Sheaf-like Manganese-Doped Zinc Silicate with Enhanced Photoluminescence Performance
Abstract
:1. Introduction
2. Experimental Section
2.1. Sample Synthesis
2.2. Characterization
3. Results and Discussion
3.1. Crystal Phase and Lattice Distortion
3.2. X-ray Photoemission Spectroscopy (XPS) Analysis
3.3. Morphology and Microstructure
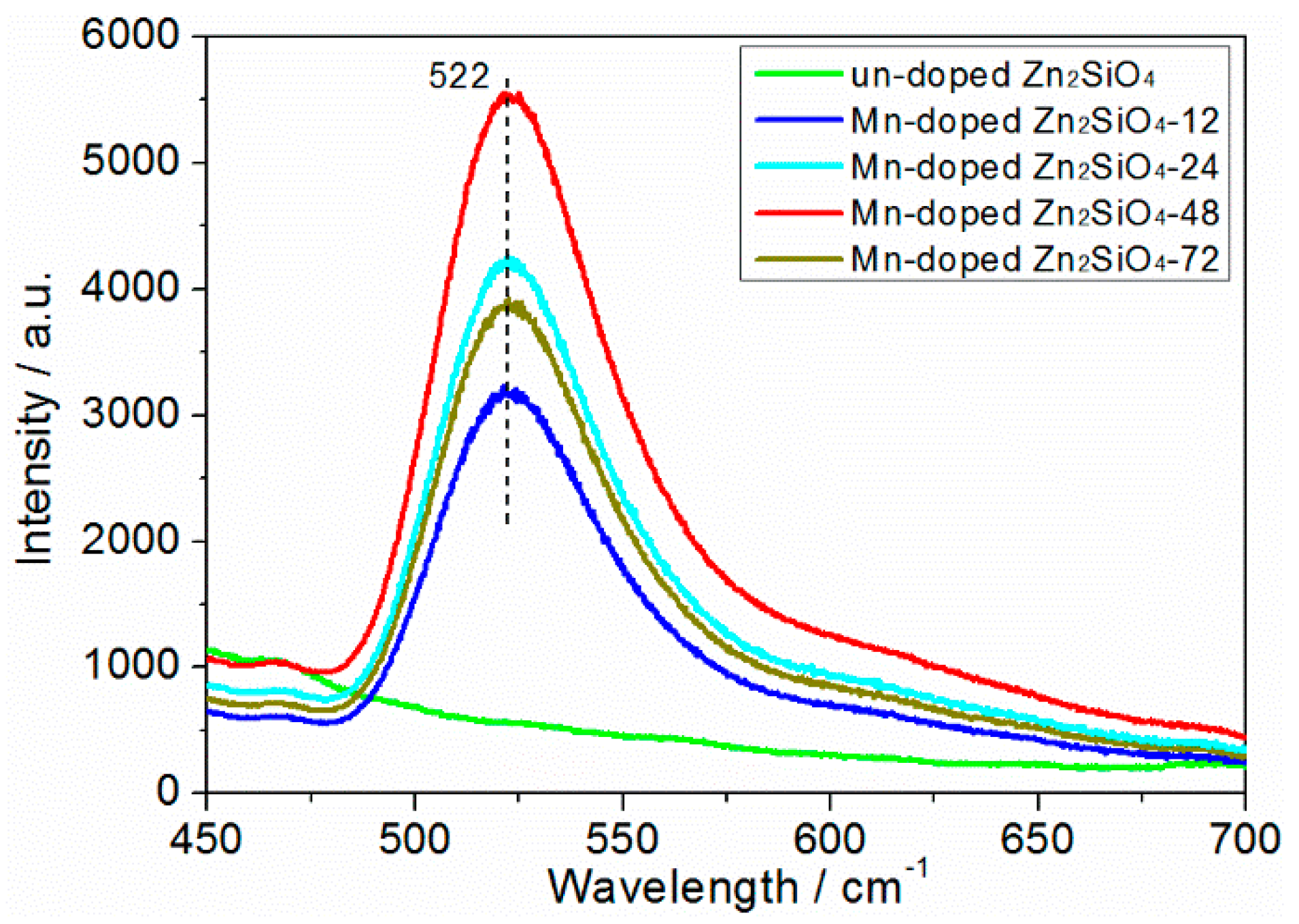
3.4. Luminescence Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kong, D.; Zhang, K.; Tian, J.; Yin, L.; Sheng, X. Biocompatible and biodegradable light-emitting materials and devices. Adv. Mater. Technol. 2022, 7, 2100006. [Google Scholar] [CrossRef]
- Wang, Q.; Liao, M.; Lin, Q.; Xiong, M.; Mu, Z.; Wu, F. A review on fluorescence intensity ratio thermometer based on rare-earth and transition metal ions doped inorganic luminescent materials. J. Alloy Compd. 2021, 850, 156744. [Google Scholar] [CrossRef]
- Wang, D.; Chen, L.; Jiang, L.; Yu, Y.; Lu, Y.; Li, H.; Li, Y.; Meng, Z.; Su, Q.; Zhang, Y.; et al. A high temperature macroscopically flexible inorganic CaYAl3O7:Eu3+ nanofiber luminescent membrane. J. Mater. Chem. C 2022, 10, 7594. [Google Scholar] [CrossRef]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Chen, D. Synthesis of Mn2+: Zn2SiO4-Eu3+: Gd2O3 nanocomposites for highly sensitive optical thermometry through the synergistic luminescence from lanthanide-transition metal ions. J. Mater. Chem. C 2017, 5, 5176–5182. [Google Scholar] [CrossRef]
- Shen, C.; Yang, Y.; Jin, S.; Ming, J. Luminous characteristics and thermal stability of BaMgAl10O17: Eu2+ phosphor for white light-emitting diodes. Phys. B Condens. Matter. 2010, 405, 1045–1049. [Google Scholar] [CrossRef]
- Polisadova, E.; Valiev, D.; Vaganov, V.; Oleshko, V.; Han, T.; Zhang, C.; Burachenko, A.; Popov, A.I. Time-resolved cathodoluminescence spectroscopy of YAG and YAG: Ce3+ phosphors. Opt. Mater. 2019, 96, 109289. [Google Scholar] [CrossRef]
- Karipbayev, Z.T.; Lisitsyn, V.M.; Mussakhanov, D.A.; Alpyssova, G.K.; Popov, A.I.; Polisadova, E.F.; Elsts, E.; Akilbekov, A.T.; Kukenova, A.B.; Kemere, M.; et al. Time-resolved luminescence of YAG: Ce and YAGG: Ce ceramics prepared by electron beam assisted synthesis. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2020, 479, 222–228. [Google Scholar] [CrossRef]
- Xie, B.; Lei, L.; Liu, E.; Bai, G.; Ye, R.; Xu, S. Blue-LED-excited Ce3+-doped alkaline-earth sulfide luminescent nanocrystals for selective and sensitive Fe3+ ions sensing. J. Lumin. 2021, 230, 117740. [Google Scholar] [CrossRef]
- Zhao, D.; Zhang, S.-R.; Fan, Y.-P.; Liu, B.-Z.; Li, Y.-N.; Shi, L.-Y.; Dai, S.-J. Two-Site Occupancy Induced a Broad-Band Emission in Phosphor K2YZr (PO4)3: Eu2+ for White-Light-Emitting Diode Applications. ACS Sustain. Chem. Eng. 2020, 8, 18992–19002. [Google Scholar] [CrossRef]
- Changshuai, G.O.G.; Xuyan, X.U.; Xiaowen, F.E.G.; Jiantong, W.A.G.; Bowen, W.A.G.; Xuejiao, W.A.G. UP-conversion photo luminescence of (La0.88Yb0.10Ho0.02)2W2O9 phosphor and dual-mode thermometry. J. Funct. Mater. 2023, 3, 03179–03186. [Google Scholar]
- Lacorre, P.; Goutenoire, F.; Bohnke, O.; Retoux, R.; Laligant, Y. Designing fast oxide-ion conductors based on La2Mo2O9. Nature 2000, 404, 856–858. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Jayasimhadri, M. Color tunable photo luminescence properties in Eu3+ doped calcium bismuth vanadate phosphors for luminescent devices. Ceram. Int. 2019, 45, 15385–15393. [Google Scholar] [CrossRef]
- Chernov, S.A.; Trinkler, L.; Popov, A.I. Photo-and thermo-stimulated luminescence of CsI-Tl crystal after UV light irradiation at 80 K. Radiat. Eff. Defects Solids 1998, 143, 345–355. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Du, H. Combustion synthesis and luminescence properties of blue NaBaPO4:Eu3+ phosphor. J. Rare Earths 2012, 30, 118–122. [Google Scholar] [CrossRef]
- Kozhevnikova, N.M.; Batueva, S.Y. Synthesis of a LiZnSc(MoO4)3:Eu3+-based red phosphor. Inorg. Mater. 2019, 55, 59–63. [Google Scholar] [CrossRef]
- Aleksandrova, L.; Iordanovaa, R.; Dimitrievbetal, Y.; Georgiev, N.; Komatsu, T. Eu3+ doped 1La2O3:2WO3:1B2O3 glass and glass–ceramic. Opt. Mater. 2014, 36, 1366–1372. [Google Scholar] [CrossRef]
- Yu, M.; Lin, J.; Fang, J. Silica spheres coated with YVO4: Eu3+ layers via sol-gel process: A simple method to obtain spherical core-shell phosphors. Chem. Mater. 2005, 17, 1783–1791. [Google Scholar] [CrossRef]
- Quan, L.N.; Rand, B.P.; Friend, R.H.; Mhaisalkar, S.G.; Lee, T.W.; Sargent, E.H. Perovskites for next-generation optical sources. Chem. Rev. 2019, 119, 7444–7477. [Google Scholar] [CrossRef]
- Liu, X.K.; Xu, W.; Bai, S.; Jin, Y.; Wang, J.; Friend, R.H.; Gao, F. Metal Halide Perovskites for Light⁃Emitting Diodes. Nat. Mater. 2021, 20, 10–21. [Google Scholar] [CrossRef]
- Yao, J.S.; Wang, J.J.; Yang, J.N.; Yao, H.B. Modulation of Metal Halide Structural Units for Light Emission. Acc. Chem. Res. 2021, 54, 441–451. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Zhang, H.; Xie, F.; Tao, C.; Xu, H.; Zhong, S. Enhanced afterglow performance of Zn2SiO4:Mn2+ by Pr3+ doping and mechanism. Ceram. Int. 2022, 48, 19358–19366. [Google Scholar] [CrossRef]
- Uegaito, K.; Hosokawa, S.; Inoue, M. Effect of heat treatments on the luminescence properties of Zn2SiO4: Mn2+ phosphors prepared by glycothermal methods. J. Lumin. 2012, 132, 64–70. [Google Scholar] [CrossRef]
- Barthou, C.; Benoit, J.; Benalloul, P.; Morell, A. Mn2+ concentration effect on the optical properties of Zn2SiO4: Mn phosphors. J. Electrochem. Soc. 1994, 141, 524. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Kato, H.; Kakihana, M. Synthesis of Zn2SiO4:Mn2+ by homogeneous precipitation using propylene glycol-modified silane. J. Mater. Chem. 2012, 22, 17272–17277. [Google Scholar] [CrossRef]
- Liu, H.; Moronta, D.; Li, L.; Yue, S.; Wong, S.S. Synthesis, properties, and formation mechanism of Mn-doped Zn2SiO4 nanowires and associated heterostructures. Phys. Chem. Chem. Phys. 2018, 20, 10086–10099. [Google Scholar] [CrossRef] [PubMed]
- Bharti, D.K.; Verma, R.; Rani, S.; Agarwal, D.; Mehra, S.; Gangwar, A.K.; Gupta, B.K.; Singh, N.; Srivastava, A.K. Synthesis and characterization of highly crystalline Bi-functional Mn-doped Zn2SiO4 nanostructures by low-cost sol-gel process. Nanomaterials 2023, 13, 538. [Google Scholar] [CrossRef]
- Kim, J.S.; Kwon, A.K.; Park, Y.H.; Choi, J.C.; Park, H.L.; Kim, G.C. Luminescent and thermal properties of full-color emitting X3MgSi2O8:Eu2+, Mn2+ (X=Ba, Sr, Ca) phosphors for white LED. J. Lumin. 2007, 122–123, 583–586. [Google Scholar] [CrossRef]
- Zhao, M.; Xia, Z.; Huang, X.; Ning, L.; Gautier, R.; Molokeev, M.S.; Zhou, Y.; Chuang, Y.C.; Zhang, Q.; Liu, Q.; et al. Li substituent tuning of LED phosphors with enhanced efficiency, tunable photoluminescence, and improved thermal stability. Sci. Adv. 2019, 5, 0363. [Google Scholar] [CrossRef]
- Song, E.H.; Zhou, Y.Y.; Wei, Y.; Han, X.X.; Tao, Z.R.; Qiu, R.L.; Xia, Z.G.; Zhang, Q.Y. A thermally stable narrow-band green-emitting phosphor MgAl2O4:Mn2+ for wide color gamut backlight display application. J. Mater. Chem. C 2019, 7, 8192–8198. [Google Scholar] [CrossRef]
- Hu, T.; Lin, H.; Xu, J.; Wang, B.; Wang, J.; Wang, Y. Color-tunable persistent luminescence in oxyfluoride glass and glass ceramic containing Mn2+: α-Zn2SiO4 nanocrystals. J. Mater. Chem. C 2017, 5, 1479–1487. [Google Scholar] [CrossRef]
- El Mir, L. Sol-gel synthesis and luminescence of undoped and Mn-doped zinc orthosilicate phosphor nanocomposites. J. Lumin. 2014, 148, 82–88. [Google Scholar]
- Lin, L.; Feiyan, X.; Dekang, X.; Chaochao, T.; Hualan, X.; Zhong, S. Zn2SiO4: Mn2+, Yb3+ long afterglow materials prepared employing Zn-based coordination polymer as precursor: Properties, Mechanism and Application. J. Lumin. 2023, 255, 119601. [Google Scholar]
- Omri, K.; Lemine, O.M.; El Mir, L. Mn doped zinc silicate nanophosphor with bifunctionality of green-yellow emission and magnetic properties. Ceram. Int. 2017, 43, 6585–6591. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, P.; Li, J. Structure and luminescence properties of Mn-doped Zn2SiO4 prepared with extracted mesoporous silica. Mater. Res. Bull. 2011, 46, 791–795. [Google Scholar] [CrossRef]
- El Ghoul, J.; El Mir, L. Synthesis by sol-gel process, structural and luminescence of V and Mn doped a-Zn2SiO4. J Mater. Sci. Mater. Electron. 2015, 26, 3550–3557. [Google Scholar] [CrossRef]
- Su, K.; Tilley, T.D.; Sailor, M.J. Molecular and polymer precursor routes to manganese-doped zinc orthosilicate phosphors. J. Am. Chem. Soc. 1996, 118, 3459–3468. [Google Scholar] [CrossRef]
- Yoo, J.B.; Yang, H.J.; Hur, N.H. Controlled synthesis of luminescent Mn-doped Zn2SiO4 microspheres by thermal hydrolysis of urea. J. Lumin. 2022, 243, 118608. [Google Scholar] [CrossRef]
- Xu, G.Q.; Xu, H.T.; Zheng, Z.X.; Wu, Y.C. Preparation and characterization of Zn2SiO4: Mn phosphors with hydrothermal methods. J. Lumin. 2010, 130, 1717–1720. [Google Scholar] [CrossRef]
- Cho, J.S.; Lee, S.M.; Jung, K.Y.; Kang, Y.C. Large-scale production of fine-sized Zn2SiO4: Mn phosphor microspheres with a dense structure and good photoluminescence properties by a spray-drying process. RSC Adv. 2014, 4, 43606–43611. [Google Scholar] [CrossRef]
- Zhang, S.; Ren, L.; Peng, S. Zn2SiO4 urchin-like microspheres: Controlled synthesis and application in lithium-ion batteries. CrystEngComm 2014, 16, 6195–6202. [Google Scholar] [CrossRef]
- Xiong, L.; Shi, J.; Gu, J.; Shen, W.; Dong, X.; Chen, H.; Zhang, L.; Gao, J.; Ruan, M. Directed growth of well-aligned zinc silicate nanowires along the channels of surfactant-assembled mesoporous silica. Small 2005, 1, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Chen, X.; Wang, Z.; Mu, L.; Qian, Y. One-dimensional rice-like Mn-doped Zn2SiO4: Preparation, characterization, luminescent properties and its stability. J. Cryst. Growth 2005, 280, 239–243. [Google Scholar] [CrossRef]
- Wang, H.; Ma, Y.; Yi, G.; Chen, D. Synthesis of Mn-doped Zn2SiO4 rodlike nanoparticles through hydrothermal method. Mater. Chem. Phys. 2003, 82, 414–418. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Haase, M.; Weller, H. Low-temperature synthesis of pure and Mn-doped willemite phosphor (Zn2SiO4: Mn) in aqueous medium. Mater. Res. Bull. 2000, 35, 1869–1879. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, E. X-ray Diffraction Procedures: For Polycrystalline and Amorphous Materials; Wiley-VCH: Weinheim, Germany, 1974; Volume 5, p. 992. ISBN 0-471-49369-4. [Google Scholar]
- Hafeez, M.; Ali, A.; Manzoora, S.; Bhatti, A.S. Anomalous optical and magnetic behavior of multi-phase Mn doped Zn2SiO4 nanowires: A new class of dilute magnetic semiconductors. Nanoscale 2014, 6, 14845–14855. [Google Scholar] [CrossRef]
- Peng, Z.A.; Peng, X.G. Mechanisms of the shape evolution of CdSe nanocrystals. J. Am. Chem. Soc. 2001, 123, 1389–1395. [Google Scholar] [CrossRef]
- Ma, H.; Zhang, S.Y.; Ji, W.Q.; Tao, Z.L.; Chen, J. α-CuV2O6 nanowires: Hydrothermal synthesis and primary lithium battery application. J. Am. Chem. Soc. 2008, 130, 5361–5367. [Google Scholar] [CrossRef]
- Zhu, G.; Sushko, M.L.; Loring, J.S.; Legg, B.A.; Song, M.; Soltis, J.A.; Huang, X.; Rosso, K.M.; De Yoreo, J.J. Self-similar mesocrystals form via interface-driven nucleation and assembly. Nature 2021, 590, 416–422. [Google Scholar] [CrossRef]
- Lee, D.H.; Wang, W.; Gutu, T.; Jeffryes, C.; Rorrer, G.L.; Jiao, J.; Chang, C.H. Biogenic silica based Zn2SiO4: Mn2+ and Y2SiO5: Eu3+ phosphor layers patterned by inkjet printing process. J. Mater. Chem. 2008, 18, 3633–3635. [Google Scholar] [CrossRef]
- Bhalla, R.; White, E.W. Cathodoluminescence Characteristics of Mn2+-Activated Willemite (Zn2SiO4) Single Crystals. J. Electrochem. Soc. 1972, 119, 740–743. [Google Scholar] [CrossRef]
- Wu, B.; Li, J.; Li, Q. Preparation and photoluminescence behavior of Mn-doped nano-ZnO. Optik 2019, 188, 205–211. [Google Scholar] [CrossRef]
- Bulyk, L.I.; Vasylechko, L.; Mykhaylyk, V.; Tang, C.; Zhydachevskyy, Y.; Hizhnyi, Y.A.; Nedilko, S.G.; Klyui, N.I.; Suchocki, A. Mn2+ luminescence of Gd (Zn, Mg) B5O10 pentaborate under high pressure. Dalton Trans. 2020, 49, 14268–14279. [Google Scholar] [CrossRef]
- Gavrilović, T.; Periša, J.; Papan, J.; Vuković, K.; Smits, K.; Jovanović, D.J.; Dramićanin, M.D. Particle size effects on the structure and emission of Eu3+: LaPO4 and EuPO4 phosphors. J. Lumin. 2018, 195, 420–429. [Google Scholar] [CrossRef]
- Hou, Z.Y.; Li, G.G.; Lian, H.Z.; Liu, J. One-dimensional luminescent materials derived from the electrospinning process: Preparation, characteristics and application. J. Mater. Chem. 2012, 22, 5254–5276. [Google Scholar] [CrossRef]
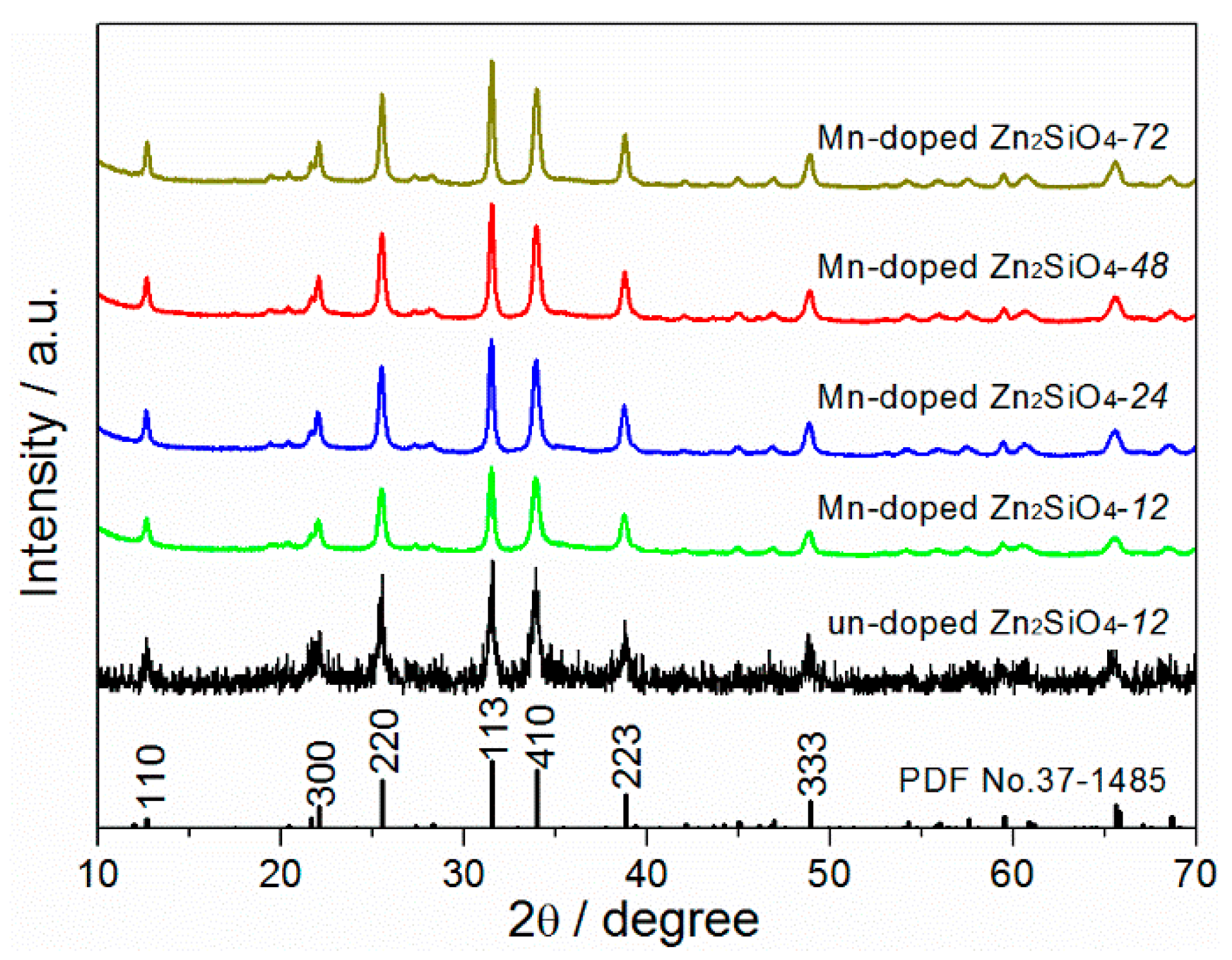

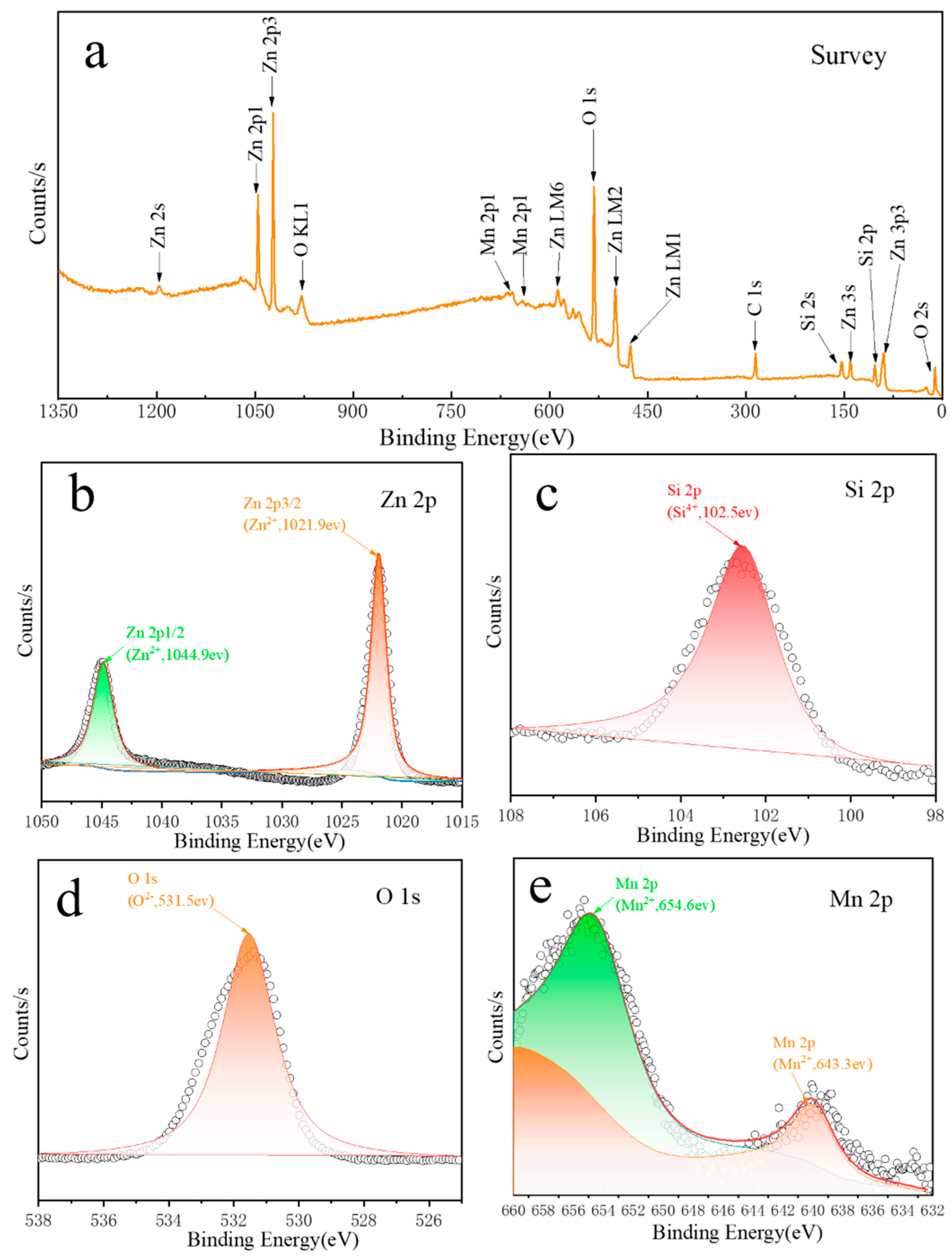
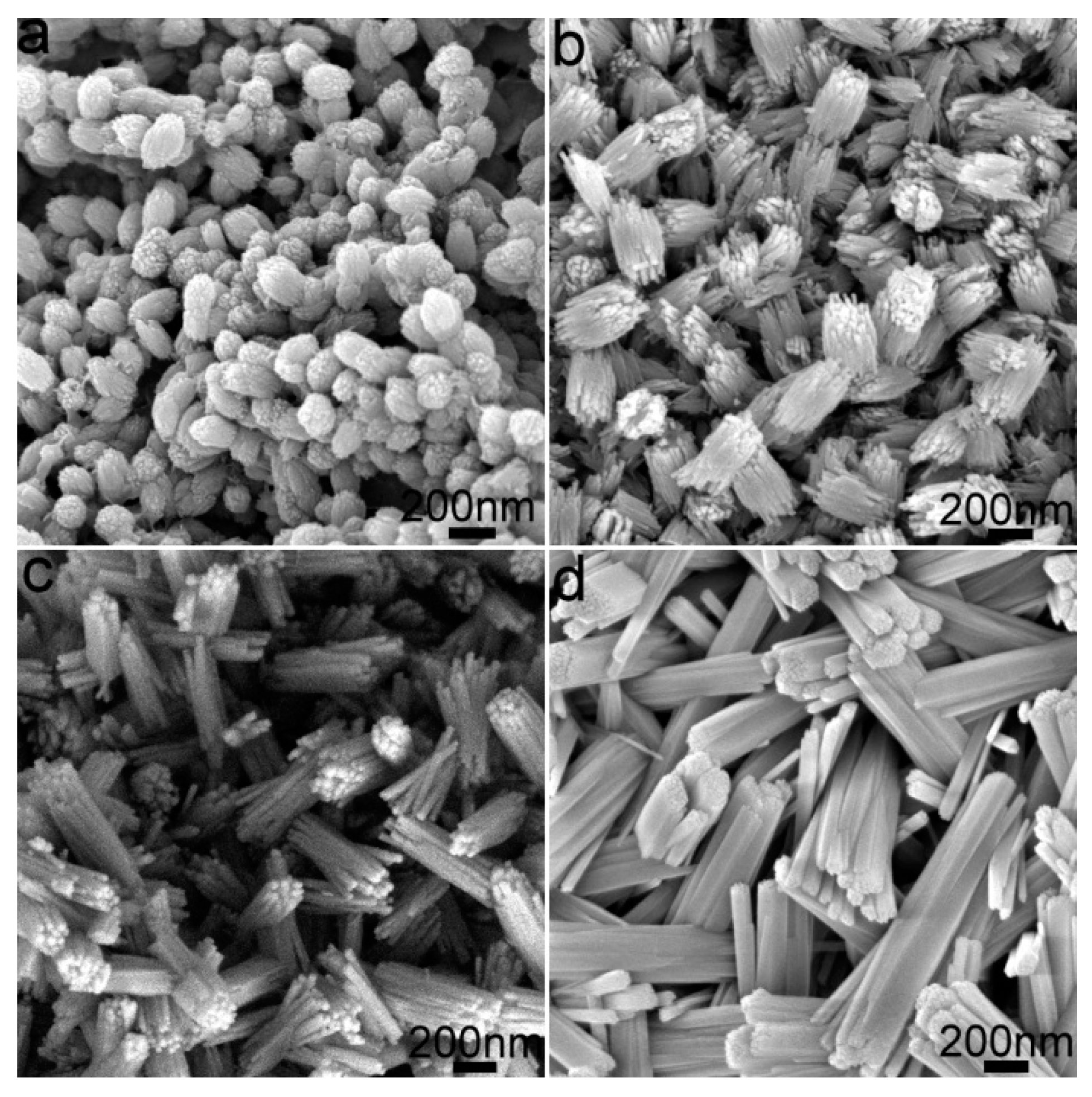


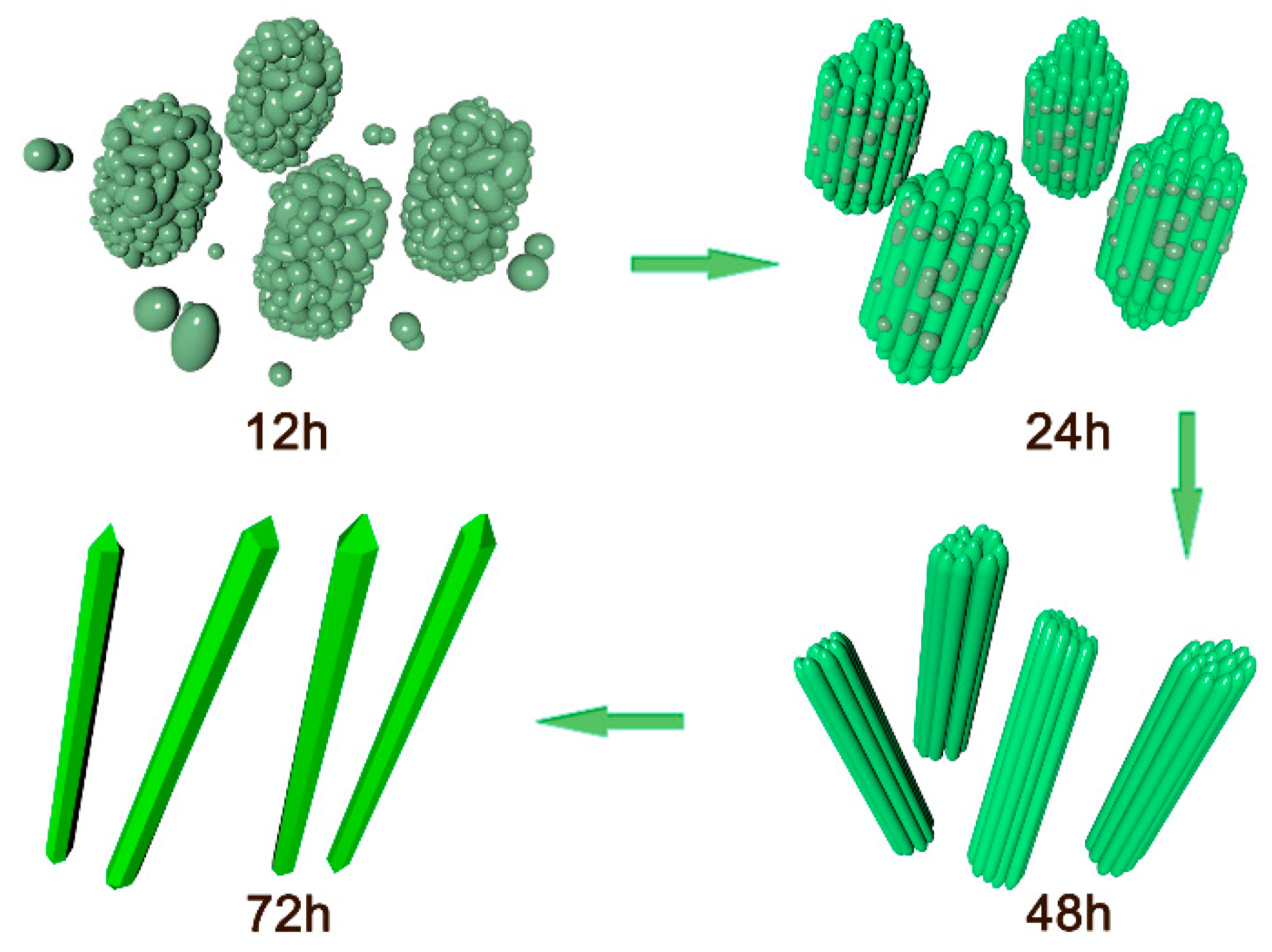
| Samples | Peak Position | L/nm | E×1000 | a, b (Å) | c (Å) | Cell Volume (Å3) |
|---|---|---|---|---|---|---|
| JCPDS No. 37-1485 | / | / | / | 13.9381 | 9.310 | 1566.3 |
| x = 12 | (110) and (220) | 31.04 | 4.16 | 13.9273 | 9.309 | 1563.8 |
| x = 24 | (110)and (220) | 34.96 | 4.43 | 13.9242 | 9.306 | 1562.6 |
| x = 48 | (110)and (220) | 38.87 | 4.98 | 13.9188 | 9.305 | 1561.2 |
| x = 72 | (110) and (220) | 41.58 | 4.02 | 13.9136 | 9.297 | 1558.7 |
| Element | Peak BE | Height CPS | Area (P) CPS.eV | Atomic % |
|---|---|---|---|---|
| C 1s | 284.80 | 14,204.3 | 36,722.61 | 17.64 (±1.00) |
| Mn 2p | 654.60 | 2960.43 | 29,177.89 | 1.46 (±0.10) |
| O 1s | 531.54 | 89,455.65 | 249,147.24 | 49.5 (±2.5) |
| Si 2p | 102.52 | 11,412.33 | 31,207.52 | 14.93 (±1.00) |
| Zn 2p | 1021.94 | 128,160.39 | 541,679.32 | 16.82 (±1.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhang, X.; Yu, Y.; Wang, L.; Cheng, S.; Zhan, H.; Liu, R.; Chen, R. Sheaf-like Manganese-Doped Zinc Silicate with Enhanced Photoluminescence Performance. Inorganics 2023, 11, 407. https://doi.org/10.3390/inorganics11100407
Li X, Zhang X, Yu Y, Wang L, Cheng S, Zhan H, Liu R, Chen R. Sheaf-like Manganese-Doped Zinc Silicate with Enhanced Photoluminescence Performance. Inorganics. 2023; 11(10):407. https://doi.org/10.3390/inorganics11100407
Chicago/Turabian StyleLi, Xiaohong, Xiaozhen Zhang, Yongzhi Yu, Leying Wang, Si Cheng, Hongquan Zhan, Runyuan Liu, and Renhua Chen. 2023. "Sheaf-like Manganese-Doped Zinc Silicate with Enhanced Photoluminescence Performance" Inorganics 11, no. 10: 407. https://doi.org/10.3390/inorganics11100407
APA StyleLi, X., Zhang, X., Yu, Y., Wang, L., Cheng, S., Zhan, H., Liu, R., & Chen, R. (2023). Sheaf-like Manganese-Doped Zinc Silicate with Enhanced Photoluminescence Performance. Inorganics, 11(10), 407. https://doi.org/10.3390/inorganics11100407












