Collectable Single Pure-Pd Metal Membrane with High Strength and Flexibility Prepared through Electroplating for Hydrogen Purification
Abstract
1. Introduction
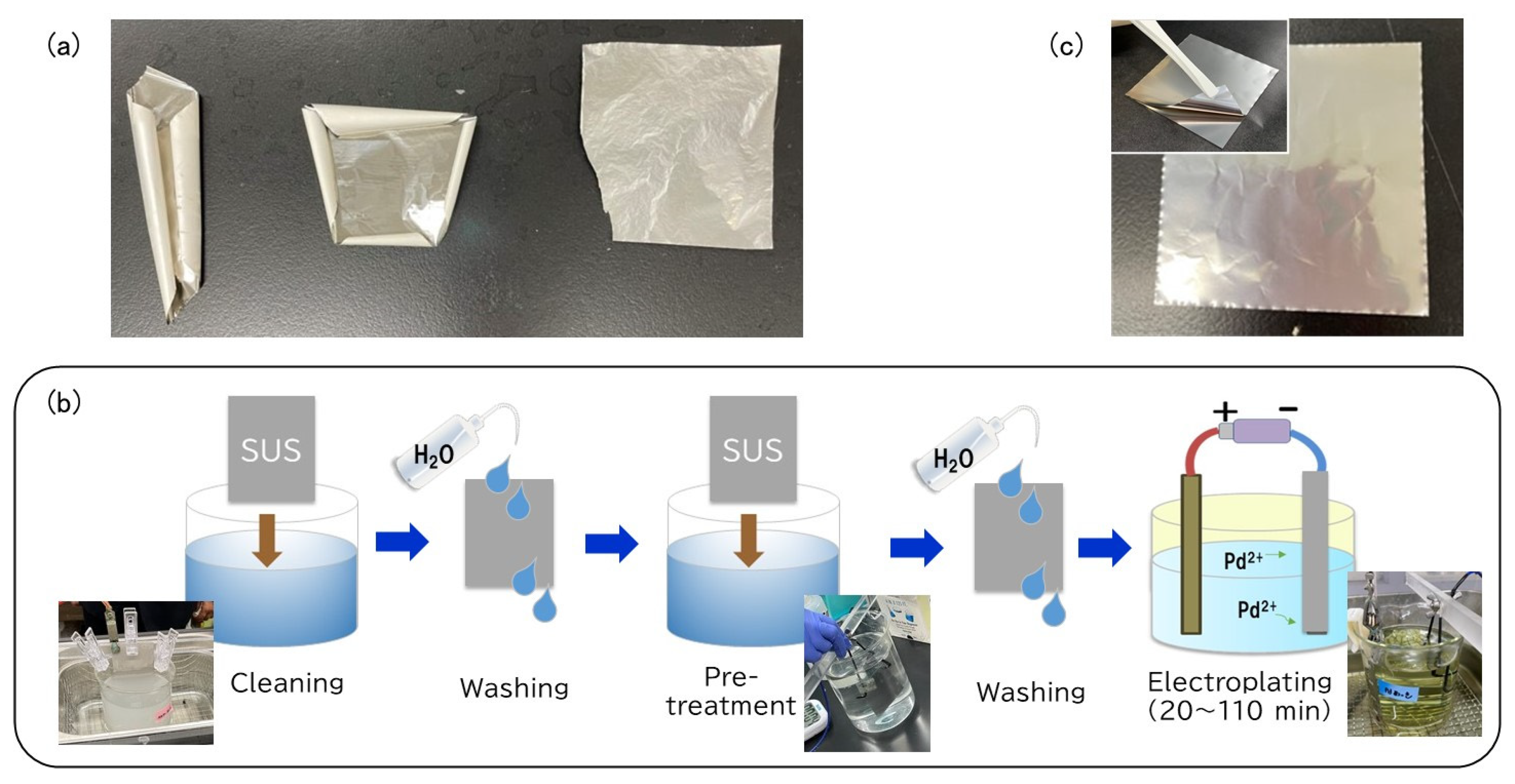
2. Materials and Methods
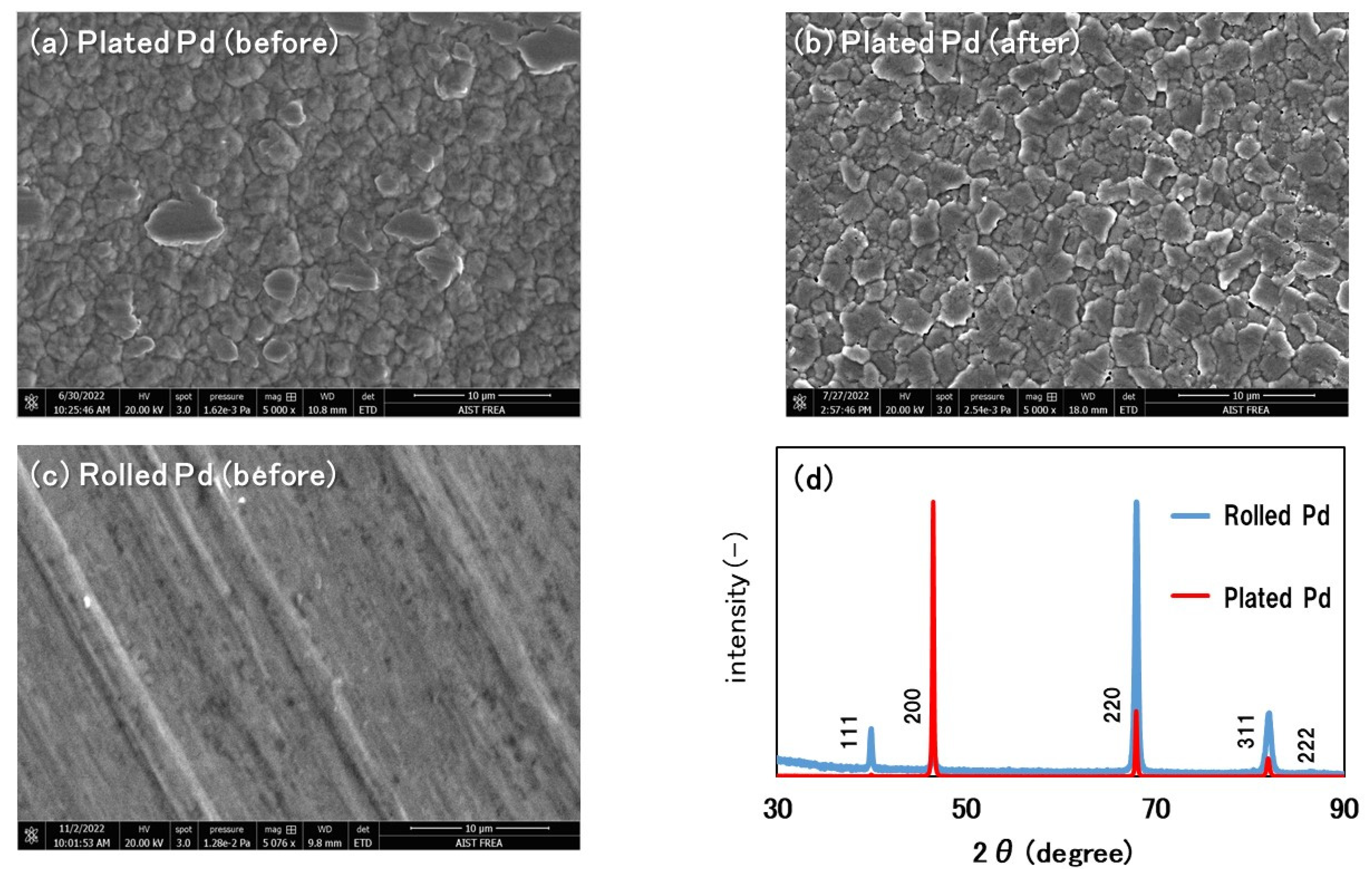
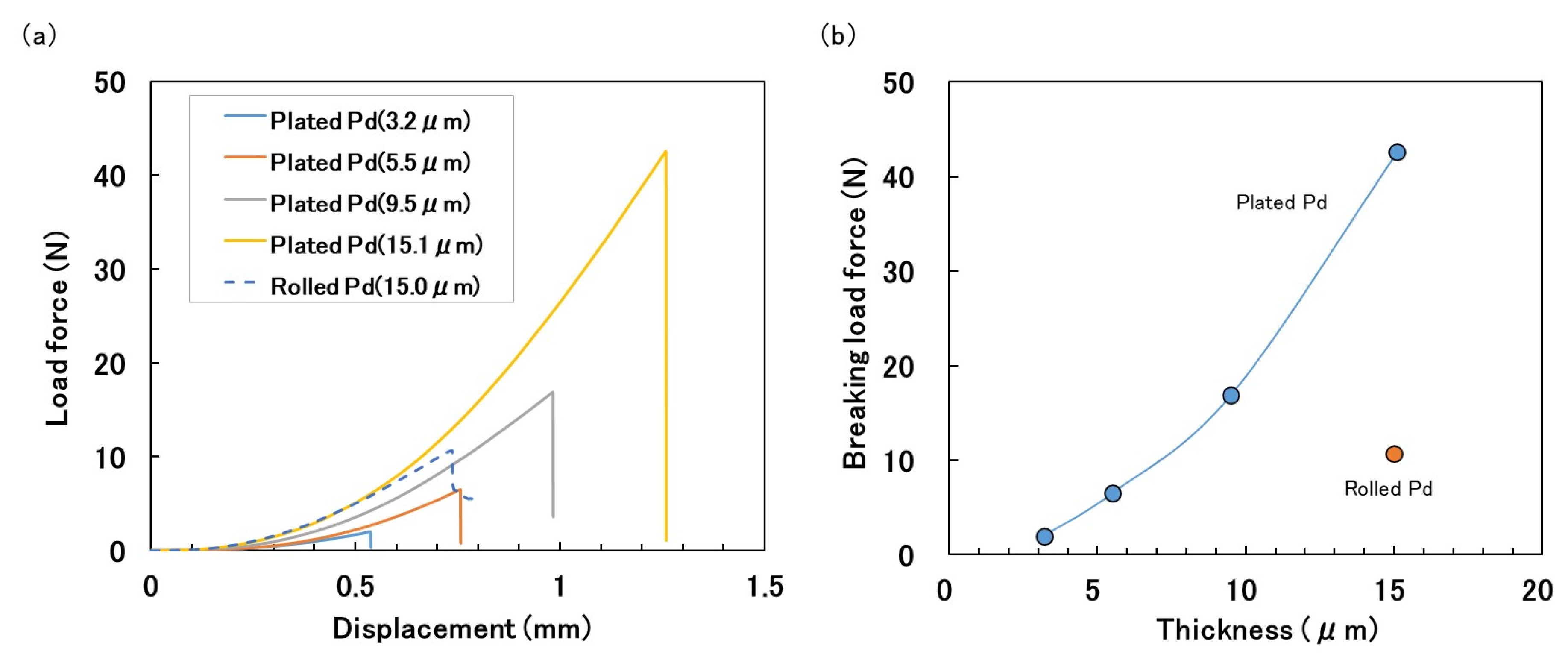
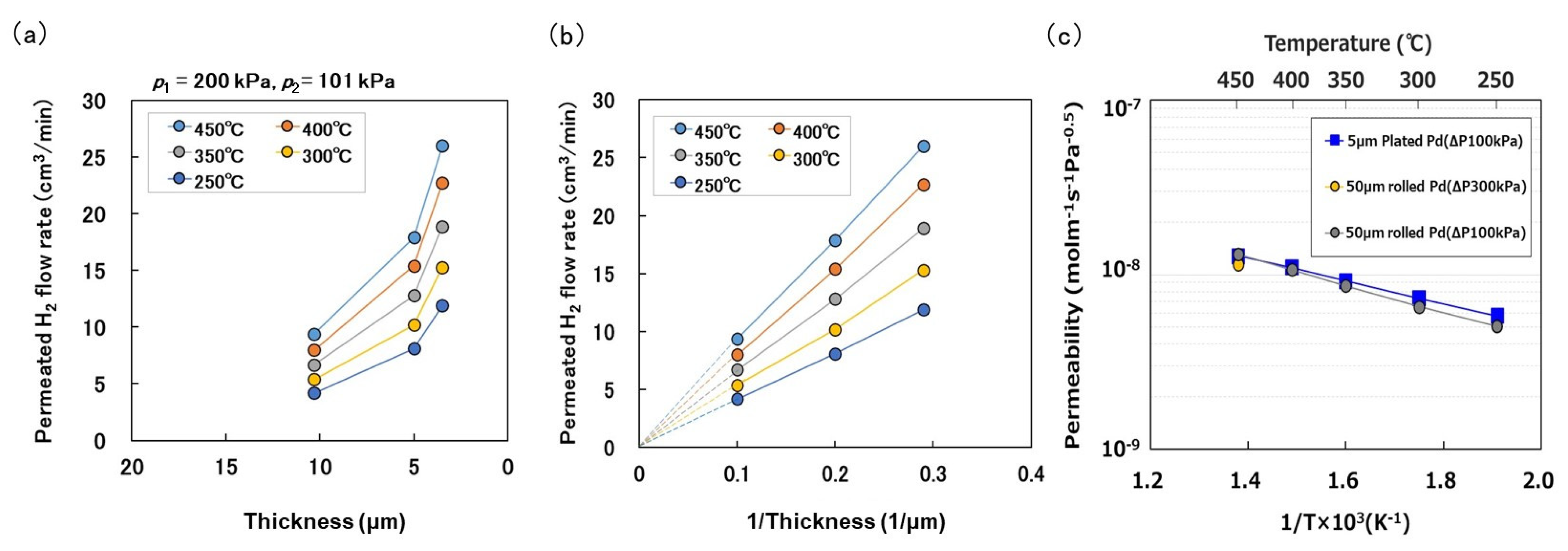
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shu, J.; Grandjean, B.P.A.; Van Neste, A.V.; Kaliaguine, S. Catalytic Palladium-Based Membrane Reactors: A Review. Can. J. Chem. Eng. 1991, 69, 1036–1060. [Google Scholar] [CrossRef]
- Basile, A.; Gallucci, F.; Tosti, S. Synthesis, Characterization, and Applications of Palladium Membranes. Membr. Sci. Technol. 2008, 13, 255–323. [Google Scholar] [CrossRef]
- Paglieri, S.N.; Way, J.D. Innovations in Palladium Membrane Research. Sep. Purif. Methods 2002, 31, 1–169. [Google Scholar] [CrossRef]
- Yun, S.; Ted Oyama, S.T. Correlations in Palladium Membranes for Hydrogen Separation: A Review. J. Membr. Sci. 2011, 375, 28–45. [Google Scholar] [CrossRef]
- Conde, J.J.; Maroño, M.; Sánchez-Hervás, J.M. Pd-Based Membranes for Hydrogen Separation: Review of Alloying Elements and Their Influence on Membrane Properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Zhang, Y.; Komaki, M.; Nishimura, C. Morphological Study of Supported Thin Pd and Pd–25Ag Membranes upon Hydrogen Permeation. J. Membr. Sci. 2005, 246, 173–180. [Google Scholar] [CrossRef]
- Kikuchi, E. Membrane Reactor Application to Hydrogen Production. Catal. Today 2000, 56, 97–101. [Google Scholar] [CrossRef]
- Sonwane, C.G.; Wilcox, J.; Ma, Y.H. Solubility of Hydrogen in PdAg and PdAu Binary Alloys Using Density Functional Theory. J. Phys. Chem. B 2006, 110, 24549–24558. [Google Scholar] [CrossRef]
- Shi, L.; Goldbach, A.; Zeng, G.; Xu, H. Preparation and Performance of Thin-Layered PdAu/Ceramic Composite Membranes. Int. J. Hydrogen Energy 2010, 35, 4201–4208. [Google Scholar] [CrossRef]
- Zeng, G.; Goldbach, A.; Shi, L.; Xu, H. On Alloying and Low-Temperature Stability of Thin, Supported PdAg Membranes. Int. J. Hydrogen Energy 2012, 37, 6012–6019. [Google Scholar] [CrossRef]
- Millet, P.; Ngameni, R.; Decaux, C.; Grigoriev, S.A. Hydrogen Sorption by Pd77Ag23 Metallic Membranes. Role of Hydrogen Content, Temperature and Sample Microstructure. Int. J. Hydrogen Energy 2011, 36, 4262–4269. [Google Scholar] [CrossRef]
- Montesinos, H.; Julián, I.; Herguido, J.; Menéndez, M. Effect of the Presence of Light Hydrocarbon Mixtures on Hydrogen Permeance through Pd–Ag Alloyed Membranes. Int. J. Hydrogen Energy 2015, 40, 3462–3471. [Google Scholar] [CrossRef]
- Kamakoti, P.; Morreale, B.D.; Ciocco, M.V.; Howard, B.H.; Killmeyer, R.P.; Cugini, A.V.; Sholl, D.S. Prediction of Hydrogen Flux through Sulfur-Tolerant Binary Alloy Membranes. Science 2005, 307, 569–573. [Google Scholar] [CrossRef]
- Decaux, C.; Ngameni, R.; Solas, D.; Grigoriev, S.; Millet, P. Time and Frequency Domain Analysis of Hydrogen Permeation across PdCu Metallic Membranes for Hydrogen Purification. Int. J. Hydrogen Energy 2010, 35, 4883–4892. [Google Scholar] [CrossRef]
- Zhang, K.; Way, J.D. Palladium-Copper Membranes for Hydrogen Separation. Sep. Purif. Technol. 2017, 186, 39–44. [Google Scholar] [CrossRef]
- Zhao, C.; Goldbach, A.; Xu, H. Low-Temperature Stability of Body-Centered Cubic PdCu Membranes. J. Membr. Sci. 2017, 542, 60–67. [Google Scholar] [CrossRef]
- Phair, J.W.; Donelson, R. Developments and Design of Novel (Non-palladium-Based) Metal Membranes for Hydrogen Separation. Ind. Eng. Chem. Res. 2006, 45, 5657–5674. [Google Scholar] [CrossRef]
- Dolan, M.D. Non-Pd BCC Alloy Membranes for Industrial Hydrogen Separation. J. Membr. Sci. 2010, 362, 12–28. [Google Scholar] [CrossRef]
- Dolan, M.D.; Song, G.; Liang, D.; Kellam, M.E.; Chandra, D.; Lamb, J.H. Hydrogen Transport through V85Ni10M5 Alloy Membranes. J. Membr. Sci. 2011, 373, 14–19. [Google Scholar] [CrossRef]
- Buxbaum, R.E.; Marker, T.L. Hydrogen Transport through Non-porous Membranes of Palladium-Coated Niobium, Tantalum and Vanadium. J. Membr. Sci. 1993, 85, 29–38. [Google Scholar] [CrossRef]
- Jayaraman, V.; Lin, Y.S. Synthesis and Hydrogen Permeation Properties of Ultrathin Palladium-Silver Alloy Membranes. J. Membr. Sci. 1995, 104, 251–262. [Google Scholar] [CrossRef]
- McCool, B.; Xomeritakis, G.; Lin, Y.S. Composition Control and Hydrogen Permeation Characteristics of Sputter Deposited Palladium-Silver Membranes. J. Membr. Sci. 1999, 161, 67–76. [Google Scholar] [CrossRef]
- Xomeritakis, G.; Lin, Y.S. Fabrication of a Thin Palladium Membrane Supported in a Porous Ceramic Substrate by Chemical Vapor Deposition. J. Membr. Sci. 1996, 120, 261–272. [Google Scholar] [CrossRef]
- Kikuchi, E.; Nemoto, Y.; Kajiwara, M.; Uemiya, S.; Kojima, T. Steam Reforming of Methane in Membrane Reactors: Comparison of Electroless-Plating and CVD Membranes and Catalyst Packing Modes. Catal. Today 2000, 56, 75–81. [Google Scholar] [CrossRef]
- Huang, Y.; Li, X.; Fan, Y.; Xu, N. Palladium-Based Composite Membranes: Principle, Preparation and Characterization. Prog. Chem. 2006, 18, 230–237. [Google Scholar]
- Thoen, P.M.; Roa, F.; Way, J.D. High Flux Palladium–Copper Composite Membranes for Hydrogen Separations. Desalination 2006, 193, 224–229. [Google Scholar] [CrossRef]
- Tosti, S. Supported and Laminated Pd-Based Metallic Membranes. Int. J. Hydrogen Energy 2003, 28, 1445–1454. [Google Scholar] [CrossRef]
- Dittmeyer, R.; Höllein, V.; Daub, K. Membrane Reactors for Hydrogenation and Dehydrogenation Processes Based on Supported Palladium. J. Mol. Catal. A Chem. 2001, 173, 135–184. [Google Scholar] [CrossRef]
- Tong, J.; Shirai, R.; Kashima, Y.; Matsumura, Y. Preparation of a Pinhole-Free Pd–Ag Membrane on a Porous Metal Support for Pure Hydrogen Separation. J. Membr. Sci. 2005, 260, 84–89. [Google Scholar] [CrossRef]
- Bryden, K.J.; Ying, J.Y. Electrodeposition Synthesis and Hydrogen Absorption Properties of Nanostructured Palladium–Iron Alloys. Nanostruct. Mater. 1997, 9, 485–488. [Google Scholar] [CrossRef]
- Gade, S.K.; Thoen, P.M.; Way, J.D. Unsupported Palladium Alloy Foil Membranes Fabricated by Electroless Plating. J. Membr. Sci. 2008, 316, 112–118. [Google Scholar] [CrossRef]
- Endo, N.; Furukawa, Y.; Goshome, K.; Yaegashi, S.; Mashiko, K.-i.; Tetsuhiko, M. Characterization of Mechanical Strength and Hydrogen Permeability of a PdCu Alloy Film Prepared by One-Step Electroplating for Hydrogen Separation and Membrane Reactors. Int. J. Hydrogen Energy 2019, 44, 8290–8297. [Google Scholar] [CrossRef]
- Kato, Y.; Maeda, T.; Endo, N.; Yaegashi, S.; Furukawa, Y.; Dezawa, N. Hydrogen Permeable Membranes and Their Preparation Methods. Japan Patent P6695929, 24 April 2020. (In Japanese). [Google Scholar]
- Knapton, A.G. Palladium Alloys for Hydrogen Diffusion Membranes. Platin. Met. Rev. 1977, 21, 44–50. [Google Scholar]
- Raub, C.J. Electroplating of Palladium for Electrical Contacts. Platin. Met. Rev. 1982, 26, 158–166. [Google Scholar]
- Yasumura, K. Palladium and Palladium Alloy Bath. J. Surf. Finish. Soc. Jpn. 2004, 55, 640–645. [Google Scholar] [CrossRef]
- Snavely, C.A. A Theory for the Mechanism of Chromium Plating; A Theory for the Physical Characteristics of Chromium Plate. J. Electrochem. Soc. 1947, 92, 537. [Google Scholar] [CrossRef]
- Yaegashi, S.; Endo, N.; Kumakawa, M.; Suzuki, S.; Maeda, T. Method and Apparatus for Evaluating Flexibility of Sheet-Type Testing Materials. Japan Patent P6265196, 24 January 2018. [Google Scholar]
- Endo, N.; Yaegashi, S.; Maehata, T.; Kumakawa, M.; Suzuki, S.; Mashiko, K.-i.; Maeda, T. High Thermal Stability and Flexibility of Thin Porous Ni Metal Support Prepared by Electroplating Deposition for Pd Alloy Membranes. Mater. Trans. 2017, 58, 1093–1096. [Google Scholar] [CrossRef]
- Nishimura, C.; Komaki, M.; Amano, M. Hydrogen Permeation Characteristics of Vanadium-Nickel Alloys. Mater. Trans. JIM 1991, 32, 501–507. [Google Scholar] [CrossRef]
- Arblaster, J.W. Crystallographic Properties of Palladium. Platin. Met. Rev. 2012, 56, 181–189. [Google Scholar] [CrossRef]
- Amano, M.; Nishimura, C.; Komaki, M. Effect of High Concentration CO and CO2 on Hydrogen Permeation through the Palladium Membrane. Mater. Trans. JIM 1990, 31, 404–408. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Endo, N.; Kaneko, Y.; Dezawa, N.; Komo, Y.; Higuchi, M. Collectable Single Pure-Pd Metal Membrane with High Strength and Flexibility Prepared through Electroplating for Hydrogen Purification. Inorganics 2023, 11, 111. https://doi.org/10.3390/inorganics11030111
Endo N, Kaneko Y, Dezawa N, Komo Y, Higuchi M. Collectable Single Pure-Pd Metal Membrane with High Strength and Flexibility Prepared through Electroplating for Hydrogen Purification. Inorganics. 2023; 11(3):111. https://doi.org/10.3390/inorganics11030111
Chicago/Turabian StyleEndo, Naruki, Yumi Kaneko, Norikazu Dezawa, Yasuhiro Komo, and Masanobu Higuchi. 2023. "Collectable Single Pure-Pd Metal Membrane with High Strength and Flexibility Prepared through Electroplating for Hydrogen Purification" Inorganics 11, no. 3: 111. https://doi.org/10.3390/inorganics11030111
APA StyleEndo, N., Kaneko, Y., Dezawa, N., Komo, Y., & Higuchi, M. (2023). Collectable Single Pure-Pd Metal Membrane with High Strength and Flexibility Prepared through Electroplating for Hydrogen Purification. Inorganics, 11(3), 111. https://doi.org/10.3390/inorganics11030111






