Abstract
Platinum (Pt) drugs have developed rapidly in clinical applications because of their broad and highly effective antitumor effects. In recent years, with the rapid development of immunotherapy, Pt-based antitumor agents have gained new challenges and opportunities. Since the discovery of their pharmacological effects in immunotherapy and tumor microenvironment regulation, research into Pt drugs has progressed to multi-ligand and multi-functional Pt precursors and their own shortcomings have been further highlighted. With the development of antitumor immunotherapy and the rise of combination therapy, the development of Pt-based drugs has started to move in the direction of multi-targeting, nanocarrier modification, immunotherapy and photodynamic therapy. In this paper, we first overview the recent applications of Pt-based drugs in antitumor inorganic chemistry, with a focus on summarizing the application of Pt-based drugs and their precursors in the anticancer immune response. The paper also provides a reasonable outlook on the future development of Pt-based drugs from the chemical and immunological perspectives, relying on the existing content and problems of Pt-based drug development. On the basis of the gathered information, joint multidisciplinary programs on implementing comprehensive immune analyses for the future development of novel anticancer metal compounds should be initiated.
1. Introduction
Since Rosenberg’s discovery of cisplatin in 1965, cisplatin has quickly reached the peak of its clinical use on account of its potent antitumor properties [1,2]. Generally speaking, Pt(IV) would be reduced into Pt(II) via the reductive microenvironment of tumors. Compared to Pt(IV)-based antitumor drugs, Pt(II)-based ones perform better cytotoxic ability. Cisplatin, for example, has been used extensively in therapies for ovarian, prostate, testicular, lung, nasopharyngeal, esophageal and other cancers through the direct binding of DNA within tumor cells. However, this kind of DNA binding is non-targeted, causing damage to other “healthy” organs and resulting in limitations of its clinical use. Cisplatin has a straightforward structure and a clear mechanism, and it can have some therapeutic effects on many tumor types. However, the substantial side effects (nephrotoxicity, ototoxicity, etc.) and drug resistance brought on by platinum (Pt)-based therapy have progressively come to light, and as the clinical application continues to advance, they have also grown to be a significant barrier to its advancement [3,4,5]. As a result, second- and third-generation Pt-based medications with the active ingredients carboplatin and oxaliplatin have appeared. Compared to cisplatin, oxaliplatin has moderate adverse effects and could be used for patients with hepatic dysfunction. It is commonly used in the treatment of colorectal cancer. Third-generation and second-generation Pt-based medications can treat resistance brought by previous-generation Pt-based therapies and have superior stability and fewer side effects compared to cisplatin [6,7,8]. In summary, the overall development of Pt-based medicines is still constrained by a unique combination of side effects, and the limitations cannot be ignored. [4].
Pt medicines primarily attach to DNA and damage it to have anticancer effects in vivo. For instance, the chloride ion in the structure of the drug cisplatin is activated by hydrolysis as soon as it enters the cell, resulting in the creation of an electrophilic molecule that may covalently attach to the nitrogen atom in the purine residue to damage DNA. Chloride anions improve the reduction potential and ability to receive electrons from some ligands such as DAD [9]. However, the existence of chloride anions could probably improve the cytotoxicity of Pt-based drugs [10]. Pt medications can all stimulate the p53 signaling pathway, caspases and cellular autophagy [11,12]. One of the main factors restricting the therapeutic usage of cisplatin has been its side effects. As a result, in the following years, attention was diverted from the development of Pt(II) to that of its prodrug, Pt(IV) [6]. It had been thought that Pt(IV), which has a gentler and less poisonous structural profile than Pt(II), would serve as the foundation for the creation of new Pt-based medications that might successfully replace Pt(II). Theoretically, reducing biomolecules including glutathione (GSH) and ascorbic acid (ASA) are thought to participate in intracellular reduction processes of Pt(IV) prodrugs to liberate the Pt(IV) parent drug and exert anticancer activity. The slower rate of DNA binding to Pt(IV) compared to Pt(II) is the main reason for the lower in vitro toxicity of Pt(IV) [6,13,14]. The reduction efficiency of Pt(IV) is considered to be a key step in the activation of Pt(IV) complexes during its antitumor activity in vivo.
The development of photoactive Pt-based drugs, immunotherapies and nanomaterials, among other things, as existing Pt-based medications which can reduce with time has received extensive interest [15,16,17]. In order to lessen side effects, the continual refinement of functional Pt-based drugs is primarily driven by 1. Reducing adverse responses and improving the selectivity of Pt-based medications 2 can boost effectiveness and synergism and mobilize immunity.
This article focuses on the advancement of Pt-based medications in recent years, listing how these medications have been used to treat malignancies through structural modifications in chemotherapy, photochemotherapy and other treatments. Due to the discovery of the actions of Pt medications on immune cells, including macrophages, their impacts and applications in tumor immunological processes are studied. Nanomaterials, metals, agonists and antagonists are employed in combination with other fields in order to lessen their own hazardous side effects and increase antitumor effectiveness. At the same time, the current advancement of pharmaceuticals based on Pt is coupled with other fields, such as drug design, to offer fresh viewpoints on emerging patterns. Overall, this paper summarizes the development of various Pt-based drugs in recent years, in order to lay some good prerequisites for the research and development of novel Pt-based drugs (Figure 1).
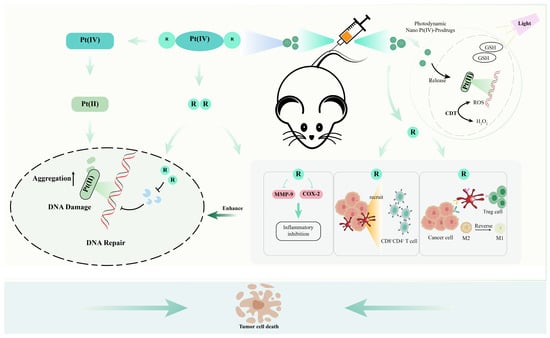
Figure 1.
Multiple pathways to increase the antitumor effect of Pt-based drugs. The breakdown of nanomaterials or Pt prodrugs to yield Pt(II) drugs, which damage DNA, increase drug aggregation in cells or reduce DNA repair activity, can enhance tumor inhibition or reduce drug resistance. PTT/PDT can facilitate the targeted release of Pt-based drugs to enhance antitumor efficiency or reduce toxic side effects. In addition to their adjuvant role, axial ligands of Pt drugs can be involved in the immune response in vivo. They enhance the antitumor effects of Pt-based drugs by reversing macrophage polarization, recruiting immune cells and inhibiting tumor-associated inflammatory responses.
2. Multiple Pathways to Enhance Targeting Properties of Pt-Based Antitumor Drugs
2.1. Improving Drug Targeting by Structural Modification of Pt(II) and Pt(IV) Prodrugs
In order to make Pt-based drugs more selective in their action on tumor cells, and at the same time reduce the impact of the adverse effects brought about by the treatment process, many researchers have started to target Pt(II) and Pt(IV) structures by linking them to different groups.
One of the primary methods for creating antitumor medications is to structurally alter Pt-based medicines to match the properties of tumor cells in order to achieve antitumor goals. Solid tumors are characterized by severe hypoxia that is primarily brought on by the hormone hypoxia-inducible factor-1 (HIF-1). Zichen Xu’s team [18] used HIF-1 as their target and created a variety of structures that target Pt(IV) alterations. They discovered that YCC-2 (1) (Figure 2) greatly reduced HIF-1 expression and improved the effects of cisplatin on HCT-116. Similarly, asparagine synthase plays an important role in tumor growth and metastasis, and as a molecular target, Di Hu‘s group [19] designed complex 2, [(bis-NHC)Pt(bt)]PF6 1α (Figure 2), which reduces cellular asparagine levels and effectively inhibits tumor cell proliferation, and a complex that modifies the resistance of cancer cells to cisplatin. Furthermore, heat shock protein 70+ can serve as a recognition site for targeted therapeutics and is closely linked to tumor aggressiveness and therapeutic resistance [20]. Couples 1–5 (Figure 2), designed by A.M. McKeon’s group, can target membrane-bound heat shock protein 70+ in cancer cells to increase cytotoxicity [21].
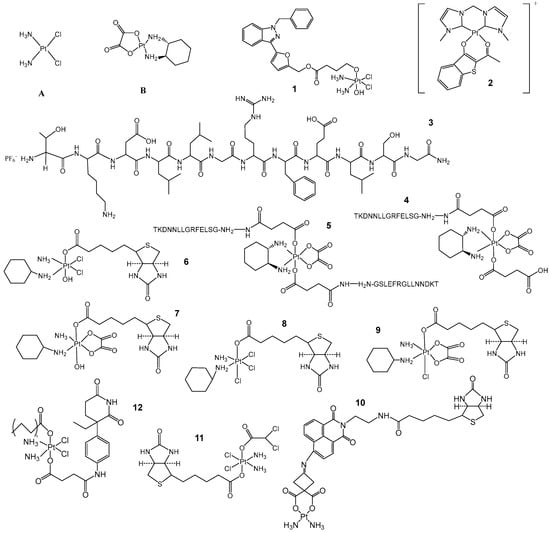
Figure 2.
Improving drug targeting properties by structural modification of Pt(II) and Pt(IV) prodrugs. (A) Structure of cisplatin. (B) Structure of oxaliplatin. (1) YCC-2. (2) Complex [(bis-NHC)Pt(bt)]PF6 1α (3, 4, 5) compounds designed by A.M. Mckeon’s group to target membrane surface heat shock protein 70+. (6, 7, 8, 9) Pt(IV) complexes designed by Gao’s group with biotin as the axial group. (10) Biotin-modified Pt(IV) complex designed by Chen’s group. (11) DPB structure. (12) Dual-targeted Pt(IV) complexes designed by Liu‘s group.
The addition of biotin to the structure can lead to more precise targeting of Pt-based drugs to cancer cells after structural modifications to increase accumulation in the cells. In 2020, researchers developed four Pt(IV) complexes, 6, 7, 8 and 9 (Figure 2), with biotin as the axial group, which were found to have a multiplicative increase in cytotoxicity compared to cisplatin in vitro, as well as a reversal of cisplatin resistance [22]. Xing Wang’s group [23] also synthesized Pt(II) complex 10 (Figure 2) to target Pt-based drugs with biotin modifications, which enhanced the antitumor activity. In addition to the increased targeting of biotin, Suxing Jin’s group [24] designed and synthesized a new Pt(IV) complex 11, DPB (Figure 2), which introduced dichloroacetic acid to enhance lipophilicity and cytotoxicity and at the same time impeded the growth of cancer cells with active glycolysis, demonstrating the importance of dual targeting for anticancer effects. The importance of dual targeting for anticancer effects was strongly demonstrated.
Not coincidentally, Xiaomeng Liu’s group [25] also relied on dual targeting to design a Pt(IV) prodrug 12 (Figure 2), which was found to enhance intracellular aggregation, thus significantly inducing DNA damage, inhibiting tumor cell migration and effectively suppressing the nephrotoxicity associated with Pt-based drugs. This also improves the strategy for the treatment of advanced postmenopausal breast cancer.
2.2. Nanoparticulate Pt-Based drug Delivery System to Upgrade Intracellular Accumulation
Although great progress in nanoparticle-based drug deliveries has been achieved in the past few decades, the toxicity and limitations should not be ignored. In nanoparticle-based drug carriers, liposomes are characterized by self-assembling, and drugs are assembled to be liposome nanoparticles (LNPs). However, liposomes tend to accumulate in the liver, spleen and bone marrow, as well as the mononuclear phagocytic system (MPS) in the human body. Liposomes accumulated in MPS could probably result in serious side effects and toxicity. To address these limitations, improving targeting ability and eliminating influences on other organisms would be the key. Louzhen Fan et al. [26] summarized the perspectives of nanomaterials such as liposomes, proteins and carbon quantum dots as carriers and illustrated the progress of nanoparticle-based drugs in cancer medications.
Based on the above, mesoporous silica nanoparticles incorporating Pt(IV) predrugs were prepared by Zigui Wang et al. [27]. Taking advantage of the liver-targeting properties of lactobionic acid (LA), the nanocarrier enhanced the circulation time while increasing the aggregation effect of the drug in hepatic tumor cells, and as it was in a reducing environment, the bound Pt(IV) could be rapidly reduced for its effect. Li Li’s group [28] combined an oxaliplatin prodrug with polyethylene glycol-modified nanosomes and found an enhanced tumor-targeting effect. Because of specific binding to epidermal growth factor receptor (EGFR) nanosomes, accumulation was found to be more pronounced in tumors than in normal cells. In addition, hydrophilic materials and peptide fragments and fluorescent dyes were used as nanocarriers for carboplatin (CRGD), and the complexes were found to be more cytotoxic than carboplatin and to have an appreciable targeting uptake capacity [29].
The combination of targeted therapy and chemotherapy is the main tool used to increase the induction of tumor cell death. It has been clinically found that panitumumab and Pt-based drugs have difficulty accumulating into high concentrations at the tumor site both alone and in combination, which is the main reason for the lack of efficacy. The investigators found that NanoPt-PAN (Figure 3), a nanomedicine made by combining the two agents, was able to improve this phenomenon and was more actively targeted than the single agent [30]. In addition, the authors found that NanoPt-PAN also had good anti-CRC effects, making it a candidate nanomedicine for the treatment of colorectal cancer. Also making nanopolymers out of existing combination regimens is Jianfeng Guo’s group [31]: as FNA, 5-FU and oxaliplatin each have low efficacy, high toxicity and long treatment cycles, nanoprecipitation technology was used to design NanoFOLOX, which was found to improve the adverse effects of the three drugs, facilitating blood circulation and the aggregation of Pt-based drugs in tumor cells. The new nanostructures were found to improve the adverse effects of the three drugs and enhance blood circulation and the aggregation of Pt-based drugs within the tumor cells. Furthermore, the authors combined the nanoparticles with a variety of other drugs and found that FOLOX nanoparticles have great potential as the basis for combination therapeutic strategies in the treatment of CRC. Nanoparticles are ideal vehicles for multiple combination strategies due to their multiple drug-carrying capabilities. A novel nanogel system was made by combining CRGDFK-modified nanogels with the sodium channel inhibitors lidocaine and cisplatin, and it was found that the adverse effects produced by the system were alleviated by the targeted release of cisplatin. Due to the introduction of high-affinity peptide fragments, the nanogel showed a significant increase in enrichment at the tumor site, which was more beneficial to the inhibition of primary tumor growth [32].
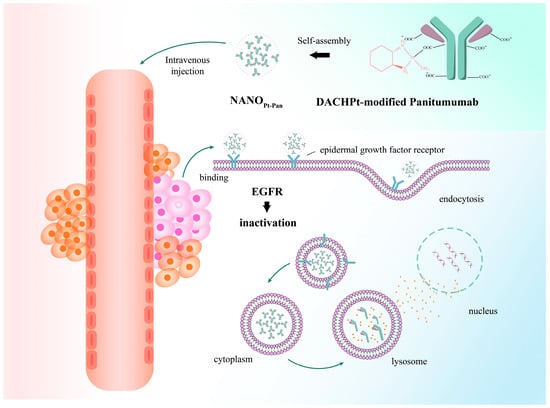
Figure 3.
Scheme of nanoparticulate Pt-based drug delivery system to upgrade intracellular accumulation. Nanoparticles prevent drug degradation and reduce toxic side effects by delivering the right dose of the drug at the right time and to the right target. Due to the specificity of nanoparticles, researchers are focusing on combining them with Pt-based drugs in order to achieve targeted delivery and increase the accumulation of drugs in tumor cells to achieve the desired efficacy. Reprinted with permission from Ref. [30].
In the case of brain tumors, such as glioma, the fundamental limitation is the presence of a blood–brain barrier in the brain, which prevents normal drug entry into brain tumor cells and inhibits therapeutic efficacy [33]. Tao Sun’s group [34] has developed a novel nanocarrier that has been found to have enhanced targeting and the ability to cross the blood–brain barrier both in vitro and in vivo. The combined studies suggest that this therapeutic strategy could be a new dosage form for Pt-based drugs, particularly for the treatment of clinical gliomas.
In previous studies, it was shown that nanocarriers with tumor acidic activation sites and conversion capabilities have important potential in targeted drug delivery, exhibiting neutral or negative charges in circulation to prolong circulation and promoting cellular internalization for targeted drug delivery. Due to the slow charge reversal at the surface of tumor tissue, Liu et al. [35] prepared UCC-NP/Pt nanocarriers, which can undergo rapid charge conversion at tumor acidity to achieve targeted drug delivery and significant anticancer effects in cisplatin-resistant cells.
In general, the structural modification of existing Pt-based drugs and the use of nanomaterials as drug carriers are promising. The goal is to improve the targeting of the drug and increase the degree of aggregation in tumor cells, thereby providing better antitumor effects or reducing the adverse effects and resistance of Pt-based drugs in clinical use.
3. Synergistic Involvement of Pt-Based Drugs in Immunotherapy
The immune system has a role in recognizing and killing tumor cells in antitumor therapy (Figure 4) [36]. Similarly, tumor cells can inhibit the action of immune cells, for example by releasing immunosuppressive factors, or avoid the effect of immune cells by avoiding or weakening their recognition [37]. With the rise of therapeutic approaches targeting immune checkpoints [38], the previous belief that resistance to chemotherapy drugs was due to suppression or disruption of immune system function needs to be re-evaluated. In response to the problems that have arisen in the clinical management of conventional Pt-based drugs, it is hoped that a new range of antitumor Pt-based drugs can be developed and designed to target the immune system. These Pt-based drugs could inhibit the immune escape that occurs during conventional drug therapy, slowing tumor growth and reducing the development of drug resistance. The combination of chemotherapy and immunotherapy will be a promising strategy in the design of this class of drugs.
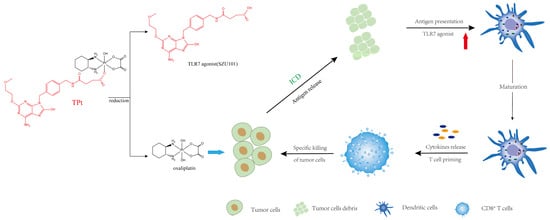
Figure 4.
Scheme of combinations with immune agonists to improve T-cell responses. Immune agonists, such as a TLR7 agonist, conjugated with oxaliplatin, can accelerate T-cell maturation, activating prime T cells into mature T cells. Mature T cells release cytokines and exert specific killing effects on tumor cells. Antigens, released by tumor cells going through immunogenic cell death, will perform a synergistic effect with immune agonists to improve T-cell responses to tumor cells. Reprinted with permission from Ref. [36].
3.1. Recruiting Immune Cells to Enhance Immunotherapy Effects
Tryptophan 2,3-dioxygenase (TDO) is an immunosuppressive enzyme that may be involved in the immune escape of tumor cells and the potential for tolerance in vivo [39,40]. Therefore, a Pt(IV) antitumor drug containing a TDO inhibitor was designed to reverse tumor immunosuppression [41]. Flow cytometry suggested that this complex 13 (Figure 5) could induce cell inactivation via the mitochondria-dependent apoptotic pathway and inhibit the TDO enzyme to block the kynurenine pathway downstream, thereby enhancing the immune response of T cells.
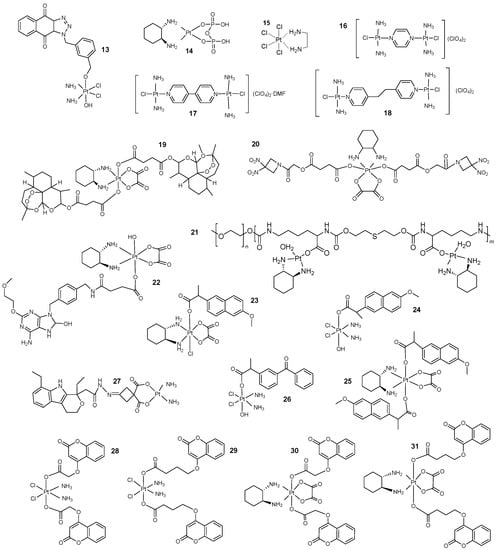
Figure 5.
Synergistic involvement of Pt-based drugs in immunotherapy. (13) Pt(IV) complexes containing TDO inhibitors. (14) PT-112. (15, 16, 17, 18) Four Pt complexes designed by Milos’s group. (19) The structure of OPA. (20) OXA-NO-enhanced immunotherapy designed by Liu’s group. (21) ROS-response micelles PKS. (22) Novel immunochemotherapeutic agents for the combination of TLR7 agonists with oxaliplatin. (23) Naproxen Pt(IV) complexes designed by Han’s group. (24, 25) DNP and NP structures. (26) Ketoprofen Pt(IV) complex. (27) IA-1 structure. (28, 29, 30, 31) A family of coumarin derivatives designed by Wang et al.
Analysis by Takahiro Yamazaki et al. [42] for complex 14, PT-112 (Figure 5), a novel Pt-based coupling, revealed that the cytotoxic response to PT-112 was associated with exposure of calreticulin on the surface of dying cells, secretion of ATP and HMGB1 and other danger signals that also promoted anticancer immunity. In addition, the authors also found that when combined with an immune checkpoint inhibitor, PT-112 controlled cancer in mice through the systemic immune function of recruiting immunoreactive cells in TME while exerting cytotoxicity, limiting the growth of distant lesions.
Milos’s team [43] developed four newly designed Pt-based complexes, 15, 16, 17 and 18 (Figure 5), and compared their antitumor effects with cisplatin in vivo and in vitro. The results suggest that [PtCl4(en)] (en = ethylenediamine) increased the number of CD45+ cells and led to a reduction in metastatic lung lesions in tumor-bearing mice, also highlighting the potential of this structure in antitumor action.
3.2. A Promising Combination of ROS and Macrophages to Direct Polarization Strategy
Tumor-associated macrophages (TAMs) account for the largest proportion of the tumor immune microenvironment (TME) and play a key role in tumorigenesis and progression [44]. Evidence suggests that tumor-associated macrophages accumulate in tumors and lead to drug resistance, whereas cisplatin inhibits the clearance of EGF by tumor-associated macrophages during antitumor therapy, thereby inhibiting tumor progression or recurrence [45]. Andrulis’s group [46] studied the treatment of the cisplatin analog “poly-plat”, SSP and SAP and found that macrophages treated with “poly-plat” and SSP showed cytoplasmic elongation after stimulation.
M1 macrophages exert their antitumor function through directly mediated cytotoxicity and antibody-dependent cell-mediated cytotoxicity (ADCC) [47]. Cisplatin-loaded umbilical cord-derived exosomes were designed by Xiaohui Zhang’s group and found to be several times more toxic and cell-sensitive in drug-resistant cells when compared to chemotherapy alone [48]. Tao Yang et al. [49] found that the Pt-based complex OPA 19 (Figure 5) exerts chemoimmunotherapeutic effects on tumors mainly by blocking DNA replication and inhibiting trigger receptors expressed on myeloid cells 2 (TREM2), while also promoting the polarization of macrophages from M2 to M1. It also stimulates dendritic cells, cytotoxic T cells and natural killer cells to act on cancer cells. In addition, OPA alters the tumor microenvironment to reverse resistance to Pt-based drugs.
Similarly, Zhuang Liu’s group designed an epigenetic Pt(IV) complex 20 (Figure 5) to enhance cancer chemoimmunotherapy [50]. This structure also promotes the polarization of macrophages from the M2 to M1 phenotype, thereby reversing the immune microenvironment and reducing immunosuppression, demonstrating better tolerance and inhibition of tumor growth compared to traditional Pt-based drugs. Of course, the investigators found that it is not feasible to rely on immunotherapy alone to inhibit tumor progression and that it needs to be combined with other therapies to maximize the effect of immunotherapy. Therefore, Chun-Liang Lo’s group [51] designed an ROS-responsive micelle 21 (Figure 5) based on the restricted regulation of ROS levels in tumor tissue and the importance of ROS in the tumor microenvironment and in the cancer treatment process. It can release Pt-based drugs into the cytoplasm of macrophages and cancer cells, increasing the level of ROS in the tumor and inducing the polarization process towards M1-type macrophages and phagocytosis of cancer cells by macrophages, achieving the dual effect of complementary chemotherapy and immunotherapy.
3.3. Combination with Immune Agonists to Improve T-Cell Responses
Myeloid-derived suppressor cells (MDSCs) play a major coordinating role in cancer-associated inflammation, dynamically promoting a differentially polarized inflammatory program in tumor progression that facilitates tumor development and resistance to therapy [52]. As mentioned above, Tao Yang’s group found that OPA could alter the tumor microenvironment to reverse drug resistance while inhibiting the expression of TREM2, thus resulting in reduced antitumor responses in mice, including less immunosuppressive macrophages, more secretion of immunostimulatory molecules and improved T-cell responses [49].
The synergistic effect of Pt-based drugs and immunotherapy was even more effective in drug-resistant cancer models, and Zhigang Wang’s group [53] designed a novel immunotherapeutic agent 22 (Figure 5) by binding TLR7 agonists to oxaliplatin prodrugs, which induced immunogenic death of 4T1 cells and activated dendritic cells to secrete proinflammatory factors such as IFN-γ, TNF-α, IL-6 and IL-12. The mechanism suggests that intratumor cytotoxic T cells are activated, thereby increasing antitumor efficiency.
3.4. Pt-Based Drugs Combined with NSAIDs to Repress Tumor-Related Inflammation
Inflammation is an important feature of cancer progression and is associated with the development, progression and metastasis of malignancies; therefore, the inhibition of the associated inflammatory response plays an important role in antineoplastic chemotherapy [54,55]. The introduction of NSAIDs into Pt-based regimens has been shown to significantly improve the efficacy of NSAIDs compared to cancer treatment alone [56,57,58]. Han’s group [59] used naproxen in combination with Pt(IV) and found that compound 23 (Figure 5) had better antitumor properties, with a Pt-based fragment in its structure causing DNA damage and naproxen as a non-steroidal anti-inflammatory agent inhibiting COX-2 and reducing the associated inflammatory response, while both significantly inhibited MMP-9 activity in tumors, thereby helping to reduce the growth and metastasis of aggressive tumors. Therefore, in line with the trend towards the development of nanodrug delivery systems in oncology therapy, Linming Li et al. [60] developed new bovine serum protein nanoparticles based on naproxen Pt-based complexes, which were found to have better antitumor effects and lower toxic side effects than the free compounds. In addition, the addition of nanoparticles reduced tumor inflammation targeting COX-2, MMP-9 and iNOS, which was more beneficial to the antitumor capacity and provided a new clinical approach to overcome the shortcomings of Pt-based drugs. In addition, Wang’s group [61] prepared two Pt-based complexes, DNP 24 (Figure 5) and NP 25 (Figure 5), with naproxen as the axial phase ligand and found that DNP showed potent antitumor activity and reduced toxicity in triple-negative breast cancer mice. DNP also reduced prostaglandin secretion and inhibited c-Myc expression, showing that the complexes could interfere with the inflammatory and metastatic processes of breast cancer.
As another NSAID approach, Zuojie Li and Qingpeng Wang et al. [62] designed and prepared a ketoprofen Pt(IV) complex 26 (Figure 5) which, in addition to the several effects described previously, notably inhibited PD-L1 expression, improving immune responses and CD8+ T-cell infiltration in tumor tissues. In 2022, Gou’s group [56] designed and found, through etodolac binding to Pt(II), that LA-1 27 (Figure 5) was significantly cytotoxic and inhibited metastasis of A2780 cells by inhibiting the COX-2/JAK2/STAT3 axis.
Since the structure of coumarin confers anti-inflammatory effects on its derivatives by inhibiting COX activity, Bingquan Wang et al. [63] designed a series of Pt-based coumarin derivatives, 28, 29, 30 and 31 (Figure 5), and found that in addition to their respective structural effects, they could also induce apoptosis by upregulating the expression of caspase 3 and caspase 9. The group also evaluated the anticancer activity of bifunctional 7-hydroxycoumarin Pt(IV) and found that the derivatives could release Pt(II) compounds to attack DNA and inhibit COX activity, which has great potential to overcome Pt(II) resistance [64].
4. Multi-Targeting Structure Modification to Actualize Diverse Antitumor Actions
Tumor tissues are often generated by kinds of mutations, and there are significant differences between samples of the same type of tumor from the same patient, so scholars have tried to achieve more satisfactory results by combining therapies that act on multiple cancer targets or by structurally modifying a drug [65]. For example, clinical attempts to use “cocktail therapy” for the treatment of AIDS beginning as early as the last century and the combination of chemotherapy and immunotherapy to improve antitumor outcomes as described above have the same aim of using multiple drugs with different effects in a more comprehensive and synergistic manner to achieve optimal efficacy for a particular condition. This section focuses more on the recent work on structural modifications of Pt-based drugs to achieve dual- or multi-targeting effects and provides more data to support the enhancement of the antitumor effects of Pt-based drugs.
Kogularamanan Suntharalingam et al. [66] reported a planar Pt(II) complex [Pt(BDI(QQ))]Cl, which the authors found to have a dual targeting ability, acting on both DNA and mitochondria. [Pt(BDI(QQ))]Cl induces DNA damage and acts selectively on cancer cells; it can also accumulate in mitochondria to cause direct damage. The article also suggests that p53 is not a determinant of complex activity and therefore can be targeted to cancers with a p53-deficient state. In the same year, the group [67] also synthesized two Pt(IV) complexes, 32 and 33 (Figure 6), of vitamin E and a-TOS and found that OET has a dual targeting effect in killing cancer cells, with the Pt-based group causing DNA damage and the axial ligand causing mitochondrial dysfunction, further validating the value of a dual targeting strategy. Weike Su’s group [68] designed and synthesized four novel Pt(IV) complexes and found that compound 34 (Figure 6) could release Pt(II) and DCA within the tumor cells, leading to DNA damage as well as disruption of mitochondrial membrane potential, illustrating the great potential of 34 in dual-targeted antitumor action. In addition, Mariafrancesca Hyeraci et al. [69] have prepared Pt(II) complexes 32 and 33 with a triphenylphosphorus fraction based on previous studies. This makes the trans-[PTBR2(NHRR’)(PPH3)] proposed in this article an option for the design of multi-targeted oncology drugs.
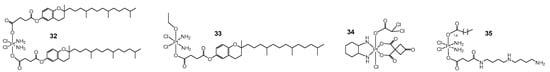
Figure 6.
Multi-targeting structure modification to actualize diverse antitumor actions. (32, 33) Pt(IV) complexes of vitamin E and a-TOS. (34) Structure of compound designed by Su’s group. (35) Polyamine-Pt(IV) prodrug.
In addition to targeting DNA damage and mitochondrial damage in tumor cells, Jin-Lei Tian et al. [70] designed a series of dual-targeted monofunctional Pt(II) complexes based on the aspect of improving drug delivery and targeting during antitumor therapy. This series of compounds can be used as one of the targets to rapidly enter the cell through the upregulation of Gluts in tumor cells leading to increased glucose uptake, and then to induce apoptosis through lysosomal damage caused by the production of large amounts of reactive oxygen species (ROS) through the localization of the P-gp protein.
Polyamines play an important role in tumor growth, progression and migration and have also been found to be highly expressed in tumor cells. Targeting polyamines to block their metabolism has become one of the popular anticancer drug targets [71]. Summarizing the previous studies, Liu’s group [72] has designed polyamine-Pt(IV) prodrug 35 (Figure 6), which can effectively inhibit tumor growth and reverse resistance to cisplatin. In addition to targeting DNA, complex 35 can also alter the high-polyamine environment and inhibit tumor cell growth by upregulating SSAT and PAO and downregulating putrescine, spermine and spermidine concentrations.
5. Activation of Pt-Based Prodrugs via Thermal/Invisible Light Stimuli
Photothermal and photodynamic therapies have become a popular area of interest in oncology treatment in recent years. Researchers have experimented with different structures of photosensitive materials and nanomaterials and have demonstrated in a variety of cancer cells and tissues that PDT and PTT are among the factors to be considered in the antitumor process due to their uniquely targeted low toxicity and high stability.
5.1. NIR-Based Photothermal Therapy Using Pt-Based Drugs
Near-infrared fluorescence imaging (Figure 7) [73] has features such as deep penetration and low photodamage among current imaging techniques [74]. Therefore, combining it with Pt-based drugs is a preferred choice for the preparation of novel photothermal therapy drugs. Near-infrared nanodrug encapsulation is a new technique for reversing tumor resistance to Pt-based drugs. Chengwei Zhang et al. [75] investigated the use of melanin-like nanoparticles (MENPs) in combination with Pt(IV) to obtain targeted therapy for prostate cancer. The authors found that the nanomaterials had appreciable biocompatibility, antitumor activity and conversion efficiency. The synergistic effect of the two resulted in a significant improvement in the antitumor effect. Zhang’s group [76] proposed a way to overcome cisplatin resistance through NIR photothermal therapy by means of a prepared nanosystem F-Pt-NPS 36, 37 (Figure 8). The authors found that NIR laser-induced nanocomplexes could promote drug uptake while accelerating GSH depletion to activate cisplatin; they also found that these nanocomplexes could increase cisplatin’s cross-linking to DNA and inhibit DNA repair. In addition, they showed good tumor inhibition in vitro and in vivo and the reversal of cisplatin resistance, suggesting an important idea of using infrared light-induced mild heat therapy to address cascade resistance.
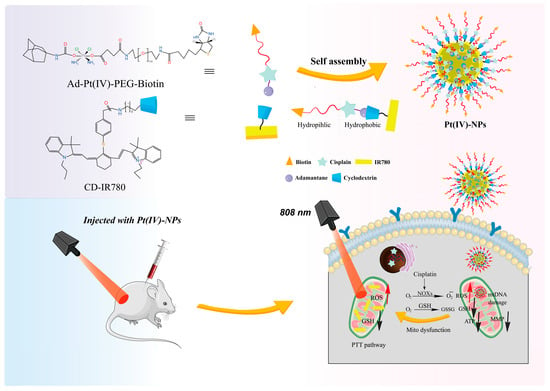
Figure 7.
Mechanism of NIR-based photothermal therapy in Pt-based drugs. This structure has both hydrophilic and hydrophobic ends, which is the structural basis module for this hybrid drug’s self-assembling into nanoparticles, making the intracellular accumulation easier. Via NIR Irradiation, this basic module will be degraded into 5 parts, which then target the nucleus and mitochondrion, resulting in ROS enhancement and GSH reduction. In addition, there also exists mt DNA damage and MMP decline in the mitochondrion. Reprinted with permission from Ref. [73].
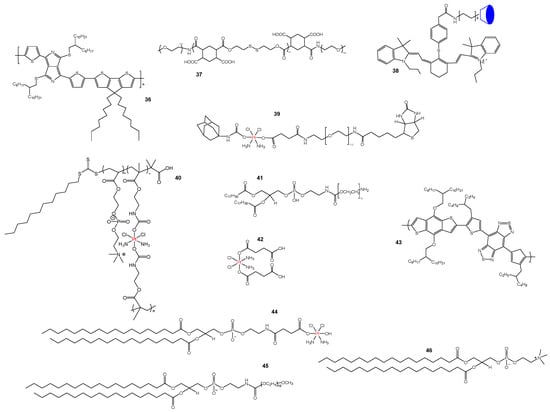
Figure 8.
Structures of Pt-based prodrugs activated via thermal/invisible light stimuli. (36, 37) The Zhang group obtained the prepared nanocomplexes by systematically combining P1 (36) with DAP-F (37) and Pt. (38, 39) Pt(IV)-NPs are mainly composed of IR1780 (38) and Ad-Pt(IV)-PEG-biotin (39). (40) The structure of prodrug. (41, 42, 43) CPNP-Fc/Pt consists mainly of DSPE-PEG500-NH2 (41), Pt(IV) prodrug (42) and CP (43). (44, 45, 46) (CAT)@Pt(IV)-liposome consisting of DSPE-Pt(IV) (44), DSPE-PEG5K (45) and DPPC (46).
Furthermore, in order to improve drug targeting effect, Yiyun Cheng et al. [77] reported a nanoparticle that has the anticancer activity of phytic acid (PA) while maintaining the photothermal effect of Pt-based nanoparticles by using natural PA-modified Pt-based nanoparticles with bone targeting properties combined with hydroxyapatite. Under NIR light irradiation, the growth of bone tumors and tumor-associated osteolysis were effectively inhibited. Also to increase targeting, Lianshuai Gu et al. [78] synthesized folic acid-modified cisplatin-loaded ICG lipid–polymer hybrid nanoparticles, FCINPS, known to be FDA-approved near-infrared fluorescent dyes. Induced apoptosis and necrosis also provide good support for tumor-targeted therapeutic nanopharmaceutical formulations.
Mao’s group [79] has developed a biotin-labeled Pt(IV) prodrug 38, 39 (Figure 8) to address cisplatin resistance in tumor cells through mitochondrial targeting and photothermal therapy. Pt(IV)-NPs were able to enhance mitochondrial targeting to induce maximal mitochondrial DNA damage and increase the intracellular accumulation of Pt, while also reducing GSH levels and inhibiting DNA repair, with significant inhibitory effects on A549cisR cells in the presence of targeted chemotherapeutic–photothermal synergistic treatment.
5.2. Pt-Based Drugs for Oxygen-Dependent Photodynamic Therapy
The hypoxic environment of solid tumors can limit the production of reactive oxygen species (ROS) by photodynamic therapy, thereby limiting its effect. Dongbo Guo et al. were the first to polymerize Pt(IV) complex precursor monomer (PPM) with 2-methacryloyloxyethyl phosphorylcholine (MPC) to form a nanoprecursor 40 (Figure 8) that could be reduced to Pt(II) under light irradiation, demonstrating that high levels of ROS could be produced in the absence of endogenous oxygen. The article also demonstrates that this structure has a long half-life and high aggregation in the antitumor process and can downregulate the expression of multidrug resistance-associated protein 1 (MRP1), thereby reversing the drug resistance problem in tumor cells. Chemodynamic therapy relies on the involvement of transition metal ions and endogenous H2O2, but low levels of endogenous H2O2 can also affect the efficacy of CDT [80]. The Ju group [81] developed CPNP-Fc/Pt 41, 42, 43 (Figure 8), a nanoparticle used to increase local oxygen levels and enhance the effects of CDT. The article illustrates that the main mechanism is that the release of Pt(II) from glutathione simultaneously triggers a cascade reaction of NADPH oxidase and superoxide dismutase to increase H2O2 levels to ensure an effective supply of H2O2, showing good CDT effects and inhibiting tumor growth. Zhuang Liu et al. [82] encapsulated CAT in Pt(IV) phospholipids to make CAT@Pt(IV)-liposome 44, 45, 46 (Figure 8) for enhancing the effect of tumor chemotherapy. The authors found that the use of this liposome resulted in good protection of enzymatic activity and triggered the breakdown of H2O2 in tumor cells to alleviate hypoxia in the environment, with good synergistic effects, providing a novel option for the clinical treatment of tumors. Similarly, to enhance the H2O2 generation of ROS to diminish cancer cells, some scholars used endogenous H2O2 for the conversion of reactive oxygen species to enhance the antitumor effect by using Fenton chemistry [83]. In this paper, an organic nanomedicine PTCG NPs was designed using EGCG, a phenolic Pt(IV) prodrug (Pt-OH) and a polyphenol-modified block copolymer (PEG-b-PPOH) as a backbone to achieve efficient drug release after cellular internalization. After being activated by the release, the cisplatin in the nanomedicine acts as an artificial enzyme involved in a cascade reaction to produce H2O2 for CDT and catalyzes the generation of highly toxic reactive oxygen species in the Fenton reaction to produce good anticancer effects. In addition, the avoidance of toxic side effects of Pt-based drugs provides a powerful strategy for cascade cancer therapy with nanomedicines.
6. Complexes with DNA Expression and Histone Post-Translation Depressants
Conventional Pt-based drugs achieve their antitumor effects primarily by acting on DNA damage. However, tumor cells can also reduce DNA damage or repair DNA through strategies such as translesion synthesis (TLS), nucleotide excision repair (NER), homologous recombination (HR) and other pathways in addition to increasing exocytosis [84,85]. Tumor cells can develop resistance to antitumor drugs through the repair of DNA damage [86]. While the effects of drug-resistant tumors of increasing accumulation in tumor cells and acting on immune pathways to reverse tumor cell immune escape have been described previously, this section focuses on the reduction of tumor cell resistance by blocking the DNA repair process and inhibiting genetic materials.
6.1. Histone Acetylation (HDAC) Inhibitors to Reverse Drug Resistance in Tumor Cells
Post-translational modifications of histones are not only involved in dynamic processes such as transcription and DNA repair in cells but are also associated with the maintenance of chromatin stability. Alterations in histones can affect DNA repair, mitosis and meiosis, and the inhibition of histones can be used to target DNA in tumor cells [87,88]. Therefore, histone acetylase inhibitors (HDACi) are novel anticancer drugs that inhibit histone acetylation.
A series of HDACi-containing Pt(IV) prodrugs, 47, 48, 49 and 50 (Figure 9), were designed and prepared by Zichen Xu et al. for multiple targeting of genomic DNA, histone acetylase and PARP-1 [89]. The article illustrates the significant antiproliferative activity of the prepared Pt(IV) prodrugs against cisplatin-resistant tumor cells. These compounds mainly resulted in increased DNA damage in chromatin and inhibited the repair process. Dan Gibson’s group prepared a series of double- and triple-acting Pt(IV) analogs 51, 52 and 53 (Figure 9) based on the currently more effective Pt(IV) complex satraplatin, cct-[Pt(NH3)(c-hexylamine)Cl2(OAc)2], in order to overcome satraplatin analog drug resistance, most of which exhibited improved water solubility in the analogs [90,91]. They found that one of the triple-acting compounds, 51 (Figure 9), was active in all cell lines, causing DNA damage to induce apoptosis. In addition, targeting HDAC and nuclear DNA is a promising therapeutic strategy.
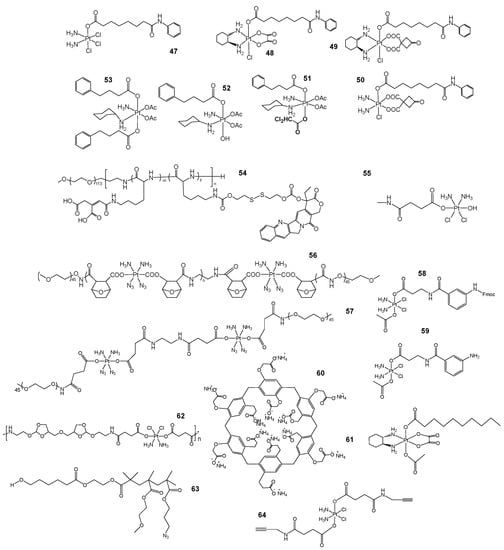
Figure 9.
Structures of complexes with DNA expression/histone post-translation related depressants. (47, 48, 49, 50) Pt(IV) prodrug structure containing HDACi. (51, 52, 53) Three satraplatin analogs designed by Dan Gibson’s group. (54, 55) A prodrug for the simultaneous activation of Pt as well as the redox polymer of camptothecin. (55, 56) Structures of DDNPs (55) and SNNPs (56). (57, 58) Two Pt(IV) prodrugs containing PARPi3-aminobenzamide (3-ABA) fragments. (59, 60) The supramolecular combination chemotherapy system (DOX@PtC10-CP6A) was prepared from CP6A (59) and Pt(IV) to obtain an amphiphilic aggregate (PtC10-CP6A) (60) which was then wrapped in DOX. (61) Octahedral coordination of Pt(IV) predrugs. (63, 64) Components of dual drug co-delivery systems.
6.2. DNA Expression Inhibitors of Various Levels to Enlighten Novel Modifications
Based on the shortcomings of existing nanomedicines, combined with the selective cytotoxicity of camptothecin on cells with S-phase DNA, Qixian Chen’s group [92] proposed the selective and simultaneous activation of Pt-based and camptothecin redox-reactive polymeric predrugs in the cytoplasm 54, 55 (Figure 9), and these drugs are self-assembled into a single nanoparticle by appropriate chemical methods. In the cytoplasm, multiple predrugs are activated and released in close proximity to the drug targets to exert an optimal therapeutic effect. The redox effect of the predrugs also depletes endogenous GSH and promotes greater sensitivity of tumor cells to chemotherapeutic agents. Hongtong Lu and Shasha He et al. [93] designed DDNPs 56 (Figure 9) and SNNPs 57 (Figure 8) as light-activated Pt-based synergistic chemotherapeutic dual-sensitive dual precursor drug nanoparticles, which are photosensitive and can activate Pt(IV) to Pt(II) under UVA light while producing a small amount of N3, which contributes to the lysosomal escape of DNNPs to achieve better photoactivated chemotherapy. In addition, the acid-sensitive protein phosphatase 2A inhibitor desmethyldeoxorubicin (DMC) in an acidic microenvironment can block DNA repair pathways and reverse resistance to Pt-based drugs.
PARP inhibitors, the first clinically approved antitumor drugs that utilize synthetic lethality, primarily target poly ADP-ribose polymerase [94]. This inhibitor can exert antitumor effects by inhibiting reparation of DNA damage and promoting apoptosis in tumor cells, and one of the reasons for resistance to CDDP is the enhanced DNA repair activity of tumor cells [95,96]. Mauro Ravera’s group [97] combined the two structures and introduced two Pt(IV) prodrugs 58, 59 (Figure 9) containing PARPi3-aminobenzamide (3-ABA) fragments and found their therapeutic effect was superior to that of CDDP, and the mechanism of action was found to be a combination of DNA damage by CDDP and inhibition of the repair process by PARPi resulting in a potent antitumor effect.
6.3. Conjugations of Doxorubicin (DOX) and Pt-Based Drugs to Reduce Drug Resistance
DOX is an inhibitor of RNA and DNA synthesis in tumor cells. Chen’s group prepared amphiphilic aggregates (PtC10-CP6A) using supramolecular carboxylated columnar aromatics (CP6A) and Pt(IV) and encapsulated DOX in them to obtain a supramolecular combination chemotherapy system (DOX@PtC10-CP6A) 60, 61 (Figure 9). The drug can be selectively released at the specific pH of the TME and was found to be non-toxic to normal cells. However, compared to CDDP and DOX, this drug not only inhibits tumor progression but also reduces the toxic effects of CDDP itself [98,99,100]. In addition, Tang’s group assembled a synergistic drug delivery system 62 (Figure 9) using Pt(IV) with octahedral coordination and DOX, which has a dual blocking effect on nuclear and mitochondrial genetic material, and the nanoshell enhances targeting and in vivo accumulation, thus significantly improving the therapeutic effect compared to CDDP and DOX [101,102]. Furthermore, Caiying Zhu and Jingjing Xiao et al. [103] developed a novel DOX and Pt(IV) dual drug co-delivery system 63, 64 (Figure 9) using the amphiphilic block copolymer PCL-b-p(OEGMA-co-AzPMA) as a nanoscale drug carrier. The substance showed a 2-5-fold increase in killing effect compared to CDDP and DOX in HeLa cells and A357 cells.
7. Conclusions and Outlook
CDDP, oxaliplatin and a series of Pt(II) class metallo-antitumor drugs have many limitations in clinical use, including low selectivity, poor accumulation in vivo, drug resistance and adverse effects caused by long-term use. Pt(IV) drugs have become a hot topic of research in recent years as modifying their axial position can reduce these problems. At present, the main research directions for Pt drugs are structural modifications and the selection of nanomaterials, and considerable progress has been made. By combining them with structures with different properties, scholars have achieved significant results in improving the efficiency of antitumor agents, reversing the resistance of tumor cells to Pt drugs and reducing adverse effects. Photodynamic and photothermal therapies, which are widely used in nanomaterials and other areas, also offer many options to improve the targeting and biostability of Pt-based drugs. Meanwhile, the development of Pt-based drugs is not limited to chemotherapy, as researchers are focusing on the synergistic effects of a combination of immunotherapies in order to achieve multiple targets on tumor cells and tissues and to explore the possibility of a comprehensive inhibition of tumor progression. Although tumorigenesis is a combination of many factors, in general, Pt-based drugs still have a large potential for development in oncology treatment. Future development of Pt-based drugs should focus on synergistic multi-treatment, with immunotherapy providing strong support for antitumor chemotherapy, effective modulation of the tumor immune microenvironment, mobilization of specific immune cells, etc., to achieve key tumor cell-targeting effects and reduce the incidence of drug resistance and adverse reactions to Pt-based drugs.
Author Contributions
Conceptualization, J.M., S.X., C.W., Y.C. (Yutong Chen), R.M., T.D. and T.L.; methodology, J.M., R.M., S.S., P.Z., Y.C. (Yutong Chen) and R.M.; software, J.L., Y.C. (Yi Cao), B.H., T.L., W.Z. and T.D.; investigation, J.L., Y.C. (Yi Cao), B.H., Z.Z., J.G., H.N., W.Z. and J.W.; writing—original draft preparation, J.L. and Y.C. (Yi Cao); writing—review and editing, J.L., Y.C. (Yi Cao) and B.H.; visualization, J.L., Y.C. (Yi Cao), B.H. and T.L.; supervision, J.M., S.X., C.W. and P.G.W.; project administration, J.M., S.X. and P.G.W.; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the China Postdoctoral Science Foundation (Grant 2021M701089), Key Scientific Re-search Projects in Henan Colleges and Universities (Grant No. 22A350002), the key scientific research projects of universities in Henan province (222102310402 and 222102310216), Key Program “New Drug Creation” of Guangdong Key Research and Development Plan (No. 2019B020202001).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no competing financial interests.
Nomenclature
| GSH | glutathione |
| ASA | ascorbic acid |
| HIF-1 | hypoxia-inducible factor-1 |
| LA | lactobionic acid |
| EGFR | epidermal growth factor receptor |
| CRGD | carboplatin |
| TDO | tryptophan 2,3-dioxygenase |
| TAM | tumor-associated macrophage |
| TME | tumor immune microenvironment |
| ADCC | antibody-dependent cell-mediated cytotoxicity |
| TREM2 | trigger receptors expressed on myeloid cells 2 |
| MDSC | myeloid-derived suppressor cell |
| ROS | reactive oxygen species |
| MENPs | melanin-like nanoparticles |
| PA | phytic acid |
| PPM | precursor monomer |
| LNP | liposome nanoparticle |
| TLS | translesion synthesis |
| NER | nucleotide excision repair |
| HR | homologous recombination |
| HDAC | histone acetylation |
| HDACi | histone acetylase inhibitors |
| PARP-1 | poly ADP-ribose polymerase-1 |
| DOX | doxorubicin |
| CP6A | carboxylated columnar aromatics |
| 3-ABA | 3-aminobenzamide |
| PDT | photodynamic therapy |
| PTT | photothermal therapy |
| CDT | chemodynamic therapy |
| UVA | ultraviolet-A |
| MRP1 | multidrug resistance-associated protein 1 |
| MPC | 2-methacryloyloxyethyl phosphorylcholine |
| MPS | mononuclear phagocytic system |
References
- Rosenberg, B.; Vancamp, L.; Trosko, J.E.; Mansour, V.H. Platinum Compounds: A New Class of Potent Antitumour Agents. Nature 1969, 222, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Muggia, F.M.; Bonetti, A.; Hoeschele, J.D.; Rozencweig, M.; Howell, S.B. Platinum Antitumor Complexes: 50 Years Since Barnett Rosenberg’s Discovery. JCO 2015, 33, 4219–4226. [Google Scholar] [CrossRef] [PubMed]
- Langer, T.; am Zehnhoff-Dinnesen, A.; Radtke, S.; Meitert, J.; Zolk, O. Understanding Platinum-Induced Ototoxicity. Trends Pharmacol. Sci. 2013, 34, 458–469. [Google Scholar] [CrossRef]
- Zhou, J.; Kang, Y.; Chen, L.; Wang, H.; Liu, J.; Zeng, S.; Yu, L. The Drug-Resistance Mechanisms of Five Platinum-Based Antitumor Agents. Front. Pharmacol. 2020, 11, 343. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Hydes, P.C.; Russell, M.J.H. Advances in Platinum Cancer Chemotherapy: Advances in the Design of Cisplatin Analogues. Cancer Metast. Rev. 1988, 7, 67–89. [Google Scholar] [CrossRef]
- Woloschuk, D.M.M.; Pruemer, J.M.; Cluxton, R.J. Carboplatin: A New Cisplatin Analog. Drug Intell. Clin. Pharm. 1988, 22, 843–849. [Google Scholar] [CrossRef]
- Sutton, E.C.; DeRose, V.J. Early Nucleolar Responses Differentiate Mechanisms of Cell Death Induced by Oxaliplatin and Cisplatin. J. Biol. Chem. 2021, 296, 100633. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Lutsenko, I.A.; Nikolaevskii, S.A.; Petrov, P.A.; Smolyaninov, I.V.; Malyants, I.K.; Shender, V.O.; Kiskin, M.A.; Sidorov, A.A.; Berberova, N.T.; et al. α-Diimine Cisplatin Derivatives: Synthesis, Structure, Cyclic Voltammetry and Cytotoxicity. Molecules 2022, 27, 8565. [Google Scholar] [CrossRef]
- Romashev, N.F.; Abramov, P.A.; Bakaev, I.V.; Fomenko, I.S.; Samsonenko, D.G.; Novikov, A.S.; Tong, K.K.H.; Ahn, D.; Dorovatovskii, P.V.; Zubavichus, Y.V.; et al. Heteroleptic Pd(II) and Pt(II) Complexes with Redox-Active Ligands: Synthesis, Structure, and Multimodal Anticancer Mechanism. Inorg. Chem. 2022, 61, 2105–2118. [Google Scholar] [CrossRef]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin Based Therapy: The Role of the Mitogen Activated Protein Kinase Signaling Pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorganic Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Hall, M.D.; Mellor, H.R.; Callaghan, R.; Hambley, T.W. Basis for Design and Development of Platinum(IV) Anticancer Complexes. J. Med. Chem. 2007, 50, 3403–3411. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.-L.; Qiao, X.; Liu, X.-M.; Song, X.-Q.; Zou, Y.-H.; Li, D.-Q.; Yu, X.-W.; Bao, W.-G.; Xu, J.-Y. Rapid DNA Interstrand Cross-Linking of Pt(IV) Compound. Eur. J. Pharmacol. 2022, 925, 174985. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Ding, W.; Zhu, X.; Li, B.; Zeng, F.; Wu, K.; Wu, X.; Wang, F. Ligand Evolution in the Photoactivatable Platinum(IV) Anticancer Prodrugs. Front. Chem. 2022, 10, 876410. [Google Scholar] [CrossRef]
- Chen, J.; Gao, C.; Zhang, Y.; Wang, T.; Qian, Y.; Yang, B.; Dong, P.; Zhang, Y. Inorganic Nano-Targeted Drugs Delivery System and Its Application of Platinum-Based Anticancer Drugs. J. Nanosci. Nanotechnol. 2017, 17, 1–17. [Google Scholar] [CrossRef]
- Rébé, C.; Demontoux, L.; Pilot, T.; Ghiringhelli, F. Platinum Derivatives Effects on Anticancer Immune Response. Biomolecules 2019, 10, 13. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, J.; Gou, S.; Xu, G. Novel Hypoxia-Targeting Pt(iv) Prodrugs. Chem. Commun. 2017, 53, 3749–3752. [Google Scholar] [CrossRef]
- Hu, D.; Yang, C.; Lok, C.-N.; Xing, F.; Lee, P.-Y.; Fung, Y.M.E.; Jiang, H.; Che, C.-M. An Antitumor Bis(N-Heterocyclic Carbene)Platinum(II) Complex That Engages Asparagine Synthetase as an Anticancer Target. Angew. Chem. Int. Ed. 2019, 131, 11030–11034. [Google Scholar] [CrossRef]
- Shevtsov, M.; Huile, G.; Multhoff, G. Membrane Heat Shock Protein 70: A Theranostic Target for Cancer Therapy. Phil. Trans. R. Soc. B 2018, 373, 20160526. [Google Scholar] [CrossRef]
- McKeon, A.M.; Noonan, J.; Devocelle, M.; Murphy, B.M.; Griffith, D.M. Platinum(iv) Oxaliplatin–Peptide Conjugates Targeting MemHsp70+ Phenotype in Colorectal Cancer Cells. Chem. Commun. 2017, 53, 11318–11321. [Google Scholar] [CrossRef]
- Zhong, Y.; Jia, C.; Zhang, X.; Liao, X.; Yang, B.; Cong, Y.; Pu, S.; Gao, C. Synthesis, Characterization, and Antitumor Activity of Novel Tumor-targeted Platinum(IV) Complexes. Appl. Organomet. Chem. 2020, 34, e5577. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Y.; Gou, S.; Chen, F. A Trifunctional Pt(II) Complex Alleviates the NHEJ/HR-Related DSBs Repairs to Evade Cisplatin-Resistance in NSCLC. Bioorg. Chem. 2020, 104, 104210. [Google Scholar] [CrossRef]
- Jin, S.; Guo, Y.; Song, D.; Zhu, Z.; Zhang, Z.; Sun, Y.; Yang, T.; Guo, Z.; Wang, X. Targeting Energy Metabolism by a Platinum(IV) Prodrug as an Alternative Pathway for Cancer Suppression. Inorg. Chem. 2019, 58, 6507–6516. [Google Scholar] [CrossRef]
- Liu, X.-M.; Li, Z.; He, X.-R.; Liu, R.-P.; Ma, Z.-Y.; Qiao, X.; Wang, S.-Q.; Xu, J.-Y. Dual-Targeting of the Aromatase Binding Domain of Heme and Androstenedione by Pt(iv) Prodrugs: A New Treatment for Postmenopausal Breast Cancer. Inorg. Chem. Front. 2022, 9, 3470–3483. [Google Scholar] [CrossRef]
- Li, S.; Su, W.; Wu, H.; Yuan, T.; Yuan, C.; Liu, J.; Deng, G.; Gao, X.; Chen, Z.; Bao, Y.; et al. Targeted Tumour Theranostics in Mice via Carbon Quantum Dots Structurally Mimicking Large Amino Acids. Nat. Biomed. Eng. 2020, 4, 704–716. [Google Scholar] [CrossRef]
- Wang, Z. Mesoporous Silica Nanoparticles with Lactose-Mediated Targeting Effect to Deliver Platinum(Iv) Prodrug for Liver Cancer Therapy. J. Mater. Chem. B 2017, 5, 7591–7597. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhu, Y.; Liu, M.; Jin, D.; Zhang, L.; Cheng, J.; Liu, Y. Conjugation of Oxaliplatin with PEGylated-Nanobody for Enhancing Tumor Targeting and Prolonging Circulation. J. Inorg. Biochem. 2021, 223, 111553. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, L.; Chen, G.; Gong, S. Carboplatin-Complexed and CRGD-Conjugated Unimolecular Nanoparticles for Targeted Ovarian Cancer Therapy. Macromol. Biosci. 2017, 17, 1600292. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.-H.; Pan, C.-H.; Peng, C.-L.; Shieh, M.-J. Panitumumab-Conjugated Pt-Drug Nanomedicine for Enhanced Efficacy of Combination Targeted Chemotherapy against Colorectal Cancer. Adv. Healthc. Mater. 2017, 6, 1700111. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Yu, Z.; Das, M.; Huang, L. Nano Codelivery of Oxaliplatin and Folinic Acid Achieves Synergistic Chemo-Immunotherapy with 5-Fluorouracil for Colorectal Cancer and Liver Metastasis. ACS Nano 2020, 14, 5075–5089. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Yang, H.; Wu, M.; Shi, K.; Zhou, C.; Peng, J.; Yang, Q. Targeting Delivery of Lidocaine and Cisplatin by Nanogel Enhances Chemotherapy and Alleviates Metastasis. ACS Appl. Mater. Interfaces 2018, 10, 25228–25240. [Google Scholar] [CrossRef]
- Wu, W.; Klockow, J.L.; Zhang, M.; Lafortune, F.; Chang, E.; Jin, L.; Wu, Y.; Daldrup-Link, H.E. Glioblastoma Multiforme (GBM): An Overview of Current Therapies and Mechanisms of Resistance. Pharmacol. Res. 2021, 171, 105780. [Google Scholar] [CrossRef]
- Sun, T.; Jiang, X.; Wang, Q.; Chen, Q.; Lu, Y.; Liu, L.; Zhang, Y.; He, X.; Ruan, C.; Zhang, Y.; et al. Substance P Mediated DGLs Complexing with DACHPt for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces 2017, 9, 34603–34617. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Iqbal, S.; Du, X.-J.; Yuan, Y.; Yang, X.; Li, H.-J.; Wang, J. Ultrafast Charge-Conversional Nanocarrier for Tumor-Acidity-Activated Targeted Drug Elivery. Biomater. Sci. 2018, 6, 350–355. [Google Scholar] [CrossRef]
- Mitra, R.; Singh, S.; Khar, A. Antitumour Immune Responses. Expert Rev. Mol. Med. 2003, 5, 1–22. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the Tumor Microenvironment in PD-L1/PD-1-Mediated Tumor Immune Escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef] [PubMed]
- Alsaab, H.O.; Sau, S.; Alzhrani, R.; Tatiparti, K.; Bhise, K.; Kashaw, S.K.; Iyer, A.K. PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome. Front. Pharmacol. 2017, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolušić, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of Tumoral Immune Resistance by Inhibition of Tryptophan 2,3-Dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef]
- Schmidt, S.K.; Müller, A.; Heseler, K.; Woite, C.; Spekker, K.; MacKenzie, C.R.; Däubener, W. Antimicrobial and Immunoregulatory Properties of Human Tryptophan 2,3-Dioxygenase. Eur. J. Immunol. 2009, 39, 2755–2764. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Chen, F.; Wang, X.; Wang, Y.; Gou, S. Pt(IV) Hybrids Containing a TDO Inhibitor Serve as Potential Anticancer Immunomodulators. J. Inorg. Biochem. 2019, 195, 130–140. [Google Scholar] [CrossRef]
- Yamazaki, T.; Buqué, A.; Ames, T.D.; Galluzzi, L. PT-112 Induces Immunogenic Cell Death and Synergizes with Immune Checkpoint Blockers in Mouse Tumor Models. OncoImmunology 2020, 9, 1721810. [Google Scholar] [CrossRef] [PubMed]
- Arsenijevic, M.; Milovanovic, M.; Jovanovic, S.; Arsenijevic, N.; Markovic, B.S.; Gazdic, M.; Volarevic, V. In Vitro and in Vivo Anti-Tumor Effects of Selected Platinum(IV) and Dinuclear Platinum(II) Complexes against Lung Cancer Cells. J. Biol. Inorg. Chem. 2017, 22, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, L.; Song, Y.; Li, W.; Xu, L. Targeting Macrophages: A Novel Treatment Strategy in Solid Tumors. J. Transl. Med. 2022, 20, 586. [Google Scholar] [CrossRef] [PubMed]
- Larionova, I.V.; Liu, T.; Riabov, V.; Mossel, D.M.; Patysheva, M.R.; Kiselev, A.M.; Kazakova, E.O.; Cherdyntseva, N.V.; Kzhyshkowska, J. The Clearance of EGF by Tumor-Associated Macrophages Is Suppressed by Chemotherapeutic Agent Cisplatin. Ann. Oncol. 2019, 30, v810. [Google Scholar] [CrossRef]
- Muenchen, H.J.; Aggarwal, S.K.; Misra, H.K.; Andrulis, P.J. Morphological and Histochemical Changes in Macrophage Activity After Novel Anti-Neoplastic Platinum Agents. Microsc. Microanal. 1997, 3, 11–12. [Google Scholar] [CrossRef]
- Pan, Y.; Yu, Y.; Wang, X.; Zhang, T. Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 2020, 11, 583084. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Tang, M.; Li, H.; Guo, X.; Yang, X. The Effects of Umbilical Cord-Derived Macrophage Exosomes Loaded with Cisplatin on the Growth and Drug Resistance of Ovarian Cancer Cells. Drug Dev. Ind. Pharm. 2020, 46, 1150–1162. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, S.; Yuan, H.; Wang, Y.; Cai, L.; Chen, H.; Wang, X.; Song, D.; Wang, X.; Guo, Z.; et al. Platinum-Based TREM2 Inhibitor Suppresses Tumors by Remodeling the Immunosuppressive Microenvironment. Angew. Chem. Int. Ed. 2022, 62, e202213337. [Google Scholar]
- Tian, L.; Shao, M.; Gong, Y.; Wei, T.; Zhu, Y.; Chao, Y.; Liu, Z. Epigenetic Platinum Complexes Breaking the “Eat Me/Don’t Eat Me” Balance for Enhanced Cancer Chemoimmunotherapy. Bioconjugate Chem. 2022, 33, 343–352. [Google Scholar] [CrossRef]
- Shueng, P.-W.; Yu, L.-Y.; Chiu, H.-C.; Chang, H.-C.; Chiu, Y.-L.; Kuo, T.-Y.; Yen, Y.-W.; Lo, C.-L. Early Phago-/Endosomal Escape of Platinum Drugs via ROS-Responsive Micelles for Dual Cancer Chemo/Immunotherapy. Biomaterials 2021, 276, 121012. [Google Scholar] [CrossRef]
- Sica, A.; Porta, C.; Amadori, A.; Pastò, A. Tumor-Associated Myeloid Cells as Guiding Forces of Cancer Cell Stemness. Cancer Immunol. Immunother. 2017, 66, 1025–1036. [Google Scholar] [CrossRef]
- Tang, L.; Cai, D.; Qin, M.; Lu, S.; Hu, M.-H.; Ruan, S.; Jin, G.; Wang, Z. Oxaliplatin-Based Platinum(IV) Prodrug Bearing Toll-like Receptor 7 Agonist for Enhanced Immunochemotherapy. ACS Omega 2020, 5, 726–734. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-Related Inflammation and Treatment Effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Karin, M.; Sun, B. Targeting Cancer-Promoting Inflammation—Have Anti-Inflammatory Therapies Come of Age? Nat. Rev. Clin. Oncol. 2021, 18, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Zang, J.; Zhang, B.; Wang, Y.; Wang, X.; Gou, S. Design, Synthesis and Biological Evaluation of Antitumor Platinum(II) Agents Conjugated with Non-Steroidal Anti-Inflammatory Drug Species. Bioorg. Chem. 2022, 120, 105633. [Google Scholar] [CrossRef]
- Ju, Z.; Li, M.; Xu, J.; Howell, D.C.; Li, Z.; Chen, F.-E. Recent Development on COX-2 Inhibitors as Promising Anti-Inflammatory Agents: The Past 10 Years. Acta Pharm. Sin. B 2022, 12, 2790–2807. [Google Scholar] [CrossRef]
- Kochel, T.J.; Goloubeva, O.G.; Fulton, A.M. Upregulation of Cyclooxygenase-2/Prostaglandin E 2 (COX-2/PGE 2) Pathway Member Multiple Drug Resistance-Associated Protein 4 (MRP4) and Downregulation of Prostaglandin Transporter (PGT) and 15-Prostaglandin Dehydrogenase (15-PGDH) in Triple-Negative Breast Cancer. Breast Cancer (Auckl) 2016, 10, BCBCR.S38529. [Google Scholar]
- Chen, Y.; Wang, Q.; Li, Z.; Liu, Z.; Zhao, Y.; Zhang, J.; Liu, M.; Wang, Z.; Li, D.; Han, J. Naproxen Platinum(iv) Hybrids Inhibiting Cycloxygenases and Matrix Metalloproteinases and Causing DNA Damage: Synthesis and Biological Evaluation as Antitumor Agents in Vitro and in Vivo. Dalton Trans. 2020, 49, 5192–5204. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Wang, Q.; Li, Z.; Liu, Z.; Hua, X.; Han, J.; Chang, C.; Wang, Z.; Li, D. Albumin-Encapsulated Nanoparticles of Naproxen Platinum(IV) Complexes with Inflammation Inhibitory Competence Displaying Effective Antitumor Activities in Vitro and in Vivo. IJN 2021, 16, 5513–5529. [Google Scholar] [CrossRef]
- Jin, S.; Muhammad, N.; Sun, Y.; Tan, Y.; Yuan, H.; Song, D.; Guo, Z.; Wang, X. Multispecific Platinum(IV) Complex Deters Breast Cancer via Interposing Inflammation and Immunosuppression as an Inhibitor of COX-2 and PD-L1. Angew. Chem. Int. Ed. 2020, 59, 23313–23321. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Q.; Li, L.; Chen, Y.; Cui, J.; Liu, M.; Zhang, N.; Liu, Z.; Han, J.; Wang, Z. Ketoprofen and Loxoprofen Platinum(IV) Complexes Displaying Antimetastatic Activities by Inducing DNA Damage, Inflammation Suppression, and Enhanced Immune Response. J. Med. Chem. 2021, 64, 17920–17935. [Google Scholar] [CrossRef]
- Li, G.; Zhang, J.; Liu, Z.; Wang, Q.; Chen, Y.; Liu, M.; Li, D.; Han, J.; Wang, B. Development of a Series of 4-Hydroxycoumarin Platinum(IV) Hybrids as Antitumor Agents: Synthesis, Biological Evaluation and Action Mechanism Investigation. J. Inorg. Biochem. 2019, 194, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, Y.; Li, G.; Liu, Z.; Ma, J.; Liu, M.; Li, D.; Han, J.; Wang, B. Synthesis and Evaluation of Bi-Functional 7-Hydroxycoumarin Platinum(IV) Complexes as Antitumor Agents. Bioorg. Med. Chem. 2019, 27, 2112–2121. [Google Scholar] [CrossRef]
- Zheng, W.; Zhao, Y.; Luo, Q.; Zhang, Y.; Wu, K.; Wang, F. Multi-Targeted Anticancer Agents. CTMC 2017, 17, 3084–3098. [Google Scholar] [CrossRef]
- Suntharalingam, K.; Wilson, J.J.; Lin, W.; Lippard, S.J. A Dual-Targeting, P53-Independent, Apoptosis-Inducing Platinum(ii) Anticancer Complex, [Pt(BDI QQ)]Cl. Metallomics 2014, 6, 437–443. [Google Scholar] [CrossRef]
- Suntharalingam, K.; Song, Y.; Lippard, S.J. Conjugation of Vitamin E Analog α-TOS to Pt(Iv) Complexes for Dual-Targeting Anticancer Therapy. Chem. Commun. 2014, 50, 2465. [Google Scholar] [CrossRef]
- Liu, F.; Dong, X.; Shi, Q.; Chen, J.; Su, W. Improving the Anticancer Activity of Platinum(iv) Prodrugs Using a Dual-Targeting Strategy with a Dichloroacetate Axial Ligand. RSC Adv. 2019, 9, 22240–22247. [Google Scholar] [CrossRef]
- Hyeraci, M.; Scalcon, V.; Folda, A.; Labella, L.; Marchetti, F.; Samaritani, S.; Rigobello, M.P.; Dalla Via, L. New Platinum(II) Complexes Affecting Different Biomolecular Targets in Resistant Ovarian Carcinoma Cells. ChemMedChem 2021, 16, 1956–1966. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shao, J.; Wang, J.; Gong, X.-J.; Liu, W.-X.; Wang, S.; Zhang, Y.; Yang, S.; Zhang, Q.-S.; Wei, J.-X.; et al. Antitumor Effects of New Glycoconjugated Pt II Agents Dual-Targeting GLUT1 and Pgp Proteins. Dalton Trans. 2022, 51, 16082–16092. [Google Scholar] [CrossRef]
- Muth, A.; Kamel, J.; Kaur, N.; Shicora, A.C.; Ayene, I.S.; Gilmour, S.K.; Phanstiel, O. Development of Polyamine Transport Ligands with Improved Metabolic Stability and Selectivity against Specific Human Cancers. J. Med. Chem. 2013, 56, 5819–5828. [Google Scholar] [CrossRef]
- Liu, H.; Ma, J.; Li, Y.; Yue, K.; Li, L.; Xi, Z.; Zhang, X.; Liu, J.; Feng, K.; Ma, Q.; et al. Polyamine-Based Pt(IV) Prodrugs as Substrates for Polyamine Transporters Preferentially Accumulate in Cancer Metastases as DNA and Polyamine Metabolism Dual-Targeted Antimetastatic Agents. J. Med. Chem. 2019, 62, 11324–11334. [Google Scholar] [CrossRef] [PubMed]
- Marin, R. New Opportunities for Light-Based Tumor Treatment with an “Iron Fist” . LightSci. Appl. 2022, 11, 65. [Google Scholar] [CrossRef]
- Ma, L.; Yang, T.; Zhang, Z.; Yin, S.; Song, Z.; Shi, W.; Chu, D.; Zhang, Y.; Zhang, M. Cyanostilbene-Based near-Infrared Emissive Platinum(II) Metallacycles for Cancer Theranostics. Chin. Chem. Lett. 2019, 30, 1942–1946. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Guo, H. Synergic Highly Effective Photothermal-Chemotherapy with Platinum Prodrug Linked Melanin-like Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 356–363. [Google Scholar] [CrossRef]
- Wang, L.; Yu, Y.; Wei, D.; Zhang, L.; Zhang, X.; Zhang, G.; Ding, D.; Xiao, H.; Zhang, D. A Systematic Strategy of Combinational Blow for Overcoming Cascade Drug Resistance via NIR-Light-Triggered Hyperthermia. Adv. Mater. 2021, 33, 2100599. [Google Scholar] [CrossRef]
- Zhou, Z.; Fan, T.; Yan, Y.; Zhang, S.; Zhou, Y.; Deng, H.; Cai, X.; Xiao, J.; Song, D.; Zhang, Q.; et al. One Stone with Two Birds: Phytic Acid-Capped Platinum Nanoparticles for Targeted Combination Therapy of Bone Tumors. Biomaterials 2019, 194, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Shi, T.; Sun, Y.; You, C.; Wang, S.; Wen, G.; Chen, L.; Zhang, X.; Zhu, J.; Sun, B. Folate-Modified, Indocyanine Green-Loaded Lipid-Polymer Hybrid Nanoparticles for Targeted Delivery of Cisplatin. J. Biomater. Sci. Polym. Ed. 2017, 28, 690–702. [Google Scholar] [CrossRef]
- Yang, G.-G.; Pan, Z.-Y.; Zhang, D.-Y.; Cao, Q.; Ji, L.-N.; Mao, Z.-W. Precisely Assembled Nanoparticles against Cisplatin Resistance via Cancer-Specific Targeting of Mitochondria and Imaging-Guided Chemo-Photothermal Therapy. ACS Appl. Mater. Interfaces 2020, 12, 43444–43455. [Google Scholar] [CrossRef]
- Guo, D.; Xu, S.; Huang, Y.; Jiang, H.; Yasen, W.; Wang, N.; Su, Y.; Qian, J.; Li, J.; Zhang, C.; et al. Platinum(IV) Complex-Based Two-in-One Polyprodrug for a Combinatorial Chemo-Photodynamic Therapy. Biomaterials 2018, 177, 67–77. [Google Scholar] [CrossRef]
- He, Y.; Jin, X.; Guo, S.; Zhao, H.; Liu, Y.; Ju, H. Conjugated Polymer–Ferrocence Nanoparticle as an NIR-II Light Powered Nanoamplifier to Enhance Chemodynamic Therapy. ACS Appl. Mater. Interfaces 2021, 13, 31452–31461. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, X.; Liang, C.; Yi, X.; Song, G.; Chao, Y.; Yang, Y.; Yang, K.; Feng, L.; Liu, Z. Catalase-Loaded Cisplatin-Prodrug-Constructed Liposomes to Overcome Tumor Hypoxia for Enhanced Chemo-Radiotherapy of Cancer. Biomaterials 2017, 138, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Sun, S.; Sun, R.; Cui, G.; Hong, L.; Rao, B.; Li, A.; Yu, Z.; Kan, Q.; Mao, Z. A Metal–Polyphenol-Coordinated Nanomedicine for Synergistic Cascade Cancer Chemotherapy and Chemodynamic Therapy. Adv. Mater. 2020, 32, 1906024. [Google Scholar] [CrossRef]
- Li, X.; Heyer, W.-D. Homologous Recombination in DNA Repair and DNA Damage Tolerance. Cell Res. 2008, 18, 99–113. [Google Scholar] [CrossRef] [PubMed]
- Basu, A. DNA Damage, Mutagenesis and Cancer. IJMS 2018, 19, 970. [Google Scholar] [CrossRef]
- Roos, W.P.; Thomas, A.D.; Kaina, B. DNA Damage and the Balance between Survival and Death in Cancer Biology. Nat. Rev. Cancer 2016, 16, 20–33. [Google Scholar] [CrossRef]
- Audia, J.E.; Campbell, R.M. Histone Modifications and Cancer. Cold Spring Harb. Perspect. Biol. 2016, 8, a019521. [Google Scholar] [CrossRef]
- Jones, P.A.; Issa, J.-P.J.; Baylin, S. Targeting the Cancer Epigenome for Therapy. Nat. Rev. Genet. 2016, 17, 630–641. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, W.; Wang, Z.; Gou, S. Platinum(IV) Prodrugs Multiply Targeting Genomic DNA, Histone Deacetylases and PARP-1. Eur. J. Med. Chem. 2017, 141, 211–220. [Google Scholar] [CrossRef]
- Kelland, L. The Resurgence of Platinum-Based Cancer Chemotherapy. Nat. Rev. Cancer 2007, 7, 573–584. [Google Scholar] [CrossRef]
- Karmakar, S.; Poetsch, I.; Kowol, C.R.; Heffeter, P.; Gibson, D. Synthesis and Cytotoxicity of Water-Soluble Dual- and Triple-Action Satraplatin Derivatives: Replacement of Equatorial Chlorides of Satraplatin by Acetates. Inorg. Chem. 2019, 58, 16676–16688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Qian, M.; Cui, H.; Zeng, S.; Wang, J.; Chen, Q. Spatiotemporal Concurrent Liberation of Cytotoxins from Dual-Prodrug Nanomedicine for Synergistic Antitumor Therapy. ACS Appl. Mater. Interfaces 2021, 13, 6053–6068. [Google Scholar] [CrossRef]
- Lu, H.; He, S.; Zhang, Q.; Li, X.; Xie, Z.; Wang, Z.; Qi, Y.; Huang, Y. Dual-Sensitive Dual-Prodrug Nanoparticles with Light-Controlled Endo/Lysosomal Escape for Synergistic Photoactivated Chemotherapy. Biomater. Sci. 2021, 9, 7115–7123. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP Inhibitors: Synthetic Lethality in the Clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG Inhibitors in Cancer Treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- LaFargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and Comparing Adverse Events between PARP Inhibitors. Lancet Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef]
- Gabano, E.; Pinton, G.; Balzano, C.; Boumya, S.; Osella, D.; Moro, L.; Ravera, M. Unsymmetric Cisplatin-Based Pt(IV) Conjugates Containing a PARP-1 Inhibitor Pharmacophore Tested on Malignant Pleural Mesothelioma Cell Lines. Molecules 2021, 26, 4740. [Google Scholar] [CrossRef]
- Oelshlegel, F.J. Glucose-6-Phosphate Dehydrogenase Deficiency in Sickle-Cell Disease. Ann. Intern. Med. 1974, 81, 413. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, Z.; Li, Q.; Huang, X.; Kang, Z.; Dong, H.; Chen, J.; Sun, J.; Dong, Y.; Li, J.; et al. A PH Responsive Complexation-Based Drug Delivery System for Oxaliplatin. Chem. Sci. 2017, 8, 4458–4464. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, Y.; Meng, Z.; Guo, L.; Yuan, X.; Zhang, Y.; Chai, Y.; Sessler, J.L.; Meng, Q.; Li, C. Supramolecular Combination Chemotherapy: A PH-Responsive Co-Encapsulation Drug Delivery System. Chem. Sci. 2020, 11, 6275–6282. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, B.; Zhang, J.; Li, K.; Liang, Y.; Sun, Y.; Ding, Y.; Han, J. Coordinated PH/Redox Dual-Sensitive and Hepatoma-Targeted Multifunctional Polymeric Micelle System for Stimuli-Triggered Doxorubicin Release: Synthesis, Characterization and in Vitro Evaluation. Int. J. Pharm. 2016, 501, 221–235. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Sun, M.; Cheng, X.; Xu, Y.; Lv, X.; Wang, X.; Tang, R. PH/Redox Dual-Sensitive Platinum (IV)-Based Micelles with Greatly Enhanced Antitumor Effect for Combination Chemotherapy. J. Colloid Interface Sci. 2019, 541, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Xiao, J.; Tang, M.; Feng, H.; Chen, W.; Du, M. Platinum Covalent Shell Cross-Linked Micelles Designed to Deliver Doxorubicin for Synergistic Combination Cancer Therapy. IJN 2017, 12, 3697–3710. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).