BaTiO3/NixZn1−xFe2O4 (x = 0, 0.5, 1) Composites Synthesized by Thermal Decomposition: Magnetic, Dielectric and Ferroelectric Properties
Abstract
1. Introduction
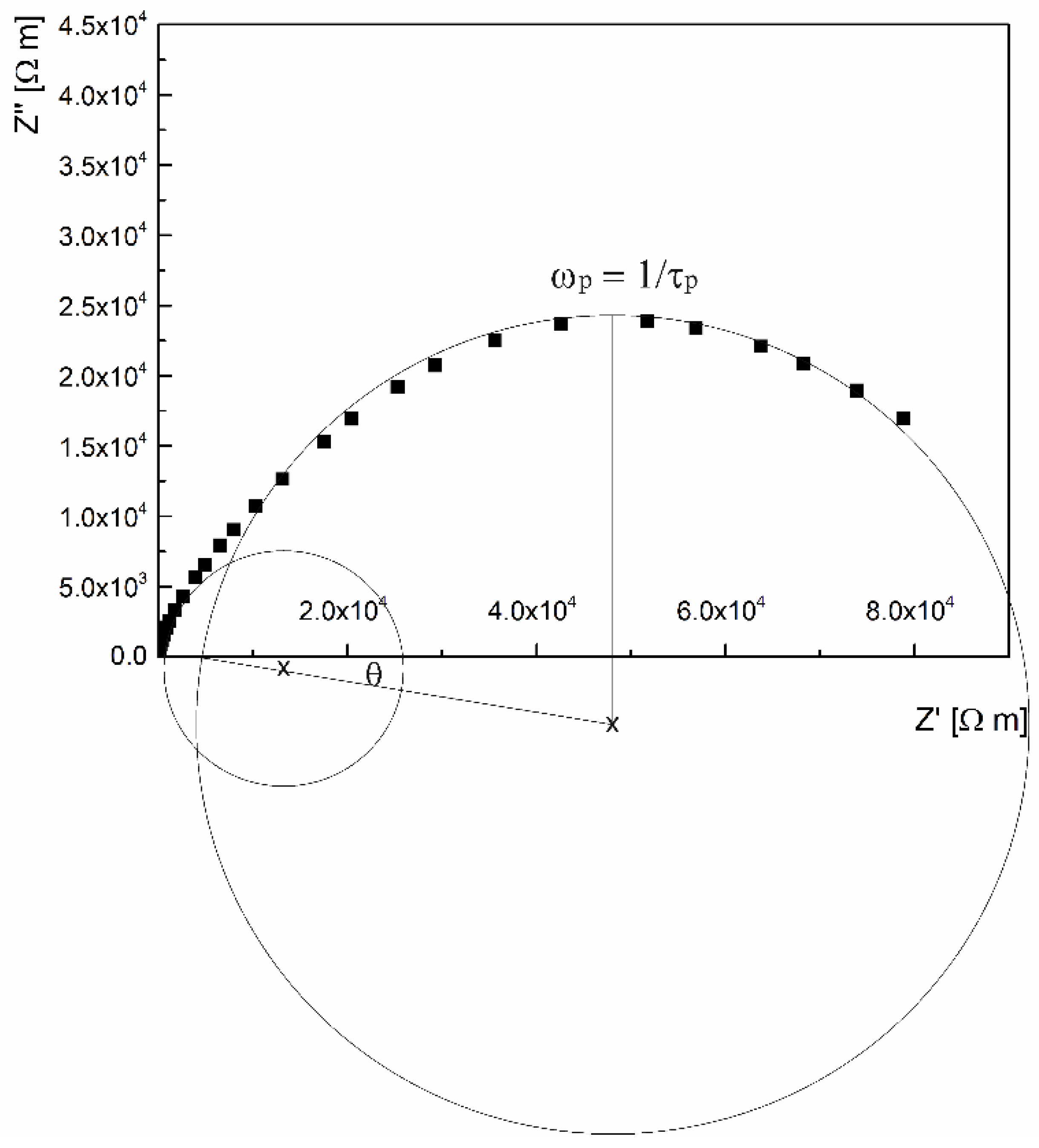
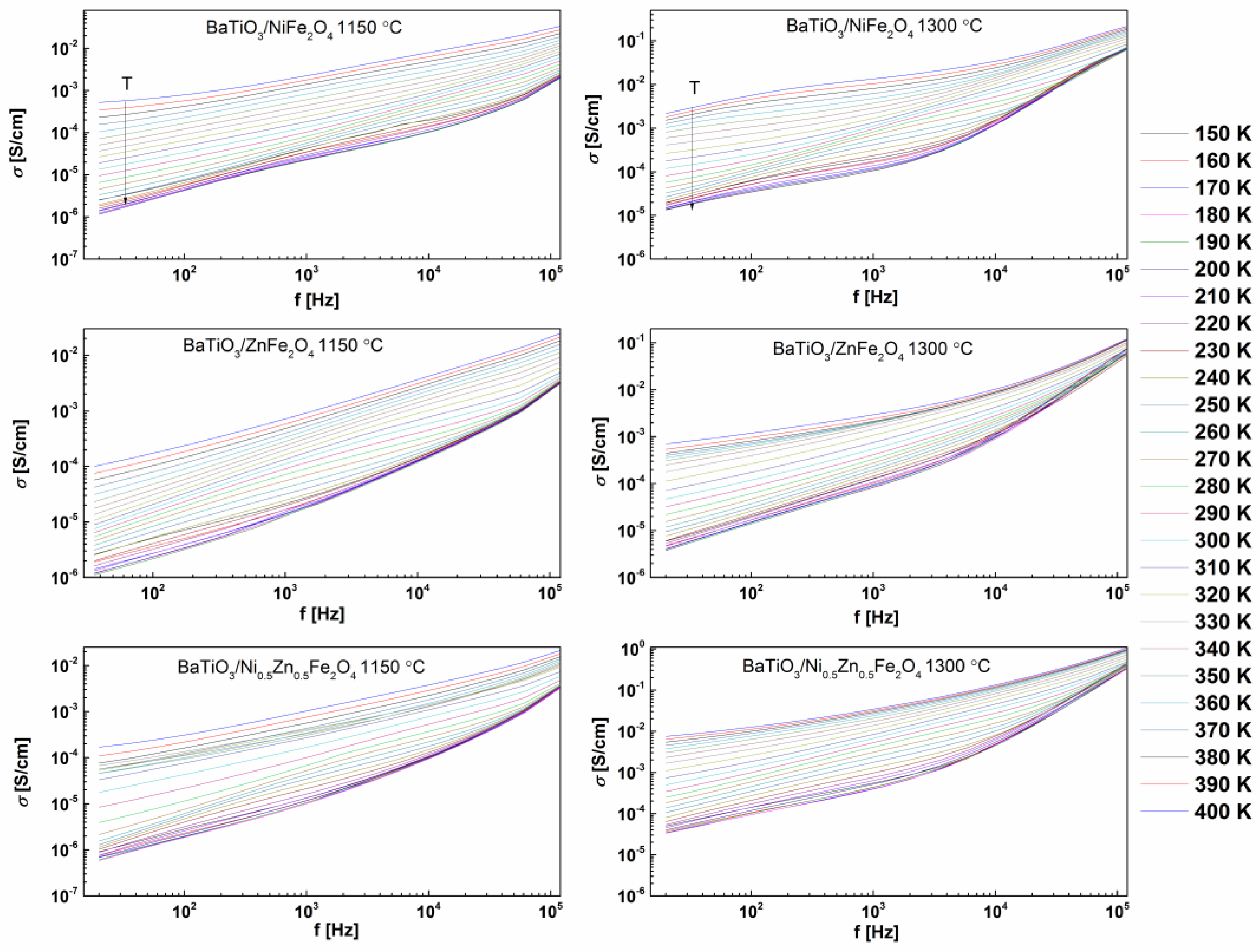
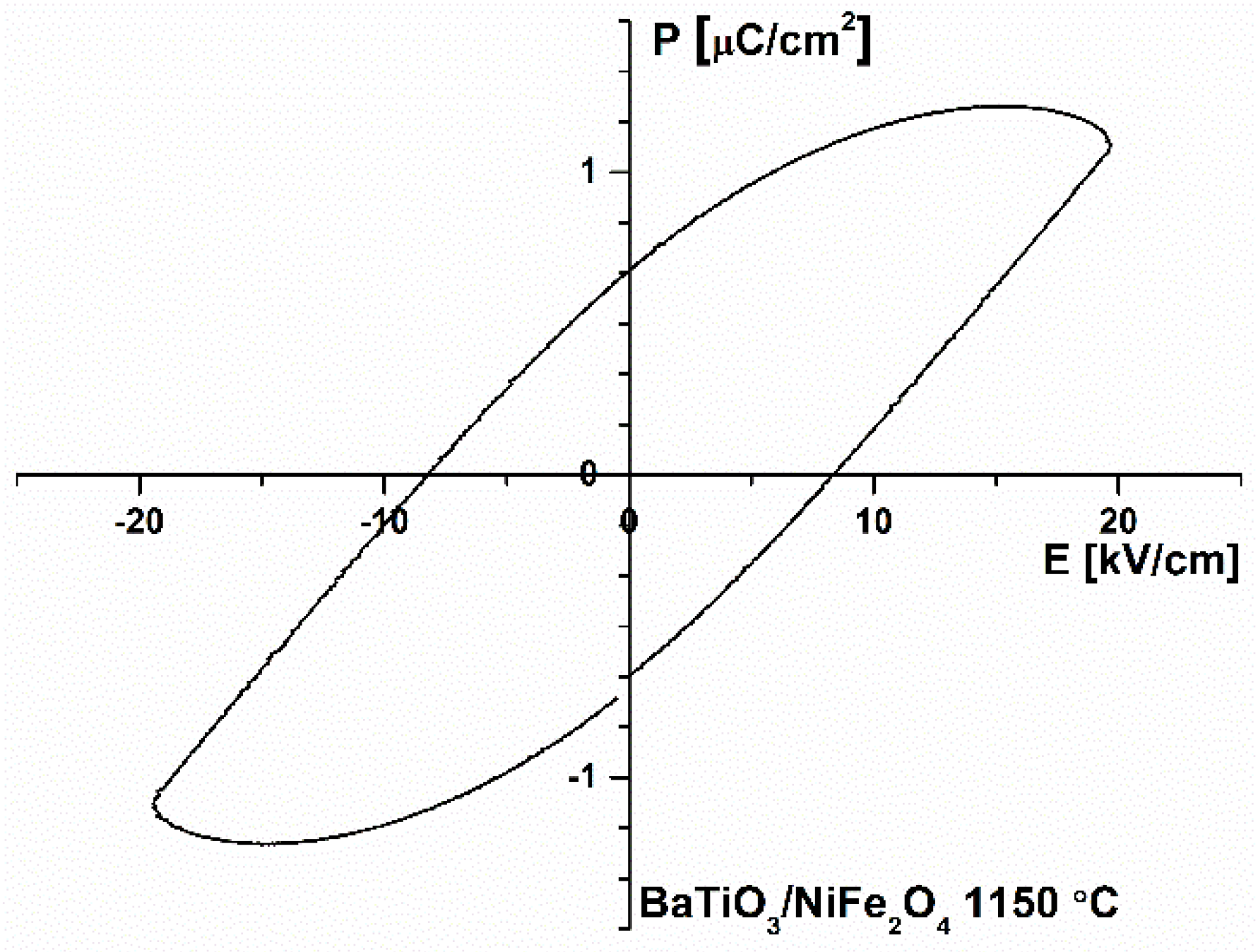
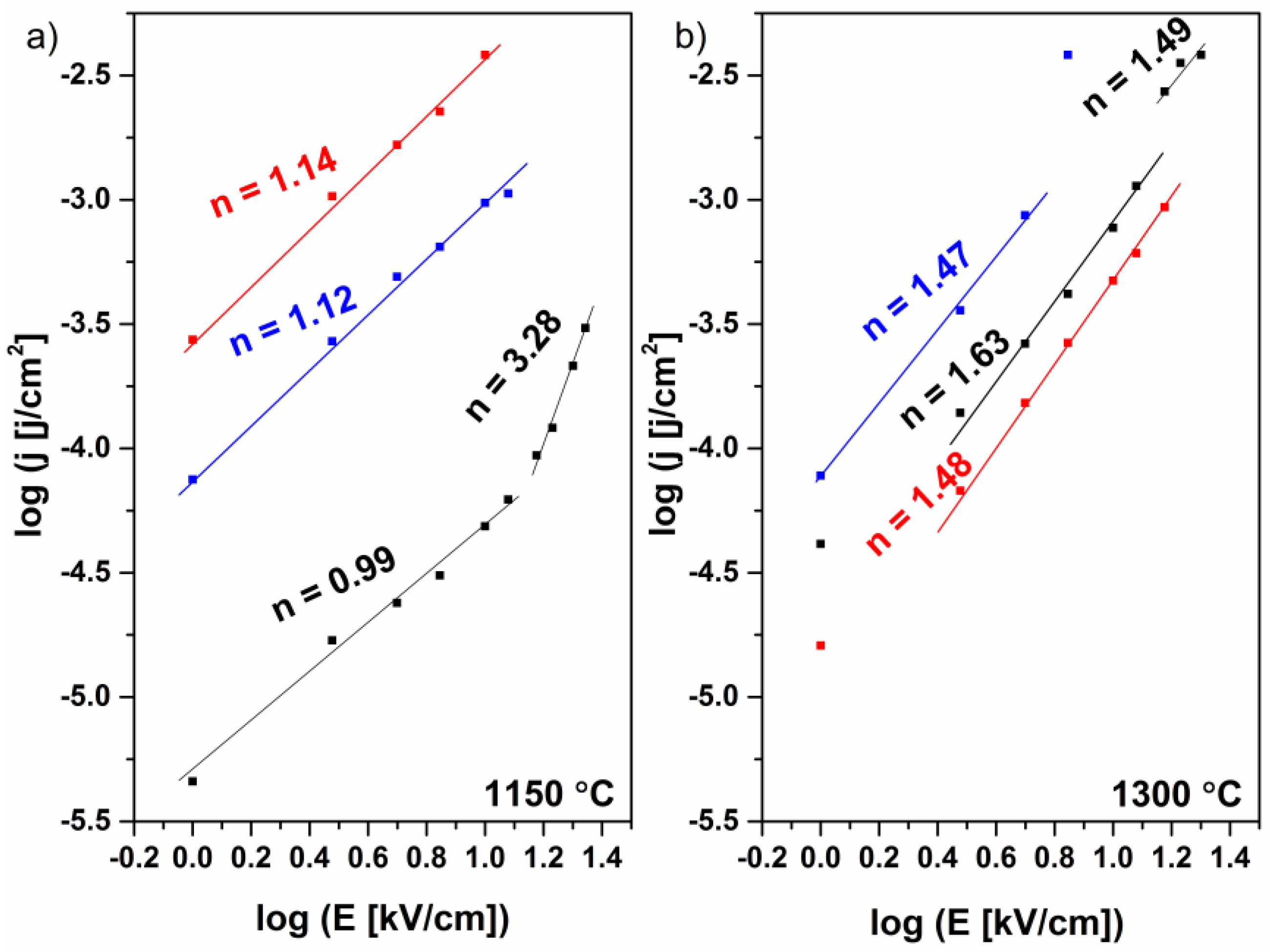
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis
3.2. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jana, B.; Ghosh, K.; Rudrapal, K.; Gaur, P.; Shihabudeen, P.K.; Roy Chaudhuri, A. Recent Progress in Flexible Multiferroics. Front. Phys. 2022, 9, 810. [Google Scholar] [CrossRef]
- Fiebig, M.; Lottermoser, T.; Meier, D.; Trassin, M. The evolution of multiferroics. Nat. Rev. Mater. 2016, 1, 16046. [Google Scholar] [CrossRef]
- Kimura, T.; Kawamoto, S.; Yamada, I.; Azuma, M.; Takano, M.; Tokura, Y. Magnetocapacitance effect in multiferroic BiMnO3. Phys. Rev. B 2003, 67, 180401. [Google Scholar] [CrossRef]
- Wang, J.; Neaton, J.B.; Zheng, H.; Nagarajan, V.; Ogale, S.B.; Liu, B.; Viehland, D.; Vaithyanathan, V.; Schlom, D.G.; Waghmare, U.V.; et al. Epitaxial BiFeO3 Multiferroic Thin Film Heterostructures. Science 2003, 299, 1719–1722. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, G. Magnetoelectric composites. Annu. Rev. Mater. Res. 2010, 40, 153–178. [Google Scholar] [CrossRef]
- Ma, J.; Hu, J.; Li, Z.; Nan, C.-W. Recent Progress in Multiferroic Magnetoelectric Composites: From Bulk to Thin Films. Adv. Mater. 2011, 23, 1062–1087. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.K.; Kumari, S.; Rack, P.D. Magnetoelectric Composites: Applications, Coupling Mechanisms, and Future Directions. Nanomaterials 2020, 10, 2072. [Google Scholar] [CrossRef]
- Mao, Q.; Wu, J.; Hu, Z.; Xu, Y.; Du, Y.; Hao, Y.; Guan, M.; Wang, C.; Wang, Z.; Zhou, Z.; et al. Magnetoelectric devices based on magnetoelectric bulk composites. J. Mater. Chem. C 2021, 9, 5594–5614. [Google Scholar] [CrossRef]
- Hu, J.-M.; Duan, C.-G.; Nan, C.-W.; Chen, L.-Q. Understanding and designing magnetoelectric heterostructures guided by computation: Progresses, remaining questions, and perspectives. Npj Comput. Mater. 2017, 3, 18. [Google Scholar] [CrossRef]
- Liu, H.; Yang, X. A brief review on perovskite multiferroics. Ferroelectrics 2017, 507, 69–85. [Google Scholar] [CrossRef]
- Dong, S.; Liu, J.-M. Recent Progress of Multiferroic Perovskite Manganites. Mod. Phys. Lett. B 2012, 26, 1230004. [Google Scholar] [CrossRef]
- Saini, J.; Sharma, A.; Sharma, M.; Kuanr, B.K. Yttrium iron garnet (YIG)/barium titanate (BTO) an engineered multiferroic nanocomposite. J. Alloys Compd. 2021, 879, 160422. [Google Scholar] [CrossRef]
- Wang, X.; Chai, Y.; Zhou, L.; Cao, H.; Cruz, C.; Yang, J.; Dai, J.; Yin, Y.; Yuan, Z.; Zhang, S.; et al. Observation of Magnetoelectric Multiferroicity in a Cubic Perovskite System: LaMn3Cr4O12. Phys. Rev. Lett. 2015, 115, 87601. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Shimano, R.; Kaneko, Y.; Murakawa, H.; Tokura, Y. Magnetoelectric resonance with electromagnons in a perovskite helimagnet. Nat. Phys. 2012, 8, 121–125. [Google Scholar] [CrossRef]
- Kopyl, S.; Surmenev, R.; Surmeneva, M.; Fetisov, Y.; Kholkin, A. Magnetoelectric effect: Principles and applications in biology and medicine—A review. Mater. Today Bio 2021, 12, 100149. [Google Scholar] [CrossRef]
- Cai, T.-Y.; Liu, S.-C.; Ju, S.; Liu, C.-Y.; Guo, G.-Y. Multiferroic Double Perovskites ScFe1−xCrO3 (1/6 ≤ x ≤ 5/6) for Highly Efficient Photovoltaics and Spintronics. Phys. Rev. Appl. 2017, 8, 34034. [Google Scholar] [CrossRef]
- Huang, W.; Harnagea, C.; Benetti, D.; Chaker, M.; Rosei, F.; Nechache, R. Multiferroic Bi2FeCrO6 based p–i–n heterojunction photovoltaic devices. J. Mater. Chem. A 2017, 5, 10355–10364. [Google Scholar] [CrossRef]
- Nechache, R.; Harnagea, C.; Li, S.; Cardenas, L.; Huang, W.; Chakrabartty, J.; Rosei, F. Bandgap tuning of multiferroic oxide solar cells. Nat. Photonics 2015, 9, 61–67. [Google Scholar] [CrossRef]
- Vavilapalli, D.S.; Srikanti, K.; Mannam, R.; Tiwari, B.; Mohan Kant, K.; Rao, M.S.R.; Singh, S. Photoactive Brownmillerite Multiferroic KBiFe2O5 and Its Potential Application in Sunlight-Driven Photocatalysis. ACS Omega 2018, 3, 16643–16650. [Google Scholar] [CrossRef]
- Sun, B.; Zhou, G.; Sun, L.; Zhao, H.; Chen, Y.; Yang, F.; Zhao, Y.; Song, Q. ABO3 multiferroic perovskite materials for memristive memory and neuromorphic computing. Nanoscale Horiz. 2021, 6, 939–970. [Google Scholar] [CrossRef]
- Plyushch, A.; Macutkevič, J.; Sokal, A.; Lapko, K.; Kudlash, A.; Adamchuk, D.; Ksenevich, V.; Bychanok, D.; Selskis, A.; Kuzhir, P.; et al. The Phosphate-Based Composite Materials Filled with Nano-Sized BaTiO3 and Fe3O4: Toward the Unfired Multiferroic Materials. Materials 2021, 14, 133. [Google Scholar] [CrossRef] [PubMed]
- Omelyanchik, A.; Antipova, V.; Gritsenko, C.; Kolesnikova, V.; Murzin, D.; Han, Y.; Turutin, A.V.; Kubasov, I.V.; Kislyuk, A.M.; Ilina, T.S.; et al. Boosting Magnetoelectric Effect in Polymer-Based Nanocomposites. Nanomaterials 2021, 11, 1154. [Google Scholar] [CrossRef] [PubMed]
- Homes, C.C.; Dordevic, S.V.; Strongin, M.; Bonn, D.A.; Liang, R.; Hardy, W.H.; Komiya, S.; Ando, Y.; Yu, G.; Kaneko, N.; et al. A universal scaling relation in high-temperature superconductors. Nature 2004, 430, 539–541. [Google Scholar] [CrossRef]
- Martins, P.; Larrea, A.; Gonçalves, R.; Botelho, G.; Ramana, E.V.; Mendiratta, S.K.; Sebastian, V.; Lanceros-Mendez, S. Novel Anisotropic Magnetoelectric Effect on δ-FeO(OH)/P(VDF-TrFE) Multiferroic Composites. ACS Appl. Mater. Interfaces 2015, 7, 11224–11229. [Google Scholar] [CrossRef]
- Corral-Flores, V.; Bueno-Baqués, D.; Ziolo, R.F. Synthesis and characterization of novel CoFe2O4–BaTiO3 multiferroic core–shell-type nanostructures. Acta Mater. 2010, 58, 764–769. [Google Scholar] [CrossRef]
- Chermahini, M.D.; Shahraki, M.M.; Kazazi, M. Multiferroic properties of novel lead-free KNN-LT/20NZCFO magneto-electric composites. Mater. Lett. 2018, 233, 188–190. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, J.; Lin, Y.; Nan, C.-W. Multiferroic magnetoelectric composite nanostructures. NPG Asia Mater. 2010, 2, 61–68. [Google Scholar] [CrossRef]
- Jayachandran, K.P.; Guedes, J.M.; Rodrigues, H.C. Solutions for maximum coupling in multiferroic magnetoelectric composites by material design. Sci. Rep. 2018, 8, 4866. [Google Scholar] [CrossRef]
- Feng, M.; Wang, J.; Hu, J.-M.; Wang, J.; Ma, J.; Li, H.-B.; Shen, Y.; Lin, Y.-H.; Chen, L.-Q.; Nan, C.-W. Optimizing direct magnetoelectric coupling in Pb(Zr,Ti)O3/Ni multiferroic film heterostructures. Appl. Phys. Lett. 2015, 106, 72901. [Google Scholar] [CrossRef]
- Gupta, A.; Chatterjee, R. Dielectric and magnetoelectric properties of BaTiO3–Co0.6Zn0.4Fe1.7Mn0.3O4 composite. J. Eur. Ceram. Soc. 2013, 33, 1017–1022. [Google Scholar] [CrossRef]
- Gorige, V.; Kati, R.; Yoon, D.H.; Kumar, P.S.A. Strain mediated magnetoelectric coupling in a NiFe2O4–BaTiO3 multiferroic composite. J. Phys. D Appl. Phys. 2016, 49, 405001. [Google Scholar] [CrossRef]
- Yang, S.-C.; Kumar, A.; Petkov, V.; Priya, S. Room-temperature magnetoelectric coupling in single-phase BaTiO3-BiFeO3 system. J. Appl. Phys. 2013, 113, 144101. [Google Scholar] [CrossRef]
- Patil, D.; Kim, J.-H.; Chai, Y.S.; Nam, J.-H.; Cho, J.-H.; Kim, B.-I.; Kim, K.H. Large Longitudinal Magnetoelectric Coupling in NiFe2O4–BaTiO3 Laminates. Appl. Phys. Express 2011, 4, 73001. [Google Scholar] [CrossRef]
- Martínez-Pérez, J.P.; Bolarín-Miró, A.M.; Cortés-Escobedo, C.A.; Sánchez-De Jesús, F. Magnetodielectric coupling in barium titanate–cobalt ferrite composites obtained via thermally-assisted high-energy ball milling. Ceram. Int. 2022, 48, 9527–9533. [Google Scholar] [CrossRef]
- Dzunuzovic, A.S.; Petrovic, M.M.V.; Stojadinovic, B.S.; Ilic, N.I.; Bobic, J.D.; Foschini, C.R.; Zaghete, M.A.; Stojanovic, B.D. Multiferroic (NiZn) Fe2O4–BaTiO3 composites prepared from nanopowders by auto-combustion method. Ceram. Int. 2015, 41, 13189–13200. [Google Scholar] [CrossRef]
- Etier, M.; Schmitz-Antoniak, C.; Salamon, S.; Trivedi, H.; Gao, Y.; Nazrabi, A.; Landers, J.; Gautam, D.; Winterer, M.; Schmitz, D.; et al. Magnetoelectric coupling on multiferroic cobalt ferrite–barium titanate ceramic composites with different connectivity schemes. Acta Mater. 2015, 90, 1–9. [Google Scholar] [CrossRef]
- Hossain, S.; Hossain, S. Magnetic and Optical Characterization of Cobalt Ferrite–Barium Titanate Core–Shell for Biomedical Applications. IEEE Trans. Magn. 2022, 58, 2501208. [Google Scholar] [CrossRef]
- Sreenivasulu, G.; Qu, H.; Srinivasan, G. Multiferroic oxide composites: Synthesis, characterisation and applications. Mater. Sci. Technol. 2014, 30, 1625–1632. [Google Scholar] [CrossRef]
- Ryu, J.; Carazo, A.V.; Uchino, K.; Kim, H.-E. Piezoelectric and magnetoelectric properties of lead zirconate titanate/Ni-ferrite particulate composites. J. Electroceramics 2001, 7, 17–24. [Google Scholar] [CrossRef]
- Hrib, L.M.; Caltun, O.F. Effects of the chemical composition of the magnetostrictive phase on the dielectric and magnetoelectric properties of cobalt ferrite–barium titanate composites. J. Alloys Compd. 2011, 509, 6644–6648. [Google Scholar] [CrossRef]
- Grigalaitis, R.; Vijatović Petrović, M.M.; Bobić, J.D.; Dzunuzovic, A.; Sobiestianskas, R.; Brilingas, A.; Stojanović, B.D.; Banys, J. Dielectric and magnetic properties of BaTiO3-NiFe2O4 multiferroic composites. Ceram. Int. 2014, 40, 6165–6170. [Google Scholar] [CrossRef]
- El-Shater, R.E.; Atlam, A.S.; Elnimr, M.K.; Assar, S.T.; Tishkevich, D.I.; Zubar, T.I.; Trukhanov, S.V.; Trukhanov, A.V.; Zhou, D.; Darwish, M.A. AC measurements, impedance spectroscopy analysis, and magnetic properties of Ni0.5Zn0.5Fe2O4/BaTiO3 multiferroic composites. Mater. Sci. Eng. B 2022, 286, 116025. [Google Scholar] [CrossRef]
- De Leo, C.T.; Dannangoda, G.C.; Hobosyan, M.A.; Held, J.T.; Samghabadi, F.S.; Khodadadi, M.; Litvinov, D.; Mkhoyan, K.A.; Martirosyan, K.S. Carbon combustion synthesis of Janus-like particles of magnetoelectric cobalt ferrite and barium titanate. Ceram. Int. 2021, 47, 5415–5422. [Google Scholar] [CrossRef]
- Matutes-Aquino, J.A.; Botello-Zubiate, M.E.; Corral-Flores, V.; Frutos, J.D.E.; Cebollada, F.; Menéndez, E.; Jiménez, F.J.; González, A.M. Synthesis and Characterization of Nickel Ferrite-Barium Titanate Ceramic Composites. Integr. Ferroelectr. 2008, 101, 22–28. [Google Scholar] [CrossRef]
- Safi Samghabadi, F.; Chang, L.; Khodadadi, M.; Martirosyan, K.S.; Litvinov, D. Scalable, cost-efficient synthesis and properties optimization of magnetoelectric cobalt ferrite/barium titanate composites. APL Mater. 2021, 9, 21104. [Google Scholar] [CrossRef]
- Majid, F.; Rauf, J.; Ata, S.; Bibi, I.; Malik, A.; Ibrahim, S.M.; Ali, A.; Iqbal, M. Synthesis and characterization of NiFe2O4 ferrite: Sol–gel and hydrothermal synthesis routes effect on magnetic, structural and dielectric characteristics. Mater. Chem. Phys. 2021, 258, 123888. [Google Scholar] [CrossRef]
- Majid, F.; Rauf, J.; Ata, S.; Bibi, I.; Yameen, M.; Iqbal, M. Hydrothermal Synthesis of Zinc Doped Nickel Ferrites: Evaluation of Structural, Magnetic and Dielectric Properties. Z. Für Phys. Chemie 2019, 233, 1411–1430. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, A.A.; Moreno-Trejo, M.B.; Meléndez-Zaragoza, M.J.; Collins-Martínez, V.; López-Ortiz, A.; Martínez-Guerra, E.; Sánchez-Domínguez, M. Spinel-type ferrite nanoparticles: Synthesis by the oil-in-water microemulsion reaction method and photocatalytic water-splitting evaluation. Int. J. Hydrogen Energy 2019, 44, 12421–12429. [Google Scholar] [CrossRef]
- Atiq, S.; Majeed, M.; Ahmad, A.; Abbas, S.K.; Saleem, M.; Riaz, S.; Naseem, S. Synthesis and investigation of structural, morphological, magnetic, dielectric and impedance spectroscopic characteristics of Ni-Zn ferrite nanoparticles. Ceram. Int. 2017, 43, 2486–2494. [Google Scholar] [CrossRef]
- Thakur, P.; Taneja, S.; Chahar, D.; Ravelo, B.; Thakur, A. Recent advances on synthesis, characterization and high frequency applications of Ni-Zn ferrite nanoparticles. J. Magn. Magn. Mater. 2021, 530, 167925. [Google Scholar] [CrossRef]
- Shahane, G.S.; Kumar, A.; Arora, M.; Pant, R.P.; Lal, K. Synthesis and characterization of Ni–Zn ferrite nanoparticles. J. Magn. Magn. Mater. 2010, 322, 1015–1019. [Google Scholar] [CrossRef]
- Gajbhiye, N.S.; Prasad, S. Thermal decomposition of hexahydrated nickel iron citrate. Thermochim. Acta 1996, 285, 325–336. [Google Scholar] [CrossRef]
- Zhang, C.-Y.; Shen, X.-Q.; Zhou, J.-X.; Jing, M.-X.; Cao, K. Preparation of spinel ferrite NiFe2O4 fibres by organic gel-thermal decomposition process. J. Sol-Gel Sci. Technol. 2007, 42, 95–100. [Google Scholar] [CrossRef]
- Itoh, H.; Takeda, T.; Naka, S. Preparation of nickel and Ni-Zn ferrite films by thermal decomposition of metal acetylacetonates. J. Mater. Sci. 1986, 21, 3677–3680. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, M.; Shin, H.-S.; Ju, B.-K.; Chun, M. Structural and Magnetic Properties of NiZn Ferrite Nanoparticles Synthesized by a Thermal Decomposition Method. Appl. Sci. 2020, 10, 6279. [Google Scholar] [CrossRef]
- Itoh, H.; Uemura, T.; Yamaguchi, H.; Naka, S. Chemical vapour deposition of epitaxial Ni-Zn ferrite films by thermal decomposition of acetylacetonato complexes. J. Mater. Sci. 1989, 24, 3549–3552. [Google Scholar] [CrossRef]
- Stoia, M.; Barvinschi, P.; Tudoran, L.B.; Barbu, M.; Stefanescu, M. Synthesis of nanocrystalline nickel ferrite by thermal decomposition of organic precursors. J. Therm. Anal. Calorim. 2012, 108, 1033–1039. [Google Scholar] [CrossRef]
- Jeremić, D.; Andjelković, L.; Milenković, M.R.; Šuljagić, M.; Ristović, M.Š.; Ostojić, S.; Nikolić, A.S.; Vulić, P.; Brčeski, I.; Pavlović, V. One-pot combustion synthesis of nickel oxide and hematite: From simple coordination compounds to high purity metal oxide nanoparticles. Sci. Sinter. 2020, 52, 481–490. [Google Scholar] [CrossRef]
- Iacob, M.; Racles, C.; Tugui, C.; Stiubianu, G.; Bele, A.; Sacarescu, L.; Timpu, D.; Cazacu, M. From iron coordination compounds to metal oxide nanoparticles. Beilstein J. Nanotechnol. 2016, 7, 2074–2087. [Google Scholar] [CrossRef]
- Jesus, J.C.D.; González, I.; Quevedo, A.; Puerta, T. Thermal decomposition of nickel acetate tetrahydrate: An integrated study by TGA, QMS and XPS techniques. J. Mol. Catal. A Chem. 2005, 228, 283–291. [Google Scholar] [CrossRef]
- Kumar, N.; Kachroo, P.L.; Kant, R. Thermal decomposition of some N-oxide complexes of cobalt(II), nickel(II) and copper(II) carboxylates. J. Therm. Anal. 1979, 17, 81–85. [Google Scholar] [CrossRef]
- Dollimore, D.; Pearce, J. Changes in the surface characteristics of residues from the thermal decomposition of nickel oxysalts. J. Therm. Anal. 1974, 6, 321–333. [Google Scholar] [CrossRef]
- Sun, Z.-L. Characteristics of thermal decomposition products of rare earth, alkali earth metal and transition metal p toluenesulfonates. J. Therm. Anal. Calorim. 2005, 79, 731–735. [Google Scholar] [CrossRef]
- García, A.R.; Laverat, A.G.; Prudencio, C.V.R.; Méndez, A.J. Synthesis and thermal decomposition of Co(II), Ni(II), Cu(II), Zn(II), Cd(II), and Pb(II) m-benzenedisulphonates. Thermochim. Acta 1993, 213, 199–210. [Google Scholar] [CrossRef]
- Fereshteh, Z.; Salavati-Niasari, M. Effect of ligand on particle size and morphology of nanostructures synthesized by thermal decomposition of coordination compounds. Adv. Colloid Interface Sci. 2017, 243, 86–104. [Google Scholar] [CrossRef]
- Šuljagić, M.; Vulić, P.; Jeremić, D.; Pavlović, V.; Filipović, S.; Kilanski, L.; Lewinska, S.; Slawska-Waniewska, A.; Milenković, M.R.; Nikolić, A.S.; et al. The influence of the starch coating on the magnetic properties of nanosized cobalt ferrites obtained by different synthetic methods. Mater. Res. Bull. 2021, 134, 111117. [Google Scholar] [CrossRef]
- Andjelković, L.; Jeremić, D.; Milenković, M.R.; Radosavljević, J.; Vulić, P.; Pavlović, V.; Manojlović, D.; Nikolić, A.S. Synthesis, characterization and in vitro evaluation of divalent ion release from stable NiFe2O4, ZnFe2O4 and core-shell ZnFe2O4@NiFe2O4 nanoparticles. Ceram. Int. 2020, 46, 3528–3533. [Google Scholar] [CrossRef]
- Kilanski, L.; Lewinska, S.; Slawska-Waniewska, A.; Pavlović, V.B.; Filipović, S. Attempts to obtain BaTiO3/Fe2O3 core-shell type structures: The role of iron oxide nanoparticle formation and agglomeration. Inorg. Chem. Commun. 2022, 145. [Google Scholar] [CrossRef]
- Syazwan, M.M.; Hapishah, A.N.; Azis, R.S.; Abbas, Z.; Hamidon, M.N. Grain growth effects on magnetic properties of Ni0.6Zn0.4Fe2O4 material prepared using mechanically alloyed nanoparticles. Results Phys. 2018, 9, 842–850. [Google Scholar] [CrossRef]
- Ranga Mohan, G.; Ravinder, D.; Ramana Reddy, A.V.; Boyanov, B.S. Dielectric properties of polycrystalline mixed nickel–zinc ferrites. Mater. Lett. 1999, 40, 39–45. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, N.; Bhargava, R.; Sahni, M.; Sung, K.; Jung, J.H. Magnetodielectric effect in BaTiO3/ZnFe2O4 core/shell nanoparticles. J. Alloys Compd. 2014, 587, 437–441. [Google Scholar] [CrossRef]
- Yu, Z.; Ang, C. Maxwell–Wagner polarization in ceramic composites BaTiO3–(Ni0.3Zn0.7)Fe2.1O4. J. Appl. Phys. 2001, 91, 794–797. [Google Scholar] [CrossRef]
- Bammannavar, B.K.; Naik, L.R. Electrical properties and magnetoelectric effect in (x)Ni0.5Zn0.5Fe2O4+(1−x)BPZT composites. Smart Mater. Struct. 2009, 18, 85013. [Google Scholar] [CrossRef]
- Curecheriu, L.P.; Buscaglia, M.T.; Buscaglia, V.; Mitoseriu, L.; Postolache, P.; Ianculescu, A.; Nanni, P. Functional properties of BaTiO3–Ni0.5Zn0.5Fe2O4 magnetoelectric ceramics prepared from powders with core-shell structure. J. Appl. Phys. 2010, 107, 104106. [Google Scholar] [CrossRef]
- Macdonald, J.R. Impedance Spectroscopy: Emphasizing Solid Materials and Systems; Wiley: Hoboken, NY, USA, 1987. [Google Scholar]
- Nuzhnyy, D.; Bovtun, V.; Savinov, M.; Kempa, M.; Petzelt, J.; Kaman, O.; Klementová, M.; Kuličková, J.; Jirák, Z. Synthesis and broadband dielectric-infrared spectroscopy of La1−xSrxMnO3@BaTiO3 nanocomposite. Mater. Res. Bull. 2021, 144, 111459. [Google Scholar] [CrossRef]
- Petzelt, J.; Nuzhnyy, D.; Bovtun, V.; Savinov, M.; Kempa, M.; Rychetsky, I. Broadband dielectric and conductivity spectroscopy of inhomogeneous and composite conductors. Phys. Status Solidi Appl. Mater. Sci. 2013, 210, 2259–2271. [Google Scholar] [CrossRef]
- Jonscher, A.K. A new understanding of the dielectric relaxation of solids. J. Mater. Sci. 1981, 16, 2037–2060. [Google Scholar] [CrossRef]
- Dzunuzovic, A.S.; Petrovic, M.M.V.; Bobic, J.D.; Ilic, N.I.; Stojanovic, B.D. Influence of ferrite phase on electrical properties of the barium zirconium titanate based multiferroic composites. J. Electroceramics 2021, 46, 57–71. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lai, B.C. Chapter 1—The electrical properties of high-dielectric-constant and ferroelectric thin films for very large scale integration circuits. In Handbook of Thin Films; Singh Nalwa, H., Ed.; Academic Press: Burlington, VT, USA, 2002; pp. 1–98. ISBN 978-0-12-512908-4. [Google Scholar]
- Kundu, T.K.; Lee, J.Y. Thickness-Dependent Electrical Properties of Pb(Zr,Ti)O3 Thin Film Capacitors for Memory Device Applications. J. Electrochem. Soc. 2000, 147, 326. [Google Scholar] [CrossRef]
- Andjelković, L.; Šuljagić, M.; Lakić, M.; Jeremić, D.; Vulić, P.; Nikolić, A.S. A study of the structural and morphological properties of Ni–ferrite, Zn–ferrite and Ni–Zn–ferrites functionalized with starch. Ceram. Int. 2018, 44, 14163–14168. [Google Scholar] [CrossRef]
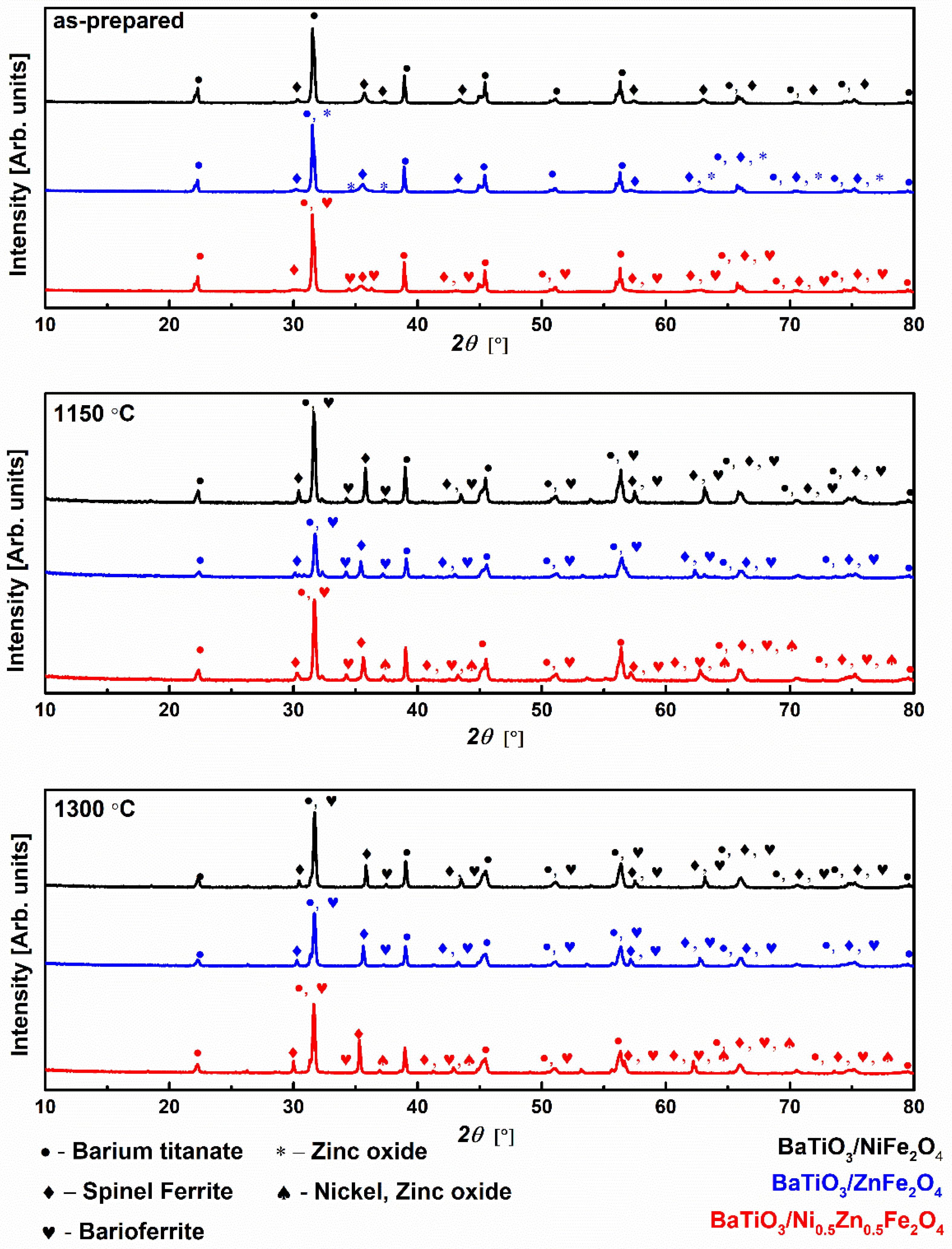
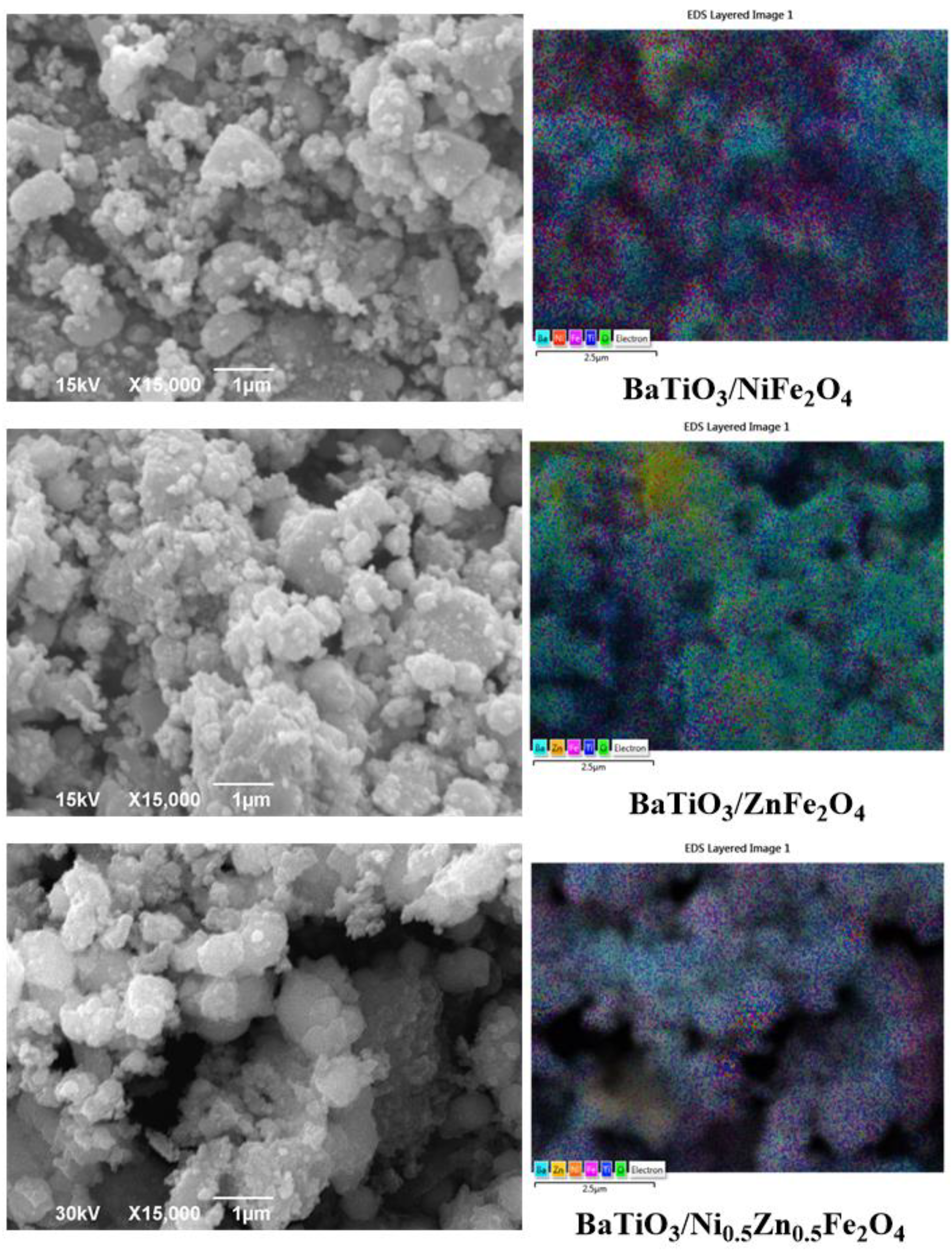
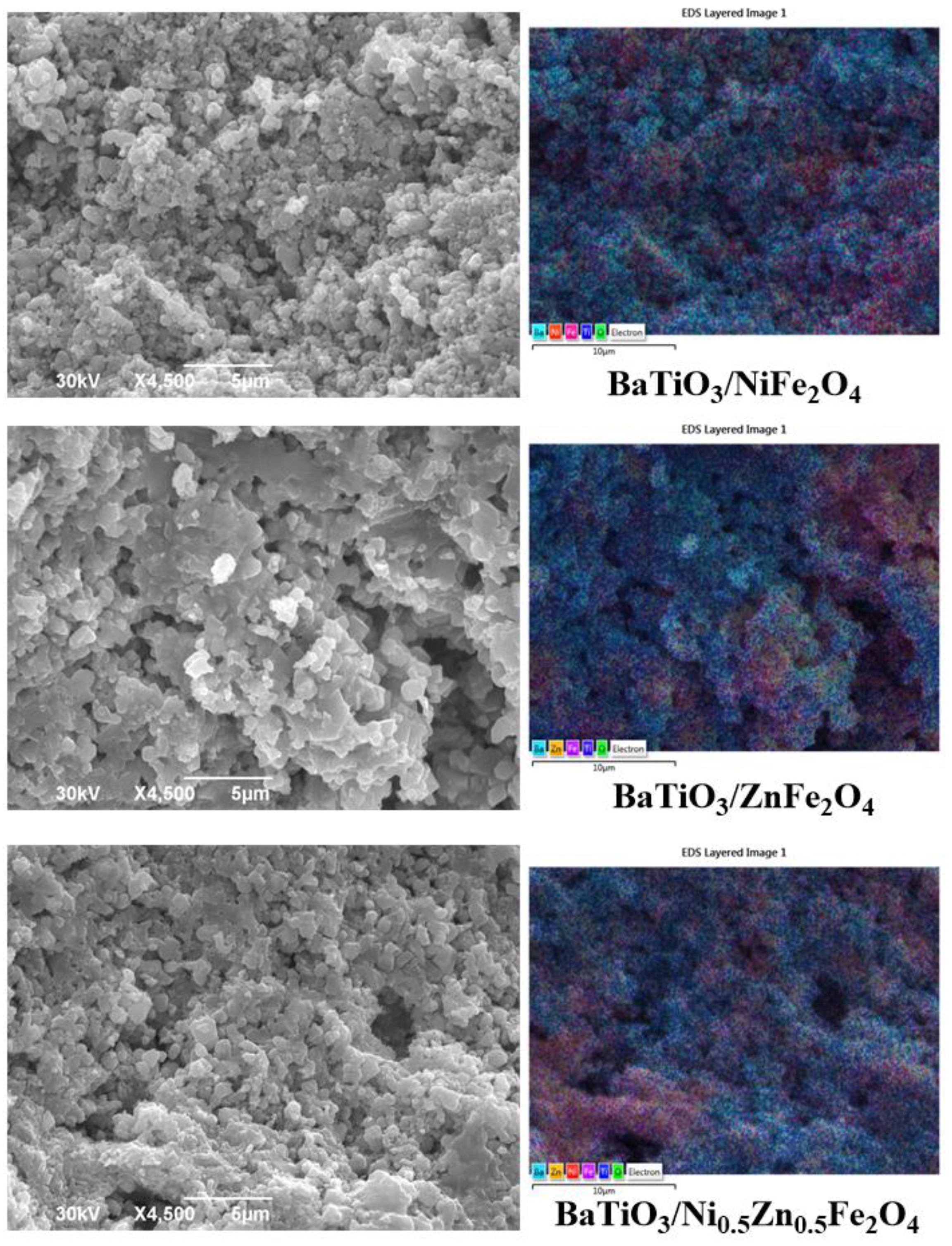

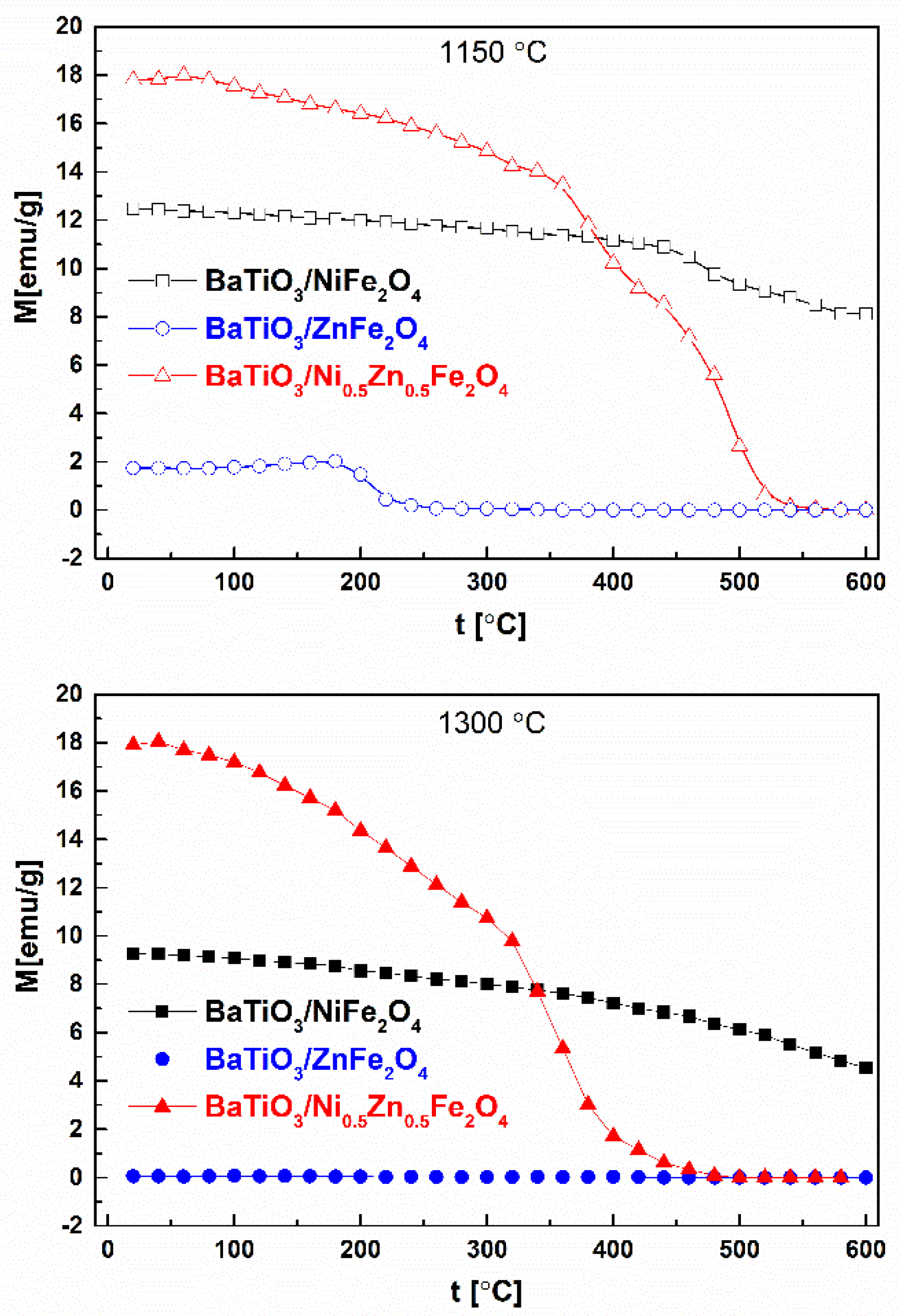




| Samples | Phase | ||
|---|---|---|---|
| BaTiO3/NiFe2O4 as-prepared | BaTiO3 Tetragonal (P4mm) PDF # 01-078-2738 57(2)% | Ni1.3Fe1.7O4 Spinel (Fd-3m) PDF # 01-080-0072 43(2)% | / |
| BaTiO3/ZnFe2O4 as-prepared | BaTiO3 Tetragonal (P4mm) PDF # 01-081-8524 66.3(6)% | ZnFe2O4 Spinel (Fd-3m) PDF # 01-083-442 29.4(5)% | ZnO Wurtzite (P63mc) PDF # 01-070-8070 4.3(2)% |
| BaTiO3/Ni0.5Zn0.5Fe2O4 as-prepared | BaTiO3 Tetragonal (P4mm) PDF # 01-083-8300 60(1)% | NixZn1−xFe2O4 Spinel (Fd-3m) PDF # 01-080-4511 40(1)% | BaTiNiFe10O19 (P63/mmc) PDF # 00-054-0776 >1% |
| BaTiO3/NiFe2O4 1150 °C | BaTiO3 Tetragonal (P4mm) PDF # 01-083-8300 77(2)% | Ni1.25Fe1.85O4 Spinel (Fd-3m) PDF # 01-088-0380 19(2)% | BaFe11.9O19/ BaTiNiFe10O19 PDF # 01-079-1742/00-054-0776 (P63/mmc) 4(1)% |
| BaTiO3/ZnFe2O4 1150 °C | BaTiO3 Tetragonal (P4mm) PDF # 00-005-0626 50(5)% | ZnFe2O4 Spinel (Fd-3m) PDF # 01-078-5429 46(4)% | BaTi0.636Fe0.364O2.804 PDF # 01-089-4607 4(1)% BaTiZnFe10O19 PDF # 00-054-1246 >1% |
| BaTiO3/Ni0.5Zn0.5Fe2O4 1150 °C | BaTiO3 Tetragonal (P4mm) PDF # 01-074-4540 40(1)% | NixZn1−xFe2O4 Spinel (Fd-3m) PDF # 01-077-9652 24(1)% | BaTiNiFe10O19 (P63/mmc) PDF # 00-054-0776 >1% Ni0.8Zn0.2O PDF # 01-071-6735 36(1)% |
| BaTiO3/NiFe2O4 1300 °C | BaTiO3 Tetragonal (P4/mmm) PDF # 01-079-2264 71.1(4)% | Ni0.4Fe2.6O4 Spinel (Fd-3m) PDF # 01-087-2335 23.5(3)% | BaFe0.67Ti0.33O2.952 PDF # 01-089-0949 (P63/mmc) 5.4(5)% |
| BaTiO3/ZnFe2O4 1300 °C | BaTiO3 Tetragonal (P4/mmm) PDF # 01-079-2264 63.5(3)% | ZnFe2O4 Spinel (Fd-3m) PDF # 01-078-5429 29.6(2)% | BaFe0.125Ti0.875O2.92 (P63/mmc) PDF # 01-089-4605 6.9(3)%. |
| BaTiO3/Ni0.5Zn0.5Fe2O4 1300 °C | BaTiO3 Tetragonal (P4/mmm) PDF # 01-079-2264 56(3)% | Ni0.8Zn0.2Fe2O4 Spinel (Fd-3m) PDF # 01-077-9719 34(3)% | BaFe0.25Ti0.75O2.888 (P63/mmc) PDF # 01-089-4604 9(1)% Ni0.02Zn0.98O PDF # 01-080-3507 1.0(1)% |
| Sample | M [emu/g] |
|---|---|
| BaTiO3/NiFe2O4 1150 °C | 12.46 |
| BaTiO3/ZnFe2O4 1150 °C | 1.74 |
| BaTiO3/Ni0.5Zn0.5Fe2O4 1150 °C | 17.26 |
| BaTiO3/NiFe2O4 1300 °C | 9.26 |
| BaTiO3/ZnFe2O4 1300 °C | 0.06 |
| BaTiO3/Ni0.5Zn0.5Fe2O4 1300 °C | 17.91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šuljagić, M.; Petronijević, I.; Mirković, M.M.; Kremenović, A.; Džunuzović, A.; Pavlović, V.B.; Kalezić-Glišović, A.; Andjelković, L. BaTiO3/NixZn1−xFe2O4 (x = 0, 0.5, 1) Composites Synthesized by Thermal Decomposition: Magnetic, Dielectric and Ferroelectric Properties. Inorganics 2023, 11, 51. https://doi.org/10.3390/inorganics11020051
Šuljagić M, Petronijević I, Mirković MM, Kremenović A, Džunuzović A, Pavlović VB, Kalezić-Glišović A, Andjelković L. BaTiO3/NixZn1−xFe2O4 (x = 0, 0.5, 1) Composites Synthesized by Thermal Decomposition: Magnetic, Dielectric and Ferroelectric Properties. Inorganics. 2023; 11(2):51. https://doi.org/10.3390/inorganics11020051
Chicago/Turabian StyleŠuljagić, Marija, Ivan Petronijević, Miljana M. Mirković, Aleksandar Kremenović, Adis Džunuzović, Vladimir B. Pavlović, Aleksandra Kalezić-Glišović, and Ljubica Andjelković. 2023. "BaTiO3/NixZn1−xFe2O4 (x = 0, 0.5, 1) Composites Synthesized by Thermal Decomposition: Magnetic, Dielectric and Ferroelectric Properties" Inorganics 11, no. 2: 51. https://doi.org/10.3390/inorganics11020051
APA StyleŠuljagić, M., Petronijević, I., Mirković, M. M., Kremenović, A., Džunuzović, A., Pavlović, V. B., Kalezić-Glišović, A., & Andjelković, L. (2023). BaTiO3/NixZn1−xFe2O4 (x = 0, 0.5, 1) Composites Synthesized by Thermal Decomposition: Magnetic, Dielectric and Ferroelectric Properties. Inorganics, 11(2), 51. https://doi.org/10.3390/inorganics11020051







