Synthesis and Characterization of Pt(II) and Pd(II) Complexes with Planar Aromatic Oximes
Abstract
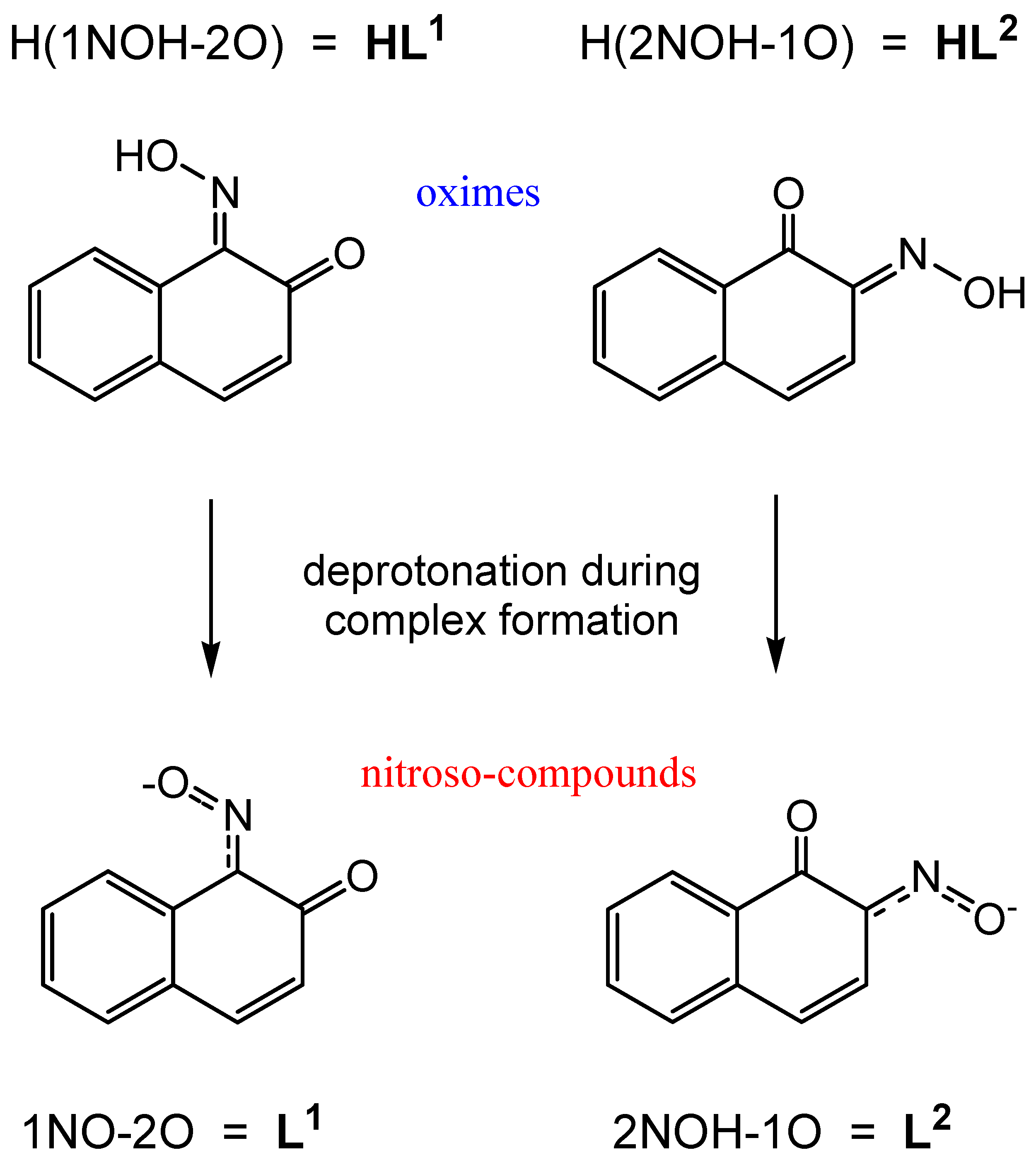
1. Introduction
2. The Experimental Part
2.1. General Considerations
2.2. Syntheses of Coordination Compounds
2.3. Spectroscopy
2.4. Thermal Analysis
2.5. Crystallographic Work
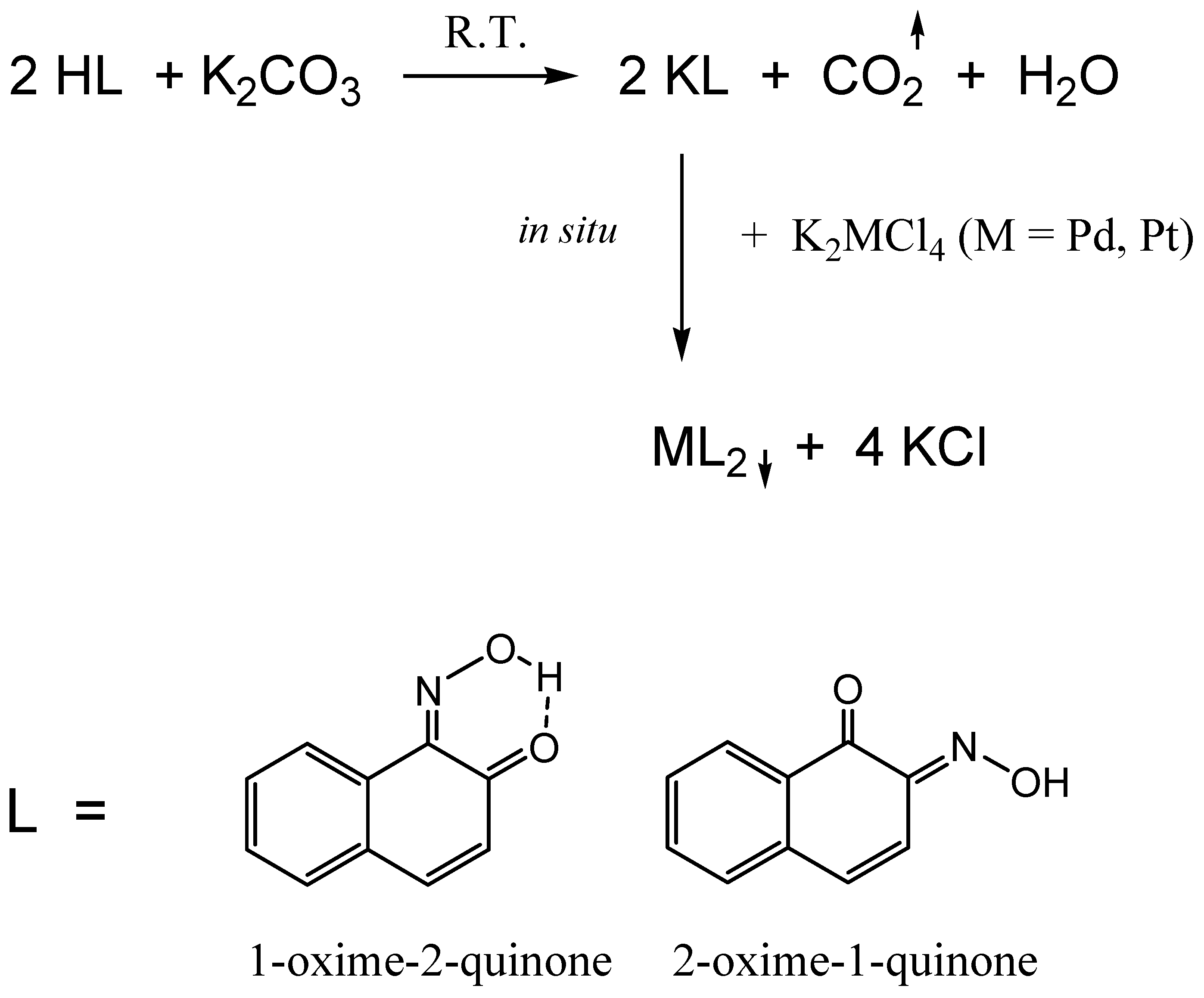
3. Results and Discussion
3.1. Thermal Analysis
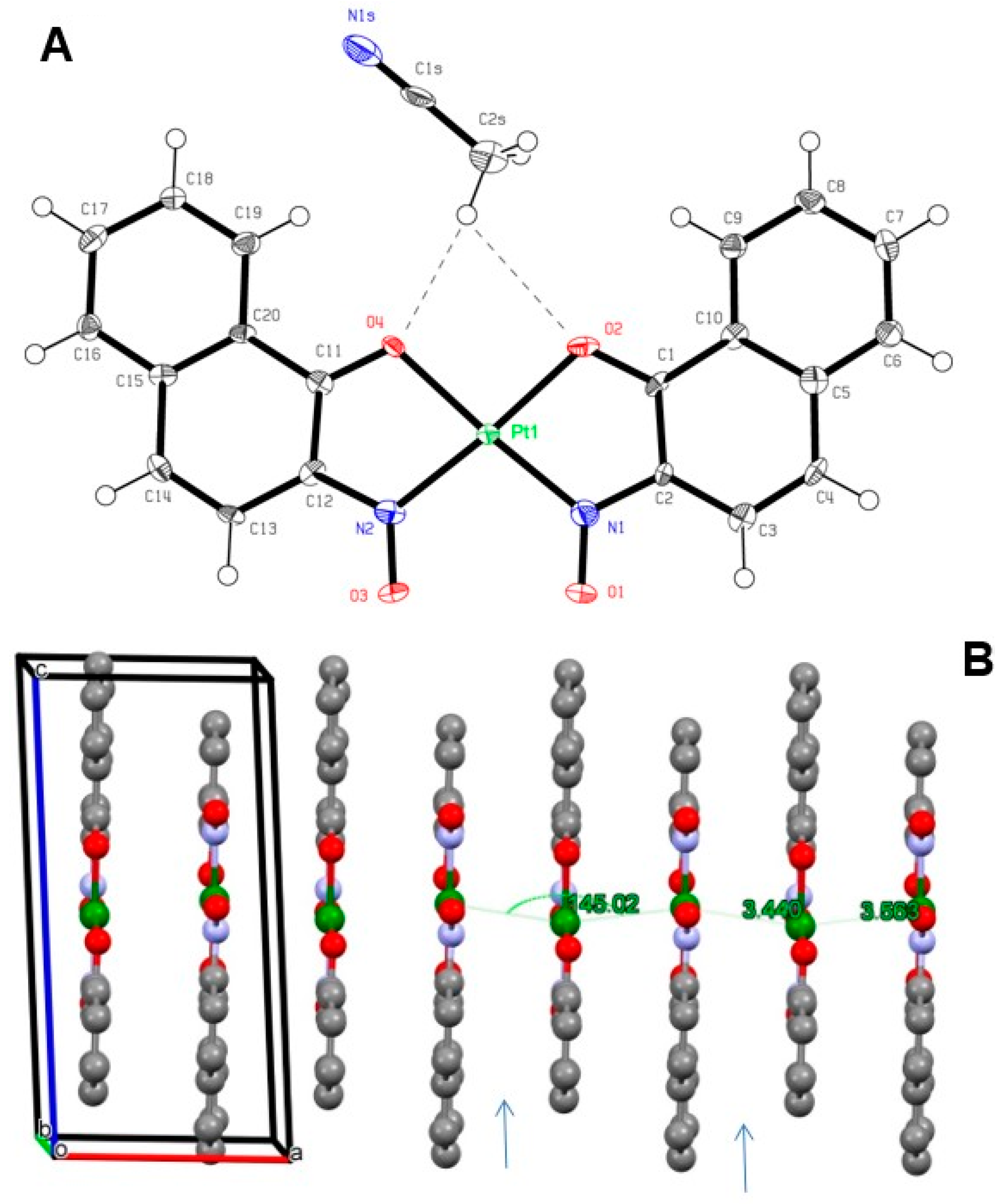
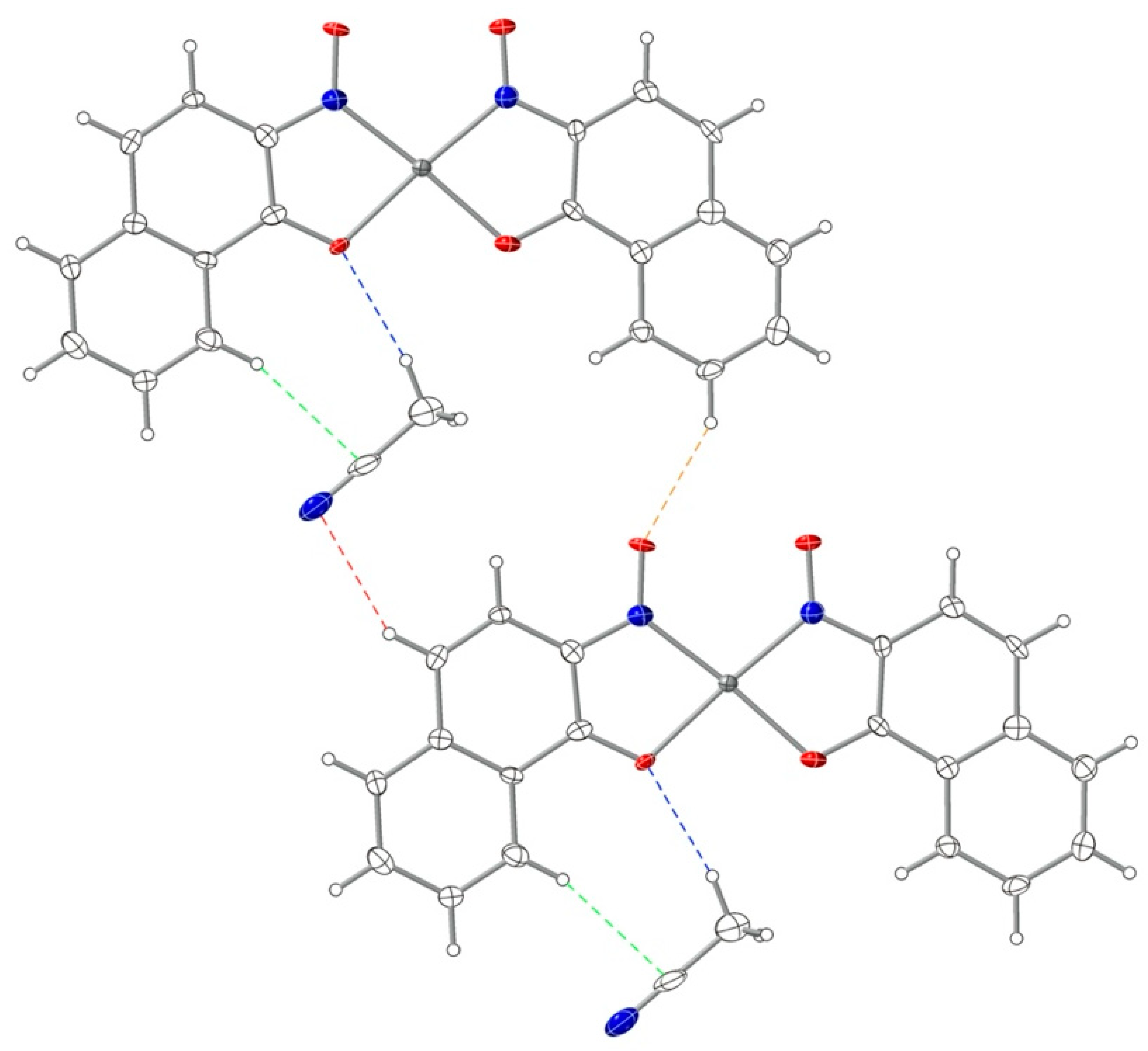
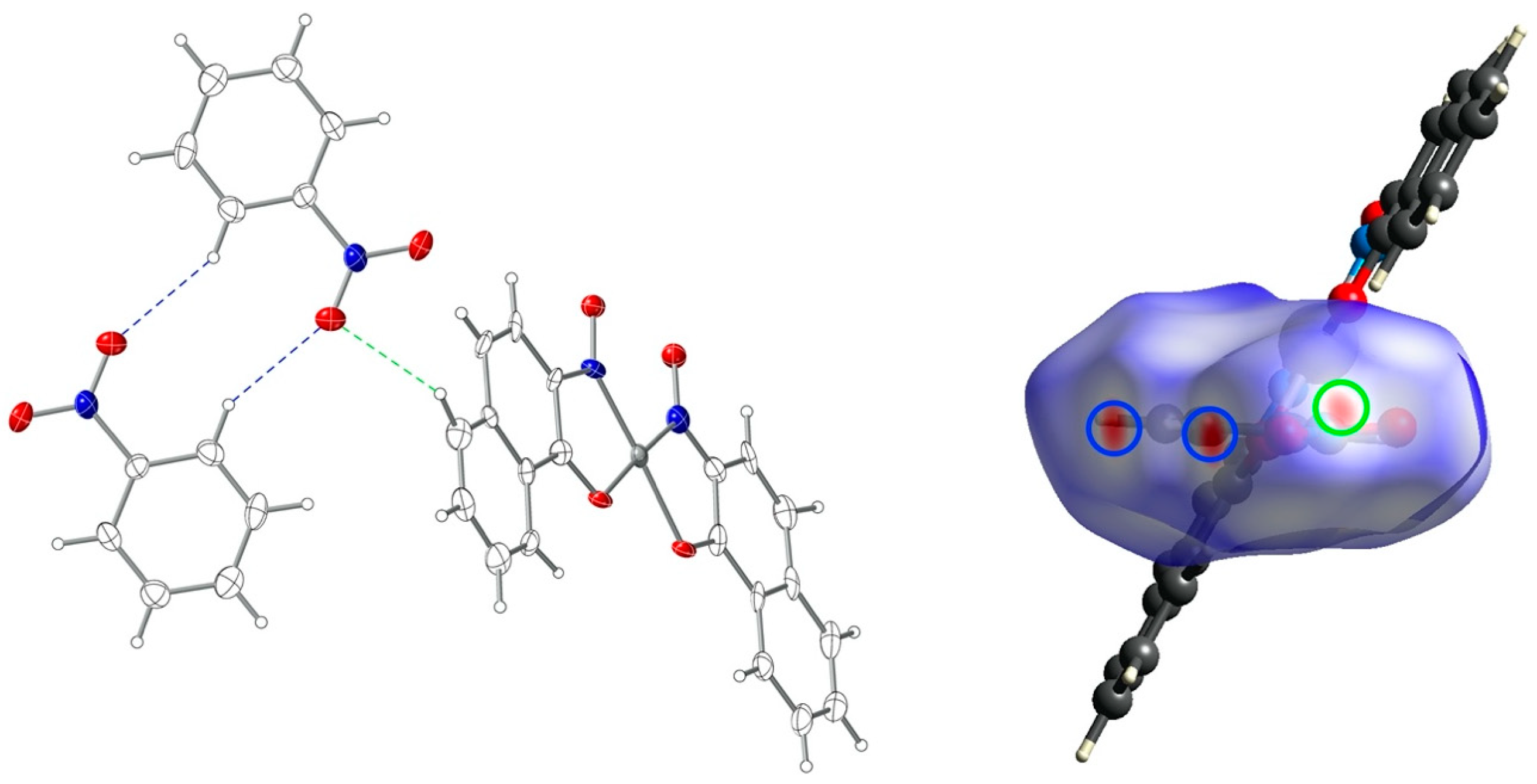
3.2. Crystal Structures

3.3. Spectroscopic Studies
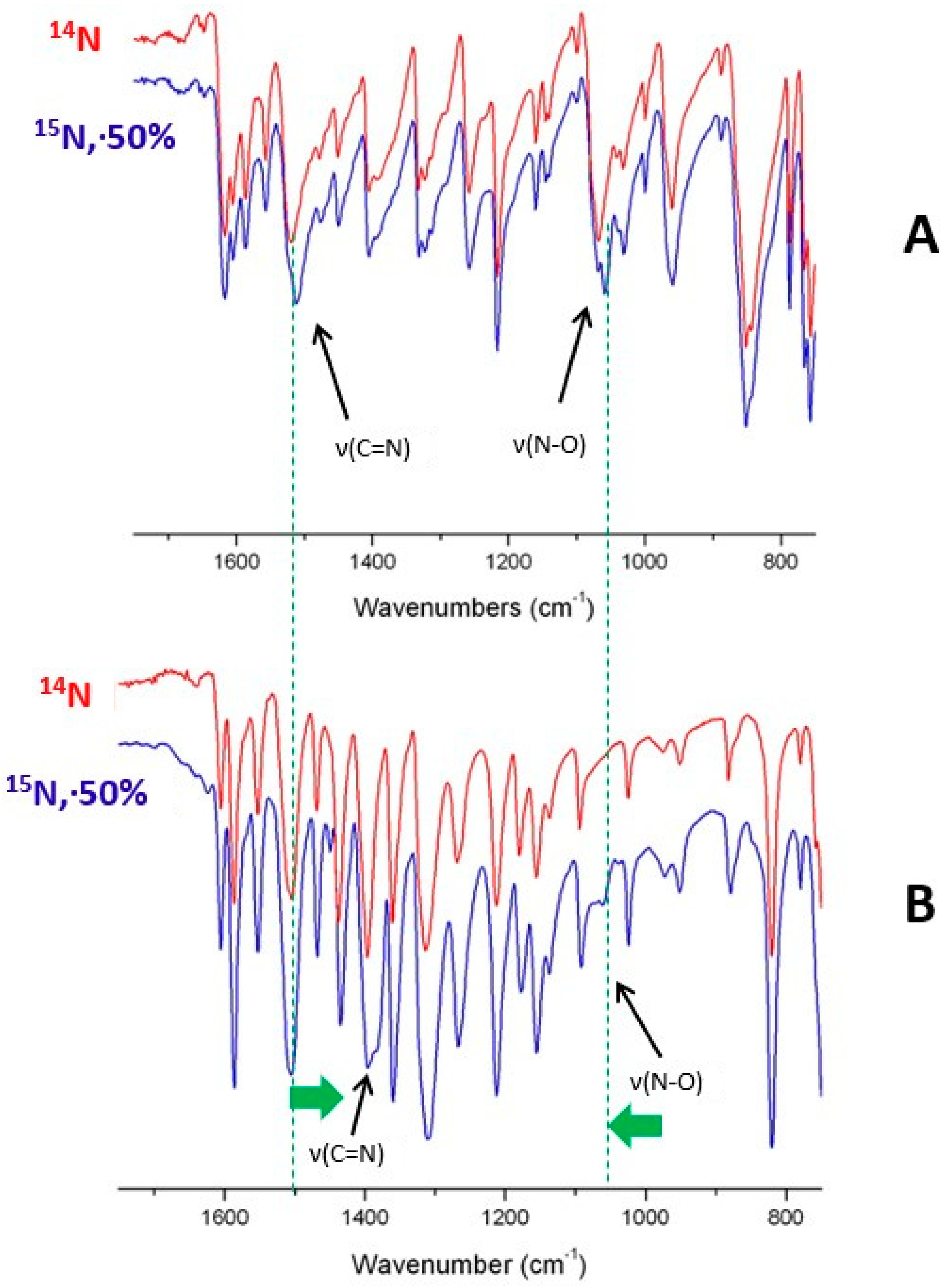
3.3.1. Vibrational Spectra
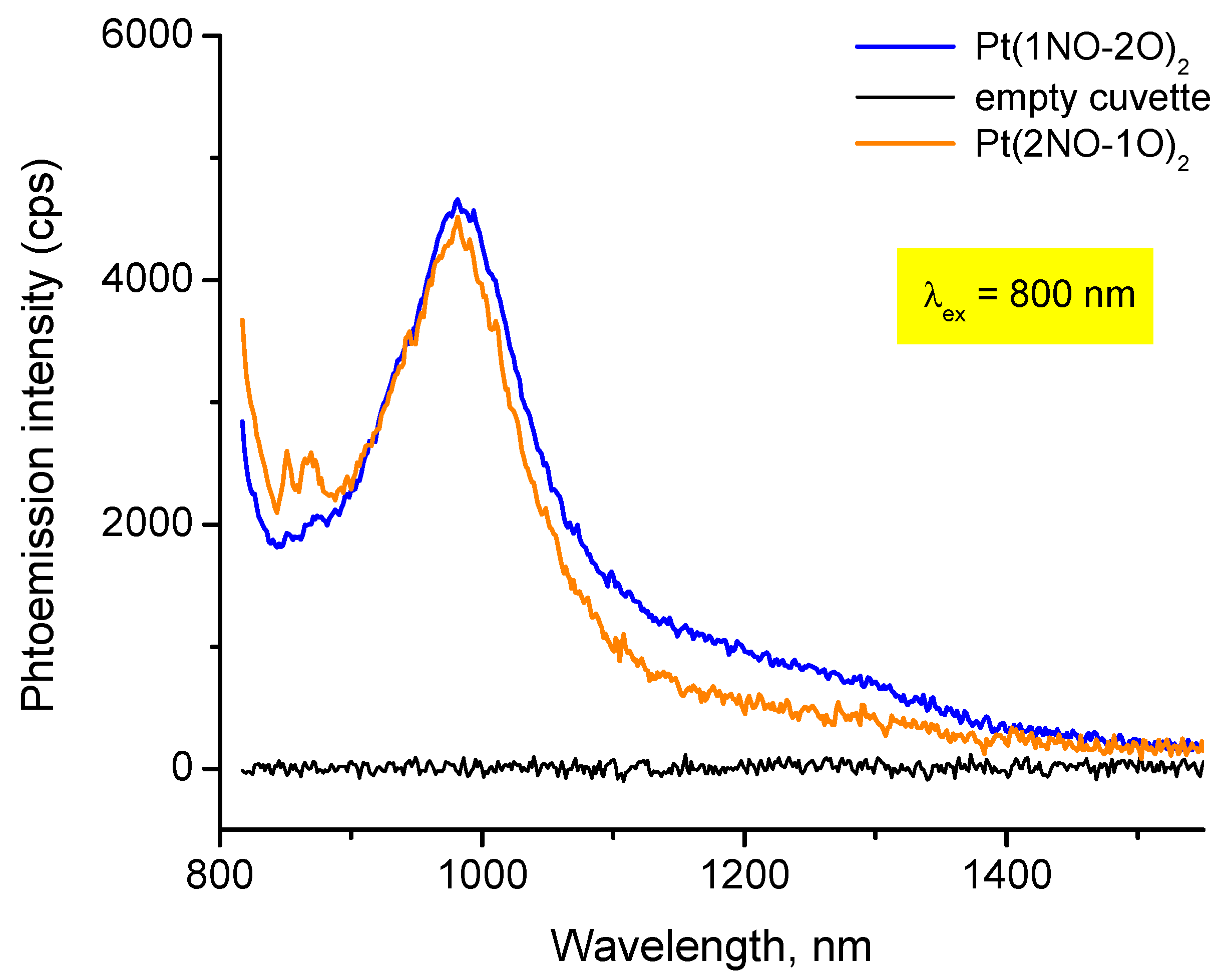
3.3.2. Electronic Spectra
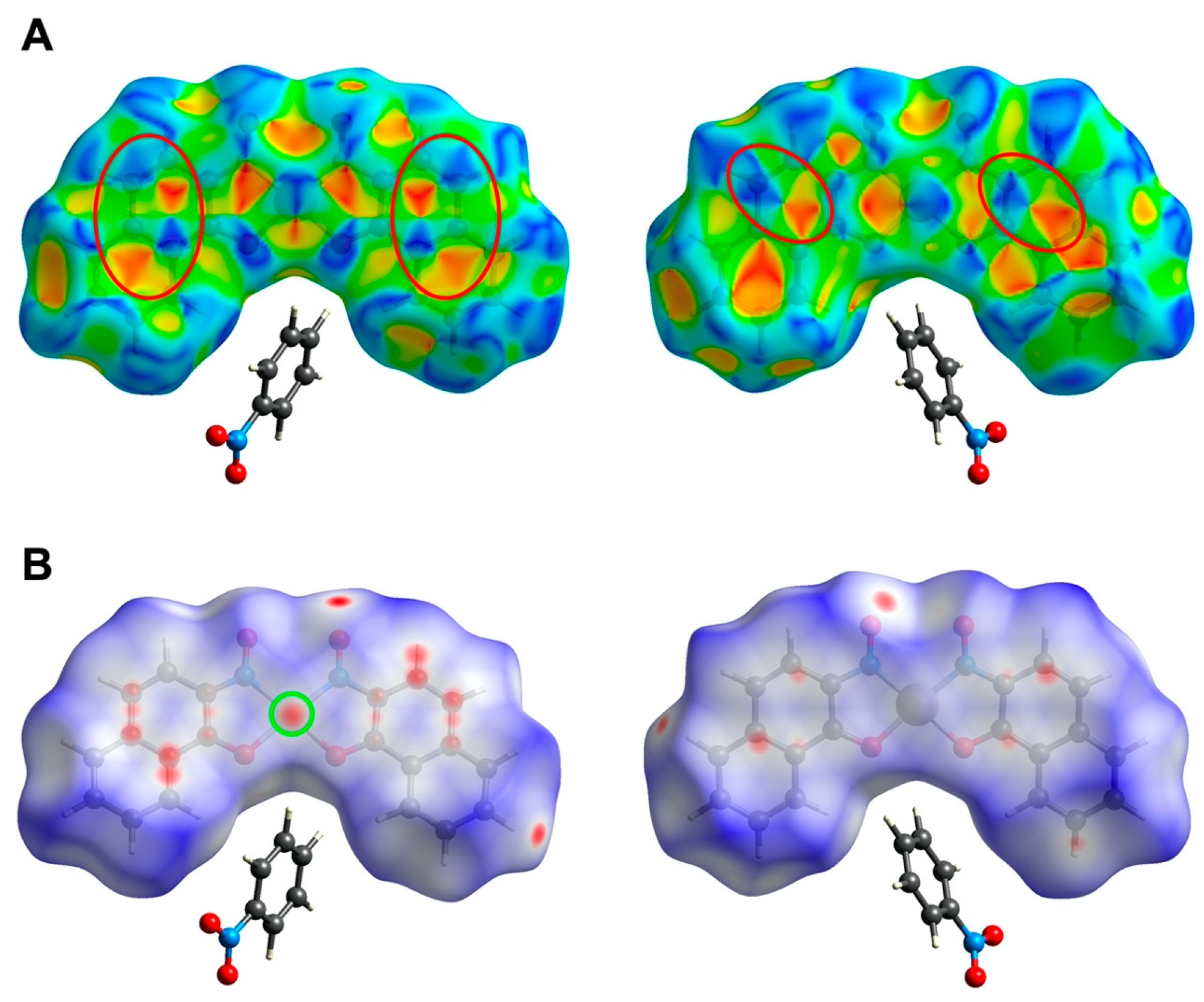
3.4. Hirshfeld Surface Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chakravorty, A. Structural Chemistry of Transition Metal Complexes of Oximes. Coord. Chem. Rev. 1974, 13, 1–46. [Google Scholar] [CrossRef]
- Knight, J.A.; Robertson, G.; Wu, J.T. The Chemical Basis and Specificity of the Nitrosonaphthol Reaction. Clin Chem. 1983, 29, 1969–1971. [Google Scholar] [CrossRef] [PubMed]
- Burger, K.; Belcher, R.; Freiser, H. Organic Reagents in Metal Analysis; Elsevier: Amsterdam, The Netherlands, 1973; Volume 54. [Google Scholar]
- Kukushkin, V.Y.; Pombeiro, A.J.L. Oxime and Oximate Metal Complexes: Unconventional Synthesis and Reactivity. Coord. Chem. Rev. 1999, 181, 147–175. [Google Scholar] [CrossRef]
- Chernov’yants, M.; Gus’kova, T.; Bagdasarov, K.; Nikitenko, I. Spectrophotometric Studies of the Reaction of Heteroarylcyanoximes with Fe(II) in Solutions. Russ. J. Anal. Chem. 1984, 39, 798–800. [Google Scholar]
- Chernov’yants, M.; Chernoivanova, T.; Bagdasarov, K.; Chernoivanov, V.; Vladimirova, E. Method of Photometric Detection of Fe(II) in Solutions. Russ. J. Analyt. Chem. 1979, 34, 236–240. [Google Scholar]
- Rollins, O.W.; Oldham, M.M. Spectrophotometric Determination of Rhodium with Nitroso R. Formula for the Rh(III)-nitroso R Complex. Anal. Chem. 1971, 43, 146–148. [Google Scholar] [CrossRef]
- Cannon, G. (Ed.) Oximes: Structure, Properties, and Applications; EBSCO: Ipswich, MA, USA, 2020; ISBN 978-1-53617-358-1. [Google Scholar]
- Botella, L.; Najera, C. A Convenient Oxime-carbapalladocycle-catalyzed Suzuki Cross-coupling of Arylchlorides in Water. Angew. Chem. Int. Ed. 2002, 41, 179–181. [Google Scholar] [CrossRef]
- Kolmel, D.K.; Kool, E.T. Oximes and Hydrazones in Bioconjugation: Mechanism and Catalysis. Chem. Rev. 2017, 117, 10358–10376. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Zhang, D.; Liu, Q.; Dong, Y.; Liu, H. Transition Metal-catalyzed Reactions Involving Oximes. Adv. Synth. Catal. 2017, 359, 710–771. [Google Scholar] [CrossRef]
- Abele, E.; Abele, R.; Lukevics, E. Pyridine Oximes: Synthesis, Reactions, and Biological Activity (Review). Chem. Heterocycl. Comp. 2003, 39, 825–865. [Google Scholar] [CrossRef]
- Dhuguru, J.; Zviagin, E.; Skouta, R. FDA-approved Oximes and their Significance in Medicinal Chemistry. Pharmaceuticals 2022, 15, 66. [Google Scholar] [CrossRef]
- Ramon Subirós-Funosas, S.N.K. Lidia Nieto-Rodríguez, Ayman El-Faham, Fernando Albericio, Advances in Acylation Methodologies Enabled by Oxyma-Based Reagents. Aldrichim. Acta 2013, 46, 21–40. [Google Scholar]
- Gerasimchuk, N.; Guzei, I.; Sipos, P. Structural Peculiarities of Cyanoximes and their Anions: Co-crystallization of Two Diastereomers and Formation of Acid-salts. Curr. Inorg. Chem. 2015, 5, 38–63. [Google Scholar] [CrossRef]
- Dean, J.A. Lange’s Handbook of Chemistry, 15th ed.; McGraw-Hill: New York, NY, USA, 1998. [Google Scholar]
- CRC Handbook of Chemistry and Physics; CRC Press, Chemical Rubber Co.: Boca Raton, FL, USA, 1974; Volume 56.
- Lada, Z.; Polyzou, C.D.; Nika, V.; Stamatatos, T.C.; Konidaris, K.F.; Perlepes, S.P. Adventures in the Coordination Chemistry of 2-pyridyl Oximes: On the Way to 3d/4f Metal Coordinated Clusters. Inorg. Chim. Acta 2022, 539, 120954. [Google Scholar] [CrossRef]
- Mokhir, A.A.; Domasevich, K.V.; Kent Dalley, N.; Kou, X.; Gerasimchuk, N.N.; Gerasimchuk, O.A. Syntheses, Crystal Structures and Coordination Compounds of Some 2-hetarylcyanoximes. Inorg. Chim. Acta 1999, 284, 85–98. [Google Scholar] [CrossRef]
- Ilinski, M.; Knorre, G.V. Uber eine neue Methode zur Trennung von Eisen und Aluminum. Ber. Deutsch. Ges. 1885, 18, S.2728–S.2734. [Google Scholar] [CrossRef]
- Barkovsii, V.F.; Solonenko, V.G. Uses of 1-nitroso-2-naphthol (Review) [Russ]. Proc. Ural. Res. Center Acad. Sci. USSR 1974, 27, 3–13. [Google Scholar]
- Irving, H.M.N.H. Coordination Compounds in Analytical Chemistry. Pure Appl. Chem. 1978, 50, 1129–1146. [Google Scholar] [CrossRef]
- Marczenko, M.B.Z. Separation, Preconcentration and Spectrophotometry in Inorganic Analysis; Elsewier Science: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Kržan, A.; Mavri, J. Nitroso-naphthol quinone-monooxime Tautomeric Equilibrium Revisited: Evidence for Oximo Group Isomerization. Chem. Phys. 2002, 277, 71–76. [Google Scholar] [CrossRef]
- Shchavlev, A.E.; Pankratov, A.N.; Enchev, V. Intramolecular Hydrogen-Bonding Interactions in 2-Nitrosophenol and Nitrosonaphthols: Ab Initio, Density Functional, and Nuclear Magnetic Resonance Theoretical Study. J. Phys. Chem. A 2007, 111, 7112–7123. [Google Scholar] [CrossRef]
- Dyrssen, D.; Johansson, E. Studies of the Extraction of Metal Complexes. Acta Chim. Scand. 1955, 9, 763–770. [Google Scholar] [CrossRef]
- French, H.S.; Perkins, D.J. A Study of the Tautomeric Equilibria of Nitrosonaphthol-Naphthoquinoneoxime Systems1. J. Am. Chem. Soc. 1937, 59, 1182–1186. [Google Scholar] [CrossRef]
- Baltazzi, E. Sur l’equilibre Tautomerique des Alfa-nitrosonaphtols. Comptes Rendus 1951, 5, 116–123. [Google Scholar]
- Ivanova, G.; Enchev, V. Does Tautomeric Equilibrium Exist in Ortho-nitrosonaphthols? Chem. Phys. 2001, 264, 235–244. [Google Scholar] [CrossRef]
- Tyukhtenko, S.I.; Hilton, M.; Gerasimchuk, N. Classic Isomeric 1,2- and 2,1-Nitrosonaphthols are Oximes in Solid State and Solutions. Curr. Inorg. Chem. 2015, 5, 120–136. [Google Scholar] [CrossRef]
- Gerasimchuk, N.N.; Tyukhtenko, S.I. Inorganic Synthesis. Laboratory Manual; Cambridge Scholars Publisher: Newcastle upon Tyne, UK, 2019; p. 386. ISBN 978-1-5275-3920-4. [Google Scholar]
- Eddings, D.; Barnes, C.; Gerasimchuk, N.; Durham, P.; Domasevich, K. First Bivalent Palladium and Platinum Cyanoximates: Synthesis, Characterization and Biological Activity. Inorg. Chem. 2004, 43, 3894–3909. [Google Scholar] [CrossRef] [PubMed]
- George Sheldrick in Bruker APEX2 and APEX3 Software Suits; Bruker AXS, Inc.: Madison, WI, USA, 2016.
- Farrugia, L.J. ORTEP-3 for Windows—A Version of ORTEP-III with a Graphical User Interface (GUI). J. Appl. Cryst. 1997, 30, 565. [Google Scholar] [CrossRef]
- Mercury Software, 4.2 ed.; CCDC (Cambridge Crystal Data Centre, Cambridge): Cambridge, UK, 2016.
- Opalade, A.A.; Gomez-Garcia, C.J.; Gerasimchuk, N. New Route to Polynuclear Ni(II) and Cu(II) Complexes with Bridging Oxime Groups That Are Inaccessible by Conventional Preparations. Cryst. Growth Des. 2019, 19, 678–693. [Google Scholar] [CrossRef]
- McDaniel, P. Preparation and Characterization of Lead(II) Cyanoximates. Master’s Thesis, Missouri State University, Springfield, MO, USA, 2022; p. 145. [Google Scholar]
- Cheadle, C.; Ratcliff, J.; Berezin, M.; Pal’shin, V.; Nemykin, V.N.; Gerasimchuk, N. Shortwave Infrared Luminescent Pt-nanowires: A Mechanistic Study of Emission in Solution and in the Solid State. Dalton Trans. 2017, 46, 13562–13581. [Google Scholar] [CrossRef]
- Dannen, S.D.; Cornelison, L.; Durham, P.; Morley, J.E.; Shahverdi, K.; Du, J.; Zhou, H.; Sudlow, L.C.; Hunter, D.; Wood, M.D.; et al. New in vitro Highly Cytotoxic Platinum and Palladium Cyanoximates with Minimal Side Effects in vivo. J. Inorg. Biochem. 2020, 208, 111082. [Google Scholar] [CrossRef] [PubMed]
- Klaus, D.R.; Keene, M.; Silchenko, S.; Berezin, M.; Gerasimchuk, N. 1D Polymeric Platinum Cyanoximate: A Strategy toward Luminescence in the Near-Infrared Region beyond 1000 nm. Inorg. Chem. 2015, 54, 1890–1900. [Google Scholar] [CrossRef] [PubMed]
- Opalade, A.A.; Labadi, I.; Gomez-Garcia, C.; Hietsoi, O.; Gerasimchuk, N. Nickel(II) Aqua Complexes with Chelating Ligands: What Happens When Water Is Gone? Cryst. Growth Des. 2022, 22, 6168–6182. [Google Scholar] [CrossRef]
- Ponomareva, V.V.; Domasevich, K.V.; Kou, X.; Gerasimchuk, N.N.; Dalley, N.K.; Skopenko, V.V. Synthesis, Spectroscopic Investigation and Crystal Structures of Coordination Compounds of Cu(II) with Benzoylcyanoxime. Russ. J. Inorg. Chem. 1997, 42, 53–60. [Google Scholar]
- Domasevitch, K.V.; Lindeman, S.V.; Struchkov, Y.; Gerasimchuk, N.N.; Zhmurko, O.A. Synthesis and Investigation of Copper(II) Complexes with N,N-dimethylacetamid-cyanoximate-ion: ONC(CN)C(O)N(CH3)2-. Russ. J. Inorg. Chem. 1993, 38, 98–103. [Google Scholar]
- Owen, T.; Grandjean, F.; Long, G.J.; Domasevitch, K.V.; Gerasimchuk, N. Synthesis and Characterization of Two Intensely Colored tris(benzoylcyanoxime)iron(II) Anionic Complexes. Inorg. Chem. 2008, 47, 8704–8713. [Google Scholar] [CrossRef]
- Marcano, D.C.; Lindeman, S.V.; Pyrkosz-Bulska, M.; Gumienna-Kontecka, E.; Lengyel, A.; Kuzmann, E.; Röminger, F.; Gerasimchuk, N. The 2-Pyridylcyanoxime and its Complexes. Curr. Inorg. Chem. 2015, 5, 98–113. [Google Scholar] [CrossRef]
- Kolbe, A.; Köhler, H. Zum IR-spectrum des Nitrosodicyanmethanid-ions [ONC(CN)2]−. Z. Anorg. Allg. Chem. 1970, 373, 230–236. [Google Scholar] [CrossRef]
- Köhler, H.; Bolelij, V.F.; Skopenko, V.V. Diaminkomplexe des Cobalt(II), Nickel(II)- und Kupfer(II)-dicyanamids und -nitrosodicyanmethanids. Z. Anorg. Allg. Chem. 1980, 468, 179–184. [Google Scholar] [CrossRef]
- Marcano, D.; Gerasimchuk, N.; Nemykin, V.; Silchenko, S. Synthesis Characterization and Studies of Coordination Polymers With Isomeric Pyridylcyan- oximes: Route to Metal Ribbons With Very Short Tl···Tl separations. Cryst. Growth Des. 2012, 12, 2877–2889. [Google Scholar] [CrossRef]
- Glower, G.; Gerasimchuk, N.; Biagioni, R.; Domasevitch, K.V. Monovalent K, Cs, Tl and Ag Nitrosodicyanmethanides: Completely different 3D networks with useful properties of Luminescent materials and nonelectric sensors for gases. Inorg. Chem. 2009, 48, 2371–2382. [Google Scholar]
- Gerasimchuk, N.; Goeden, L.; Durham, P.; Barnes, C.; Cannon, J.F. Synthesis and Characterization of the First Disubstituted Arylcyanoximes and their Several Metal Complexes. Inorg. Chim. Acta 2008, 361, 1983–2001. [Google Scholar] [CrossRef]
- Komiya, N.; Okada, M.; Fukumoto, K.; Kaneta, K.; Yoshida, A.; Naota, T. Vaulted Trans-Bis(salicylaldiminato)platinum(II) Crystals: Heat-Resistant, Chromatically Sensitive Platforms for Solid-State Phosphorescence at Ambient Temperature. Chem. Eur. J. 2013, 19, 4798–4811. [Google Scholar] [CrossRef] [PubMed]
- Sicilia, V.; Fornieś, J.; Casas, J.-M.; Martín, A.; López, J.A.; Larraz, C.; Borja, P.; Ovejero, C.; Tordera, D.; Bolink, H. Highly Luminescent Half-Lantern Cyclometalated Platinum(II) Complex: Synthesis, Structure, Luminescence Studies, and Reactivity. Inorg. Chem. 2012, 51, 3427–3435. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Chan, C.W.M.; Zhu, N.; Che, C.-M.; Li, C.; Hui, Z. Structural and Spectroscopic Studies on Pt···Pt and π–π Interactions in Luminescent Multinuclear Cyclometalated Platinum(II) Homologues Tethered by Oligophosphine Auxiliaries. J. Am. Chem. Soc. 2004, 126, 7639–7651. [Google Scholar] [CrossRef] [PubMed]
- Ikeshita, M.; Ito, M.; Naota, T. Variations in the Solid-State Emissions of Clothespin-shaped Binuclear trans-Bis(salicylaldiminato)platinum(II) with Halogen Functionalities. Eur. J. Inorg. Chem. 2019, 31, 3561–3571. [Google Scholar] [CrossRef]
- Riddles, C.N.; Whited, M.; Lotlikar, S.R.; Still, K.; Patrauchan, M.; Silchenko, S.; Gerasimchuk, N. Synthesis and Characterization of Two Cyanoxime Ligands, Their Precursors, and Light Insensitive Antimicrobial Silver(I) Cyanoximates. Inorg. Chim. Acta 2014, 412, 94–103. [Google Scholar] [CrossRef]
- Dragoi, M. Investigation of emission profiles of micelles of Pt-based cyanoximates. In Proceedings of the Oral Talk at the MWRM-2022, Iowa City, IA, USA, 22–24 October 2022. [Google Scholar]
- Berry, J. Extended Metal Atoms Chains, 3rd ed.; Springer Science and Business Media, Inc.: Berlin/Heidelberg, Germany, 2005. [Google Scholar]
- Krogmann, K. Planar Complexes Containing Metal-metal Bonds. Angew. Chem. Int. Ed. Engl. 1969, 8, 35. [Google Scholar] [CrossRef]
- Williams, J.M.; Schultz, A.; Underhill, A.; Carneiro, K. The Synthesis, Structure, Electrical Conduction Properties, and Theory of Divalent, Tetravalent, and One-Dimensional Partially Oxidized Tetracyanoplatinate Complexes. In Extended Linear Chain Compounds, Miller, J.S., Ed.; Springer US, Plenum Press: New York, NY, USA, 1982; pp. 73–118. [Google Scholar]
- Gerasimchuk, N.; Berezin, M. New Infrared Emitters. U.S. Patent 9,982,188, 29 May 2018. [Google Scholar]
- Lu, H.; Zheng, Y.; Zhao, X.; Wang, L.; Ma, S.; Han, X.; Xu, B.; Tian, W.; Gao, H. Highly Efficient Far Red/Near-Infrared Solid Fluorophores: Aggregation Induced Emission, Intramolecular Charge Transfer, Twisted Molecular Conformation and Bioimaging Applications. Angew. Chem. Inter. Ed. 2016, 55, 155–159. [Google Scholar] [CrossRef]
- Panchenko, P.A.; Fedorova, O.A.; Fedorov, Y.V. Fluorescent and Colorimetric Chemosemsors for Cations Based on 1,8-naphtalimide Derivatives: Design Principles and Optical Signaling Mechanisms. Russ. Chem. Rev. 2014, 83, 155–182. [Google Scholar] [CrossRef]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. University of Western Australia. Available online: www.hirshfeldsurface.net (accessed on 9 July 2015).
- Miskowski, V.M.; Houlding, V.H. Electronic Spectra and Photophysics of Platinum(II) Complexes with.alpha.-diimine ligands solid-state effects. 2. Metal-metal interaction in Double Salts and Linear Chains. Inorg. Chem. 1991, 30, 4446–4452. [Google Scholar] [CrossRef]
- Cao, D.L.; Ren, F.D.; Feng, X.Q.; Wang, J.L.; Li, Y.X.; Hu, Z.Y.; Chen, S.S. Unusual intermolecular T-shaped X–H⋅⋅⋅π interactions between CH3C≡N/CH3N≡C and H2O, NH3 or C2H2: A B3LYP and MP2 Theoretical Study. J. Mol. Struct. THEOCHEM 2008, 849, 76–83. [Google Scholar] [CrossRef]
- Nishio, M. CH/π hydrogen bonds in crystals. Cryst. Eng. Comm 2004, 6, 130–158. [Google Scholar] [CrossRef]
- Steiner, T. C–H···O Hydrogen Bonding in Crystals. Crystallogr. Rev. 2003, 9, 177–228. [Google Scholar] [CrossRef]




| Parameter | Compound | |
|---|---|---|
| PtL22∙CH3CN | PtL22∙C6H5NO2 | |
| Empirical formula | C22H15N3O4Pt | C26H17N3O6Pt |
| Formula weight, g/M | 580.46 | 662.52 |
| Temperature, K | 120(2) | 100(2) |
| Crystal system | triclinic | triclinic |
| Color/habitus | metallic black needle | green-black plate |
| Crystal size (mm) | 0.04 × 0.05 × 0.302 | 0.042 × 0.226 × 0.408 |
| Space group | P-1, #2 | P-1, #2 |
| Cell constants, Å/° | ||
| a | 6.679(8) | 6.707(2) |
| b | 10.897(13) | 12.203(4) |
| c | 13.866(17) | 14.016(5) |
| α | 97.279(14) | 92.739(5) |
| β | 93.082(16) | 103.808(5) |
| γ | 92.877(14) | 96.875(5) |
| Volume (Å3) | 998(2) | 1102.5(6) |
| Z | 2 | 2 |
| ρcalc (g/cm3) | 1.932 | 1.996 |
| μ (mm−1) | 7.065 | 6.413 |
| F(000) | 556 | 640 |
| 2Θ range for data (°) | 2.96 to 50.00 | 3.38 to 53.1 |
| Index ranges: | −8 < h < 8 | −8 < h < 8 |
| −13 < k < 13 | −15 < k < 15 | |
| 0 < l < 17 | −17 < l < 17 | |
| Reflections collected | 3234 | 13115 |
| Independent | 3234 | 4553 [R(int) = 0.079] |
| Data/restrains/parameters | 3234/48/272 | 4553 / 0 / 323 |
| Goodness-of-fit on F2 | 0.762 | 0.973 |
| Final R indices: | 1883 [I>2σ(I)]; R1 = 0.0590 | 4553 [I>2σ(I)]; R1 = 0.0572 |
| wR2 = 0.1153 | wR2 = 0.1430 | |
| All data: | R1 = 0.1030; wR2 = 0.1264 | R1 = 0.0683; wR2 = 0.1515 |
| Peak/hole difference, e(A−3) | 2.279 and −1.68 | 5.340 and −3.981 |
| Volume taken, A3 (%) | 638.5 (63.9) | 742.53 (67.9) |
| Compound | F.W. | Exo-Peak (T °C) | E, Released (kJ/mol) | Time Interval (min) | Heat Release (J/sec) |
|---|---|---|---|---|---|
| HL1 | 173.2 | 156.7 | 192.1 | ~9.3 | 344 |
| HL2 | 173.2 | 168.9 | 98.9 | ~2 | 825 |
| PtL22 | 539.4 | 322.8 | 112.3 | ~3.8 | 492 |
| PdL12 | 450.7 | 304.3 | 119.6 | ~3 | 665 |
| PdL22 | 450.7 | 309.3 | 140.5 | ~2 | 2342 |
| Compound | Bonds, Å | Angles, ° |
|---|---|---|
| HL2 * | C1-N1 = 1.304 | N1-C1-C2 = 113.4 |
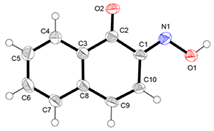 | 1.370 | C1-N1-O1 = 112.8 |
| 1.227 | N1-O2-H(O) = 109.6 | |
| 1.481 | O2-C2-C1 = 121.1 | |
| 1.454 | O2-C2-C3 = 121.9 | |
| 1.339 | N1-C1-C10 = 126.0 | |
| PtL22∙CH3CN | C1-O1 = 1.326(11) | O2-C1-C2 = 122.0(13) |
| C11-O4 = 1.302(15) | O2-C1-C10 = 119.6(13) | |
| C1-C2 = 1.45(2) | O1-N1-C2 = 117.8(11) | |
| C2-C3 = 1.428(19) | C3-C2-N1 = 124.6(12) | |
| C3-C4 = 1.397(19) | N1-C2-C1 = 111.4(12) | |
| C2-N1 = 1.431(17) | O4-C11-C12 = 119.8(13) | |
| C11-O4 = 1.302(15) | O4-C11-C20 = 122.8(12) | |
| C11-C12 = 1.450(19) | N2-C12-C11 = 112.4(12) | |
| C12-N2 = 1.383(17) | N2-C12-C13 = 124.8(12) | |
| C12-C13 = 1.474(19) | O3-N2-C12 = 119.8(11) | |
| C13-C14 = 1.386(19) | ||
| N1-O1 = 1.274(14) | ||
| N2-O3 = 1.301(14) | ||
| PtL22∙C6H5NO2 | C1-O2 = 1.326(11) | O1-C1-C2 = 118.3(9) |
| C11-O4 = 1.338(11) | O1-C1-C10 = 121.9(9) | |
| N1-O2 = 1.249(11) | O2-N1-C2 = 119.3(8) | |
| N2-O3 = 1.242(10) | N1-C2-C3 = 126.0(9) | |
| C1-C2 = 1.430(14) | N1-C2-C1 = 113.8(8) | |
| C11-C12 = 1.436(13) | O4-C11-C12 = 117.4(8) | |
| C2-N1 = 1.357(13) | O4-C11-C20 = 123.2(8) | |
| C12-N2 = 1.391(12) | N2-C12-C11 = 112.6(8) | |
| C2-C3 = 1.427(13) | N2-C12-C13 = 126.7(9) | |
| C3-C4 = 1.346(15) | O3-N2-C12 = 117.0(8) | |
| C11-C12 = 1.436(13) | ||
| C12-C13 = 1.411(13) |
| Compound | Structure | Solvent | Geometrical Parameters, Å | Refs. | |
|---|---|---|---|---|---|
| Pt---Pt | N-O---H-C(solv) | ||||
| a [Pt(MCO)2]2 | dimer | DMSO | 3.133 | 2.526, 2.535 | [37] |
| b [Pt(PyrCO)2]2 | dimer | DMSO | 3.207 | 2.737, 2.743 | [38] |
| [PtL22] | 1D polymer | CH3CN | 3.440, 3.563 | 2.543, 2.857 | this work |
| [PtL22] | 1D polymer | C6H5NO2 | 3.301, 3.536 | 3.845, 2.994 | this work |
| Tentative Band Assignment | ||||
|---|---|---|---|---|
| Complex | ν (C-H, aryl) | ν (C=C) | ν (C=N) | ν (N-O) |
| HL1 | 3065, 3020 | 1617, 1605 | 1520 (1505) | 1067 (1057) |
| HL2 | 3085, 2973 | 1622 | 1548 | 1062 |
| PdL12 | 3047, 2918 | 1607, 1589 | 1394 | 1088 |
| PdL22 | 3058 | 1603, 1587 | 1396 | 1089 |
| PtL22 | 3065 | 1606, 1587 | 1406 (1395) | 1100 |
| PtL12 | 3058 | 1611 | 1402 (1392) | 1093 (1079) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meadows, M.; Yang, L.; Turner, C.; Berezin, M.; Tyukhtenko, S.; Gerasimchuk, N. Synthesis and Characterization of Pt(II) and Pd(II) Complexes with Planar Aromatic Oximes. Inorganics 2023, 11, 116. https://doi.org/10.3390/inorganics11030116
Meadows M, Yang L, Turner C, Berezin M, Tyukhtenko S, Gerasimchuk N. Synthesis and Characterization of Pt(II) and Pd(II) Complexes with Planar Aromatic Oximes. Inorganics. 2023; 11(3):116. https://doi.org/10.3390/inorganics11030116
Chicago/Turabian StyleMeadows, Mikala, Lei Yang, Cody Turner, Mikhail Berezin, Sergiy Tyukhtenko, and Nikolay Gerasimchuk. 2023. "Synthesis and Characterization of Pt(II) and Pd(II) Complexes with Planar Aromatic Oximes" Inorganics 11, no. 3: 116. https://doi.org/10.3390/inorganics11030116
APA StyleMeadows, M., Yang, L., Turner, C., Berezin, M., Tyukhtenko, S., & Gerasimchuk, N. (2023). Synthesis and Characterization of Pt(II) and Pd(II) Complexes with Planar Aromatic Oximes. Inorganics, 11(3), 116. https://doi.org/10.3390/inorganics11030116







