Thermochemistry, Structure, and Optical Properties of a New β-La2(SO4)3 Polymorphic Modification
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermochemistry of Formation and Thermal Stability
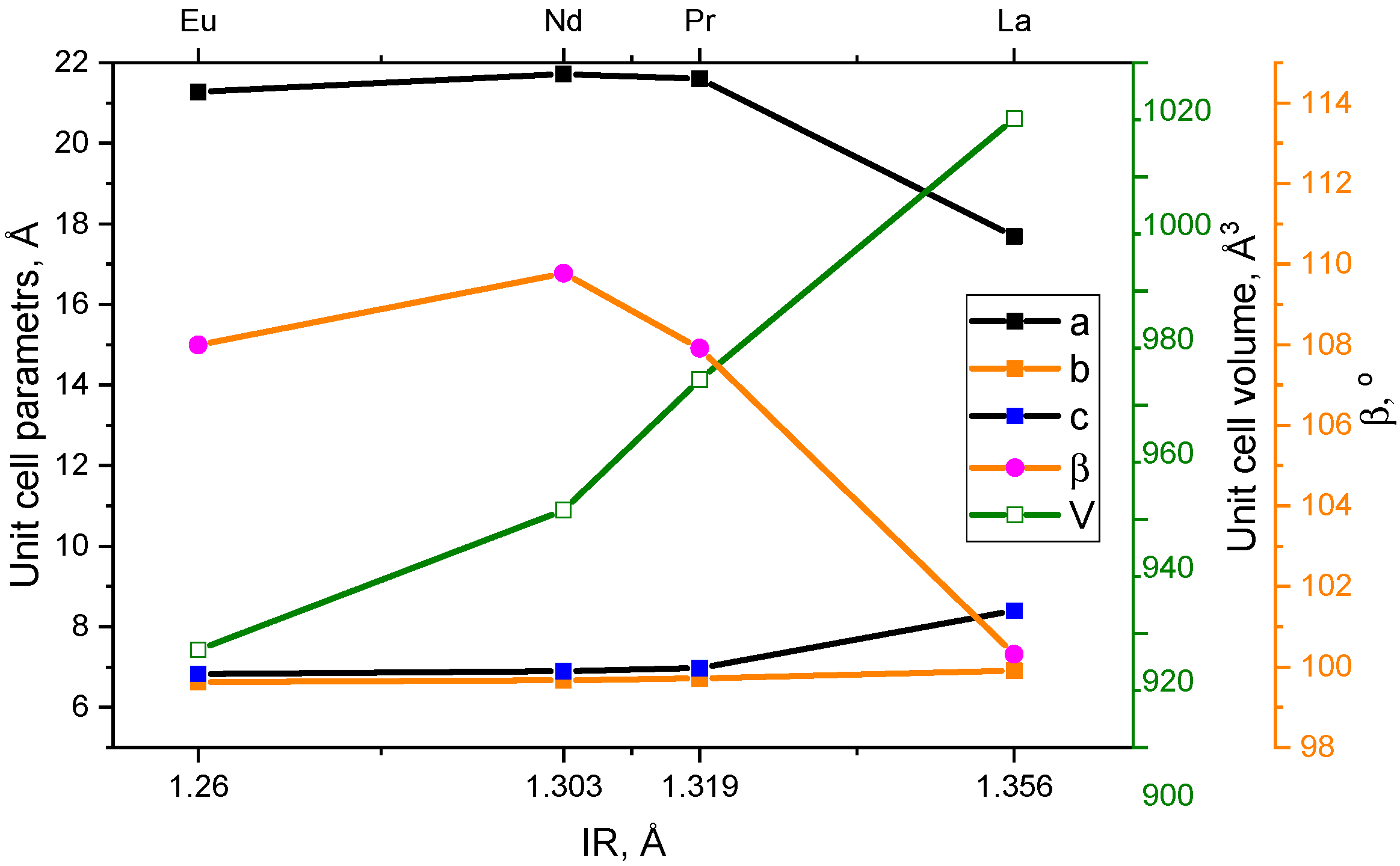
2.2. Crystal Structure
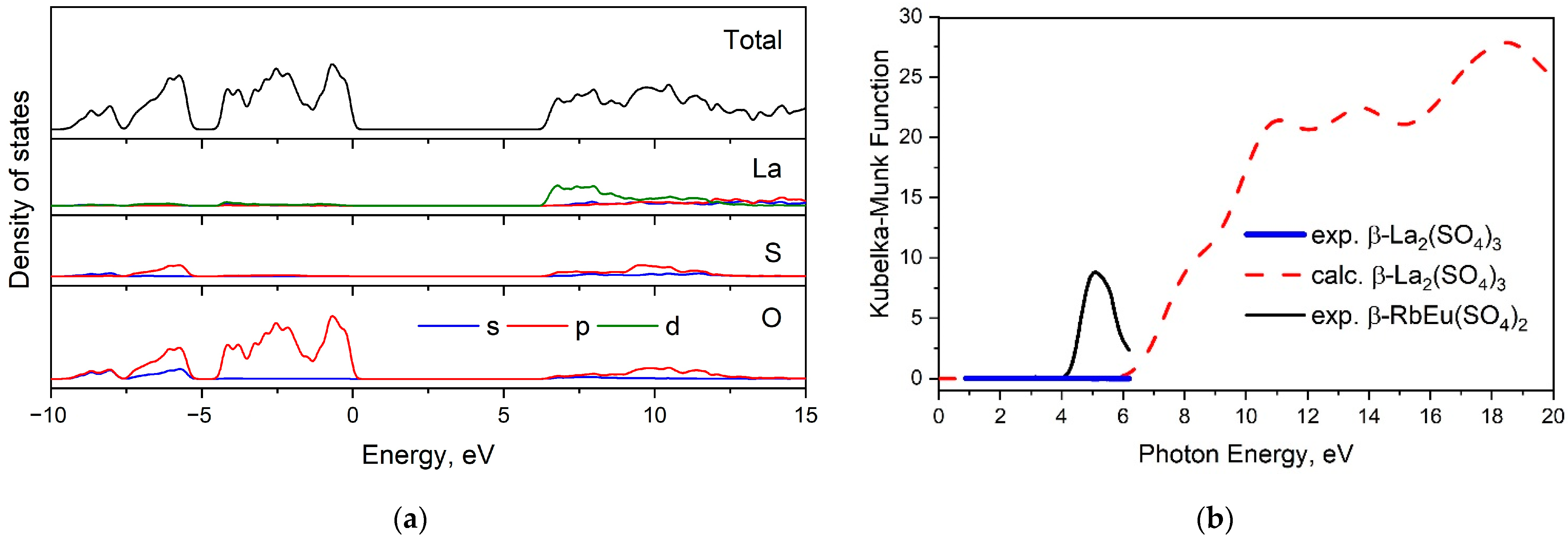
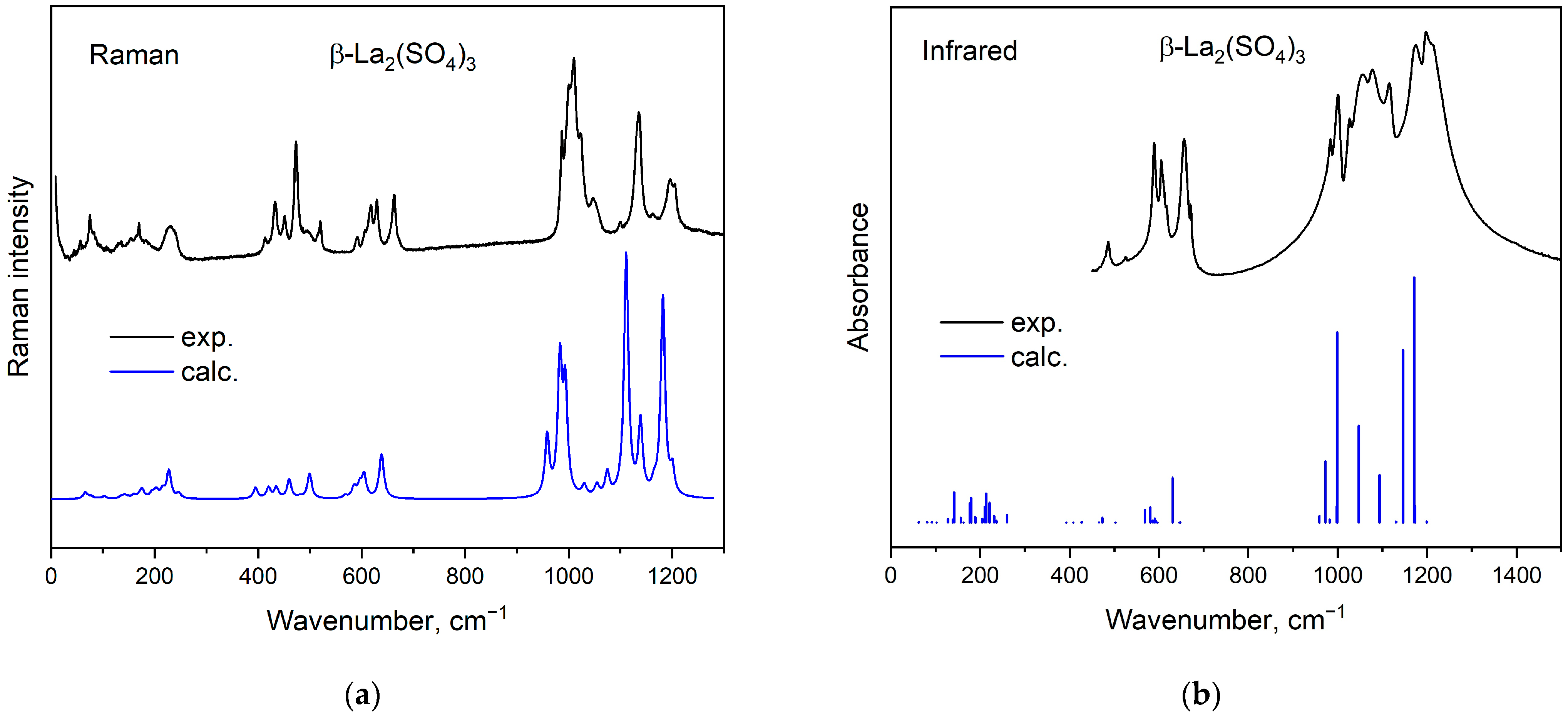
2.3. Electronic Structure and Optical Properties
3. Methods and Materials
3.1. Synthesis
3.2. Analysis Methods
3.3. Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Han, K.N. Characteristics of precipitation of rare earth elements with various precipitants. Minerals 2020, 10, 178. [Google Scholar] [CrossRef]
- Amato, A.; Becci, A.; Birloaga, I.; De Michelis, I.; Ferella, F.; Innocenzi, V.; Ippolito, N.M.; Gomez, C.P.J.; Vegliò, F.; Beolchini, F. Sustainability analysis of innovative technologies for the rare earth elements recovery. Renew. Sustain. Energy Rev. 2019, 106, 41–53. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Gerven, T.V.; Yang, Y.; Walton, A.; Buchert, M. Recycling of rare earths: A critical review. J. Clean. Prod. 2013, 51, 1–22. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, T.A.; Dreisinger, D.; Doyle, F. A critical review on solvent extraction of rare earths from aqueous solutions. Miner. Eng. 2014, 56, 10–28. [Google Scholar] [CrossRef]
- Bao, W.; Liu, D.; Li, P.; Duan, Y. Structural properties, elastic anisotropies and thermal conductivities of tetragonal LnB2C2 (Ln = Rare Earth) compounds from first-principles calculations. Ceram. Int. 2019, 45, 1857–1867. [Google Scholar] [CrossRef]
- Guo, D.; Moreno-Ramírez, L.M.; Law, J.-Y.; Zhang, Y.; Franco, V. Excellent cryogenic magnetocaloric properties in heavy rare-earth based HRENiGa2 (HRE = Dy, Ho, or Er) compounds. Sci. China Mater. 2023, 66, 249–256. [Google Scholar] [CrossRef]
- Gupta, P.; Mahapatra, P.K.; Choudhary, R.N.P. Structural and electrical characteristics of rare-earth modified bismuth layer structured compounds. J. Alloys Compd. 2021, 863, 158457. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Z.; Xu, X.; Mao, X.; Xiong, J.; Yang, Y.; Han, K. All-inorganic rare-earth halide double perovskite single crystals with highly efficient photoluminescence. Adv. Opt. Mater. 2021, 9, 2100689. [Google Scholar] [CrossRef]
- Batchu, N.K.; Li, Z.; Verbelen, B.; Binnemans, K. Structural effects of neutral organophosphorus extractants on solvent extraction of rare-earth elements from aqueous and non-aqueous nitrate solutions. Sep. Purif. Technol. 2021, 255, 117711. [Google Scholar] [CrossRef]
- Silva, R.G.; Morais, C.A.; Teixeira, L.V.; Oliveira, É.D. Selective precipitation of high-quality rare earth oxalates or carbonates from a purified sulfuric liquor containing soluble impurities. Min. Metall. Explor. 2019, 36, 967–977. [Google Scholar] [CrossRef]
- Geneyton, A.; Foucaud, Y.; Filippov, L.O.; Menad, N.-E.; Renard, A.; Badawi, M. Synergistic adsorption of lanthanum ions and fatty acids for efficient rare-earth phosphate recovery: Surface analysis and ab initio molecular dynamics studies. Appl. Surf. Sci. 2020, 526, 146725. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, J.; Cheng, G.; Xue, X.; Yang, H. Kinetics and mechanism of hydrochloric acid leaching of rare earths from Bayan Obo slag and recovery of rare earth oxalate and high purity oxides. Hydrometallurgy 2022, 208, 105782. [Google Scholar] [CrossRef]
- Huang, W.; Chen, W.; Bai, Q.; Zhang, Z.; Feng, M.; Zheng, Z. Anion-guided stepwise assembly of high-nuclearity lanthanide hydroxide clusters. Angew. Chem. 2022, 134, e202205385. [Google Scholar] [CrossRef]
- Jiao, D.-X.; Zhang, H.-L.; Li, X.-F.; He, C.; Li, J.-H.; Wei, Q.; Yang, G.-Y. YSO4F·H2O: A Deep-Ultraviolet Birefringent Rare-Earth Sulfate Fluoride with Enhanced Birefringence Induced by Fluorinated Y-Centered Polyhedra. Inorg. Chem. 2023, 62, 17333–17340. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, Y.G.; Molokeev, M.S.; Jiang, X.; Sedykh, A.E.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Roginskii, E.M.; Zhernakov, M.A.; Heuler, D.; Seuffert, M.; et al. Negative thermal expansion in the polymorphic modification of double sulfate β-AEu(SO4)2 (A–Rb+, Cs+). Inorg. Chem. 2023, 62, 12423–12433. [Google Scholar] [CrossRef] [PubMed]
- Denisenko, Y.G.; Khritokhin, N.A.; Andreev, O.V.; Basova, S.A.; Sal’nikova, E.I.; Polkovnikov, A.A. Thermal decomposition of europium sulfates Eu2(SO4)3·8H2O and EuSO4. J. Solid State Chem. 2017, 255, 219–224. [Google Scholar] [CrossRef]
- Buyer, C.; Enseling, D.; Jüstel, T.; Schleid, T. Hydrothermal synthesis, crystal structure, and spectroscopic properties of pure and Eu3+-doped NaY[SO4]2∙H2O and its anhydrate NaY[SO4]2. Crystals 2021, 11, 575. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Peshehonova, A.O.; Lipina, O.A.; Medyankina, I.S.; Enyashin, A.N.; Chufarov, A.Y.; Tyutyunnik, A.P. Co-crystallization of red emitting (NH4)3Sc(SO4)3:Eu3+ microfibers: Structure–luminescence relationship for promising application in optical thermometry. CrystEngComm 2022, 24, 4819–4830. [Google Scholar] [CrossRef]
- Pasechnik, L.A.; Medyankina, I.S.; Yatsenko, S.P. Scandium extraction from multicomponent systems by crystallization of complex sulfates. IOP Conf. Ser. Mater. Sci. Eng. 2020, 848, 012064. [Google Scholar] [CrossRef]
- Thomsen, M.S.; Sørensen, T.J. Delicate, a study of the structural changes in ten-coordinated La (III), Ce (III), Pr (III), Nd (III), Sm (III) and Eu (III) sulfates. Dalton Trans. 2022, 51, 8964–8974. [Google Scholar] [CrossRef]
- Haghighat, M.; Naroie, A.; Rezvani, A.; Hakimi, M.; Saravani, H.; Darroudi, M.; Amini, A.; Sabaghan, M.; Khatami, M. Anticancer property of lanthanide sulfate nanostructure against neuroblastoma-neuro2a cell line. BioNanoScience 2021, 11, 696–702. [Google Scholar] [CrossRef]
- Das, G.; Lencka, M.M.; Eslamimanesh, A.; Wang, P.; Anderko, A.; Riman, R.E.; Navrotsky, A. Rare earth sulfates in aqueous systems: Thermodynamic modeling of binary and multicomponent systems over wide concentration and temperature ranges. J. Chem. Thermodyn. 2019, 131, 49–79. [Google Scholar] [CrossRef]
- Niinistö, L.; Leskelä, M. Inorganic complex compounds II. Handb. Phys. Chem. Rare Earths 1987, 9, 91–320. [Google Scholar]
- Denisenko, Y.G.; Sedykh, A.E.; Basova, S.A.; Atuchin, V.V.; Molokeev, M.S.; Aleksandrovsky, A.S.; Krylov, A.S.; Oreshonkov, A.S.; Khritokhin, N.A.; Sal’nikova, E.I.; et al. Exploration of the structural, spectroscopic and thermal properties of double sulfate monohydrate NaSm(SO4)2·H2O and its thermal decomposition product NaSm(SO4)2. Adv. Powder Technol. 2021, 32, 3943–3953. [Google Scholar] [CrossRef]
- Tunsu, C.; Petranikova, M.; Gergoric, M.; Ekberg, C.; Retegan, T. Reclaiming rare earth elements from end-of-life products: A review of the perspectives for urban mining using hydrometallurgical uni toperations. Hydrometallurgy 2015, 156, 239–258. [Google Scholar] [CrossRef]
- Zhu, Z.; Pranolo, Y.; Cheng, C.Y.; Cheng, C.Y. Separation of uranium and thorium from rare earths for rare earth production—A review. Miner. Eng. 2015, 77, 185–196. [Google Scholar] [CrossRef]
- Beltrami, D.; Deblonde, G.-P.; Bélair, S.; Weigel, V.; Bélair, S.; Weigel, V. Recovery of yttrium and lanthanides from sulfate solutions with high concentration of iron and low rare earth content. Hydrometallurgy 2015, 157, 356–362. [Google Scholar] [CrossRef]
- Diaz, L.A.; Lister, T.E.; Parkman, J.A.; Clark, G.G. Comprehensive process for the recovery of value and critical materials from electronic waste. J. Clean. Prod. 2016, 125, 236–244. [Google Scholar] [CrossRef]
- Kelley, S.P.; Nuss, J.S.; Rogers, R.D. Using crystal structures of ionic compounds to explore complexation and extraction of rare earth elements in ionic liquids. In Application of Ionic Liquids on Rare Earth Green Separation and Utilization; Springer: Berlin/Heidelberg, Germany, 2015; pp. 21–42. [Google Scholar]
- Zhao, Z.; Qiu, Z.; Yang, J.; Lu, S.; Cao, L.; Zhang, W.; Xu, Y. Recovery of rare earth elements from spent fluid catalytic cracking catalysts using leaching and solvent extraction techniques. Hydrometallurgy 2017, 167, 183–188. [Google Scholar] [CrossRef]
- Hatada, N.; Shizume, K.; Uda, T. Discovery of rapid and reversible water insertion in rare earth sulfates: A new process for thermochemical heat storage. Adv. Mater. 2017, 29, 1606569. [Google Scholar] [CrossRef]
- Wickleder, M.S. Inorganic lanthanide compounds with complex anions. Chem. Rev. 2002, 102, 2011–2088. [Google Scholar] [CrossRef]
- Choi, M.-H.; Kim, M.K.; Jo, V.; Lee, D.W.; Shim, I.-W.; Ok, K.M. Hydrothermal syntheses, structures, and characterizations of two lanthanide sulfate hydrates materials, La2(SO4)3·H2O and Eu2(SO4)3·4H2O. Bull. Korean Chem. Soc. 2010, 31, 1077–1080. [Google Scholar] [CrossRef]
- Denisenko, Y.G.; Atuchin, V.V.; Molokeev, M.S.; Sedykh, A.E.; Khritokhin, N.A.; Aleksandrovsky, A.S.; Oreshonkov, A.S.; Shestakov, N.P.; Adichtchev, S.V.; Pugachev, A.M.; et al. Exploration of the crystal structure and thermal and spectroscopic properties of monoclinic praseodymium sulfate Pr2(SO4)3. Molecules 2022, 27, 3966. [Google Scholar] [CrossRef] [PubMed]
- Sirotinkin, S.P.; Efremov, V.A.; Kovba, L.M.; Pokrovsky, A.N. Crystal structure of anhydrous neodymium sulfate Nd2(SO4)3. Kristallografiya 1977, 22, 1272–1273. [Google Scholar]
- Denisenko, Y.G.; Aleksandrovsky, A.S.; Atuchin, V.V.; Krylov, A.S.; Molokeev, M.S.; Oreshonkov, A.S.; Shestakov, N.P.; Andreev, O.V. Exploration of structural, thermal and spectroscopic properties of self-activated sulfate Eu2(SO4)3 with isolated SO4 groups. J. Ind. Eng. Chem. 2018, 68, 109–116. [Google Scholar] [CrossRef]
- Lobanov, N.N.; Kuznetsova, T.A. Crystal chemistry of lanthanide oxochlorotungstates. Russ. J. Inorg. Chem. 2008, 53, 1256–1262. [Google Scholar] [CrossRef]
- Dzhurinskii, B.F. Periodicity of properties of rare earth elements. Russ. J. Inorg. Chem. 1980, 25, 79–86. (In Russian) [Google Scholar]
- Wickleder, M.S. La(NH2SO3)3: Einkristalle eines wasserfreien Amidosulfates der Selten-Erd-Elemente durch schonende Entwässerung von La(NH2SO3)3·2,5H2O. Z. Anorg. Allg. Chem. 1999, 625, 1794–1798. [Google Scholar] [CrossRef]
- Topas, B.A. V4: General profile and structure analysis software for powder diffraction data. In User’s Manual; Bruker AXS: Karlsruhe, Germany, 2008. [Google Scholar]
- Matkovic, B.; Prodic, B.; Sljukic, M. Crystal structure of patassium dithorium triphosphate KTh2(PO4)3. Croat. Chem. Acta 1968, 40, 147–161. [Google Scholar]
- Kräußlich, J.; Höfer, S.; Zastrau, U.; Jeutter, N.; Baehtz, C. Temperature dependence of lattice parameters of langasite single crystals. Cryst. Res. Technol. 2010, 45, 490–492. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, X.; Molokeev, M.S.; Zhang, X.; Lin, Z. Zn3GaB6O12As and Zn4P6N12S: Isotropic Zero Thermal Expansion Materials Based on the “Cage-Restricting” Model. Chem. Mater. 2022, 34, 9915–9922. [Google Scholar] [CrossRef]
- Kugaenko, O.M.; Uvarova, S.S.; Krylov, S.A.; Senatulin, B.R.; Petrakov, V.S.; Buzanov, O.A.; Egorov, V.N.; Sakharov, S.A. Basic thermophysical parameters of langasite (La3Ga5SiO14), langatate (La3Ta0.5Ga5.5O14), and catangasite (Ca3TaGa3Si2O14) single crystals in a temperature range of 25 to 1000 °C. Bull. Russ. Acad. Sci. Phys. 2012, 76, 1258–1263. [Google Scholar] [CrossRef]
- Yuan, L.-D.; Deng, H.-X.; Li, S.-S.; Wei, S.-H.; Luo, J.-W. Unified theory of direct or indirect band-gap nature of conventional semiconductors. Phys. Rev. B 2018, 98, 245203. [Google Scholar] [CrossRef]
- Bartók, A.P.; Yates, J.R. Regularized SCAN Functional. J. Chem. Phys. 2019, 150, 161101. [Google Scholar] [CrossRef] [PubMed]
- Krukau, A.V.; Vydrov, O.A.; Izmaylov, A.F.; Scuseria, G.E. Influence of the Exchange Screening Parameter on the Performance of Screened Hybrid Functionals. J. Chem. Phys. 2006, 125, 224106. [Google Scholar] [CrossRef] [PubMed]
- BChoi, K.; Lockwood, D.J. Raman spectrum of Na2SO4 (phase V). Solid State Commun. 1989, 72, 133–137. [Google Scholar]
- Sherry, E.G. The structure of Pr2(SO4)3(H2O)8 and La2(SO4)3(H2O)9. J. Solid State Chem. 1976, 19, 271–279. [Google Scholar] [CrossRef]
- Blaine, R.L.; Kissinger, H.E. Homer Kissinger and the Kissinger equation. Thermochim. Acta 2012, 540, 1–6. [Google Scholar] [CrossRef]
- Llopiz, J.; Romero, M.M.; Jerez, A.; Laureiro, Y. Generalization of the Kissinger equation for several kinetic models. Thermochim. Acta 1995, 256, 205–211. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Doyle, C.D. Kinetic analysis of thermogravimetric data. J. Appl. Polym. Sci. 1961, 15, 285–292. [Google Scholar] [CrossRef]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Für Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Perdew, J.P.; Zunger, A. Self-Interaction Correction to Density-Functional Approximations for Many-Electron Systems. Phys. Rev. B 1981, 23, 5048–5079. [Google Scholar] [CrossRef]
- Ceperley, D.M.; Alder, B.J. Ground State of the Electron Gas by a Stochastic Method. Phys. Rev. Lett. 1980, 45, 566–569. [Google Scholar] [CrossRef]
- Refson, K.; Tulip, P.R.; Clark, S.J. Variational Density-Functional Perturbation Theory for Dielectrics and Lattice Dynamics. Phys. Rev. B 2006, 73, 155114. [Google Scholar] [CrossRef]
- Porezag, D.; Pederson, M.R. Infrared Intensities and Raman-Scattering Activities within Density-Functional Theory. Phys. Rev. B 1996, 54, 7830–7836. [Google Scholar] [CrossRef]
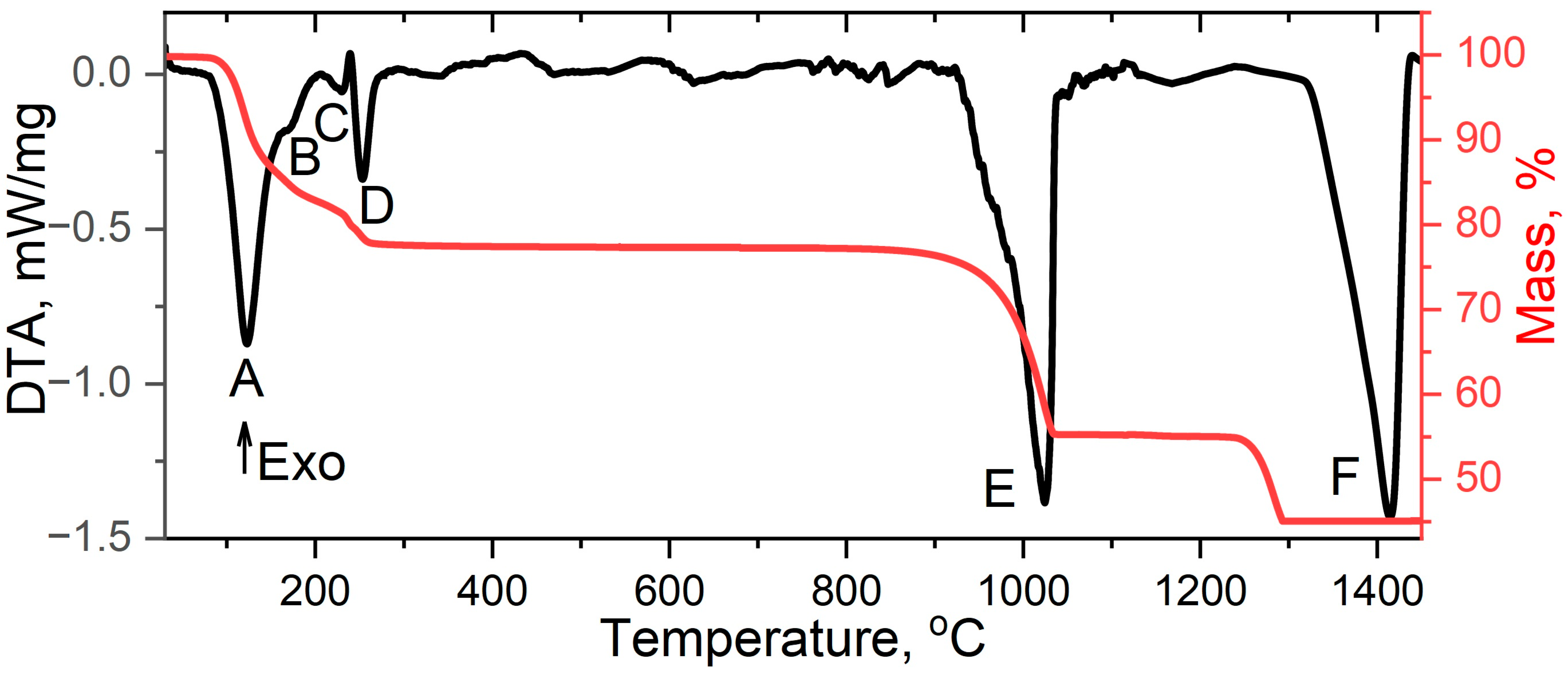
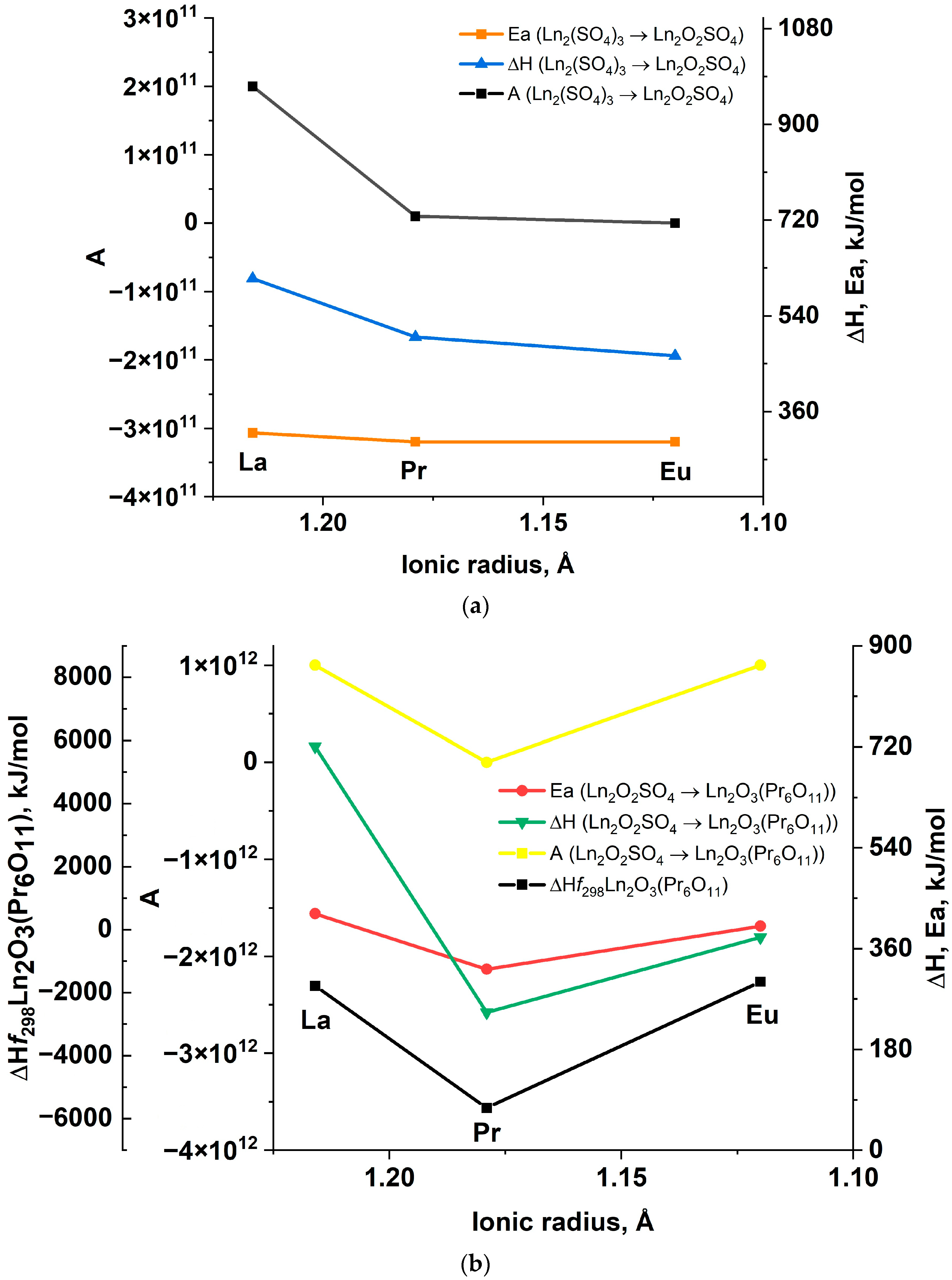
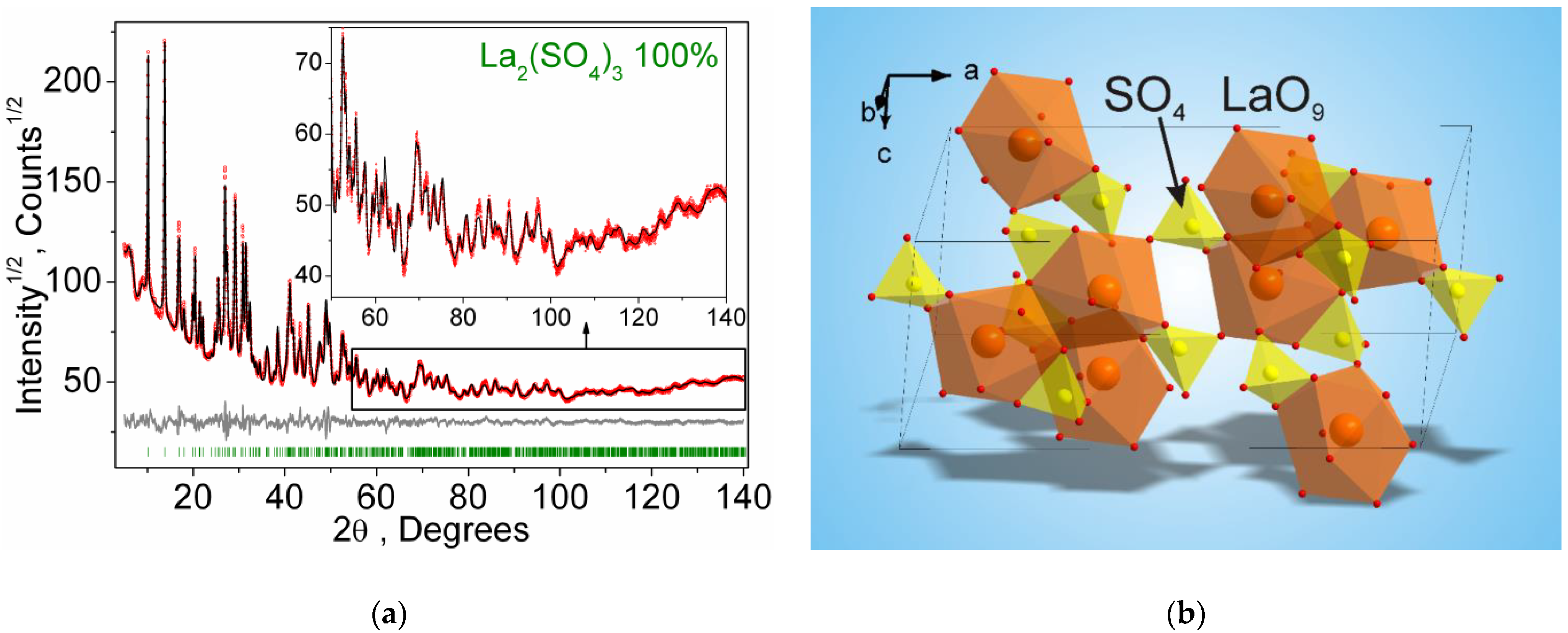
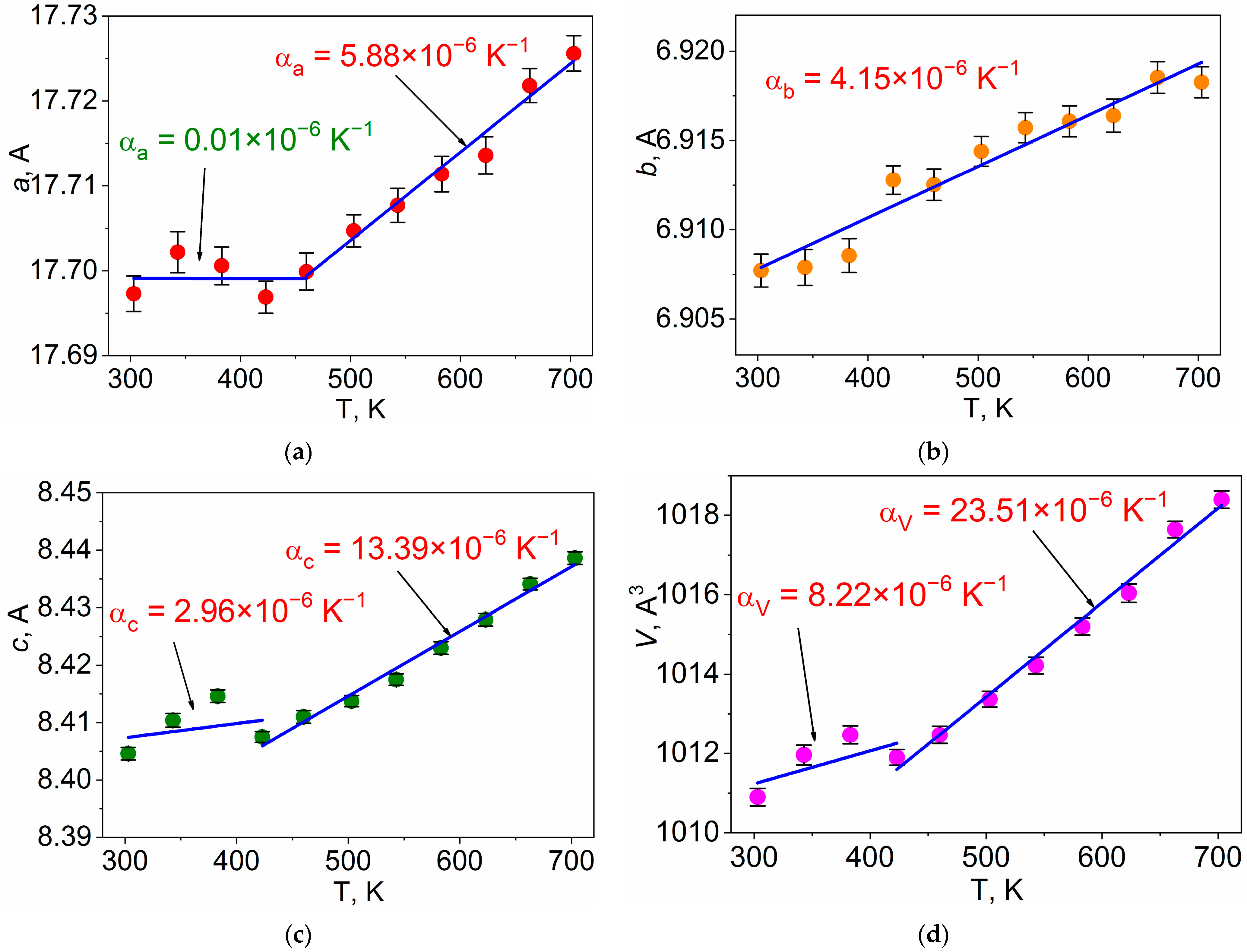

| Signal | Chemical Process | Δmexp., % | Δmtheor., % | ΔH, kJ/mol |
|---|---|---|---|---|
| A | La2(SO4)3∙9H2O → La2(SO4)3∙4H2O + 5H2O | −12.42 | −12.37 | 345.87 |
| B | La2(SO4)3∙4H2O → La2(SO4)3∙2H2O + 2H2O | −17.34 | −17.32 | 101.45 |
| C | La2(SO4)3∙2H2O → La2(SO4)3∙H2O + H2O | −19.81 | −19.79 | 12.52 |
| D | La2(SO4)3∙H2O → β-La2(SO4)3 + H2O | −22.34 | −22.41 | 56.40 |
| E | β-La2(SO4)3 → La2O2SO4 + 2SO2 + O2 | −44.22 | −44.25 | 610.22 |
| F | La2O2SO4 → La2O3 + SO2 + 1/2O2 | −55.20 | −55.25 | 721.28 |
| Chemical Process | Kinetic Parameters | ||
|---|---|---|---|
| Kissinger Equation | Ozawa-Doyle Equation | ||
| Ea, kJ/mol | A | Ea, kJ/mol | |
| La2(SO4)3∙9H2O → La2(SO4)3∙4H2O + 5H2O | 65 | 1.07 × 108 | 67 |
| La2(SO4)3∙4H2O → La2(SO4)3∙2H2O + 2H2O | 343 | 2.01 × 1040 | 315 |
| La2(SO4)3∙2H2O → La2(SO4)3∙H2O + H2O | 232 | 6.39 × 1023 | 223 |
| La2(SO4)3∙H2O → β-La2(SO4)3 + H2O | 147 | 1.15 × 1014 | 147 |
| β-La2(SO4)3 → La2O2SO4 + 2SO2 + O2 | 320 | 1.79 × 1011 | 323 |
| La2O2SO4 → La2O3 + SO2 + 1/2O2 | 422 | 1.26 × 1012 | 424 |
| Compound | β-La2(SO4)3 |
|---|---|
| Sp.Gr. | C2/c |
| a, Å | 17.6923 (9) |
| b, Å | 6.9102 (4) |
| c, Å | 8.3990 (5) |
| β, ° | 100.321 (3) |
| V, Å3 | 1010.22 (9) |
| Z | 4 |
| 2θ-interval, ° | 5–140 |
| Rwp, % | 4.66 |
| Rp, % | 3.67 |
| Rexp, % | 1.46 |
| χ2 | 3.19 |
| RB, % | 1.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basova, S.A.; Molokeev, M.S.; Oreshonkov, A.S.; Zhernakov, M.A.; Khritokhin, N.A.; Aleksandrovsky, A.S.; Krylov, A.S.; Sal’nikova, E.I.; Azarapin, N.O.; Shelpakova, N.A.; et al. Thermochemistry, Structure, and Optical Properties of a New β-La2(SO4)3 Polymorphic Modification. Inorganics 2023, 11, 434. https://doi.org/10.3390/inorganics11110434
Basova SA, Molokeev MS, Oreshonkov AS, Zhernakov MA, Khritokhin NA, Aleksandrovsky AS, Krylov AS, Sal’nikova EI, Azarapin NO, Shelpakova NA, et al. Thermochemistry, Structure, and Optical Properties of a New β-La2(SO4)3 Polymorphic Modification. Inorganics. 2023; 11(11):434. https://doi.org/10.3390/inorganics11110434
Chicago/Turabian StyleBasova, Sofia A., Maxim S. Molokeev, Aleksandr S. Oreshonkov, Maksim A. Zhernakov, Nikolay A. Khritokhin, Aleksandr S. Aleksandrovsky, Alexander S. Krylov, Elena I. Sal’nikova, Nikita O. Azarapin, Natalia A. Shelpakova, and et al. 2023. "Thermochemistry, Structure, and Optical Properties of a New β-La2(SO4)3 Polymorphic Modification" Inorganics 11, no. 11: 434. https://doi.org/10.3390/inorganics11110434
APA StyleBasova, S. A., Molokeev, M. S., Oreshonkov, A. S., Zhernakov, M. A., Khritokhin, N. A., Aleksandrovsky, A. S., Krylov, A. S., Sal’nikova, E. I., Azarapin, N. O., Shelpakova, N. A., Müller-Buschbaum, K., & Denisenko, Y. G. (2023). Thermochemistry, Structure, and Optical Properties of a New β-La2(SO4)3 Polymorphic Modification. Inorganics, 11(11), 434. https://doi.org/10.3390/inorganics11110434









