Synthesis, Characterization, and Antitumor Mechanism Investigation of Ruthenium(II)/Rhenium(I)-Daminozide Conjugates
Abstract
1. Introduction
2. Results and Discussion
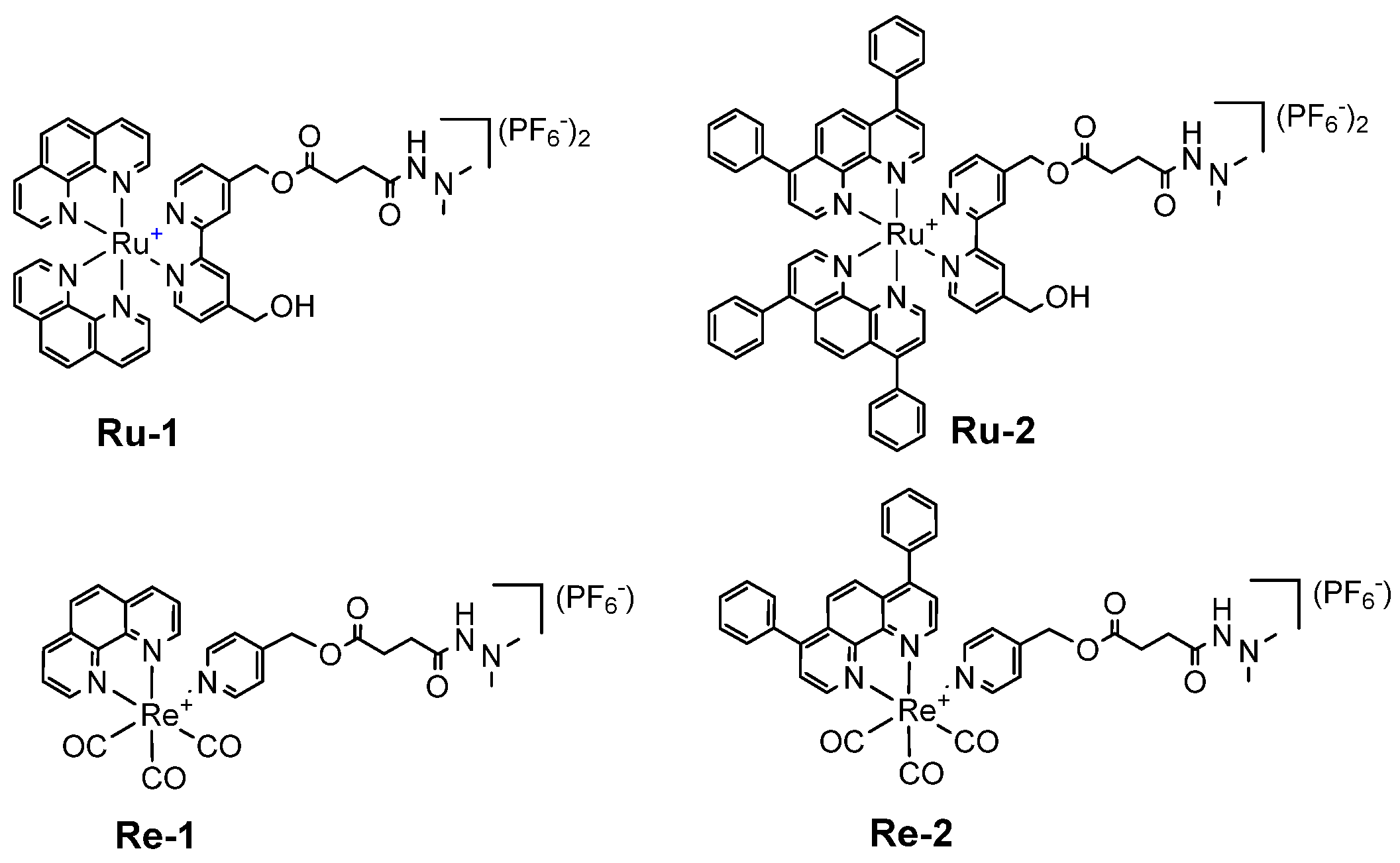
2.1. Synthesis, Photophysical Characterization
2.2. Lipophilicity and In Vitro Cytotoxicity
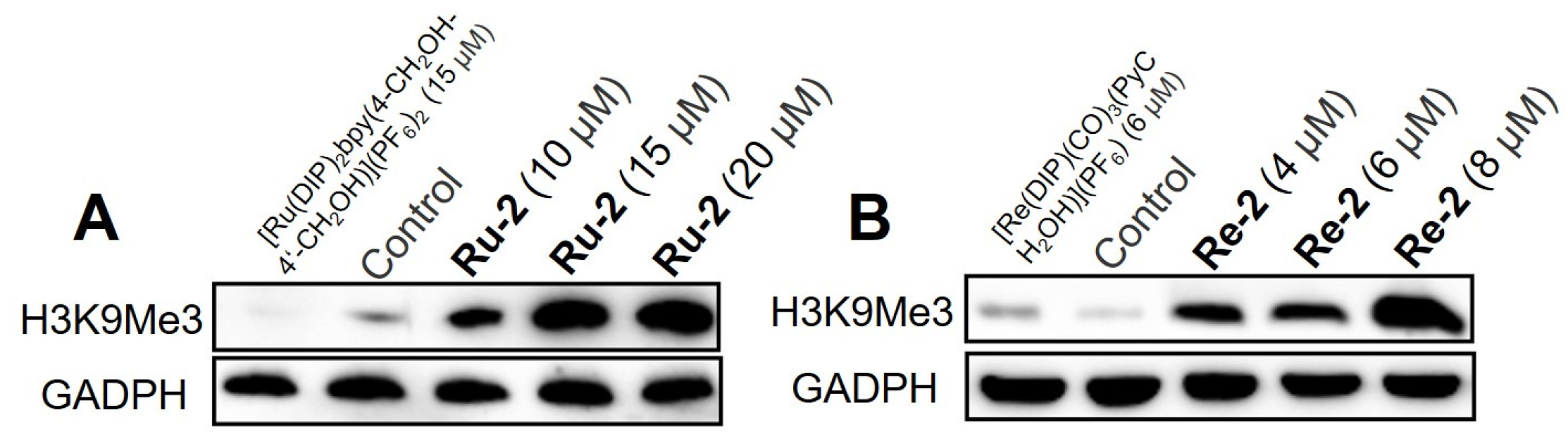
2.3. Upregulation of the Histone-Methylation Level
2.4. Cellular Localization and Uptake Mechanisms
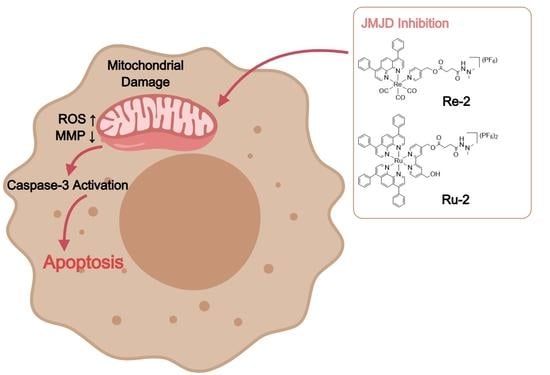
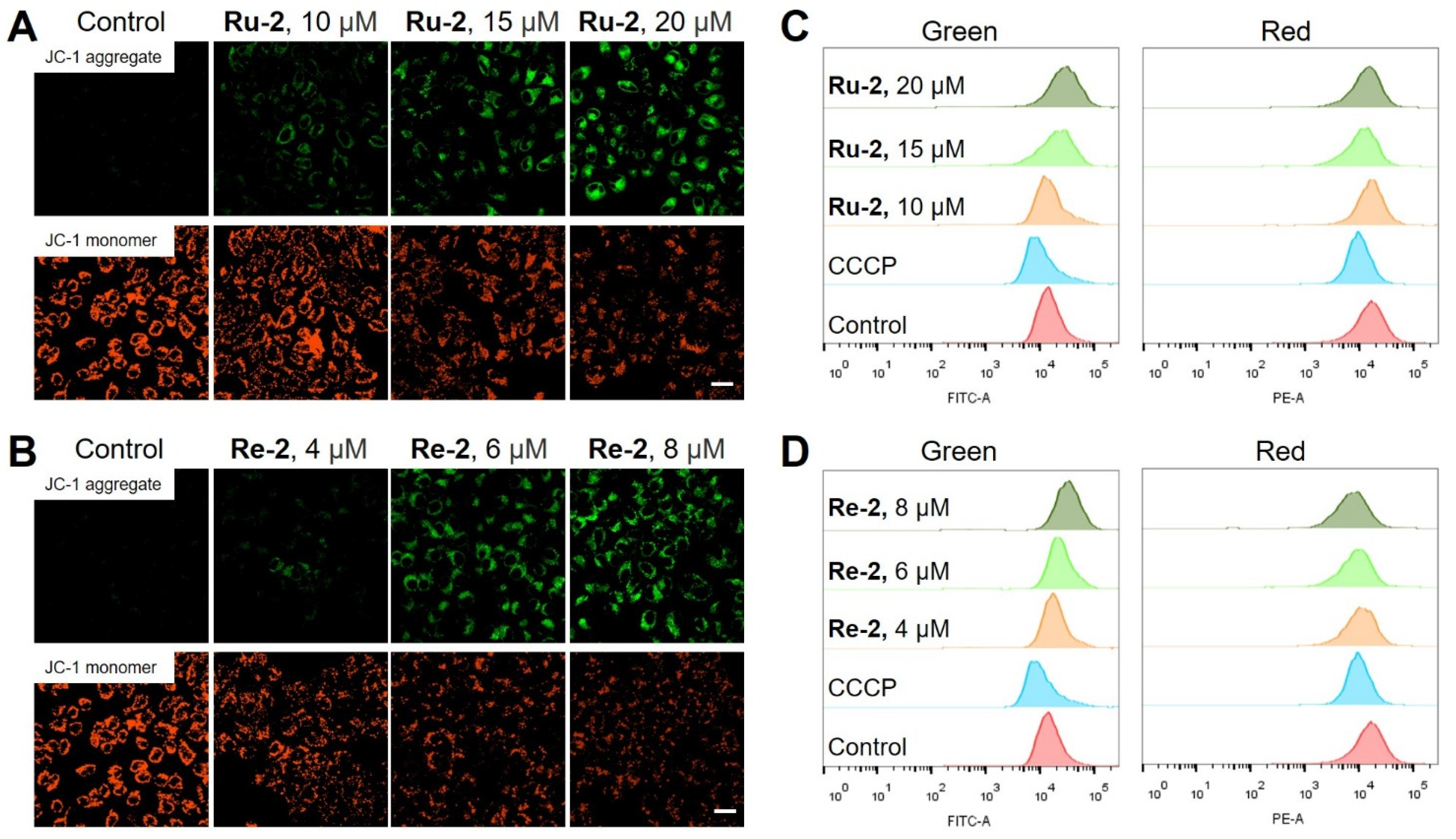
2.5. Mitochondrial Damage
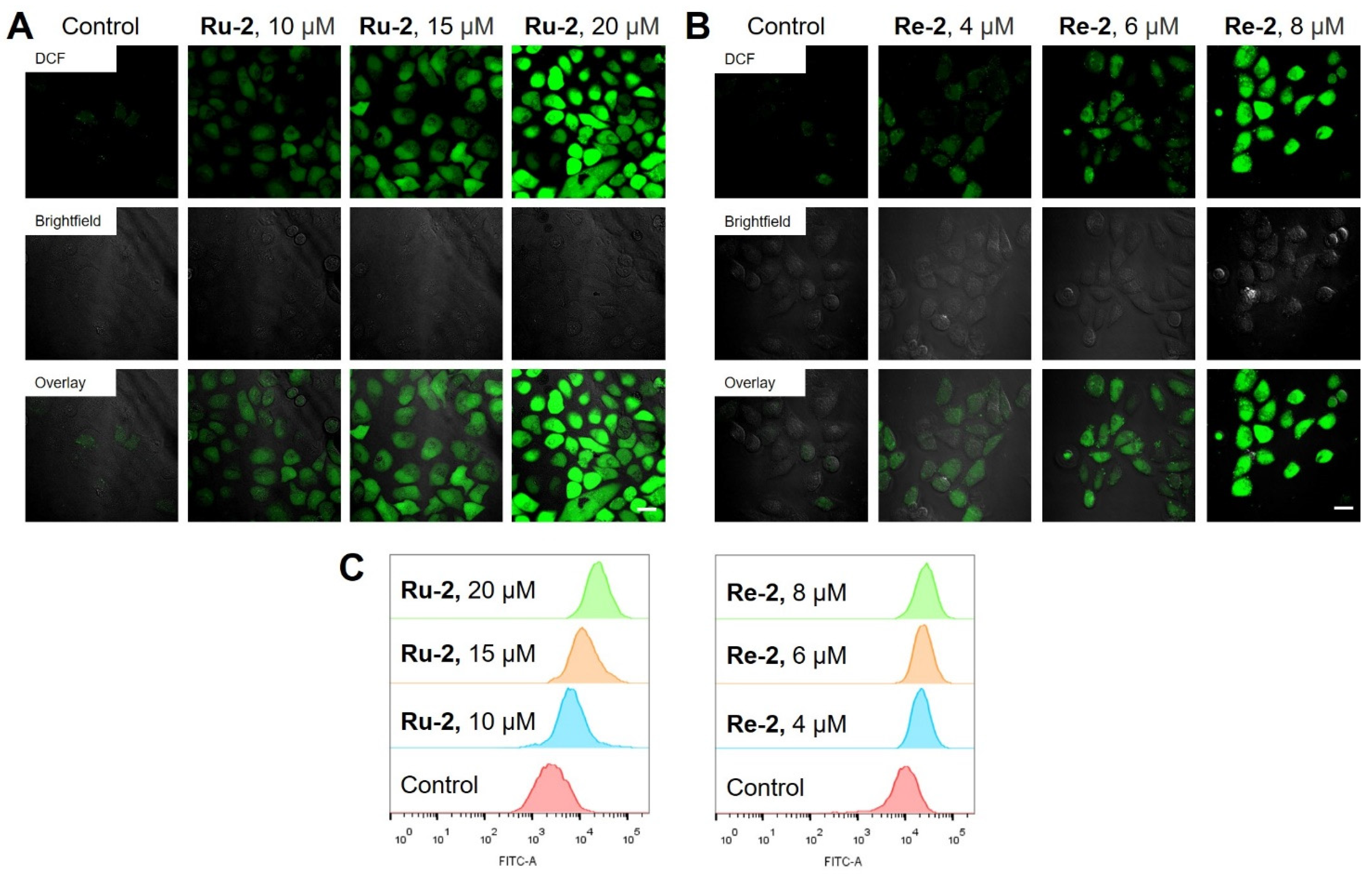
2.6. Elevation of Intracellular ROS Levels
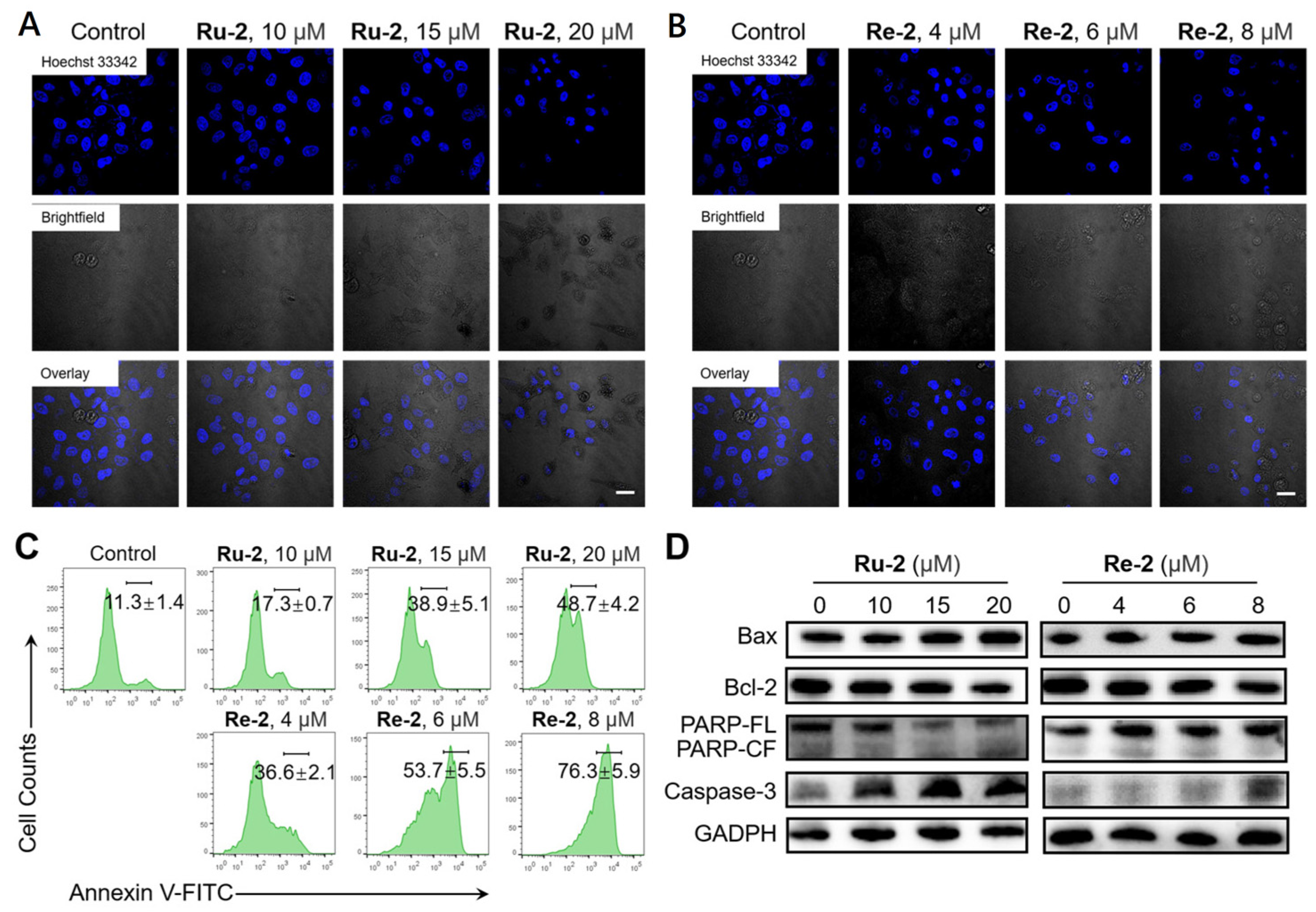
2.7. Induction of Apoptosis
2.8. Inhibition of Cell Migration and Colony Formation
3. Materials and Methods
3.1. Materials and Instruments
3.2. Preparation of Ruthenium(II) and Rhenium(I) Complexes
3.3. Cell Lines and Culture Conditions
3.4. In Vitro Cytotoxicity Assay
3.5. Cellular Localization Assay
3.6. Measurement of MMP
3.7. Measurement of Intracellular ROS
3.8. Hoechst 33342 Staining
3.9. Annexin V Staining
3.10. Wound Healing Assay
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ludwig, H.; Novis Durie, S.; Meckl, A.; Hinke, A.; Durie, B. Multiple Myeloma Incidence and Mortality around the Globe; Interrelations between Health Access and Quality, Economic Resources, and Patient Empowerment. Oncologist 2020, 25, e1406–e1413. [Google Scholar] [CrossRef] [PubMed]
- Rashedi, A.S.; De Roo, S.F.; Ataman, L.M.; Edmonds, M.E.; Silva, A.A.; Scarella, A.; Horbaczewska, A.; Anazodo, A.; Arvas, A.; Ramalho de Carvalho, B. Survey of Fertility Preservation Options Available to Patients with Cancer around the Globe. JCO Glob. Oncol. 2020, 6, 331–344. [Google Scholar] [CrossRef] [PubMed]
- Dilruba, S.; Kalayda, G.V. Platinum-Based Drugs: Past, Present and Future. Cancer Chemother. Pharmacol. 2016, 77, 1103–1124. [Google Scholar] [CrossRef]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The Side Effects of Platinum-Based Chemotherapy Drugs: A Review for Chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Yin, J.-Y.; Li, X.; Li, X.-P.; Xiao, L.; Zheng, W.; Chen, J.; Mao, C.-X.; Fang, C.; Cui, J.-J.; Guo, C.-X. Prediction Models for Platinum-Based Chemotherapy Response and Toxicity in Advanced NSCLC Patients. Cancer Lett. 2016, 377, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Leonidova, A.; Gasser, G. Underestimated Potential of Organometallic Rhenium Complexes as Anticancer Agents. ACS Chem. Biol. 2014, 9, 2180–2193. [Google Scholar] [CrossRef] [PubMed]
- He, S.-F.; Liao, J.-X.; Huang, M.-Y.; Zhang, Y.-Q.; Zou, Y.-M.; Wu, C.-L.; Lin, W.-Y.; Chen, J.-X.; Sun, J. Rhenium–Guanidine Complex as Photosensitizer: Trigger HeLa Cell Apoptosis through Death Receptor-Mediated, Mitochondria-Mediated, and Cell Cycle Arrest Pathways. Metallomics 2022, 14, mfac008. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Pan, Z.-Y.; Qin, W.-W.; Li, Y.; Tan, C.-P.; Mao, Z.-W. Impairment of the Autophagy-Related Lysosomal Degradation Pathway by an Anticancer Rhenium(I) Complex. Dalton Trans. 2019, 48, 4398–4404. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.C.-C.; Lo, K.K.-W. Strategic Design of Luminescent Rhenium(I), Ruthenium(II), and Iridium(III) Complexes as Activity-Based Probes for Bioimaging and Biosensing. Chem. Asian J. 2022, 17, e202200840. [Google Scholar] [CrossRef]
- Olelewe, C.; Awuah, S.G. Mitochondria as a Target of Third Row Transition Metal-Based Anticancer Complexes. Curr. Opin. Chem. Biol. 2023, 72, 102235. [Google Scholar] [CrossRef]
- Ye, R.-R.; Peng, W.; Chen, B.-C.; Jiang, N.; Chen, X.-Q.; Mao, Z.-W.; Li, R.-T. Mitochondria-Targeted Artesunate Conjugated Cyclometalated Iridium(III) Complexes as Potent Anti-HepG2 Hepatocellular Carcinoma Agents. Metallomics 2020, 12, 1131–1141. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.K.-W.; Zhang, K.Y. Iridium(III) Complexes as Therapeutic and Bioimaging Reagents for Cellular Applications. RSC Adv. 2012, 2, 12069–12083. [Google Scholar] [CrossRef]
- Liu, Z.; Romero-Canelón, I.; Habtemariam, A.; Clarkson, G.J.; Sadler, P.J. Potent Half-Sandwich Iridium(III) Anticancer Complexes Containing C∧N-Chelated and Pyridine Ligands. Organometallics 2014, 33, 5324–5333. [Google Scholar] [CrossRef] [PubMed]
- Hearn, J.M.; Romero-Canelón, I.; Qamar, B.; Liu, Z.; Hands-Portman, I.; Sadler, P.J. Organometallic Iridium(III) Anticancer Complexes with New Mechanisms of Action: NCI-60 Screening, Mitochondrial Targeting, and Apoptosis. ACS Chem. Biol. 2013, 8, 1335–1343. [Google Scholar] [CrossRef]
- Lu, Y.; Ma, X.; Chang, X.; Liang, Z.; Lv, L.; Shan, M.; Lu, Q.; Wen, Z.; Gust, R.; Liu, W. Recent Development of Gold(I) and Gold(III) Complexes as Therapeutic Agents for Cancer Diseases. Chem. Soc. Rev. 2022, 51, 5518–5556. [Google Scholar] [CrossRef]
- Yang, Z.; Bian, M.; Lv, L.; Chang, X.; Wen, Z.; Li, F.; Lu, Y.; Liu, W. Tumor-Targeting NHC−Au(I) Complex Induces Immunogenic Cell Death in Hepatocellular Carcinoma. J. Med. Chem. 2023, 66, 3934–3952. [Google Scholar] [CrossRef]
- Lee, L.C.-C.; Leung, K.-K.; Lo, K.K.-W. Recent Development of Luminescent Rhenium(I) Tricarbonyl Polypyridine Complexes as Cellular Imaging Reagents, Anticancer Drugs, and Antibacterial Agents. Dalton Trans. 2017, 46, 16357–16380. [Google Scholar] [CrossRef]
- Wähler, K.; Ludewig, A.; Szabo, P.; Harms, K.; Meggers, E. Rhenium Complexes with Red-Light-Induced Anticancer Activity. Eur. J. Med. Chem. 2014, 2014, 807–811. [Google Scholar] [CrossRef]
- Kastl, A.; Dieckmann, S.; Wähler, K.; Völker, T.; Kastl, L.; Merkel, A.L.; Vultur, A.; Shannan, B.; Harms, K.; Ocker, M. Rhenium Complexes with Visible-Light-Induced Anticancer Activity. ChemMedChem 2013, 8, 924–927. [Google Scholar] [CrossRef]
- Martínez-Lillo, J.; Mastropietro, T.F.; Lappano, R.; Madeo, A.; Alberto, M.E.; Russo, N.; Maggiolini, M.; De Munno, G. Rhenium (IV) Compounds Inducing Apoptosis in Cancer Cells. Chem. Commun. 2011, 47, 5283–5285. [Google Scholar] [CrossRef]
- Cheng, Y.; Qi, Y. Current Progresses in Metal-based Anticancer Complexes as Mammalian TrxR Inhibitors. Anticancer Agents Med. Chem. 2017, 17, 1046–1069. [Google Scholar] [CrossRef]
- Ye, R.; Tan, C.; Chen, B.; Li, R.; Mao, Z. Zinc-Containing Metalloenzymes: Inhibition by Metal-Based Anticancer Agents. Front. Chem. 2020, 8, 402. [Google Scholar] [CrossRef] [PubMed]
- Dörr, M.; Meggers, E. Metal complexes as structural templates for targeting proteins. Curr. Opin. Chem. Biol. 2014, 19, 76–81. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xiong, K.; Wang, L.; Guan, R.; Chen, Y.; Ji, L.; Chao, H. Iridium(III) complexes as mitochondrial topoisomerase inhibitors against cisplatin-resistant cancer cells. Chem. Commun. 2021, 57, 8308–8311. [Google Scholar] [CrossRef]
- Berry, W.L.; Janknecht, R. KDM4/JMJD2 histone demethylases: Epigenetic regulators in cancer cells. Cancer Res. 2013, 73, 2936–2942. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Xu, S.; Zhang, F.; Cui, X.; Fazli, L.; Gleave, M.; Clark, D.J.; Yang, A.; Hussain, A.; Rassool, F.; et al. Histone demethylase JMJD1A promotes expression of DNA repair factors and radio-resistance of prostate cancer cells. Cell Death Dis. 2020, 11, 214. [Google Scholar] [CrossRef]
- Morera, L.; Lubbert, M.; Jung, M. Targeting histone methyl-transferases and demethylases in clinical trials for cancer therapy. Clin. Epigenetics 2016, 8, 57. [Google Scholar] [CrossRef]
- Varier, R.A.; Timmers, H.T. Histone lysine methylation and demethylation pathways in cancer. Biochim. Biophys. Acta 2011, 1815, 75–89. [Google Scholar] [CrossRef]
- Yang, G.J.; Wu, J.; Miao, L.; Zhu, M.H.; Zhou, Q.J.; Lu, X.J.; Lu, J.F.; Leung, C.H.; Ma, D.L.; Chen, J. Pharmacological inhibition of KDM5A for cancer treatment. Eur. J. Med. Chem. 2021, 226, 113855. [Google Scholar] [CrossRef]
- Kaniskan, H.U.; Martini, M.L.; Jin, J. Inhibitors of protein methyltransferases and demethylases. Chem. Rev. 2018, 118, 989–1068. [Google Scholar] [CrossRef]
- Yang, G.-J.; Wang, W.; Mok, S.W.F.; Wu, C.; Law, B.Y.K.; Miao, X.-M.; Wu, K.-J.; Zhong, H.-J.; Wong, C.-Y.; Wong, V.K.W. Selective Inhibition of Lysine-Specific Demethylase 5A (KDM5A) Using a Rhodium(III) Complex for Triple-Negative Breast Cancer Therapy. Angew. Chem. Int. Ed. 2018, 130, 13275–13279. [Google Scholar] [CrossRef]
- Ma, X.; Lu, J.; Yang, P.; Zhang, Z.; Huang, B.; Li, R.; Ye, R. 8-Hydroxyquinoline-Modified Ruthenium(II) Polypyridyl Complexes for JMJD Inhibition and Photodynamic Antitumor Therapy. Dalton Trans. 2022, 51, 13902–13909. [Google Scholar] [CrossRef]
- Lu, J.-J.; Ma, X.-R.; Xie, K.; Chen, M.-R.; Huang, B.; Li, R.-T.; Ye, R.-R. Lysosome-Targeted Cyclometalated Iridium(III) Complexes: JMJD Inhibition, Dual Induction of Apoptosis, and Autophagy. Metallomics 2022, 14, mfac068. [Google Scholar] [CrossRef]
- Rose, N.R.; Woon, E.C.; Tumber, A.; Walport, L.J.; Chowdhury, R.; Li, X.S.; King, O.N.; Lejeune, C.; Ng, S.S.; Krojer, T. Plant Growth Regulator Daminozide Is a Selective Inhibitor of Human KDM2/7 Histone Demethylases. J. Med. Chem. 2012, 55, 6639–6643. [Google Scholar] [CrossRef]
- Chen, B.-C.; Lu, J.-J.; Jiang, N.; Ma, X.-R.; Li, R.-T.; Ye, R.-R. Synthesis, characterization and antitumor mechanism investigation of ruthenium(II) polypyridyl complexes with artesunate moiety. J. Biol. Inorg. Chem. 2021, 26, 909–918. [Google Scholar] [CrossRef]
- Ye, R.-R.; Chen, B.-C.; Lu, J.-J.; Ma, X.-R.; Li, R.-T. Phosphorescent Rhenium(I) Complexes Conjugated with Artesunate: Mitochondrial Targeting and Apoptosis-Ferroptosis Dual Induction. J. Inorg. Biochem. 2021, 223, 111537. [Google Scholar] [CrossRef] [PubMed]
- Smiley, S.T.; Reers, M.; Mottola-Hartshorn, C.; Lin, M.; Chen, A.; Smith, T.W.; Steele, G.D.; Chen, L.B. Intracellular heterogeneity in mitochondrial membrane potentials revealed by a J-aggregate-forming lipophilic cation JC-1. Proc. Natl. Acad. Sci. USA 1991, 88, 3671–3675. [Google Scholar] [CrossRef]
- Sabharwal, S.S.; Schumacker, P.T. Mitochondrial ROS in cancer: Initiators, amplifiers or an Achilles’ heel? Nat. Rev. Cancer 2014, 14, 709–721. [Google Scholar] [CrossRef] [PubMed]
- LeBel, C.P.; Ischiropoulos, H.; Bondy, S.C. Evaluation of the probe 2′,7′-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem. Res. Toxicol. 1992, 5, 227–231. [Google Scholar] [CrossRef]
- Mussunoor, S.; Murray, G.I. The role of annexins in tumour development and progression. J. Pathol. 2008, 216, 131–140. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [PubMed]
- Swanton, E.; Savory, P.; Cosulich, S.; Clarke, P.; Woodman, P. Bcl-2 regulates a caspase-3/caspase-2 apoptotic cascade in cytosolic extracts. Oncogene 1999, 18, 1781–1787. [Google Scholar] [CrossRef] [PubMed]



| Compound | IC50 (μM) | ||||
|---|---|---|---|---|---|
| HeLa | A549 | A549R | HepG2 | LO2 | |
| Ru-1 | >50 | >50 | >50 | >50 | >50 |
| Ru-2 | 5.0 ± 0.3 | 10.1 ± 2.2 | 12.3 ± 0.6 | 14.4 ± 1.6 | 18.2 ± 2.7 |
| Re-1 | >50 | >50 | >50 | >50 | >50 |
| Re-2 | 2.0 ± 0.2 | 3.8 ± 1.1 | 2.6 ± 0.2 | 3.6 ± 0.3 | 7.6 ± 2.1 |
| Cisplatin | 26.1 ± 3.7 | 27.3 ± 6.1 | >50 | 33.4 ± 4.3 | 35.5 ± 5.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, P.-X.; Xie, K.; Chen, M.-R.; Zhang, Z.; Huang, B.; Li, R.-T.; Ye, R.-R. Synthesis, Characterization, and Antitumor Mechanism Investigation of Ruthenium(II)/Rhenium(I)-Daminozide Conjugates. Inorganics 2023, 11, 142. https://doi.org/10.3390/inorganics11040142
Yang P-X, Xie K, Chen M-R, Zhang Z, Huang B, Li R-T, Ye R-R. Synthesis, Characterization, and Antitumor Mechanism Investigation of Ruthenium(II)/Rhenium(I)-Daminozide Conjugates. Inorganics. 2023; 11(4):142. https://doi.org/10.3390/inorganics11040142
Chicago/Turabian StyleYang, Pei-Xin, Kai Xie, Mei-Ru Chen, Zheng Zhang, Bo Huang, Rong-Tao Li, and Rui-Rong Ye. 2023. "Synthesis, Characterization, and Antitumor Mechanism Investigation of Ruthenium(II)/Rhenium(I)-Daminozide Conjugates" Inorganics 11, no. 4: 142. https://doi.org/10.3390/inorganics11040142
APA StyleYang, P.-X., Xie, K., Chen, M.-R., Zhang, Z., Huang, B., Li, R.-T., & Ye, R.-R. (2023). Synthesis, Characterization, and Antitumor Mechanism Investigation of Ruthenium(II)/Rhenium(I)-Daminozide Conjugates. Inorganics, 11(4), 142. https://doi.org/10.3390/inorganics11040142