Investigation on the Formation of Rare-Earth Metal Phenoxides via Metathesis
Abstract
1. Introduction
2. Results
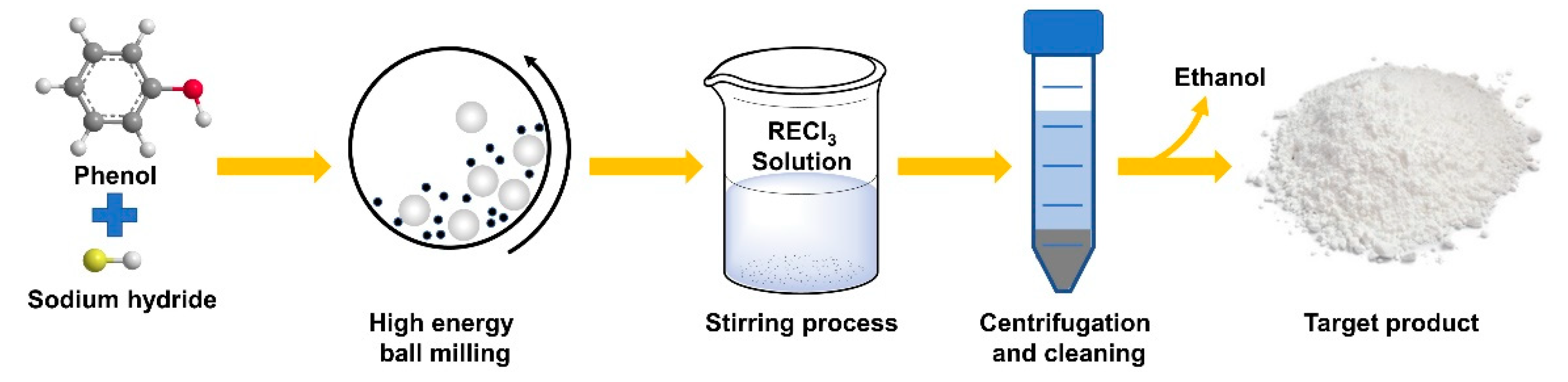
2.1. Syntheses of Rare-Earth Metal Phenoxides
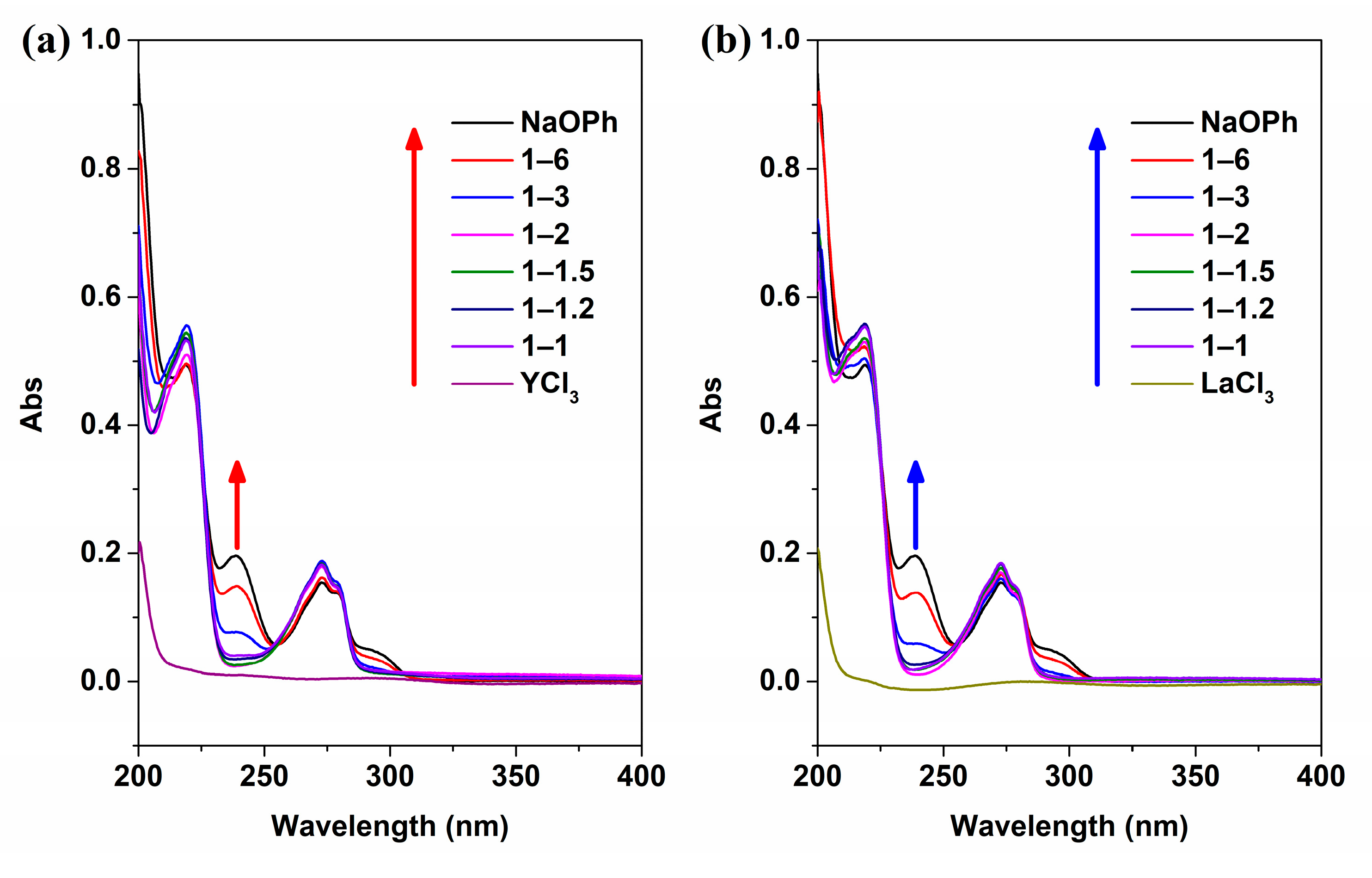
2.2. Characterizations
2.3. Molecular Structures
3. Materials and Methods
3.1. Material and Characterization
3.2. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Amin, M.; Shah, H.H.; Fareed, A.G.; Khan, W.U.; Chung, E.; Zia, A.; Rahman Farooqi, Z.U.; Lee, C. Hydrogen Production through Renewable and Non-Renewable Energy Processes and Their Impact on Climate Change. Int. J. Hydrogen Energy 2022, 47, 33112–33134. [Google Scholar] [CrossRef]
- Jiang, W.; Chen, Y.; Hu, M.; Zeng, C.; Liang, C. Rare Earth-Mg-Ni-Based Alloys with Superlattice Structure for Electro-chemical Hydrogen Storage. J. Alloys Compd. 2021, 887, 161381. [Google Scholar] [CrossRef]
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.a.H.; Rooney, D.W. Hydrogen Production, Storage, Utilisation and Environmental Impacts: A Review. Environ. Chem. Lett. 2022, 20, 153–188. [Google Scholar] [CrossRef]
- El Hajj Chehade, A.M.; Daher, E.A.; Assaf, J.C.; Riachi, B.; Hamd, W. Simulation and Optimization of Hydrogen Production by Steam Reforming of Natural Gas for Refining and Petrochemical Demands in Lebanon. Int. J. Hydrogen Energy 2020, 45, 33235–33247. [Google Scholar] [CrossRef]
- Khan, M.A.; Al-Attas, T.; Roy, S.; Rahman, M.M.; Ghaffour, N.; Thangadurai, V.; Larter, S.; Hu, J.; Ajayan, P.M.; Kibria, M.G. Seawater Electrolysis for Hydrogen Production: A Solution Looking for a Problem? Energy Environ. Sci. 2021, 14, 4831–4839. [Google Scholar] [CrossRef]
- Sultanov, F.; Daulbayev, C.; Bakbolat, B.; Daulbayev, O.; Bigaj, M.; Mansurov, Z.; Kuterbekov, K.; Bekmyrza, K. Aligned Composite SrTiO3/PAN Fibers as 1D Photocatalyst Obtained by Electrospinning Method. Chem. Phys. Lett. 2019, 737, 136821. [Google Scholar] [CrossRef]
- Akhlaghi, N.; Najafpour-Darzi, G. A Comprehensive Review on Biological Hydrogen Production. Int. J. Hydrogen Energy 2020, 45, 22492–22512. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen Carriers. Nat. Rev. Mater. 2016, 1, 16059. [Google Scholar] [CrossRef]
- Lin, H.-J.; Li, H.-W.; Shao, H.; Lu, Y.; Asano, K. In Situ Measurement Technologies on Solid-State Hydrogen Storage Materials: A Review. Mater. Today Energy 2020, 17, 100463. [Google Scholar] [CrossRef]
- Daulbayev, C.; Lesbayev, B.; Bakbolat, B.; Kaidar, B.; Sultanov, F.; Yeleuov, M.; Ustayeva, G.; Rakhymzhan, N. A Mini-Review on Recent Trends in Prospective Use of Porous 1D Nanomaterials for Hydrogen Storage. S. Afr. J. Chem. Eng. 2022, 39, 52–61. [Google Scholar] [CrossRef]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High Capacity Hydrogen Storage Materials: Attributes for Automotive Applications and Techniques for Materials Discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef] [PubMed]
- Yadav, M.; Xu, Q. Liquid-Phase Chemical Hydrogen Storage Materials. Energy Environ. Sci. 2012, 5, 9698–9725. [Google Scholar] [CrossRef]
- He, T.; Cao, H.; Chen, P. Complex Hydrides for Energy Storage, Conversion, and Utilization. Adv. Mater. 2019, 31, 1902757. [Google Scholar] [CrossRef]
- He, T.; Cao, H.; Chen, P. The Roles of Alkali/Alkaline Earth Metals in the Materials Design and Development for Hydrogen Storage. Acc. Mater. Res. 2021, 2, 726–738. [Google Scholar] [CrossRef]
- Yu, Y.; He, T.; Wu, A.; Pei, Q.; Karkamkar, A.; Autrey, T.; Chen, P. Reversible Hydrogen Uptake/Release over a Sodium Phenoxide–Cyclohexanolate Pair. Angew. Chem. Int. Ed. 2019, 58, 3102–3107. [Google Scholar] [CrossRef]
- Jing, Z.; Yu, Y.; Chen, R.; Tan, K.C.; He, T.; Wu, A.; Pei, Q.; Chua, Y.S.; Zheng, D.; Zhang, X.; et al. Sodium Anilinide–Cyclohexylamide Pair: Synthesis, Characterization, and Hydrogen Storage Properties. Chem. Commun. 2020, 56, 1944–1947. [Google Scholar] [CrossRef]
- Jing, Z.; Tan, K.C.; He, T.; Yu, Y.; Pei, Q.; Wang, J.; Wu, H.; Chen, P. Synthesis, Characterization, and Crystal Structure of Lithium Pyrrolide. Acta Phys.-Chim. Sin. 2021, 37, 2009039. [Google Scholar] [CrossRef]
- Tan, K.C.; Yu, Y.; Chen, R.; He, T.; Jing, Z.; Pei, Q.; Wang, J.; Chua, Y.S.; Wu, A.; Zhou, W.; et al. Metallo-N-Heterocycles—A New Family of Hydrogen Storage Material. Energy Storage Mater. 2020, 26, 198–202. [Google Scholar] [CrossRef]
- Jing, Z.; Yuan, Q.; Yu, Y.; Kong, X.; Tan, K.C.; Wang, J.; Pei, Q.; Wang, X.-B.; Zhou, W.; Wu, H.; et al. Developing Ideal Metalorganic Hydrides for Hydrogen Storage: From Theoretical Prediction to Rational Fabrication. ACS Mater. Lett. 2021, 3, 1417–1425. [Google Scholar] [CrossRef]
- Boyle, T.J.; Ottley, L.A.M. Advances in Structurally Characterized Lanthanide Alkoxide, Aryloxide, and Silyloxide Compounds. Chem. Rev. 2008, 108, 1896–1917. [Google Scholar] [CrossRef] [PubMed]
- Ortu, F. Rare Earth Starting Materials and Methodologies for Synthetic Chemistry. Chem. Rev. 2022, 122, 6040–6116. [Google Scholar] [CrossRef]
- Balaram, V. Rare Earth Elements: A Review of Applications, Occurrence, Exploration, Analysis, Recycling, and Environmental Impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Lin, H.-J.; Lu, Y.-S.; Zhang, L.-T.; Liu, H.-Z.; Edalati, K.; Révész, Á. Recent Advances in Metastable Alloys for Hydrogen Storage: A Review. Rare Met. 2022, 41, 1797–1817. [Google Scholar] [CrossRef]
- Sato, T.; Saitoh, H.; Utsumi, R.; Ito, J.; Nakahira, Y.; Obana, K.; Takagi, S.; Orimo, S.I. Hydrogen Absorption Reactions of Hydrogen Storage Alloy LaNi5 under High Pressure. Molecules 2023, 28, 1256. [Google Scholar] [CrossRef]
- Huggins, M.L. Electronic Structures of Atoms. J. Phys. Chem. 1922, 26, 601–625. [Google Scholar] [CrossRef]
- Xia, Q.; Cui, Y.; Yuan, D.; Wang, Y.; Yao, Y. Synthesis and Characterization of Lanthanide Complexes Stabilized by N-Aryl Substituted β-Ketoiminato Ligands and Their Application in the Polymerization of Rac-Lactide. J. Organomet. Chem. 2017, 846, 161–168. [Google Scholar] [CrossRef]
- Nie, K.; Han, Y.; Wang, C.; Cheng, X. Rare-Earth Metal-Catalyzed Hydroboration of Unsaturated Compounds. Appl. Organomet. Chem. 2022, 36, e6570. [Google Scholar] [CrossRef]
- Wei, C.; Sun, B.; Zhao, Z.; Cai, Z.; Liu, J.; Tan, Y.; Wei, H.; Liu, Z.; Bian, Z.; Huang, C. A Family of Highly Emissive Lanthanide Complexes Constructed with 6-(Diphenylphosphoryl)Picolinate. Inorg. Chem. 2020, 59, 8800–8808. [Google Scholar] [CrossRef]
- Van den Hende, J.R.; Hitchcock, P.B.; Holmes, S.A.; Lappert, M.F.; Leung, W.-P.; Mak, T.C.W.; Prashar, S. Synthesis and Characterisation of Lanthanide(II) Aryloxides Including the First Structurally Characterised Europium(II) Compound [Eu(OC6H2but2-2,6-Me-4)2(thf)3]·thf (thf = tetrahydrofuran). J. Chem. Soc. Dalton Trans. 1995, 1427–1433. [Google Scholar] [CrossRef]
- Barnhart, D.M.; Clark, D.L.; Gordon, J.C.; Huffman, J.C.; Vincent, R.L.; Watkin, J.G.; Zwick, B.D. Synthesis, Properties, and X-Ray Structures of the Lanthanide η6-Arene-Bridged Aryloxide Dimers Ln2(O-2,6-i-Pr2C6H3)6 and Their Lewis Base Adducts Ln(O-2,6-i-Pr2C6H3)3(THF)2 (Ln = Pr, Nd, Sm, Gd, Er, Yb, Lu). Inorg. Chem. 1994, 33, 3487–3497. [Google Scholar] [CrossRef]
- Thornton, D.A. Crystal Field Aspects of the Vibrational Spectra of Metal Complexes. Coord. Chem. Rev. 1984, 55, 113–149. [Google Scholar] [CrossRef]
- Galyametdinov, Y.; Athanassopoulou, M.A.; Griesar, K.; Kharitonova, O.; Soto Bustamante, E.A.; Tinchurina, L.; Ovchinnikov, I.; Haase, W. Synthesis and Magnetic Investigations on Rare-Earth-Containing Liquid Crystals with Large Magnetic Anisotropy. Chem. Mater. 1996, 8, 922–926. [Google Scholar] [CrossRef]
- Luo, Y.; Han, Y.; Lin, J. Synthesis and Luminescent Properties of Europium (III) Schiff Base Complexes Covalently Bonded to Silica Xerogels. J. Lumin. 2007, 122–123, 83–86. [Google Scholar] [CrossRef]
- Gagne, O.C.; Hawthorne, F.C. Empirical Lewis Acid Strengths for 135 Cations Bonded to Oxygen. Acta Crystallogr. Sect. B 2017, 73, 956–961. [Google Scholar] [CrossRef] [PubMed]
- Moehring, S.A.; Miehlich, M.; Hoerger, C.J.; Meyer, K.; Ziller, J.W.; Evans, W.J. A Room-Temperature Stable Y(II) Aryloxide: Using Steric Saturation to Kinetically Stabilize Y(II) Complexes. Inorg. Chem. 2020, 59, 3207–3214. [Google Scholar] [CrossRef] [PubMed]
- Spinner, E.; Late, A.B. The Electronic Spectra of Acetophenones Substituted in the Methyl Group. Spectrochim. Acta 1961, 17, 558–567. [Google Scholar] [CrossRef]
- Rowe, W.F.; Marginean, I.; Carnes, S.; Lurie, I.S. The Role of Diode Array Ultraviolet Detection for the Identification of Synthetic Cathinones. Drug Test. Anal. 2017, 9, 1512–1521. [Google Scholar] [CrossRef]
- Berger, J.; Staretz, M.E.; Wood, M.; Brettell, T.A. Ultraviolet Absorption Properties of Synthetic Cathinones. Forensic Chem. 2020, 21, 100286. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yu, H.; Guo, Y.; Zhang, Y.; Jiang, X.; Sun, B.; Fu, K.; Chen, J.; Qi, Y.; Zheng, J.; et al. Promoting Hydrogen Absorption of Liquid Organic Hydrogen Carriers by Solid Metal Hydrides. J. Mater. Chem. A 2019, 7, 16677–16684. [Google Scholar] [CrossRef]
- Zheng, X.; Li, G.; Guo, Y.; Yu, H.; Li, S.; Xiao, R.; Zheng, J.; Li, X. Yttrium Trihydride Enhanced Lithium Storage in Carbon Materials. Carbon 2020, 164, 317–323. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09 Revision A.1; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular Interactions from a Natural Bond Orbital, Donor-Acceptor Viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Pei, Q.; Yu, Y.; Cui, J.; Wang, S.; Tan, K.C.; Guo, J.; He, T.; Chen, P. Investigation on the Formation of Rare-Earth Metal Phenoxides via Metathesis. Inorganics 2023, 11, 115. https://doi.org/10.3390/inorganics11030115
Wang J, Pei Q, Yu Y, Cui J, Wang S, Tan KC, Guo J, He T, Chen P. Investigation on the Formation of Rare-Earth Metal Phenoxides via Metathesis. Inorganics. 2023; 11(3):115. https://doi.org/10.3390/inorganics11030115
Chicago/Turabian StyleWang, Jintao, Qijun Pei, Yang Yu, Jirong Cui, Shangshang Wang, Khai Chen Tan, Jiaquan Guo, Teng He, and Ping Chen. 2023. "Investigation on the Formation of Rare-Earth Metal Phenoxides via Metathesis" Inorganics 11, no. 3: 115. https://doi.org/10.3390/inorganics11030115
APA StyleWang, J., Pei, Q., Yu, Y., Cui, J., Wang, S., Tan, K. C., Guo, J., He, T., & Chen, P. (2023). Investigation on the Formation of Rare-Earth Metal Phenoxides via Metathesis. Inorganics, 11(3), 115. https://doi.org/10.3390/inorganics11030115





