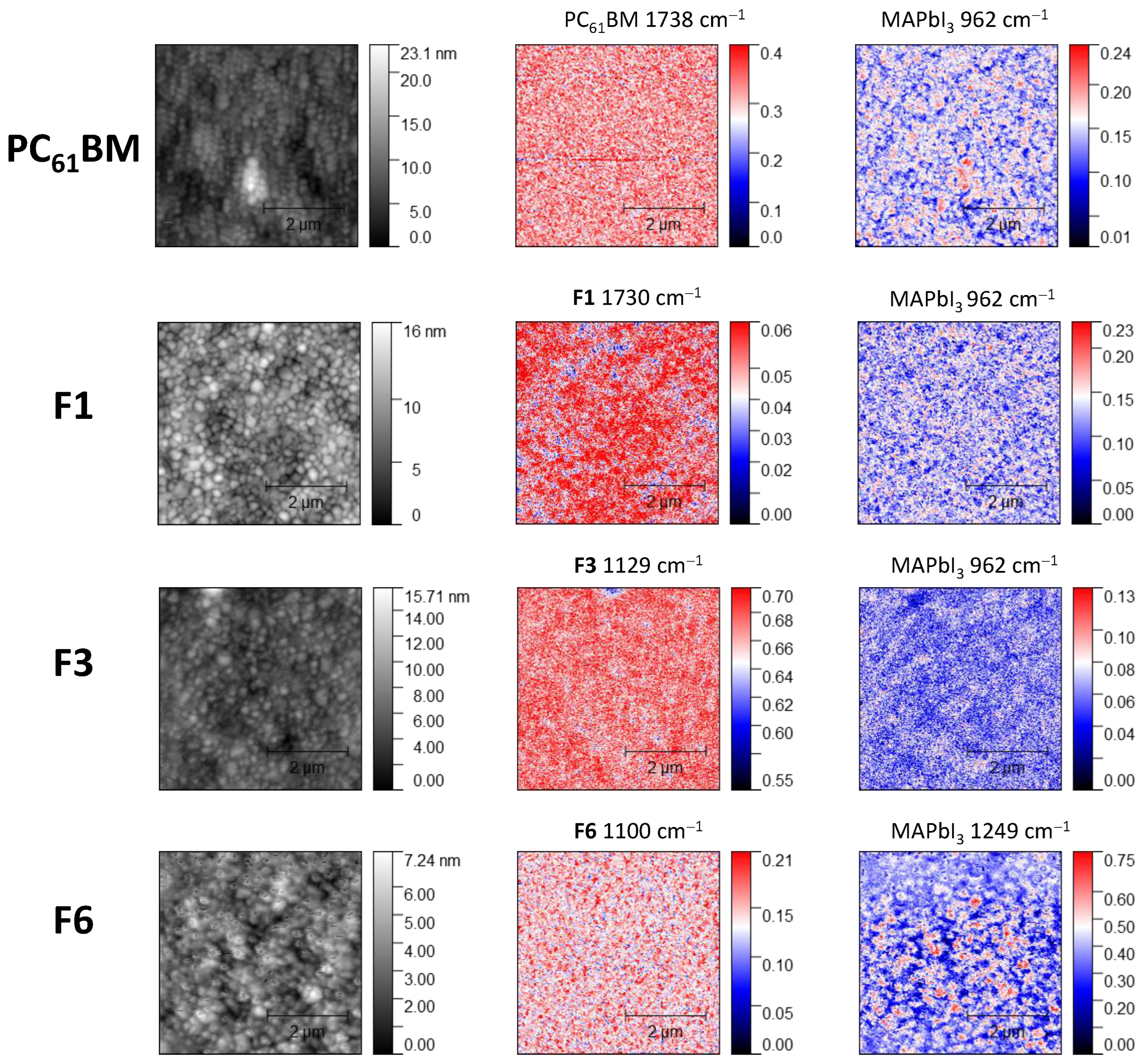

| ETL | ETL Concentration and Deposition Rate | Scan Direction | VOC, mV | JSC, mA cm−2 | FF, % | PCE, % |
|---|---|---|---|---|---|---|
| PC61BM | 30 mg mL−1, 3000 rpm | F | 936 (947 ± 16) | 21.8 (22.1 ± 0.9) | 78.5 (74.5 ± 3.4) | 16.0 (15.6 ± 0.4) |
| R | 942 (957 ± 14) | 21.8 (22.1 ± 0.9) | 79.4 (75.7 ± 2.9) | 16.3 (16.0 ± 0.3) | ||
| F1 | 20.2 mg mL−1, 1500 rpm | F | 930 (930 ± 12) | 23.0 (22.6 ± 0.4) | 77.6 (76.6 ± 1.0) | 16.6 (16.1 ± 0.3) |
| R | 939 (942 ± 7) | 23.2 (22.6 ± 0.4) | 77.8 (77.4 ± 1.0) | 16.9 (16.5 ± 0.2) | ||
| F2 | 16.2 mg mL−1, 1500 rpm | F | 900 (906 ± 18) | 22.6 (22.2 ± 0.7) | 76.2 (75.6 ± 1.2) | 15.5 (15.2 ± 0.3) |
| R | 927 (929 ± 10) | 22.8 (22.2 ± 0.8) | 78.2 (78.0 ± 1.3) | 16.5 (16.1 ± 0.6) | ||
| F3 | 30 mg mL−1, 3000 rpm | F | 922 (947 ± 15) | 23.1 (22.2 ± 0.5) | 72.8 (70.6 ± 2.0) | 15.5 (14.8 ± 0.5) |
| R | 977 (967 ± 9) | 22.1 (22.2 ± 0.4) | 75.9 (73.0 ± 2.2) | 16.4 (15.7 ± 0.6) | ||
| F4 | 30 mg mL−1, 3000 rpm | F | 942 (949 ± 11) | 22.5 (22.2 ± 0.3) | 75.5 (73.9 ± 1.7) | 16.0 (15.6 ± 0.6) |
| R | 958 (962 ± 8) | 22.6 (22.2 ± 0.2) | 75.7 (73.9 ± 1.6) | 16.4 (15.8 ± 0.5) | ||
| F5 | 17.2 mg mL−1, 1500 rpm | F | 952 (940 ± 28) | 22.0 (21.4 ± 0.6) | 66.1 (62.2 ± 4.9) | 13.8 (12.5 ± 1.3) |
| R | 961 (954 ± 30) | 22.2 (21.4 ± 0.6) | 69.5 (67.4 ± 2.5) | 14.8 (13.7 ± 1.0) | ||
| F6 | 30 mg mL−1, 3000 rpm | F | 953 (947 ± 7) | 21.2 (20.0 ± 0.9) | 67.4 (59.3 ± 4.3) | 13.6 (11.3 ± 1.2) |
| R | 957 (953 ± 5) | 21.2 (20.1 ± 0.8) | 69.1 (61.7 ± 4.5) | 14.0 (11.8 ± 1.3) | ||
| F7 | 30 mg mL−1, 3000 rpm | F | 954 (955 ± 6) | 21.2 (21.3 ± 0.2) | 72.9 (70.3 ± 1.7) | 14.7 (14.3 ± 0.3) |
| R | 963 (964 ± 4) | 21.5 (21.4 ± 0.2) | 72.0 (70.2 ± 1.4) | 14.9 (14.5 ± 0.3) | ||
| F8 | 18.8 mg mL−1, 2000 rpm | F | 950 (937 ± 21) | 22.4 (22.3 ± 0.2) | 74.1 (71.8 ± 2.9) | 15.8 (15.0 ± 0.8) |
| R | 972 (958 ± 17) | 22.5 (22.4 ± 0.3) | 74.6 (74.2 ± 0.8) | 16.3 (15.9 ± 0.4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ozerova, V.V.; Mumyatov, A.V.; Goryachev, A.E.; Khakina, E.A.; Peregudov, A.S.; Aldoshin, S.M.; Troshin, P.A. Rational Design of Fullerene Derivatives for Improved Stability of p-i-n Perovskite Solar Cells. Inorganics 2023, 11, 153. https://doi.org/10.3390/inorganics11040153
Ozerova VV, Mumyatov AV, Goryachev AE, Khakina EA, Peregudov AS, Aldoshin SM, Troshin PA. Rational Design of Fullerene Derivatives for Improved Stability of p-i-n Perovskite Solar Cells. Inorganics. 2023; 11(4):153. https://doi.org/10.3390/inorganics11040153
Chicago/Turabian StyleOzerova, Victoria V., Alexander V. Mumyatov, Andrey E. Goryachev, Ekaterina A. Khakina, Alexander S. Peregudov, Sergey M. Aldoshin, and Pavel A. Troshin. 2023. "Rational Design of Fullerene Derivatives for Improved Stability of p-i-n Perovskite Solar Cells" Inorganics 11, no. 4: 153. https://doi.org/10.3390/inorganics11040153
APA StyleOzerova, V. V., Mumyatov, A. V., Goryachev, A. E., Khakina, E. A., Peregudov, A. S., Aldoshin, S. M., & Troshin, P. A. (2023). Rational Design of Fullerene Derivatives for Improved Stability of p-i-n Perovskite Solar Cells. Inorganics, 11(4), 153. https://doi.org/10.3390/inorganics11040153