Luminescent Water-Dispersible Nanoparticles Engineered from Copper(I) Halide Cluster Core and P,N-Ligand with an Optimal Balance between Stability and ROS Generation
Abstract
1. Introduction
2. Results and Discussion
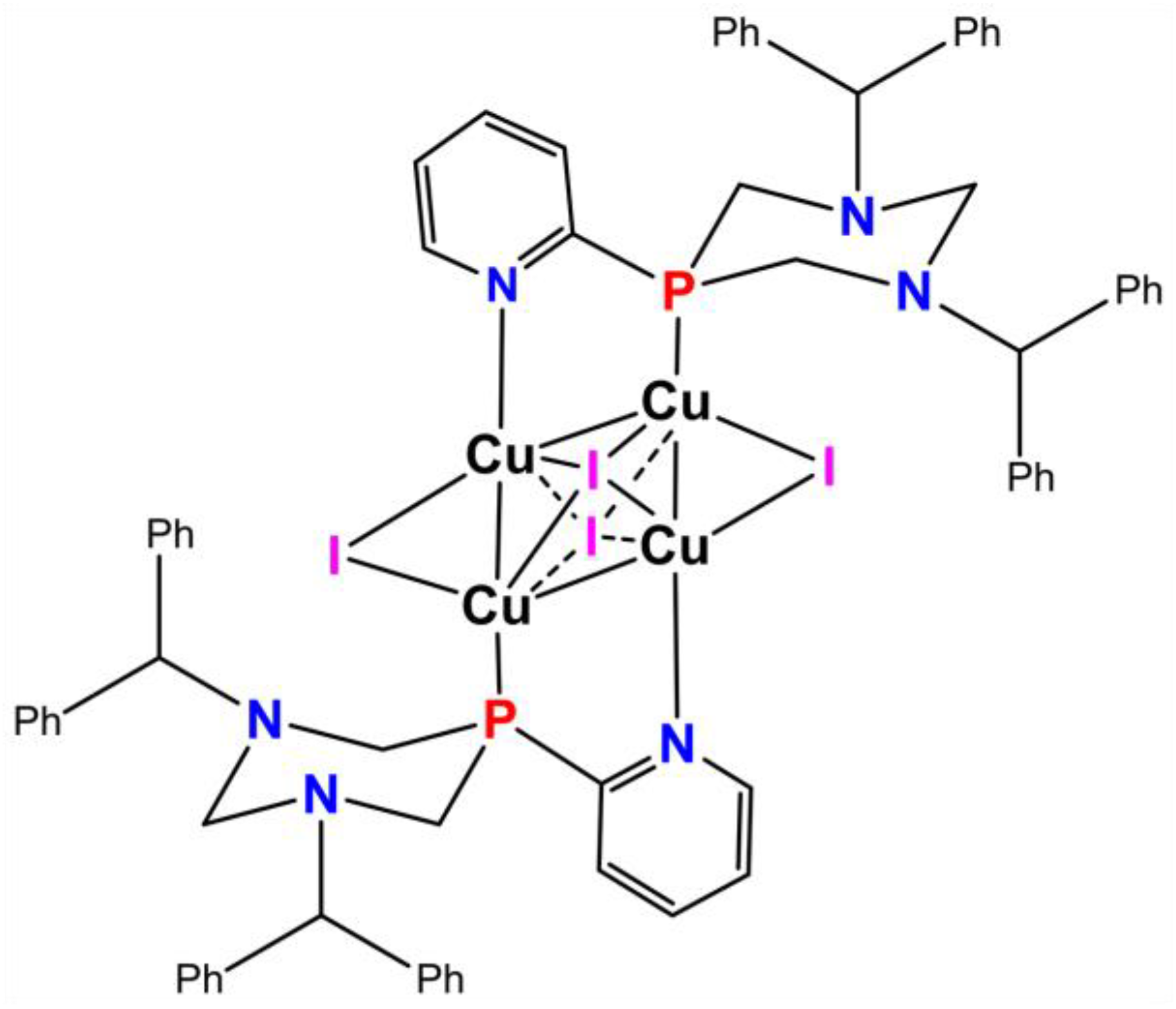
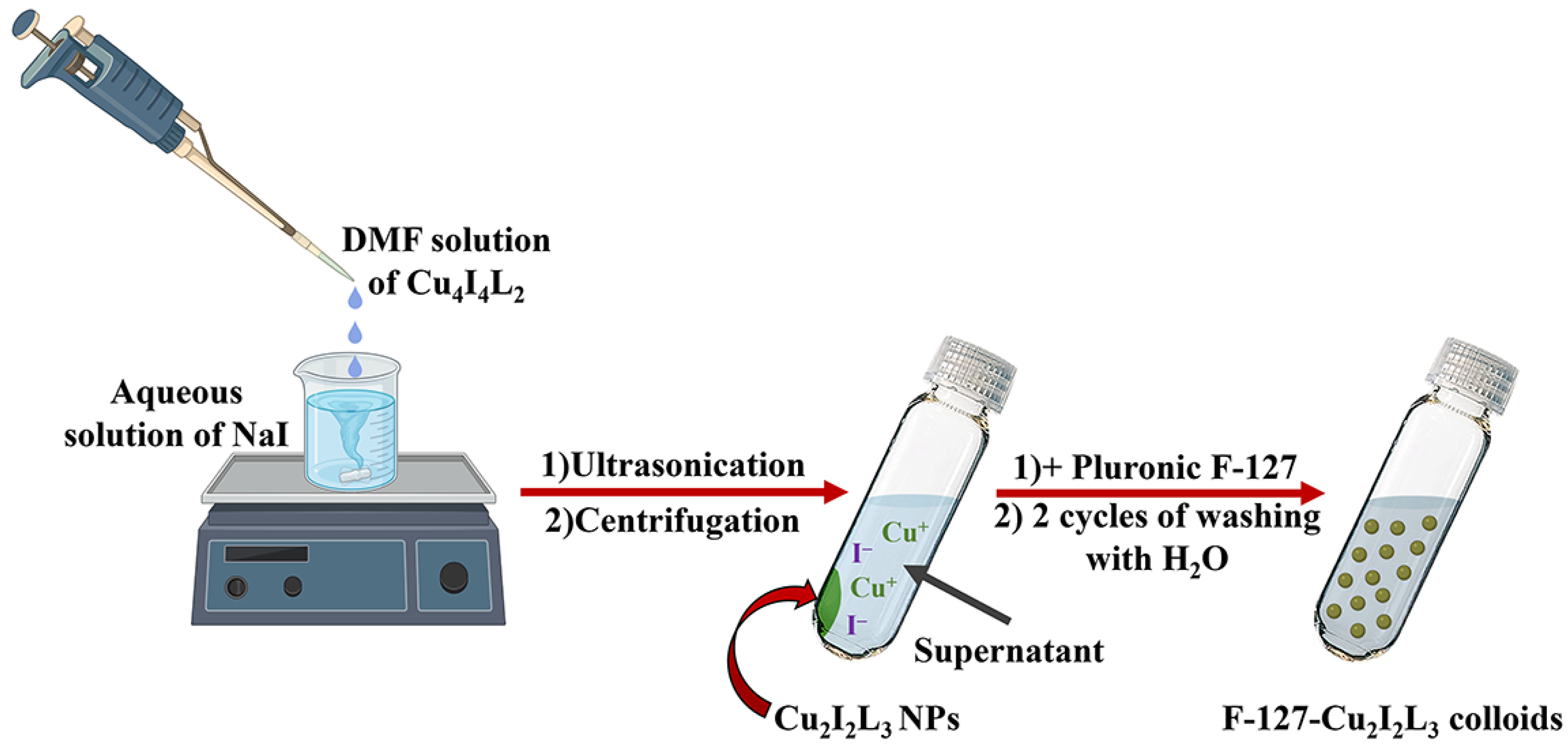
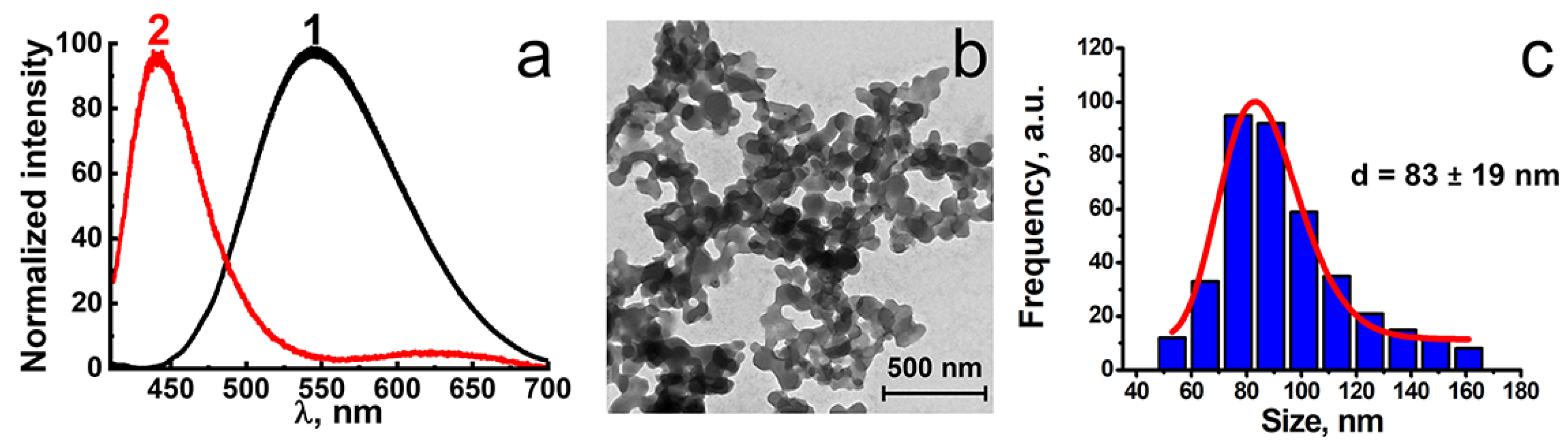
2.1. Synthesis of F-127-Cu2I2L3 Nanoparticles
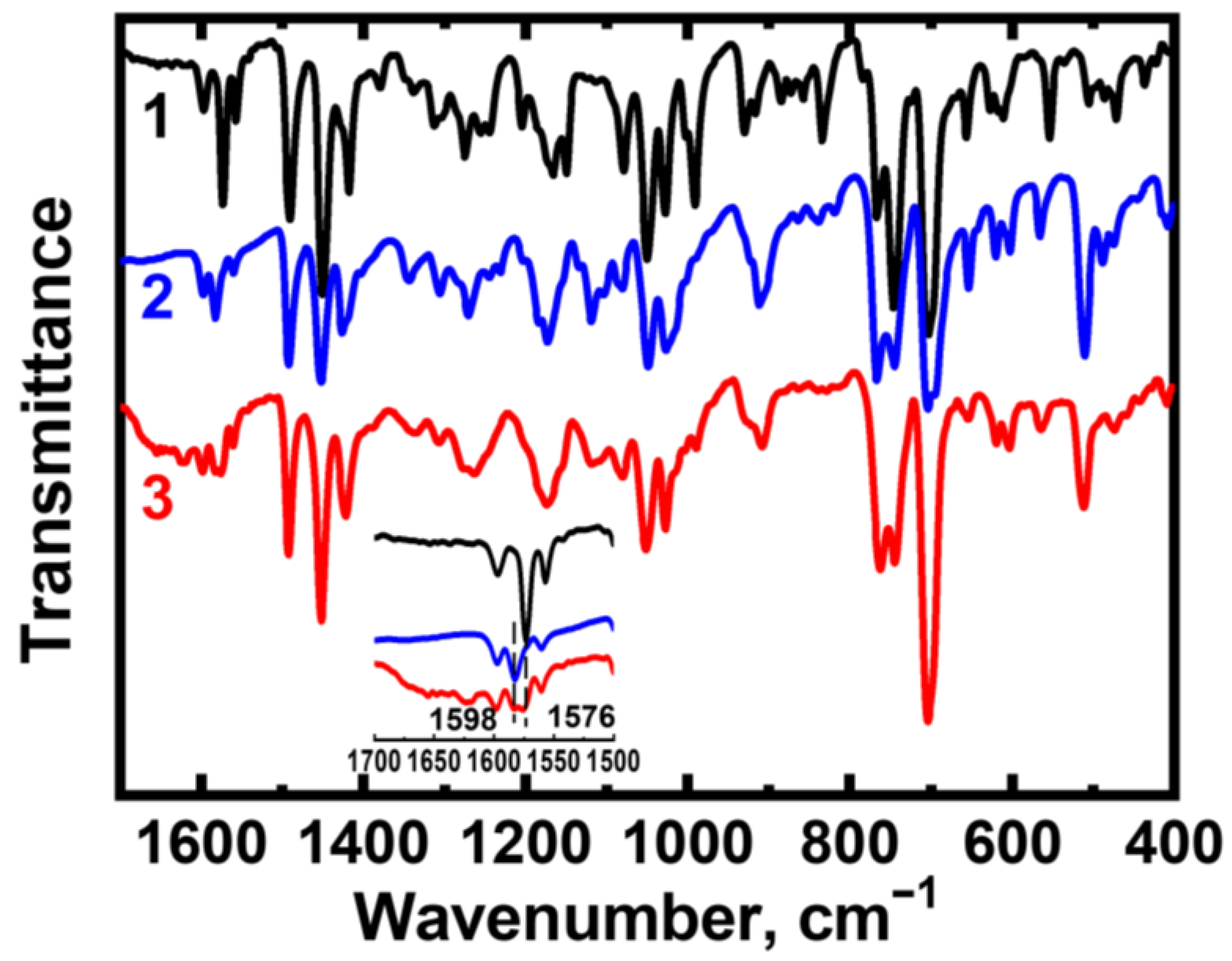
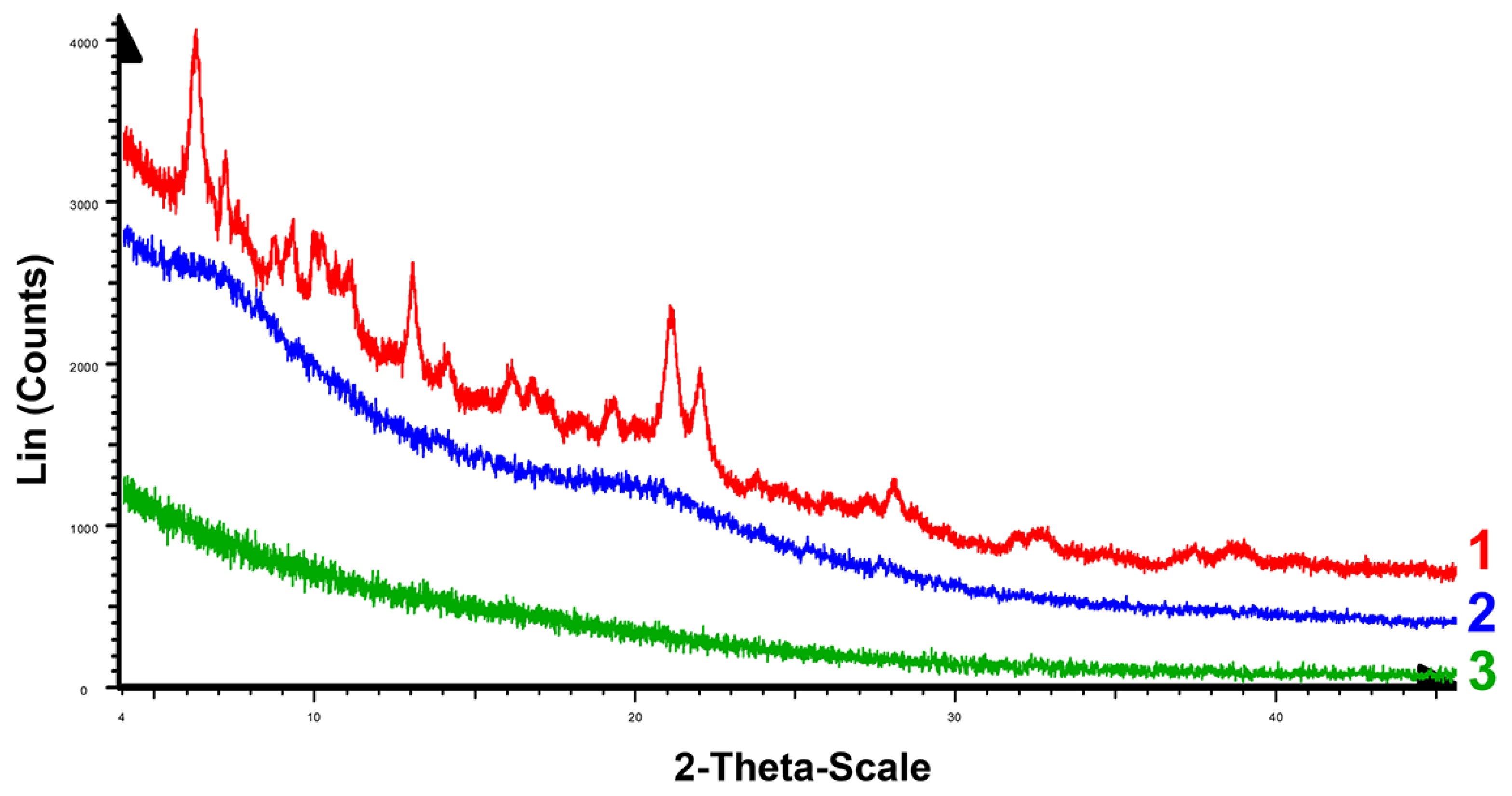
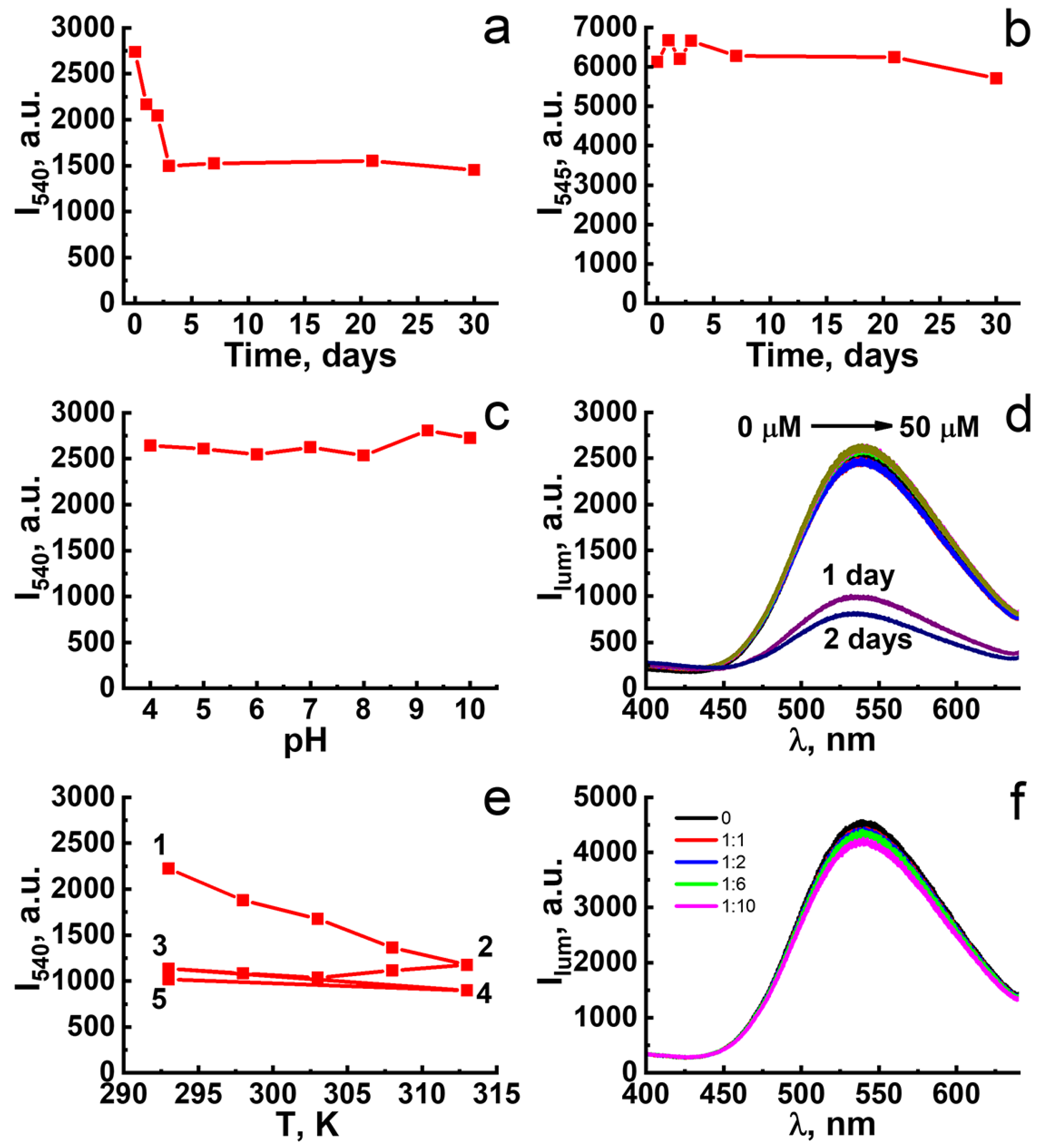
2.2. Chemical Stability of F-127-Cu2I2L3 Nanoparticles in Ambient Conditions and in Solutions Modeling Bioliquids
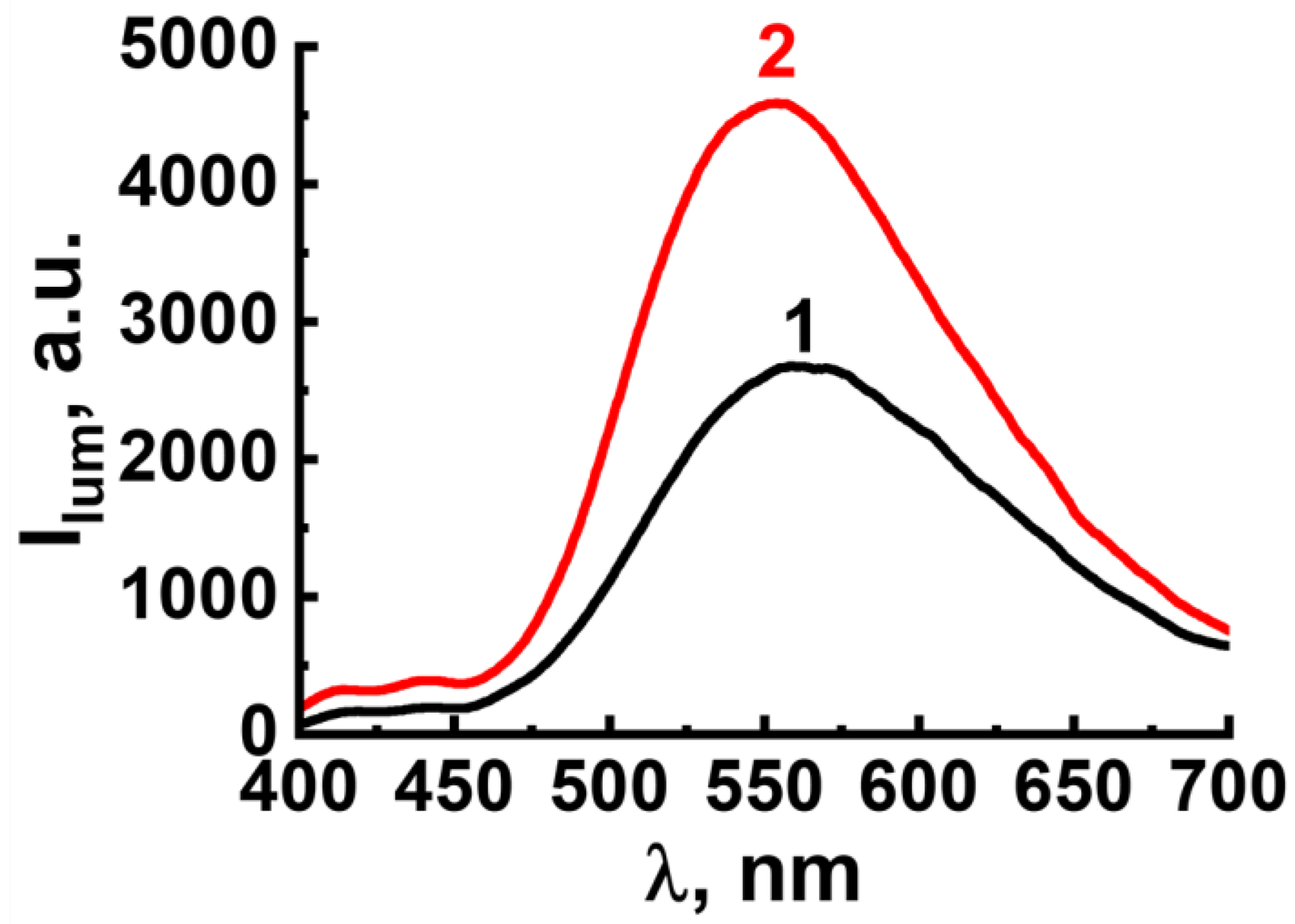
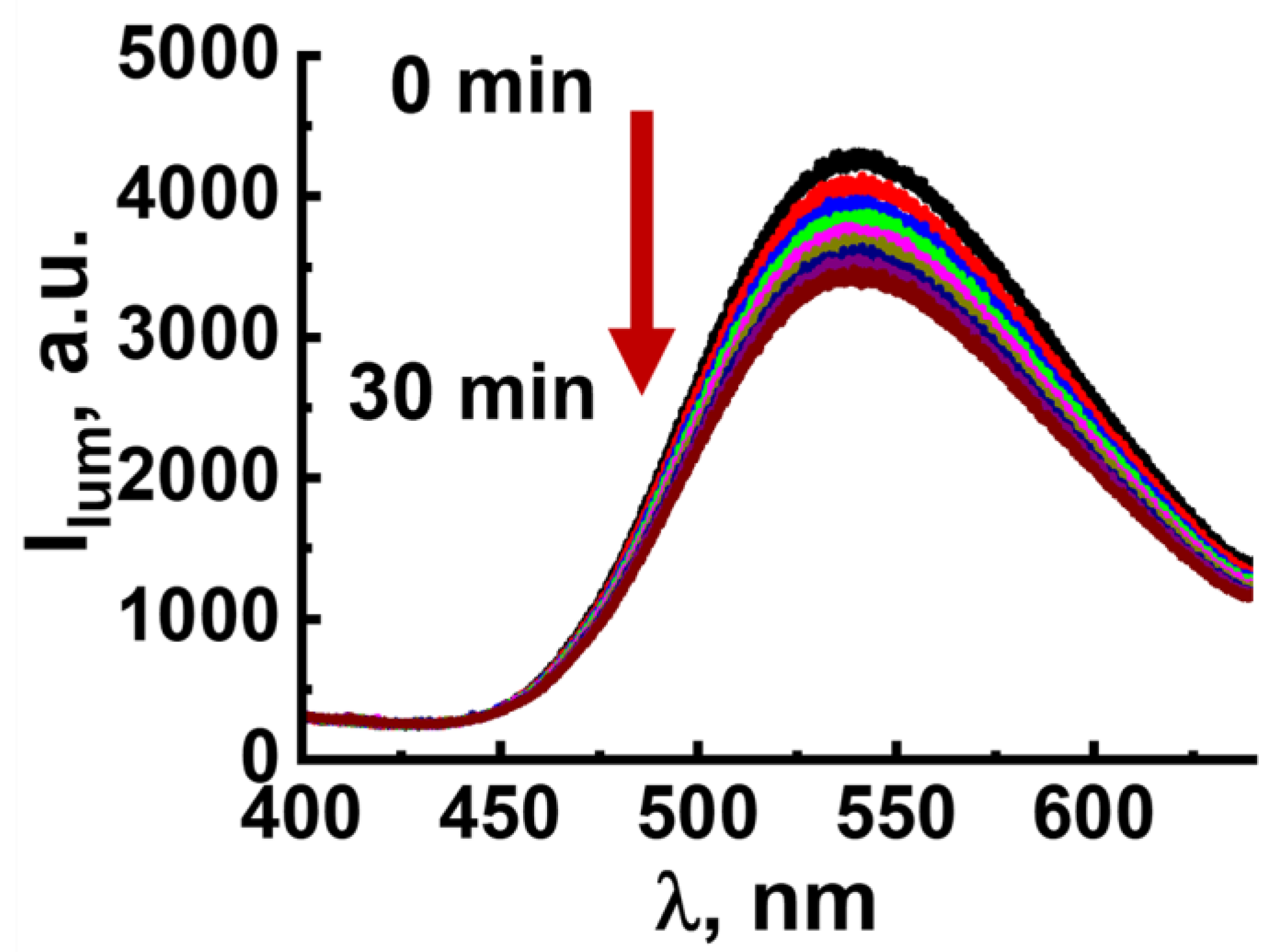
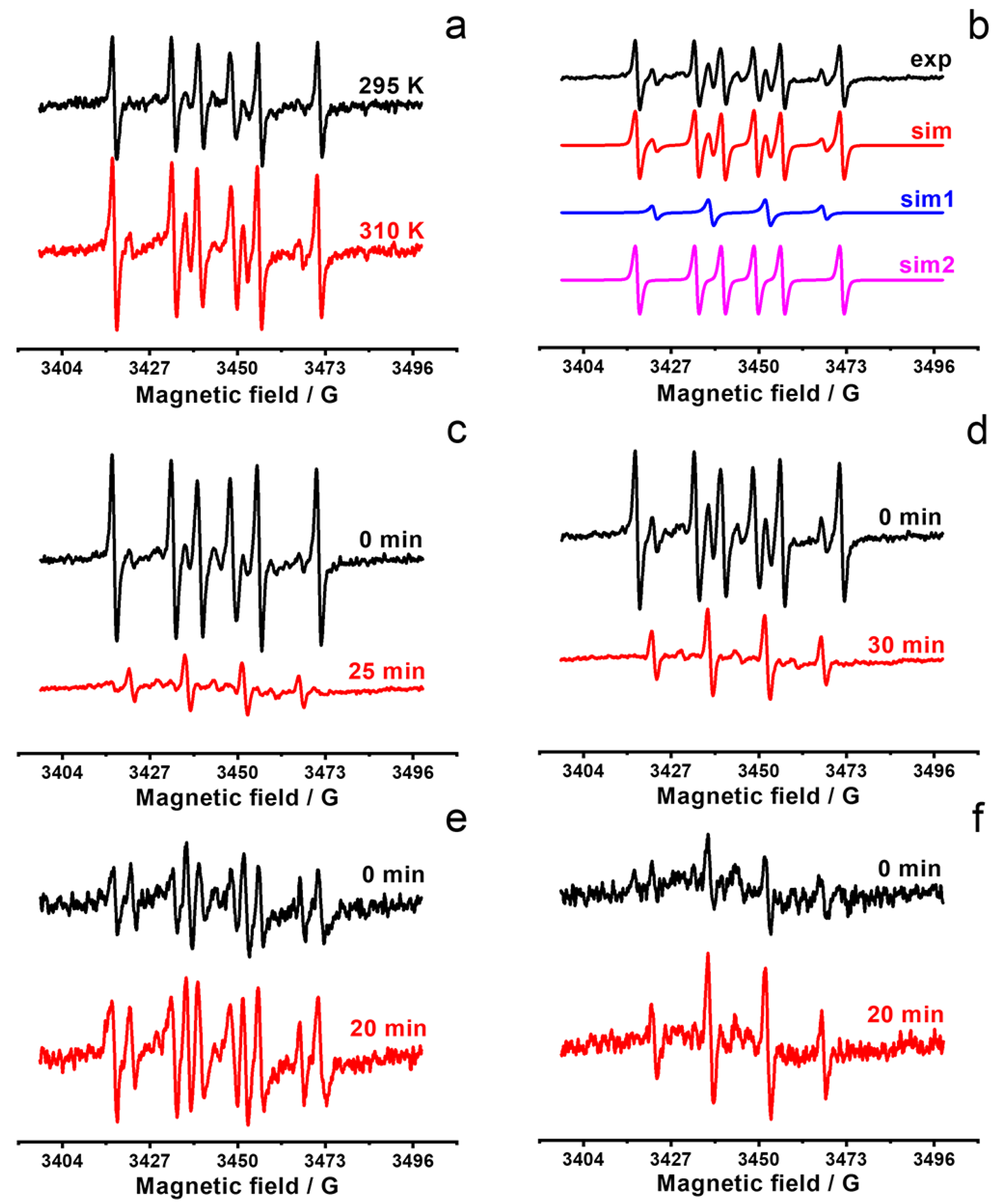
2.3. ROS Generation by F-127-Cu2I2L3 Nanoparticles
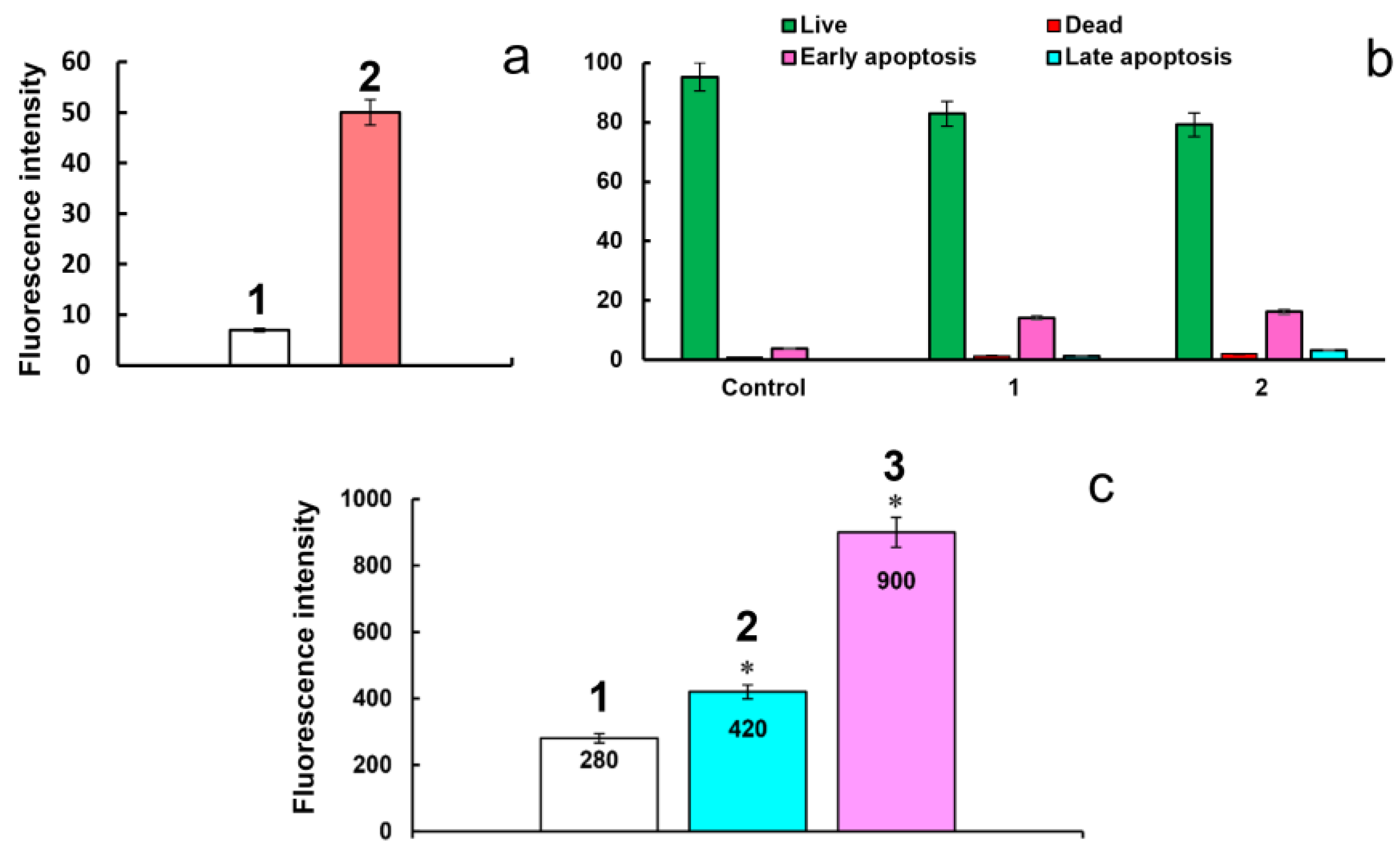
2.4. Cytotoxicity and Cell Internalization of F-127-Cu2I2L3 Nanoparticles
3. Materials and Methods
3.1. Reagents and Materials
3.2. Methods
3.2.1. Dynamic Light Scattering
3.2.2. Fluorescence Spectroscopy
3.2.3. NMR Spectroscopy
3.2.4. IR Spectroscopy
3.2.5. ICP-OES
3.2.6. ESI Measurements
3.2.7. TEM Measurements
3.2.8. Powder X-ray Diffraction (PXRD)
3.2.9. ESR Measurements
3.2.10. Cytotoxicity Assay
3.2.11. Cellular Uptake Study
3.2.12. Cell Apoptosis Analysis
3.2.13. Detection of Intracellular ROS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, S.Y.; Wang, Z.; Liu, Z.M.; Yu, H.J.; Zhang, J.H.; Wang, Y.; Mao, R.; Pan, M.; Su, C.Y. Multiresponsive UV-one-photon absorption, near-infrared-two-photon absorption, and X/γ-photoelectric absorption luminescence in one [Cu4I4] compound. Inorg. Chem. 2019, 58, 10736–10742. [Google Scholar] [CrossRef] [PubMed]
- Strelnik, I.; Shamsieva, A.; Akhmadgaleev, K.; Gerasimova, T.; Dayanova, I.; Kolesnikov, I.; Fayzullin, R.; Islamov, D.; Musina, E.; Karasik, A.; et al. Emission and Luminescent Vapochromism Control of Octahedral Cu4I4 Complexes by Conformationally Restricted P,N Ligands. Chem. Eur. J. 2022, 29, e202202864. [Google Scholar] [CrossRef]
- Artem’ev, A.V.; Baranov, A.Y.; Rakhmanova, M.I.; Malysheva, S.F.; Samsonenko, D.G. Copper(I) halide polymers derived from tris[2-(pyridin-2-yl)ethyl]phosphine: Halogen-tunable colorful luminescence spanning from deep blue to green. New J. Chem. 2020, 44, 6916–6922. [Google Scholar] [CrossRef]
- Boden, P.; Di Martino-Fumo, P.; Busch, J.M.; Rehak, F.R.; Steiger, S.; Fuhr, O.; Nieger, M.; Volz, D.; Klopper, W.; Brase, S.; et al. Investigation of Luminescent Triplet States in Tetranuclear CuI Complexes: Thermochromism and Structural Characterization. Chem. Eur. J. 2021, 27, 5439–5452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Djurovich, P.I.; Whited, M.T.; Thompson, M.E. Cu4I4 clusters supported by P∧N-type ligands: New structures with tunable emission colors. Inorg. Chem. 2012, 51, 230–236. [Google Scholar] [CrossRef]
- Liu, G.N.; Zhao, R.Y.; Xu, R.D.; Zhang, X.; Tang, X.N.; Dan, Q.J.; Wei, Y.W.; Tu, Y.Y.; Bo, Q.B.; Li, C. A novel tetranuclear copper (I) iodide metal–organic cluster [Cu4I4(Ligand)5] with highly selective luminescence detection of antibiotic. Cryst. Growth Des. 2018, 18, 5441–5448. [Google Scholar] [CrossRef]
- Xin, X.L.; Chen, M.; Ai, Y.B.; Yang, F.L.; Li, X.L.; Li, F. Aggregation-induced emissive copper (I) complexes for living cell imaging. Inorg. Chem. 2014, 53, 2922–2931. [Google Scholar] [CrossRef]
- Shamsieva, A.V.; Kolesnikov, I.E.; Strelnik, I.D.; Gerasimova, T.P.; Kalinichev, A.A.; Katsyuba, S.A.; Musina, E.I.; Lahderanta, E.; Karasik, A.A.; Sinyashin, O.G. Fresh look on the nature of dual-band emission of octahedral copper-iodide clusters—Promising ratiometric luminescent thermometers. J. Phys. Chem. C 2019, 123, 25863–25870. [Google Scholar] [CrossRef]
- Kang, Z.; Wu, C.; Dong, L.; Liu, W.; Mou, J.; Zhang, J.; Chang, Z.; Jiang, B.; Wang, G.; Kang, F.; et al. 3D porous copper skeleton supported zinc anode toward high capacity and long cycle life zinc ion batteries. ACS Sustain. Chem. Eng. 2019, 7, 3364–3371. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in copper complexes as anticancer agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Kozieł, S.; Zabierowski, P.; Kruszyński, R.; Lesiow, M.K.; Tisato, F.; Porchia, M.; Kyzioł, A. Copper (I) complexes with phosphines P(p-OCH3-Ph)2CH2OH and P(p-OCH3-Ph)2CH2SarGly. Synthesis, multimodal DNA interactions, and prooxidative and in vitro antiproliferative activity. J. Inorg. Biochem. 2020, 203, 110926. [Google Scholar] [CrossRef]
- Gandin, V.; Ceresa, C.; Esposito, G.; Indraccolo, S.; Porchia, M.; Tisato, F.; Santini, C.; Pellei, M.; Marzano, C. Therapeutic potential of the phosphino Cu (I) complex (HydroCuP) in the treatment of solid tumors. Sci. Rep. 2017, 7, 13936. [Google Scholar] [CrossRef]
- Luo, C.L.; Hu, C.X.; Shang, P.; Wen, G.Z.; Zhu, J.J.; Xuan, Y.H.; Xia, B.L.; Liu, Y.C.; Jiang, Z.H.; Dong, G.; et al. Synthesis of heteroleptic phosphine–copper (I) complexes: Fluorescence sensing and catalytic properties. New J. Chem. 2021, 45, 8910–8917. [Google Scholar] [CrossRef]
- Fanizza, E.; Mastrogiacomo, R.; Pugliese, O.; Guglielmelli, A.; De Sio, L.; Castaldo, R.; Scavo, M.P.; Giancaspro, M.; Rizzi, F.; Gentile, G.; et al. NIR-Absorbing Mesoporous Silica-Coated Copper Sulphide Nanostructures for Light-to-Thermal Energy Conversion. Nanomaterials 2022, 12, 2545. [Google Scholar] [CrossRef] [PubMed]
- Fedorenko, S.; Farvaeva, D.; Stepanov, A.; Bochkova, O.; Kholin, K.; Nizameev, I.; Drobyshev, S.; Gerasimova, T.; Voloshina, A.; Fanizza, E.; et al. Tricks for organic-capped Cu2-xS nanoparticles encapsulation into silica nanocomposites co-doped with red emitting luminophore for NIR activated-photothermal/chemodynamic therapy. J. Photochem. Photobiol. A 2022, 433, 114187. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Pucelik, B.; Wojtala, D.; Lesiów, M.K.; Stochel, G.; Kyzioł, A. Evaluation of anticancer activity in vitro of a stable copper (I) complex with phosphine-peptide conjugate. Sci. Rep. 2021, 11, 23943. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Kozieł, S.; Starosta, R.; Kyzioł, A. Selective Cu (I) complex with phosphine-peptide (SarGly) conjugate contra breast cancer: Synthesis, spectroscopic characterization and insight into cytotoxic action. J. Inorg. Biochem. 2018, 186, 162–175. [Google Scholar] [CrossRef]
- Kyzioł, A.; Cierniak, A.; Gubernator, J.; Markowski, A.; Jeżowska-Bojczuk, M.; Komarnicka, U.K. Copper (I) complexes with phosphine derived from sparfloxacin. Part III: Multifaceted cell death and preliminary study of liposomal formulation of selected copper (I) complexes. Dalton Trans. 2018, 47, 1981–1992. [Google Scholar] [CrossRef] [PubMed]
- Elistratova, J.; Faizullin, B.; Strelnik, I.; Gerasimova, T.; Khairullin, R.; Sapunova, A.; Voloshina, A.; Mukhametzyanov, T.; Musina, E.; Karasik, A.; et al. Impact of oppositely charged shell and cores on interaction of core-shell colloids with differently charged proteins as a route for tuning of the colloids cytotoxicity. Colloids Surf. B 2020, 196, 111306. [Google Scholar] [CrossRef]
- Zhang, K.; Meng, X.; Yang, Z.; Dong, H.; Zhang, X. Enhanced cancer therapy by hypoxia-responsive copper metal-organic frameworks nanosystem. Biomaterials 2020, 258, 120278. [Google Scholar] [CrossRef]
- Zhao, R.M.; Guo, Y.; Yang, H.Z.; Zhang, J.; Yu, X.Q. Zn-Promoted gene transfection efficiency for non-viral vectors: A mechanism study. New J. Chem. 2021, 45, 13549–13557. [Google Scholar] [CrossRef]
- Lin, G.; Zhang, Y.; Zhang, L.; Wang, J.; Tian, Y.; Cai, W.; Tang, S.; Chu, C.; Zhou, J.J.; Mi, P.; et al. Metal-organic frameworks nanoswitch: Toward photo-controllable endo/lysosomal rupture and release for enhanced cancer RNA interference. Nano Res. 2020, 13, 238–245. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, M.; Jin, G.; Jiang, Y.; Luan, Y. Cu-MOF chemodynamic nanoplatform via modulating glutathione and H2O2 in tumor microenvironment for amplified cancer therapy. J. Colloid Interface Sci. 2021, 587, 358–366. [Google Scholar] [CrossRef]
- Hwang, E.; Jung, H.S. Metal–organic complex-based chemodynamic therapy agents for cancer therapy. Chem. Commun. 2020, 56, 8332–8341. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Liu, F.; Zhang, S.; Duan, J.; Li, Z.; Kong, Y.; Sang, Y.; Liu, H.; Bu, W.; et al. Self-assembled copper–amino acid nanoparticles for in situ glutathione “AND” H2O2 sequentially triggered chemodynamic therapy. J. Am. Chem. Soc. 2018, 141, 849–857. [Google Scholar] [CrossRef] [PubMed]
- Enikeeva, K.R.; Shamsieva, A.V.; Strelnik, A.G.; Fayzullin, R.R.; Zakharychev, D.V.; Kolesnikov, I.E.; Dayanova, I.R.; Gerasimova, T.P.; Strelnik, I.D.; Musina, E.I.; et al. Green Emissive Copper (I) Coordination Polymer Supported by the Diethylpyridylphosphine Ligand as a Luminescent Sensor for Overheating Processes. Molecules 2023, 28, 706. [Google Scholar] [CrossRef]
- Elistratova, J.; Faizullin, B.; Shamsutdinova, N.; Gubaidullin, A.; Strelnik, I.; Babaev, V.; Kholin, K.; Nizameev, I.; Musina, E.; Khairullin, R.; et al. Synthesis of Au (I) complex-based aqueous colloids for sensing of biothiols. Inorg. Chim. Acta 2019, 485, 26–32. [Google Scholar] [CrossRef]
- Podyachev, S.N.; Zairov, R.R.; Mustafina, A.R. 1,3-Diketone calix[4]arene derivatives—A new type of versatile ligands for metal complexes and nanoparticles. Molecules 2021, 26, 1214. [Google Scholar] [CrossRef] [PubMed]
- Musina, E.I.; Shamsieva, A.V.; Strelnik, I.D.; Gerasimova, T.P.; Krivolapov, D.B.; Kolesnikov, I.E.; Grachova, E.V.; Tunik, S.P.; Bannwarth, C.; Grimme, S.; et al. Synthesis of novel pyridyl containing phospholanes and their polynuclear luminescent copper (I) complexes. Dalton Trans. 2016, 45, 2250–2260. [Google Scholar] [CrossRef] [PubMed]
- Zink, D.M.; Baumann, T.; Friedrichs, J.; Nieger, M.; Bräse, S. Copper (I) complexes based on five-membered P∧N heterocycles: Structural diversity linked to exciting luminescence properties. Inorg. Chem. 2013, 52, 13509–13520. [Google Scholar] [CrossRef]
- Zink, D.M.; Volz, D.; Baumann, T.; Mydlak, M.; Flügge, H.; Friedrichs, J.; Nieger, M.; Bräse, S. Heteroleptic, dinuclear copper (I) complexes for application in organic light-emitting diodes. Chem. Mater. 2013, 25, 4471–4486. [Google Scholar] [CrossRef]
- Mondal, R.; Lozada, I.B.; Davis, R.L.; Williams, J.G.; Herbert, D.E. Exploiting synergy between ligand design and counterion interactions to boost room temperature phosphorescence from Cu (I) compounds. J. Mater. Chem. C 2019, 7, 3772–3778. [Google Scholar] [CrossRef]
- Hofbeck, T.; Niehaus, T.A.; Fleck, M.; Monkowius, U.; Yersin, H. P∩N Bridged Cu (I) Dimers Featuring Both TADF and Phosphorescence. From Overview towards Detailed Case Study of the Excited Singlet and Triplet States. Molecules 2021, 26, 3415. [Google Scholar] [CrossRef] [PubMed]
- Zink, D.M.; Bächle, M.; Baumann, T.; Nieger, M.; Kühn, M.; Wang, C.; Klopper, W.; Monkowius, U.; Hofbeck, T.; Yersin, H.; et al. Synthesis, structure, and characterization of dinuclear copper (I) halide complexes with P^N ligands featuring exciting photoluminescence properties. Inorg. Chem. 2013, 52, 2292–2305. [Google Scholar] [CrossRef]
- Abbas, K.; Babić, N.; Peyrot, F. Use of spin traps to detect superoxide production in living cells by electron paramagnetic resonance (EPR) spectroscopy. Methods 2016, 109, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Aliaga, M.E.; López-Alarcón, C.; Bridi, R.; Speisky, H. Redox-implications associated with the formation of complexes between copper ions and reduced or oxidized glutathione. J. Inorg. Biochem. 2016, 154, 78–88. [Google Scholar] [CrossRef]
- Walsh, M.J.; Ahner, B.A. Determination of stability constants of Cu (I), Cd (II) & Zn (II) complexes with thiols using fluorescent probes. J. Inorg. Biochem. 2013, 128, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.T.; Nguyen, L.A.H.; Hancock, H.L.; Fahrni, C.J. Glutathione limits aquacopper (I) to sub-femtomolar concentrations through cooperative assembly of a tetranuclear cluster. J. Biol. Chem. 2017, 292, 21558–21567. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Xiao, C.; Li, Z.; Yang, X. Engineering nanomedicine for glutathione depletion-augmented cancer therapy. Chem. Soc. Rev. 2021, 50, 6013–6041. [Google Scholar] [CrossRef]
- Carini, M.; Aldini, G.; Orioli, M.; Facino, R.M. Electron paramagnetic resonance (EPR) spectroscopy: A versatile and powerful tool in pharmaceutical and biomedical analysis. Curr. Pharm. Anal. 2006, 2, 141–159. [Google Scholar] [CrossRef]
- Angelé-Martínez, C.; Nguyen, K.V.T.; Ameer, F.S.; Anker, J.N.; Brumaghim, J.L. Reactive oxygen species generation by copper (II) oxide nanoparticles determined by DNA damage assays and EPR spectroscopy. Nanotoxicology 2017, 11, 278–288. [Google Scholar] [CrossRef]
- Hedrick, W.R.; Webb, M.D.; Zimbrick, J.D. Spin trapping of reactive uracilyl radicals produced by ionizing radiation in aqueous solutions. Int. J. Radiat. Biol. Relat. Stud. Phys. Chem. Med. 1982, 41, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Kirino, Y.; Ohkuma, T.; Kwan, T. Spin trapping with 5,5-dimethylpyrroline-N-oxide in aqueous solution. Chem. Pharm. Bull. 1981, 29, 29–34. [Google Scholar] [CrossRef]
- Luo, B.; Chen, L.; Hong, Z.; You, X.; Huang, F.P.; Bian, H.D.; Zhang, L.; Zhao, S. A simple and feasible atom-precise biotinylated Cu (I) complex for tumor-targeted chemodynamic therapy. Chem. Commun. 2021, 57, 6046–6049. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, K.R.; Marsh, J.; Chechik, V. Formation of self-inhibiting copper (II) nanoparticles in an autocatalytic Fenton-like reaction. Dalton Trans. 2014, 43, 4745–4751. [Google Scholar] [CrossRef]
- Bykowska, A.; Komarnicka, U.K.; Jeżowska-Bojczuk, M.; Kyzioł, A. CuI and CuII complexes with phosphine derivatives of fluoroquinolone antibiotics—A comparative study on the cytotoxic mode of action. J. Inorg. Biochem. 2018, 181, 1–10. [Google Scholar] [CrossRef]
- Hu, J.; Mao, R.; Wang, R.; Ruan, H.; Zhao, X.; Li, K.; Guo, Y. Cu(I)-benzimidazole complexes with triphenylphosphine as coligand: DNA lesion and reactive oxygen-dependent mitochondrial dysfunction inducing apoptosis. Inorg. Chim. Acta 2023, 546, 121333. [Google Scholar] [CrossRef]
- Delgado, A.V.; Gonzalez-Caballero, F.; Hunter, R.J.; Koopal, L.K.; Lyklema, J. Measurement and interpretation of electro-kinetic phenomena. J. Colloid Interface Sci. 2007, 309, 194–224. [Google Scholar] [CrossRef]
- Bruker AXS. DIFFRAC Plus Evaluation Package EVA, Version 11, User’s Manual; Bruker AXS: Karlsruhe, Germany, 2005; 258p. [Google Scholar]
- Bruker AXS. TOPAS V3: General Profile and Structure Analysis Software for Powder Diffraction Data; Technical Reference; Bruker AXS: Karlsruhe, Germany, 2005; 117p. [Google Scholar]
- AAT Bioquest, Inc. Quest Graph™ IC50 Calculator. Available online: https://www.aatbio.com/tools/ic50-calculator (accessed on 1 February 2023).
| dav, nm | dnum, nm | PDI | ζ, mV | |
|---|---|---|---|---|
| H2O | 186 ± 2 | 163 ± 13 | 0.089 ± 0.024 | +9 ± 8 |
| Storage for 1.5 month: | ||||
| H2O * | 205 ± 2 | 165 ± 24 | 0.145 ± 0.006 | −20 ± 6 |
| H2O ** | 170 ± 5 | 150 ± 7 | 0.075 ± 0.009 | +11 ± 8 |
| After heating colloids up to 40 °C: | ||||
| H2O * | 202 ± 3 | 158 ± 28 | 0.214 ± 0.006 | −6 ± 4 |
| H2O ** | 186 ± 3 | 166 ± 1 | 0.129 ± 0.024 | −20 ± 6 |
| Buffered solutions: | ||||
| pH = 4 | 171 ± 4 | 151 ± 9 | 0.081 ± 0.012 | +8 ± 7 |
| pH = 4 *** | 164 ± 5 | 151 ± 5 | 0.055 ± 0.022 | +10 ± 6 |
| pH = 7 | 170 ± 1 | 146 ± 8 | 0.086 ± 0.016 | −5 ± 7 |
| pH = 7 *** | 172 ± 3 | 155 ± 2 | 0.098 ± 0.004 | −4 ± 8 |
| pH = 10 | 172 ± 4 | 144 ± 10 | 0.076 ± 0.012 | −5 ± 7 |
| pH = 10 *** | 167 ± 3 | 149 ± 6 | 0.065 ± 0.012 | −5 ± 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faizullin, B.A.; Elistratova, J.G.; Strelnik, I.D.; Akhmadgaleev, K.D.; Gubaidullin, A.T.; Kholin, K.V.; Nizameev, I.R.; Babaev, V.M.; Amerhanova, S.K.; Voloshina, A.D.; et al. Luminescent Water-Dispersible Nanoparticles Engineered from Copper(I) Halide Cluster Core and P,N-Ligand with an Optimal Balance between Stability and ROS Generation. Inorganics 2023, 11, 141. https://doi.org/10.3390/inorganics11040141
Faizullin BA, Elistratova JG, Strelnik ID, Akhmadgaleev KD, Gubaidullin AT, Kholin KV, Nizameev IR, Babaev VM, Amerhanova SK, Voloshina AD, et al. Luminescent Water-Dispersible Nanoparticles Engineered from Copper(I) Halide Cluster Core and P,N-Ligand with an Optimal Balance between Stability and ROS Generation. Inorganics. 2023; 11(4):141. https://doi.org/10.3390/inorganics11040141
Chicago/Turabian StyleFaizullin, Bulat A., Julia G. Elistratova, Igor D. Strelnik, Kamil D. Akhmadgaleev, Aidar T. Gubaidullin, Kirill V. Kholin, Irek R. Nizameev, Vasily M. Babaev, Syumbelya K. Amerhanova, Alexandra D. Voloshina, and et al. 2023. "Luminescent Water-Dispersible Nanoparticles Engineered from Copper(I) Halide Cluster Core and P,N-Ligand with an Optimal Balance between Stability and ROS Generation" Inorganics 11, no. 4: 141. https://doi.org/10.3390/inorganics11040141
APA StyleFaizullin, B. A., Elistratova, J. G., Strelnik, I. D., Akhmadgaleev, K. D., Gubaidullin, A. T., Kholin, K. V., Nizameev, I. R., Babaev, V. M., Amerhanova, S. K., Voloshina, A. D., Gerasimova, T. P., Karasik, A. A., Sinyashin, O. G., & Mustafina, A. R. (2023). Luminescent Water-Dispersible Nanoparticles Engineered from Copper(I) Halide Cluster Core and P,N-Ligand with an Optimal Balance between Stability and ROS Generation. Inorganics, 11(4), 141. https://doi.org/10.3390/inorganics11040141








