Synthesis, Characterization, and Biological Properties of the Copper(II) Complexes with Novel Ligand: N-[4-({2-[1-(pyridin-2-yl)ethylidene]hydrazinecarbothioyl}amino)phenyl]acetamide
Abstract
:1. Introduction
2. Results and Discussion
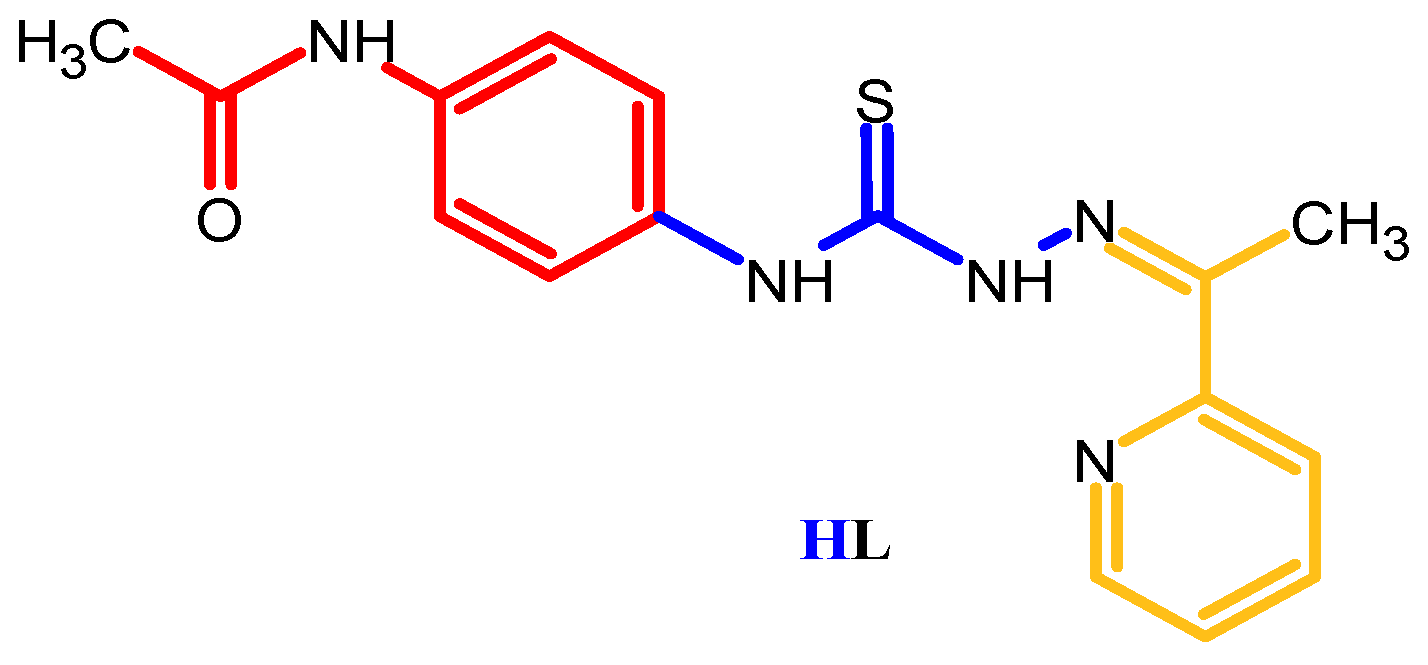
2.1. Structural Characterization of Ligand HL and Coordination Compounds 1–3
2.2. Biological Activity
2.2.1. Antimicrobial and Antifungal Activity
2.2.2. Antioxidant Activity
2.2.3. Acute In Vivo Toxicity of the Tested Compounds Assessed through Immobilization of the Crustacean Daphnia magna
3. Materials and Methods
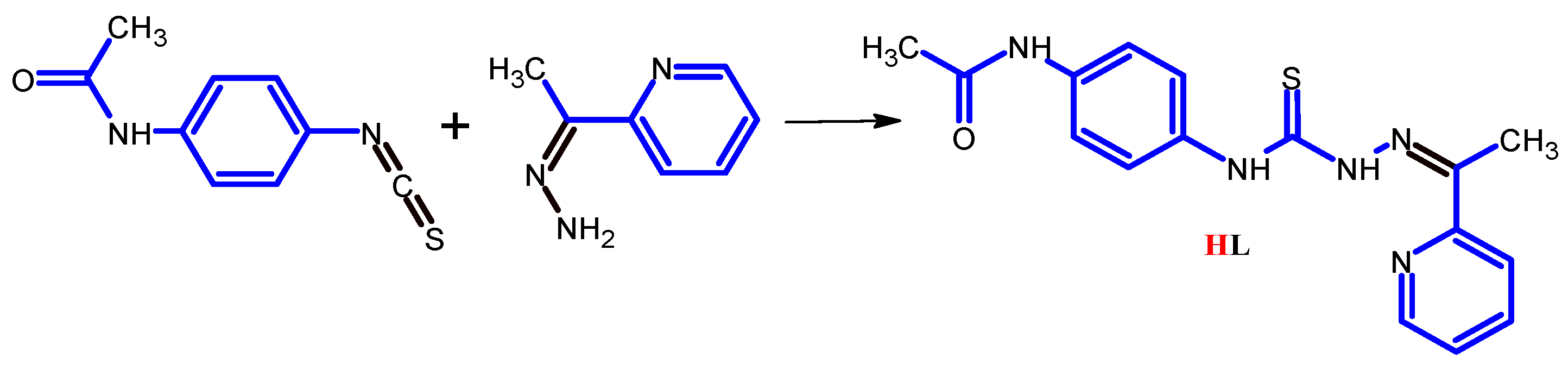
3.1. Synthesis
3.1.1. Synthesis of N-[4-({2-[1-(pyridin-2-yl)ethylidene]hydrazinecarbothioyl}amino)-phenyl]acetamide (HL)
3.1.2. Synthesis of Copper(II) Complexes (1–5)
[Cu(L)CH3COO] (1)
[{Cu(L)Cl}2]·H2O (2)
[Cu(L)H2O·DMF]NO3 (3)
[Cu(L)Br] (4)
[Cu(L)H2O]ClO4 (5)
3.2. FT-IR Spectroscopy
3.3. NMR Spectroscopy
3.4. Molar Conductivity
3.5. Melting Point
3.6. Thin Layer Chromatographic
3.7. X-ray Crystallography
3.8. EPR Study
3.9. Antibacterial and Antifungal Activity
3.10. Antioxidant Activity
3.11. Toxicity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Z.; Qiao, H.; Yang, F.; Zhou, W.; Gong, Y.; Zhang, X.; Wang, H.; Zhao, B.; Ma, L.; Liu, H.-M.; et al. Novel Thiosemicarbazone Derivatives Containing Indole Fragment as Potent and Selective Anticancer Agent. Eur. J. Med. Chem. 2019, 184, 111764. [Google Scholar] [CrossRef] [PubMed]
- Nishida, C.R.; de Montellano, P.R.O. Bioactivation of Antituberculosis Thioamide and Thiourea Prodrugs by Bacterial and Mammalian Flavin Monooxygenases. Chem. Biol. Interact. 2011, 192, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Peter, R.D.; Van Helden, P.D. Antituberculosis Chemotherapy; Bolliger, C.T., Ed.; Karger Publishers: Basel, Switzerland, 2011; ISBN 978-3-8055-9627-5. [Google Scholar]
- Souza, M.R.P.; Coelho, N.P.; Baldin, V.P.; Scodro, R.B.L.; Cardoso, R.F.; da Silva, C.C.; Vandresen, F. Synthesis of Novel (-)-Camphene-Based Thiosemicarbazones and Evaluation of Anti-Mycobacterium Tuberculosis Activity. Nat. Prod. Res. 2019, 33, 3372–3377. [Google Scholar] [CrossRef]
- Jiang, X.; Fielding, L.A.; Davis, H.; Carroll, W.; Lisic, E.C.; Deweese, J.E. Inhibition of Topoisomerases by Metal Thiosemicarbazone Complexes. Int. J. Mol. Sci. 2023, 24, 12010. [Google Scholar] [CrossRef]
- Prathima, B.; Rao, Y.S.; Reddy, S.A.; Reddy, Y.P.; Reddy, A.V. Copper (II) and nickel (II) complexes of benzyloxybenzaldehyde-4- phenyl-3-thiosemicarbazone: Synthesis, characterization and biological activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2010, 77, 248–252. [Google Scholar] [CrossRef]
- Sahoo, J.; Sahoo, C.R.; Nandini Sarangi, P.K.; Prusty, S.K.; Padhy, R.N.; Paidesetty, S.K. Molecules with Versatile Biological Activities Bearing Antipyrinyl Nucleus as Pharmacophore. Eur. J. Med. Chem. 2020, 186, 111911. [Google Scholar] [CrossRef]
- Gaber, A.; Refat, M.S.; Belal, A.A.M.; El-Deen, I.M.; Hassan, N.; Zakaria, R.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Saied, E.M. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone Complexes with Potential Biological Activity: Antimicrobial and Molecular Docking Study. Molecules 2021, 26, 2288. [Google Scholar] [CrossRef] [PubMed]
- Al-Mutairi, A.A.; Al-Alshaikh, M.A.; Al-Omary, F.A.M.; Hassan, H.M.; El-Mahdy, A.M.; El-Emam, A.A. Synthesis, Antimicrobial, and Anti-Proliferative Activities of Novel 4-(Adamantan-1-yl)-1-arylidene-3-thiosemicarbazides, 4-Arylmethyl N′-(Adamantan-1-yl)piperidine-1-carbothioimidates, and Related Derivatives. Molecules 2019, 24, 4308. [Google Scholar] [CrossRef]
- Fathy, A.; Ibrahim, A.B.M.; Abd Elkhalik, S.; Meurer, F.; Bodensteiner, M.; Abbas, S.M. Thiosemicarbazones and Derived Antimony Complexes: Synthesis, Structural Analysis, and In Vitro Evaluation against Bacterial, Fungal, and Cancer Cells. Inorganics 2022, 10, 172. [Google Scholar] [CrossRef]
- Opletalova, V.; Dolezel, J.; Kunes, J.; Buchta, V.; Vejsova, M.; Kucerova-Chlupacova, M. Synthesis and Antifungal Screening of 2-{[1-(5-Alkyl/arylalkylpyrazin-2-yl)ethylidene]hydrazono}-1,3-thiazolidin-4-ones. Molecules 2016, 21, 1592. [Google Scholar] [CrossRef]
- Khan, A.A.; Alanazi, A.M.; Alsaif, N.; Algrain, N.; Wani, T.A.; Bhat, M.A. Enhanced Efficacy of Thiosemicarbazone Derivative-Encapsulated Fibrin Liposomes against Candidiasis in Murine Model. Pharmaceutics 2021, 13, 333. [Google Scholar] [CrossRef] [PubMed]
- Kshirsagar, A.; Toraskar, M.P.; Kulkarni, V.M.; Dhanashire, S.; Kadam, V. Microwave Assisted Synthesis of Potential Anti Infective and Anticonvulsant Thiosemicarbazones. Int. J. Chem. Tech. Res. 2009, 1, 696–701. [Google Scholar]
- Tripathi, L.; Kumar, P.; Singh, R.; Stables, J.P. Design, Synthesis and Anticonvulsant Evaluation of Novel N-(4-Substituted Phenyl)-2-[4-(Substituted) Benzylidene]-Hydrazinecarbothio Amides. Eur. J. Med. Chem. 2012, 47, 153–166. [Google Scholar] [CrossRef]
- Tripathi, L.; Kumar, P. Augmentation of GABAergic Neurotransmission by Novel N-(Substituted)-2-[4- (Substituted)Benzylidene]Hydrazinecarbothioamides—A Potential Anticonvulsant Approach. Eur. J. Med. Chem. 2013, 64, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, C.; Gruene, T.; Prado-Roller, A.; Aranđelović, S.; Reynisson, J.; Arion, V.B. Latonduine-1-Amino-Hydantoin Hybrid, Triazole-Fused Latonduine Schiff Bases and Their Metal Complexes: Synthesis, X-ray and Electron Diffraction, Molecular Docking Studies and Antiproliferative Activity. Inorganics 2023, 11, 30. [Google Scholar] [CrossRef]
- Milunović, M.N.M.; Palamarciuc, O.; Sirbu, A.; Shova, S.; Dumitrescu, D.; Dvoranová, D.; Rapta, P.; Petrasheuskaya, T.V.; Enyedy, E.A.; Spengler, G.; et al. Insight into the Anticancer Activity of Copper(II) 5-Methylenetrimethylammonium-Thiosemicarbazonates and Their Interaction with Organic Cation Transporters. Biomolecules 2020, 10, 1213. [Google Scholar] [CrossRef]
- Aly, A.A.; Abdallah, E.M.; Ahmed, S.A.; Rabee, M.M.; Bräse, S. Transition Metal Complexes of Thiosemicarbazides, Thiocarbohydrazides, and Their Corresponding Carbazones with Cu(I), Cu(II), Co(II), Ni(II), Pd(II), and Ag(I)—A Review. Molecules 2023, 28, 1808. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Suntharalingam, K.; Lippard, S.J. The Next Generation of Platinum Drugs: Targeted Pt(II) Agents, Nanoparticle Delivery, and Pt(IV) Prodrugs. Chem. Rev. 2016, 116, 3436–3486. [Google Scholar] [CrossRef]
- Wenzel, M.; Casini, A. Mass Spectrometry as a Powerful Tool to Study Therapeutic Metallodrugs Speciation Mechanisms: Current Frontiers and Perspectives. Coord. Chem. Rev. 2017, 352, 432–460. [Google Scholar] [CrossRef]
- Laws, K.; Bineva-Todd, G.; Eskandari, A.; Lu, C.; O’Reilly, N.; Suntharalingam, K. A Copper(II) Phenanthroline Metallopeptide That Targets and Disrupts Mitochondrial Function in Breast Cancer Stem Cells. Angew. Chemie Int. Ed. 2018, 57, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Deka, B.; Sarkar, T.; Banerjee, S.; Kumar, A.; Mukherjee, S.; Deka, S.; Saikia, K.K.; Hussain, A. Novel Mitochondria Targeted Copper(<scp>ii</scp>) Complexes of Ferrocenyl Terpyridine and Anticancer Active 8-Hydroxyquinolines Showing Remarkable Cytotoxicity, DNA and Protein Binding Affinity. Dalt. Trans. 2017, 46, 396–409. [Google Scholar] [CrossRef]
- Zhang, J.; Fang, R.; Li, Y.; Jin, J.; Yang, F.; Chen, J. Encapsulation of Au(III) Complex Using Lactoferrin Nanoparticles to Combat Glioma. Mol. Pharm. 2023, 20, 3632–3644. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, J.; Yang, T.; Li, S.; Xu, G.; Liang, H.; Yang, F. Developing an Anticancer Platinum(II) Compound Based on the Uniqueness of Human Serum Albumin. J. Med. Chem. 2023, 66, 5669–5684. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.R.; Gholam Azad, M.; Afroz, R.; Richardson, V.; Dharmasivam, M. Thiosemicarbazones reprogram pancreatic cancer bidirectional oncogenic signaling between cancer cells and stellate cells to suppress desmoplasia. Future Med. Chem. 2022, 14, 1005–1017. [Google Scholar] [CrossRef] [PubMed]
- Laamari, Y.; Bimoussa, A.; Fawzi, M.; Oubella, A.; Rohand, T.; Van Meervelt, L.; Ait Itto, M.Y.; Morjani, H.; Auhmani, A. Synthesis, crystal structure and evaluation of anticancer activities of some novel heterocyclic compounds based on thymol. J. Mol. Struct. 2023, 1278, 134906. [Google Scholar] [CrossRef]
- Balakrishnan, N.; Haribabu, J.; Dharmasivam, M.; Jayadharini, J.P. Dhanabalan Anandakrishnan, Srividya Swaminathan, Nattamai Bhuvanesh, Cesar Echeverria, and Ramasamy Karvembu. Organometallics 2023, 42, 259–275. [Google Scholar] [CrossRef]
- Bullo, S.; Jawaria, R.; Faiz, I.; Shafiq, I.; Khalid, M.; Asghar, M.A.; Baby, R.; Orfali, R.; Perveen, S. Efficient Synthesis, Spectroscopic Characterization, and Nonlinear Optical Properties of Novel Salicylaldehyde-Based Thiosemicarbazones: Experimental and Theoretical Studies. ACS Omega 2023, 8, 13982–13992. [Google Scholar] [CrossRef]
- Colleen Moore, E.; Sartorelli, A.C. Inhibition of Ribonucleotide Reductase by α-(N)-Heterocyclic Carboxaldehyde Thiosemicarbazones. Pharmacol. Ther. 1984, 24, 439–447. [Google Scholar] [CrossRef]
- Duan, X.; Xie, Z.; Ma, L.; Jin, X.; Zhang, M.; Xu, Y.; Liu, Y.; Lou, H.; Chang, W. Selective Metal Chelation by a Thiosemicarbazone Derivative Interferes with Mitochondrial Respiration and Ribosome. Microbial Spectr. 2022, 10, e0195121. [Google Scholar] [CrossRef]
- Rosu, T.; Negoiu, M.; Pasculescu, S.; Pahontu, E.; Poirier, D.; Gulea, A. Metal-Based Biologically Active Agents: Synthesis, Characterization, Antibacterial and Antileukemia Activity Evaluation of Cu(II), V(IV) and Ni(II) Complexes with Antipyrine-Derived Compounds. Eur. J. Med. Chem. 2010, 45, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Kuznetcova, I.; Bacher, F.; Alfadul, S.M.; Tham, M.J.R.; Ang, W.H.; Babak, M.V.; Rapta, P.; Arion, V.B. Elucidation of Structure-Activity Relationships in Indolobenzazepine-Derived Ligands and Their Copper(II) Complexes: The Role of Key Structural Components and Insight into the Mechanism of Action. Inorg. Chem. 2022, 61, 10167–10181. [Google Scholar] [CrossRef] [PubMed]
- Stepanenko, I.; Babak, M.V.; Spengler, G.; Hammerstad, M.; Popovic-bijelic, A.; Shova, S.; Büchel, G.; Darvasiova, D.; Rapta, P.; Arion, V. Coumarin-based Triapine Derivatives and Their Copper(Ii) Complexes: Synthesis, Cytotoxicity and Mr2 Rnr Inhibition Activity. Biomolecules 2021, 11, 862. [Google Scholar] [CrossRef] [PubMed]
- Fuior, A.; Cebotari, D.; Haouas, M.; Marrot, J.; Minguez Espallargas, G.; Guérineau, V.; Touboul, D.; Rusnac, R.V.; Gulea, A.; Floquet, S. Synthesis, Structures, and Solution Studies of a New Class of [Mo2O2S2]-Based Thiosemicarbazone Coordination Complexes. ACS Omega 2022, 7, 16547–16560. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Fujimori, R.; Tang, J.; Ishiwata, T. FTIR Spectroscopy of NO3: Perturbation Analysis of the Ν3+ν4 State. J. Phys. Chem. A 2013, 117, 13732–13742. [Google Scholar] [CrossRef]
- Shahjahan, M.; Shalaby, A. Determination of nitrofurazone in some pharmaceutical preparations. Int. J. Pharm. 1998, 168, 169–172. [Google Scholar] [CrossRef]
- Hsin, Y.K.; Thangarajoo, T.; Choudhury, H.; Pandey, M.; Meng, L.W. Bapi Gorain, Stimuli-Responsive in situ Spray Gel of Miconazole Nitrate for Vaginal Candidiasis. J. Pharm. Sci. 2023, 112, 562–572. [Google Scholar] [CrossRef]
- Ulchina, I.; Graur, V.; Tsapkov, V.; Chumakov, Y.; Garbuz, O.; Burduniuc, O.; Ceban, E.; Gulea, A. Introducing N-Heteroaromatic Bases into Copper(II) Thiosemicarbazon Complexes: A Way to Change their Biological Activity. ChemistryOpen 2022, 11, e202200208. [Google Scholar] [CrossRef]
- Garbuz, O.; Gudumac, V.; Toderas, I.; Gulea, A. Antioxidant Properties of Synthetic Compounds and Natural Products. Action Mechanisms; Monograph; CEP USM: Chișinău, Moldova, 2023; ISBN 978-9975-62-516-6. [Google Scholar]
- Perrin, D.D.; Armarego, W.L.; Perrin, D.R. Purification of Laboratory Chemicals, 4th ed.; Butterworth-Heinemann, Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Joseph, M.; Sreekanth, A.; Suni, V.; Kurup, M.R.P. Spectral Characterization of Iron(III) Complexes of 2-Benzoylpyridine N(4)-Substituted Thiosemicarbazones. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2006, 64, 637–641. [Google Scholar] [CrossRef]
- Touchstone, J.C. Thin-Layer Chromatographic Procedures for Lipid Separation. J. Chromatogr. B Biomed. Sci. Appl. 1995, 671, 169–195. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Graur, V.; Chumakov, Y.; Garbuz, O.; Hureau, C.; Tsapkov, V.; Gulea, A. Synthesis, Structure, and Biologic Activity of Some Copper, Nickel, Cobalt, and Zinc Complexes with 2-Formylpyridine N4-Allylthiosemicarbazone. Bioinorg. Chem. Appl. 2022, 2022, 2705332. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Gulea, A.; Toderas, I.; Garbuz, O.; Ulchina, I.; Graur, V.; Railean, N. Biological Evaluation of a Series of Amine-Containing Mixed-Ligand Copper(II) Coordination Compounds with 2-(2-hydroxybenzylidene)-N-(prop-2-en-1-yl)hydrazinecarbothioamide. Microsc. Microanal. 2022, 28, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Q.; Guo, J.; Li, X.; Song, J. Transcriptomic Analysis of Diethylstilbestrol in Daphnia Magna: Energy Metabolism and Growth Inhibition. Toxics 2023, 11, 197. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Cui, Y.; Wen, M.; Ji, G. Toxic Effects of Methylene Blue on the Growth, Reproduction and Physiology of Daphnia magna. Toxics 2023, 11, 594. [Google Scholar] [CrossRef]

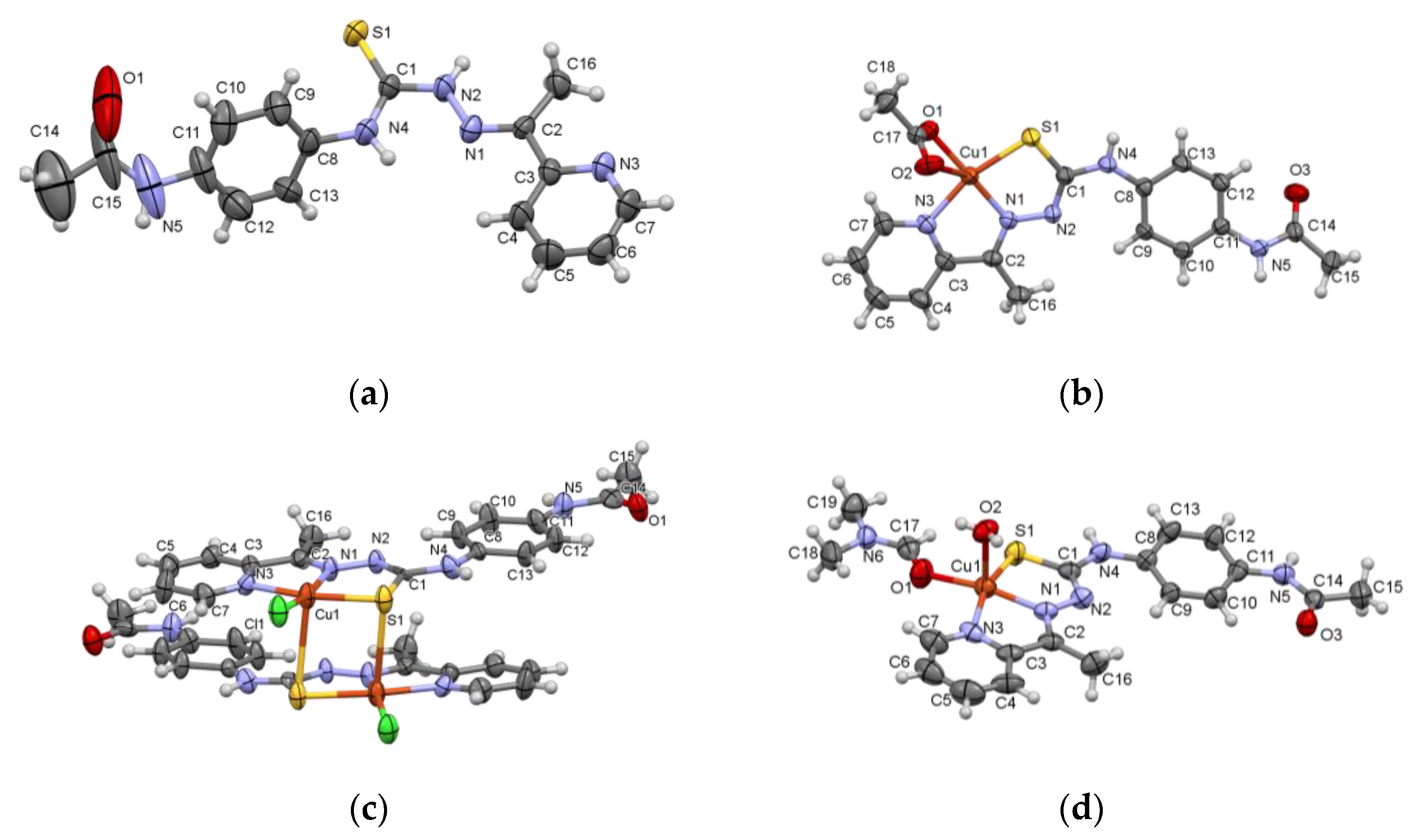

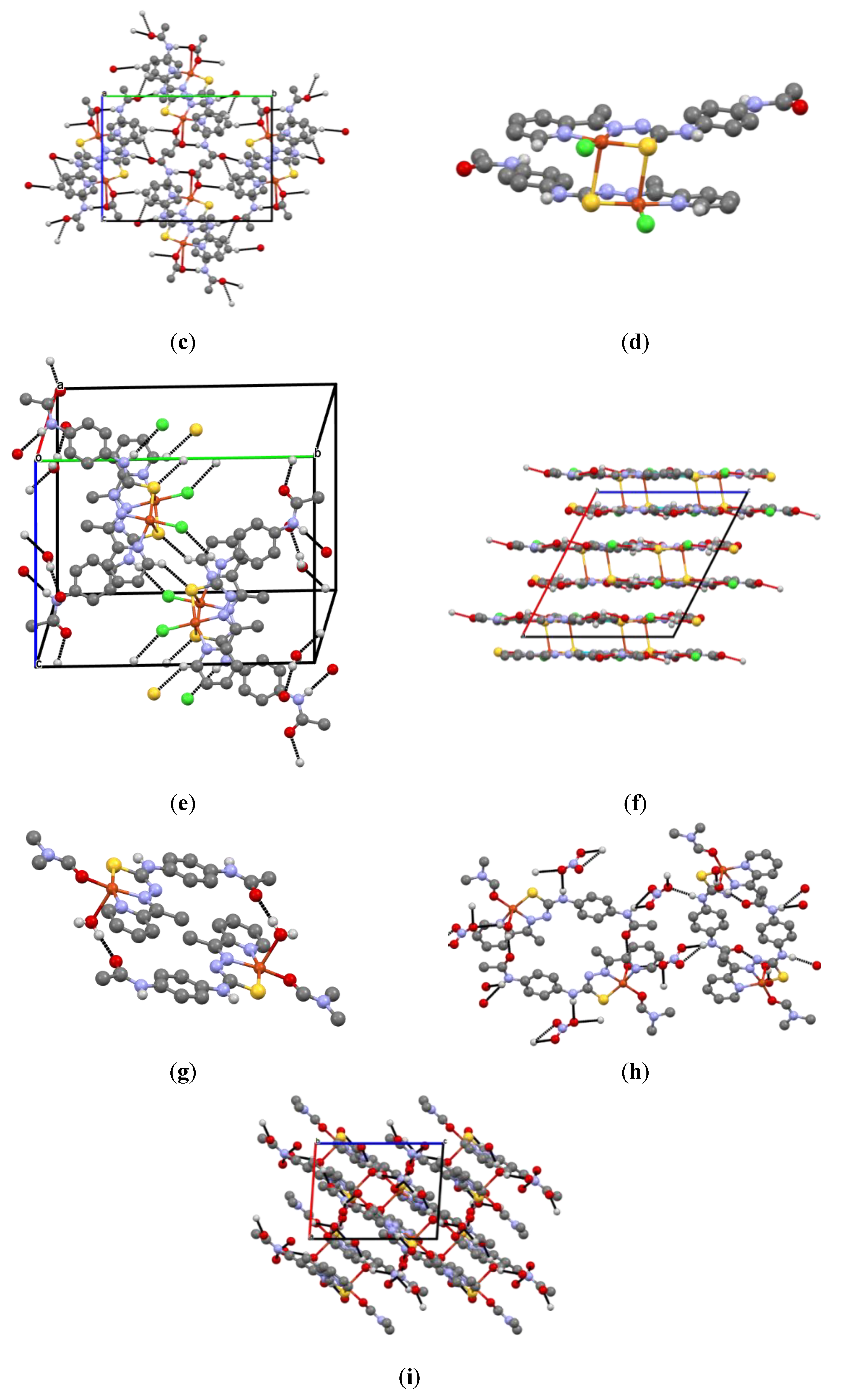
| Identification Code | HL | [Cu(L)CH3COO] (1) |
|---|---|---|
| CCDC | 2213214 | 2213213 |
| Empirical formula | C16H17N5OS | C18H19N5O3SCu |
| Formula weight | 327.40 | 448.98 |
| Temperature/K | 293(2) | 293(2) |
| Crystal system | triclinic | monoclinic |
| Space group | P-1 | P21/c |
| a/Å | 5.6303(7) | 8.5347(4) |
| b/Å | 10.2646(10) | 17.6158(5) |
| c/Å | 14.941(2) | 13.5270(5) |
| α/° | 108.622(11) | 90 |
| β/° | 92.330(11) | 105.779(5) |
| γ/° | 91.118(9) | 90 |
| Volume/Å3 | 817.11(18) | 1957.08(13) |
| Z | 2 | 4 |
| ρcalcg/cm3 | 1.331 | 1.524 |
| μ/mm−1 | 0.210 | 1.252 |
| F(000) | 344.0 | 924.0 |
| Crystal size/mm3 | 0.55 × 0.7 × 0.06 | 0.21 × 0.18 × 0.15 |
| Radiation | MoKα (λ = 0.71073) | MoKα (λ = 0.71073) |
| 2Θ range for data collection/° | 5.94 to 50.076 | 6.26 to 50.084 |
| Index ranges | −6 ≤ h ≤ 5, −12 ≤ k ≤ 9, 17 ≤ l ≤ 17 | −10 ≤ h ≤ 10, −20 ≤ k ≤ 15, −16 ≤ l ≤ 11 |
| Reflections collected | 5061 | 7038 |
| Independent reflections | 2881 [Rint = 0.0413, Rsigma = 0.1093] | 3429 [Rint = 0.0363, Rsigma = 0.0613] |
| Data/restraints/parameters | 2881/6/213 | 3429/0/256 |
| Goodness-of-fit on F2 | 1.035 | 1.013 |
| Final R indexes [I ≥2σ (I)] | R1 = 0.0821, wR2 = 0.1964 | R1 = 0.0445, wR2 = 0.0872 |
| Final R indexes [all data] | R1 = 0.1530, wR2 = 0.2358 | R1 = 0.0709, wR2 = 0.0975 |
| Largest diff. peak/hole/e Å−3 | 0.62/−0.37 | 0.33/−0.30 |
| Identification code | [{Cu(L)Cl}2]·H2O (2) | [Cu(L)H2O·DMF]NO3 (3) |
| CCDC | 2213215 | 2213216 |
| Empirical formula | C16H18N5O2SClCu | C19H25N7O6SCu |
| Formula weight | 443.40 | 543.07 |
| Temperature/K | 293(2) | N/A |
| Crystal system | monoclinic | monoclinic |
| Space group | C2/c | P21/n |
| a/Å | 15.166(3) | 8.1545(7) |
| b/Å | 18.8615(15) | 26.686(2) |
| c/Å | 14.090(2) | 10.8802(9) |
| α/° | 90 | 90 |
| β/° | 117.14(2) | 93.856(8) |
| γ/° | 90 | 90 |
| Volume/Å3 | 3586.6(12) | 2362.3(3) |
| Z | 8 | 4 |
| ρcalcg/cm3 | 1.642 | 1.5268 |
| μ/mm−1 | 1.505 | 1.063 |
| F(000) | 1816.0 | 1126.3 |
| Crystal size/mm3 | 0.32 × 0.06 × 0.01 | 0.43 × 0.18 × 0.05 |
| Radiation | MoKα (λ = 0.71073) | Mo Kα (λ = 0.71073) |
| 2Θ range for data collection/° | 6.174 to 50.5 | 5.92 to 50.5 |
| Index ranges | −18 ≤ h ≤ 11, −22 ≤ k ≤ 14, −9 ≤ l ≤ 16 | −11 ≤ h ≤ 8, −37 ≤ k ≤ 37, −15 ≤ l ≤ 15 |
| Reflections collected | 3484 | 9282 |
| Independent reflections | 2313 [Rint = 0.0852, Rsigma = 0.1628] | 4091 [Rint = 0.0634, Rsigma = 0.1693] |
| Data/restraints/parameters | 2313/0/240 | 4091/0/314 |
| Goodness-of-fit on F2 | 0.892 | 1.011 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0713, wR2 = 0.1092 | R1 = 0.0683, wR2 = 0.1090 |
| Final R indexes [all data] | R1 = 0.1564, wR2 = 0.1432 | R1 = 0.1396, wR2 = 0.1442 |
| Largest diff. peak/hole/e Å−3 | 0.45/−0.40 | 1.37/−0.82 |
| Bond | d, Å | |||
|---|---|---|---|---|
| HL1 | 1 | 2 | 3 | |
| Cu1-S1 | 2.2452(11) | 2.257(3) | 2.2458(18) | |
| Cu1-O1(Cl1) | 1.942(3) | 2.273(3) | 1.986(4) | |
| Cu1-N1 | 1.948(3) | 2.005(8) | 1.972(4) | |
| Cu1-N3 | 2.014(3) | 1.981(8) | 2.013(5) | |
| Cu1-O2(S1′) | 2.755(3) | 2.982(3) | 2.338(4) | |
| S1-C1 | 1.672(5) | 1.747(3) | 1.757(8) | 1.744(6) |
| N1-N2 | 1.367(5) | 1.372(4) | 1.370(11) | 1.376(7) |
| N1-C2 | 1.288(7) | 1.301(4) | 1.286(13) | 1.288(9) |
| N2-C1 | 1.354(7) | 1.322(4) | 1.301(11) | 1.311(7) |
| N3-C3 | 1.339(7) | 1.362(4) | 1.343(12) | 1.369(8) |
| N4-C1 | 1.339(7) | 1.343(4) | 1.378(12) | 1.351(7) |
| C2-C3 | 1.475(6) | 1.462(5) | 1.487(15) | 1.466(11) |
| Angle | ω° | |||
| S1-Cu1-O1(Cl1) | 97.24(7) | 98.16(10) | 98.65(13) | |
| S1-Cu1-N1 | 84.71(8) | 83.4(2) | 84.43(15) | |
| S1-Cu1-N3 | 164.46(8) | 163.2(2) | 162.20(16) | |
| S1-Cu1-O2(S1′) | 100.77(6) | 96.07(11) | 103.02(13) | |
| O1(Cl1)-Cu1-N1 | 174.04(11) | 168.1(3) | 170.24(18) | |
| O1(Cl1)-Cu1-N3 | 97.82(10) | 98.2(2) | 94.5(2) | |
| N1-Cu1-N3 | 80.68(11) | 79.8(3) | 80.7(2) | |
| O1(Cl1)-Cu1-O2(S1′) | 52.17(10) | 91.31(10) | 88.55(16) | |
| N1-Cu1- O2(S1′) | 133.09(10) | 100.3(3) | 99.82(16) | |
| N3-Cu1- O2(S1′) | 85.55(9) | 87.3(3) | 89.22(19) | |
| Cu1-S1-C1 | 94.78(11) | 95.5(3) | 94.86(19) | |
| Cu1-N3-C3 | 112.2(2) | 114.5(7) | 112.3(5) | |
| Cu1-N1-N2 | 123.58(19) | 124.1(6) | 122.9(3) | |
| Cu1-N1-C2 | 118.1(2) | 117.6(7) | 117.3(4) | |
| N2-N1-C2 | 118.3(4) | 118.3(3) | 118.3(8) | 119.8(5) |
| N1-N2-C1 | 119.1(4) | 111.2(2) | 111.6(6) | 111.3(4) |
| S1-C1-N2 | 119.9(3) | 125.4(2) | 125.3(7) | 126.0(4) |
| N1-C2-C3 | 115.1(4) | 113.5(3) | 114.1(8) | 114.5(6) |
| N3-C3-C2 | 116.5(4) | 115.4(3) | 114.0(9) | 115.0(6) |
| D–H⋅⋅⋅A | d(H⋅⋅⋅A) | d(D⋅⋅⋅A) | ∠(DHA) | Symmetry Transformation for Acceptor |
|---|---|---|---|---|
| HL | ||||
| N2-H... S1 | 2.86 | 3.714(4) | 174.0 | 2-x,1-y,2-z |
| N5-H... O2 | 1.85 | 2.63(2) | 150.0 | x,-1 + y,z |
| 1 | ||||
| N4-H... O3 | 2.14 | 2.937(4) | 153.0 | x,3/2-y,-1/2 + z |
| N5-H... O2 | 2.0 | 2.854(4 | 177.0 | 1-x,1-y,-z |
| C5-H... O1 | 2.57 | 3.477(4) | 164.0 | x,3/2-y,-1/2 + z |
| C13-H... O3 | 2.54 | 3.211(4) | 130.0 | 2-x,-1/2 + y,-1/2-z |
| 2 | ||||
| O2-H... O1 | 2.15 | 2.878(11) | 144.0 | x. y, z |
| N4-H... Cl1 | 2.81 | 3.666(8) | 171.0 | x,2-y,1/2 + z |
| N5-H... O2 | 2.26 | 3.105(13) | 166/0 | x,1-y,-1/2 + z |
| C7-H... S1 | 2.72 | 3.459(10) | 137/0 | x,2-y,-1/2 + z |
| 3 | ||||
| O2-H... O3 | 1.804 | 2.678(6) | 174.0 | 2-x,1-y,2-z |
| O2-H... O5 | 2.33 | 3.088(7) | 165.0 | x, y, z |
| O2-H... S1 | 2.86 | 3.214(5) | 110.0 | 1-x,1-y,1-z |
| N4-H... O5 | 2.13 | 2.985(7) | 174.0 | 1-x,1-y,1-z |
| N5-H... O4 | 2.28 | 3.133(9) | 170.0 | 3/2-x,-1/2 + y,3/2-z |
| N5-H... O6 | 2.52 | 3.227(9) | 140.5 | 3/2-x,-1/2 + y,3/2-z |
| Compound | Staphylococcus aureus (ATCC 25923) | Escherichia coli (ATCC 25922) | Candida albicans (ATCC 10231) | |||
|---|---|---|---|---|---|---|
| MIC | MBC | MIC | MBC | MIC | MFC | |
| HL | ≥500 | ≥500 | ≥500 | ≥500 | 31.3 | 190.9 |
| (1) [Cu(L)CH3COO] | 15.6 | 31.3 | ≥500 | ≥500 | ≥500 | ≥500 |
| (2) [{Cu(L)Cl}2]·H2O | 3.9 | 7.8 | 250.0 | 500 | 15.6 | 73.5 |
| (3) [Cu(L)H2O·DMF]NO3 | 3.9 | 7.8 | ≥500 | ≥500 | ≥500 | ≥500 |
| (4) [Cu(L)Br] | 3.9 | 3.9 | 62.5 | 120.6 | 31.3 | 66.5 |
| (5) [Cu(L)H2O]ClO4 | 3.9 | 7.8 | ≥500 | ≥500 | ≥500 | ≥500 |
| Nitrofurazone | 4.7 | 9.4 | 4.7 | 4.7 | - | - |
| Miconazole | - | - | - | - | 16.0 | 76.9 |
| Compound | IC50, µM |
|---|---|
| HL | 8.5 ± 1.5 |
| (1) [Cu(L)CH3COO] | 47.4 ± 1.9 |
| (2) [{Cu(L)Cl}2]·H2O | 24.3 ± 1.3 |
| (3) [Cu(L)H2O·DMF]NO3 | 23.3 ± 0.9 |
| (4) [Cu(L)Br] | 32.4 ± 1.6 |
| (5) [Cu(L)H2O]ClO4 | 10.1 ± 0.3 |
| Trolox | 33.3 ± 0.2 |
| Compound | LC50 (µM) |
|---|---|
| HL | ≥100 |
| (1) [Cu(L)CH3COO] | 3.5 ± 2.8 |
| (2) [{Cu(L)Cl}2]·H2O | ≥100 |
| (3) [Cu(L)H2O·DMF]NO3 | 8.9 ± 1.3 |
| (4) [Cu(L)Br] | 1.0 ± 0.1 |
| (5) [Cu(L)H2O]ClO4 | 65.4 ± 11.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusnac, R.; Garbuz, O.; Chumakov, Y.; Tsapkov, V.; Hureau, C.; Istrati, D.; Gulea, A. Synthesis, Characterization, and Biological Properties of the Copper(II) Complexes with Novel Ligand: N-[4-({2-[1-(pyridin-2-yl)ethylidene]hydrazinecarbothioyl}amino)phenyl]acetamide. Inorganics 2023, 11, 408. https://doi.org/10.3390/inorganics11100408
Rusnac R, Garbuz O, Chumakov Y, Tsapkov V, Hureau C, Istrati D, Gulea A. Synthesis, Characterization, and Biological Properties of the Copper(II) Complexes with Novel Ligand: N-[4-({2-[1-(pyridin-2-yl)ethylidene]hydrazinecarbothioyl}amino)phenyl]acetamide. Inorganics. 2023; 11(10):408. https://doi.org/10.3390/inorganics11100408
Chicago/Turabian StyleRusnac, Roman, Olga Garbuz, Yurii Chumakov, Victor Tsapkov, Christelle Hureau, Dorin Istrati, and Aurelian Gulea. 2023. "Synthesis, Characterization, and Biological Properties of the Copper(II) Complexes with Novel Ligand: N-[4-({2-[1-(pyridin-2-yl)ethylidene]hydrazinecarbothioyl}amino)phenyl]acetamide" Inorganics 11, no. 10: 408. https://doi.org/10.3390/inorganics11100408
APA StyleRusnac, R., Garbuz, O., Chumakov, Y., Tsapkov, V., Hureau, C., Istrati, D., & Gulea, A. (2023). Synthesis, Characterization, and Biological Properties of the Copper(II) Complexes with Novel Ligand: N-[4-({2-[1-(pyridin-2-yl)ethylidene]hydrazinecarbothioyl}amino)phenyl]acetamide. Inorganics, 11(10), 408. https://doi.org/10.3390/inorganics11100408







