Synthesis, Structure and Antimicrobial Activity of New Co(II) Complex with bis-Morpholino/Benzoimidazole-s-Triazine Ligand
Abstract
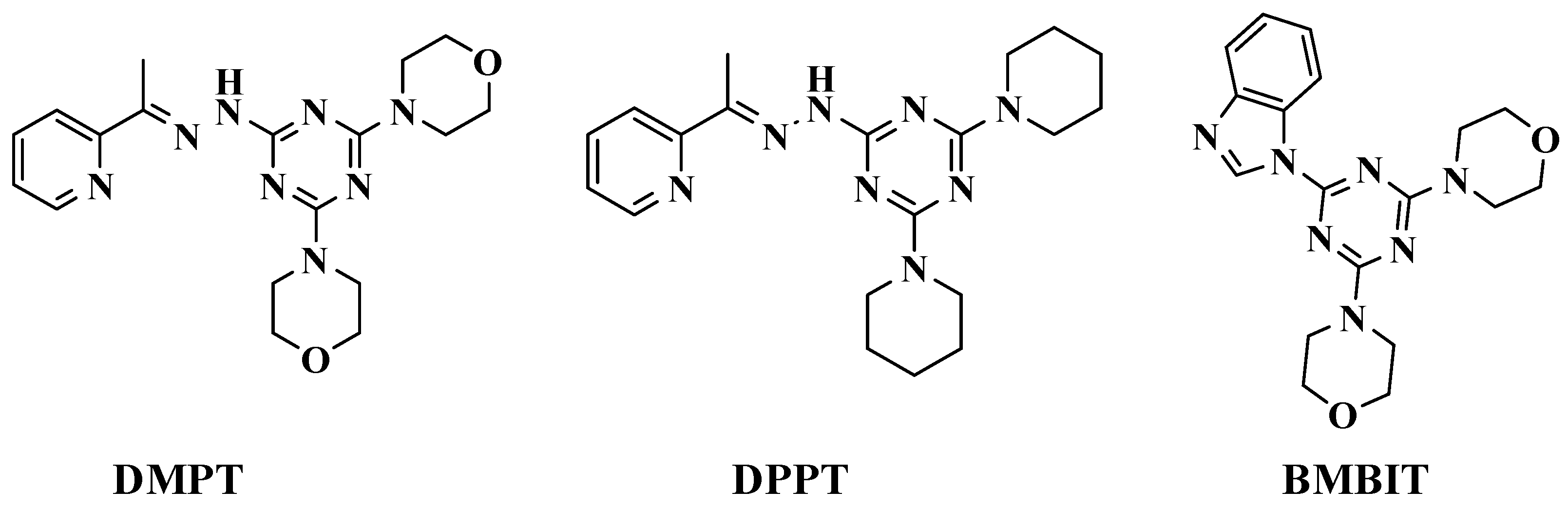
1. Introduction
2. Results
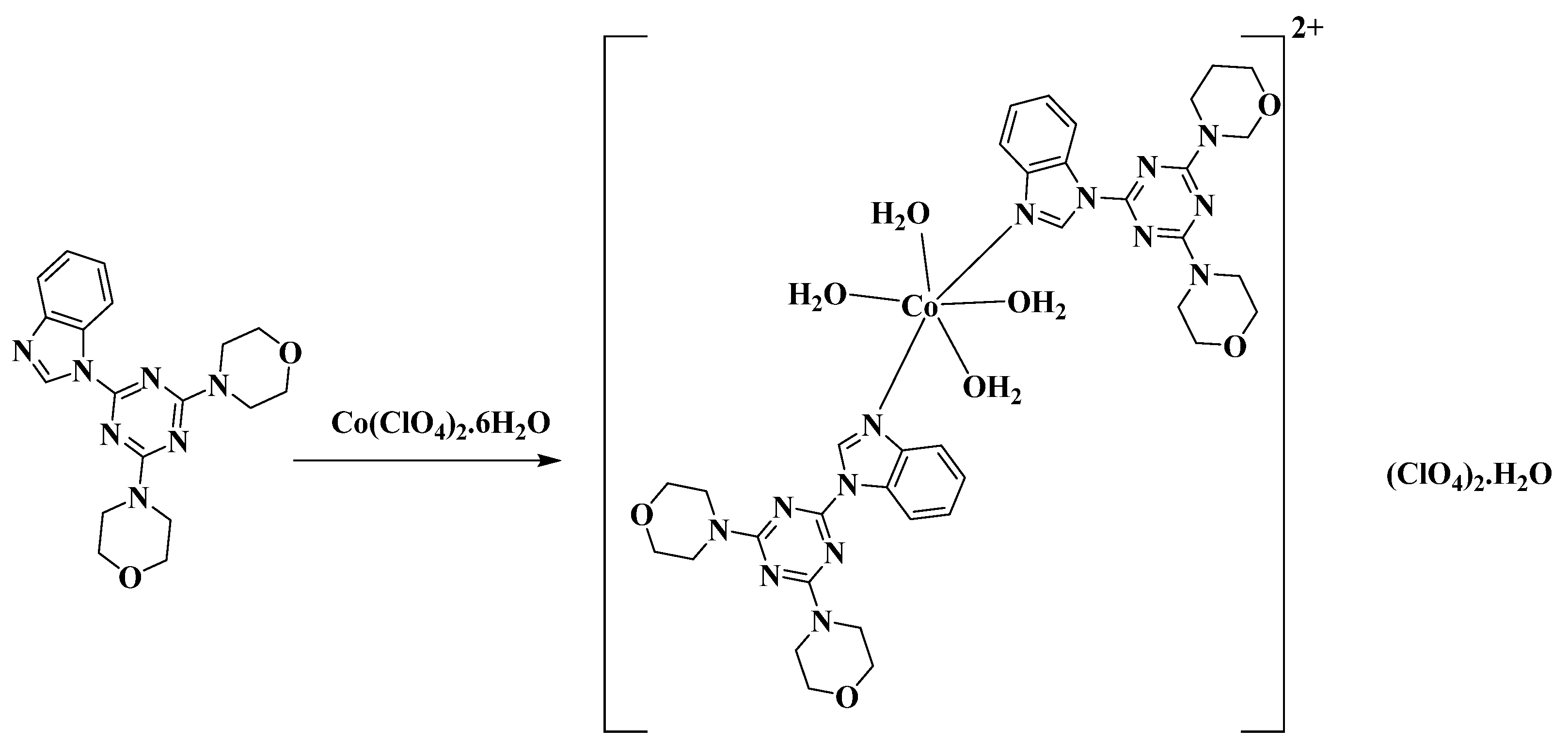
2.1. Synthesis and Characterizations
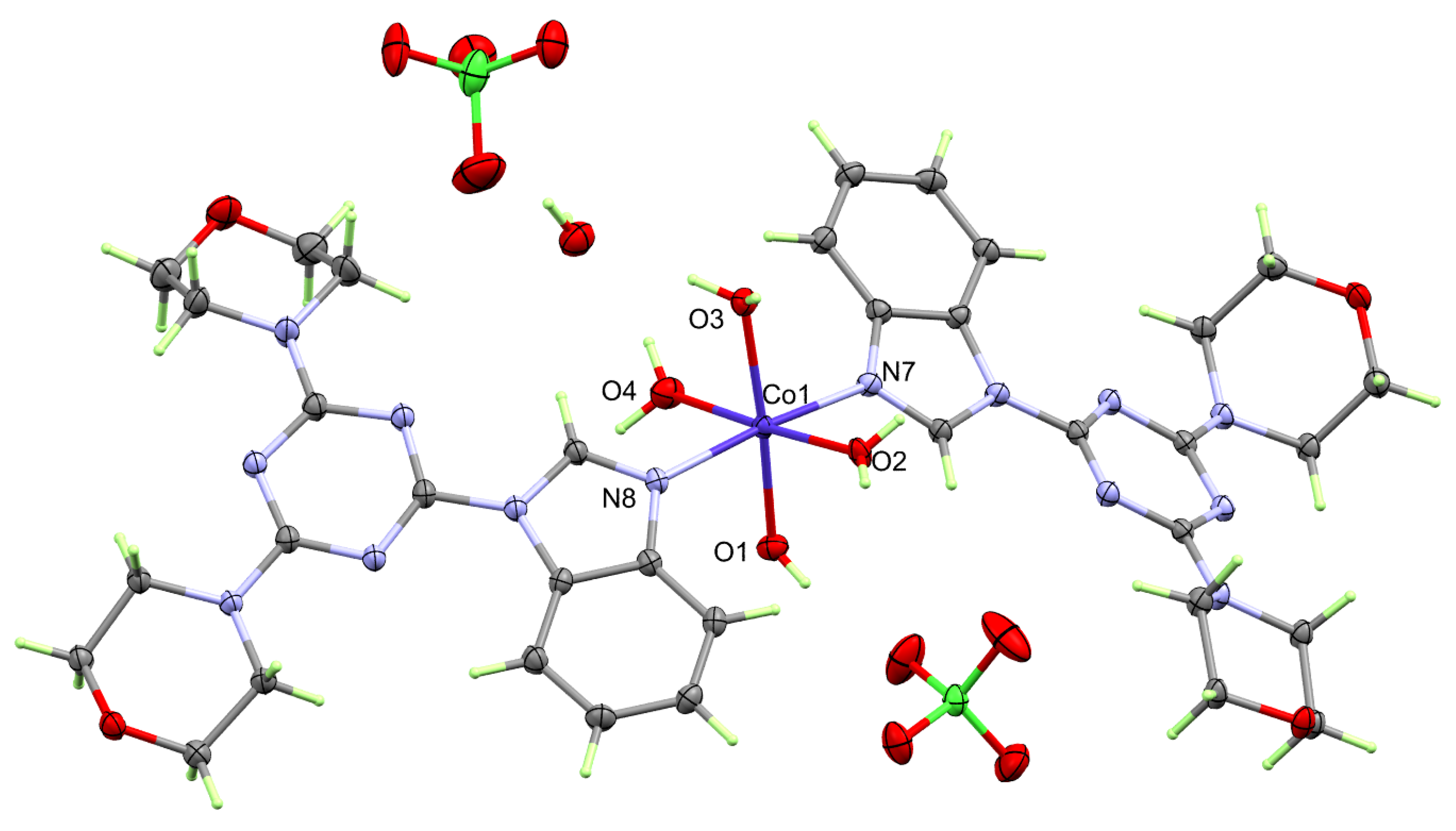
2.2. X-ray Structure Description
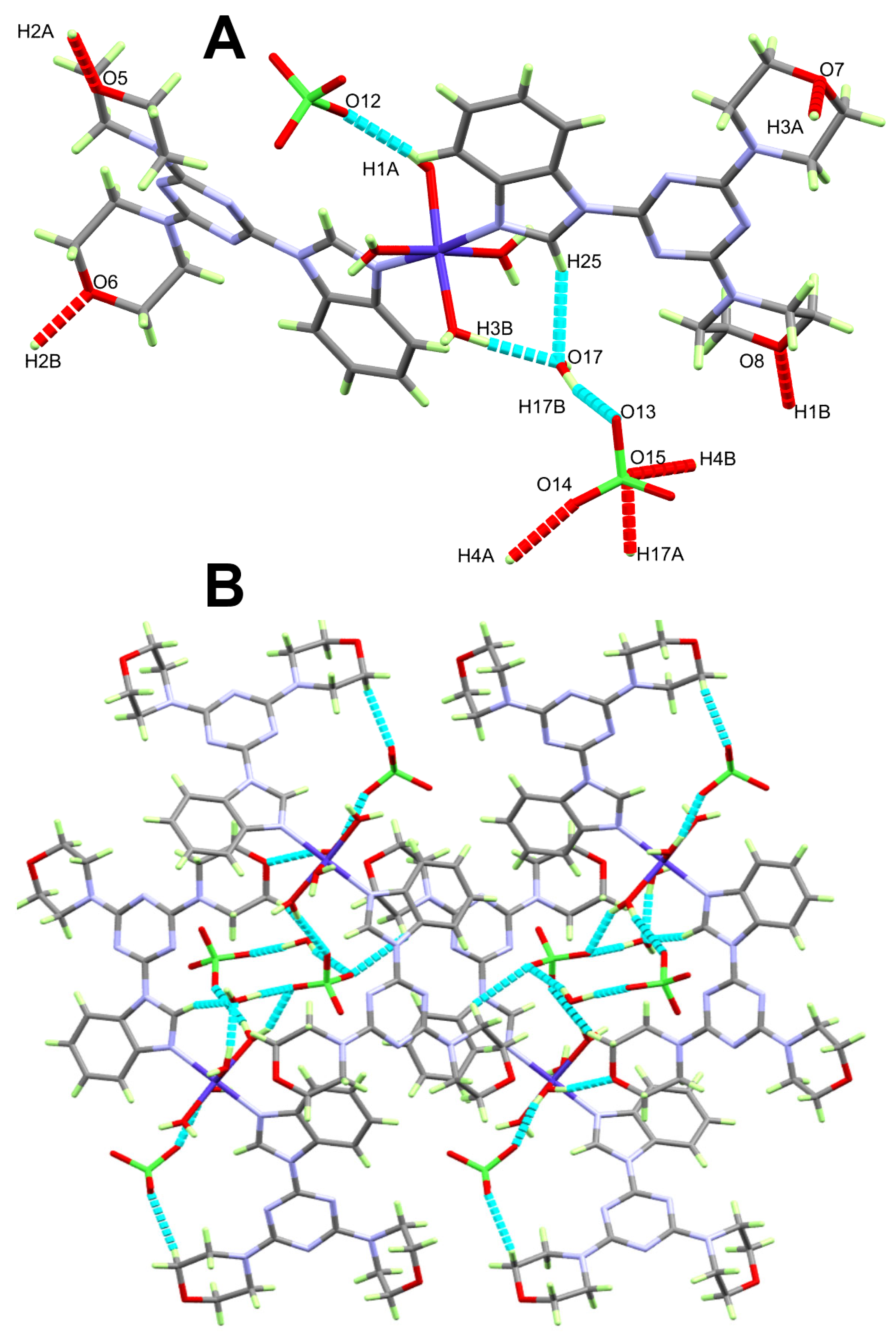
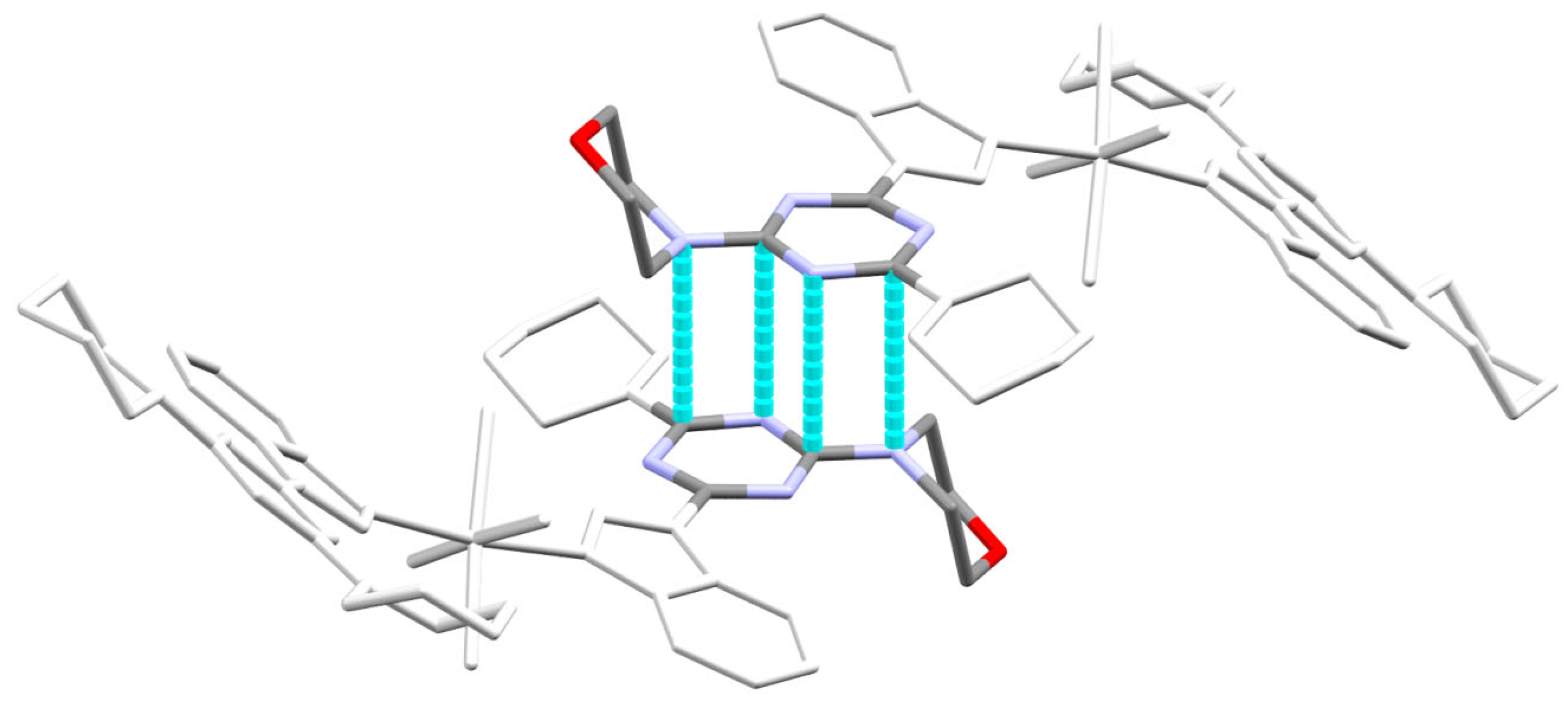
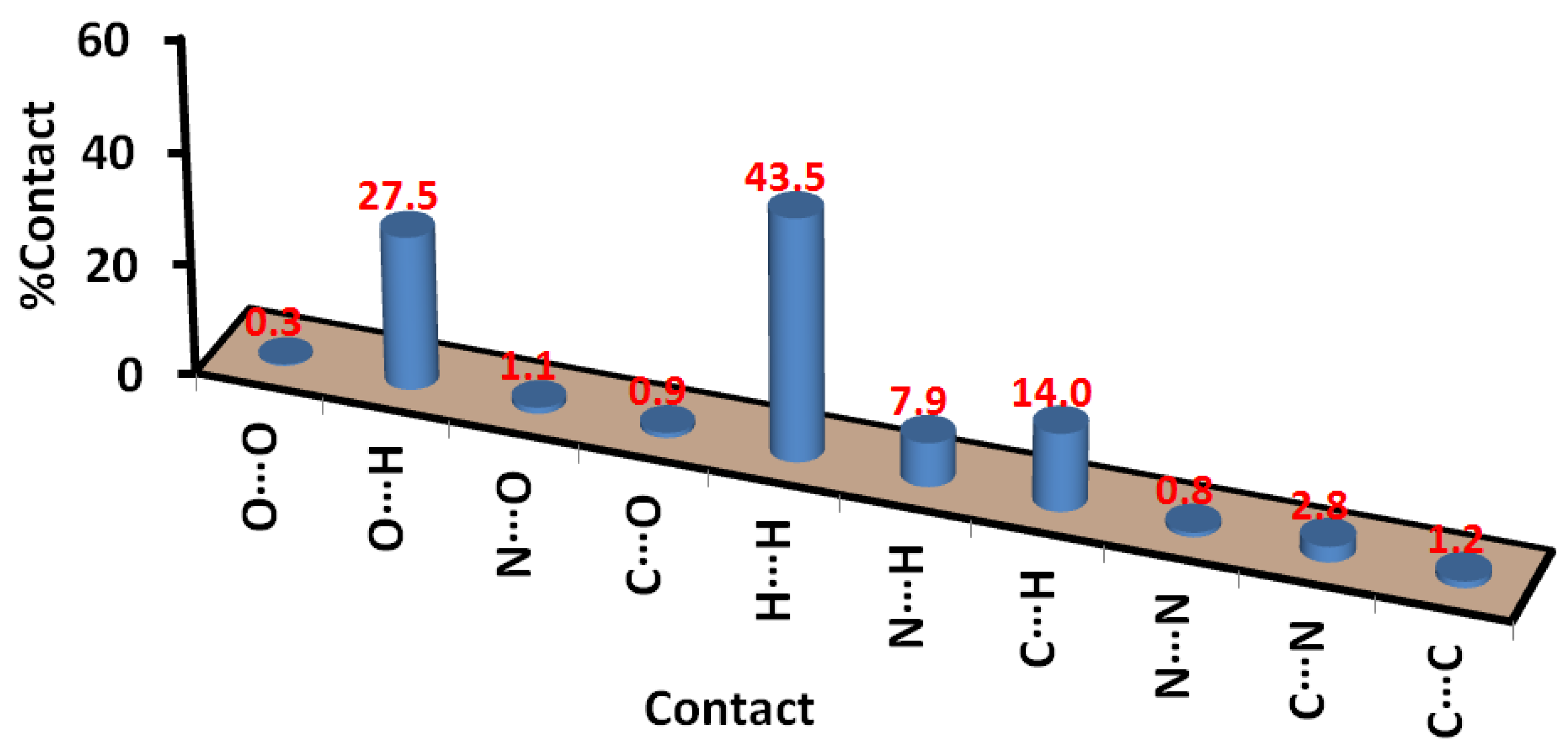
2.3. Analysis of Molecular Packing
2.4. Antimicrobial Assay
3. Materials and Methods
3.1. Physical Measurements
3.2. Synthesis of 4,4′-(6-(1H-benzo[d]imidazol-1-yl)-1,3,5-triazine-2,4-diyl)dimorpholine (BMBIT) Ligand [39]
3.3. Synthesis of [Co(BMBIT)2(H2O)4](ClO4)2*H2O Complex
3.4. Crystal Structure Determination
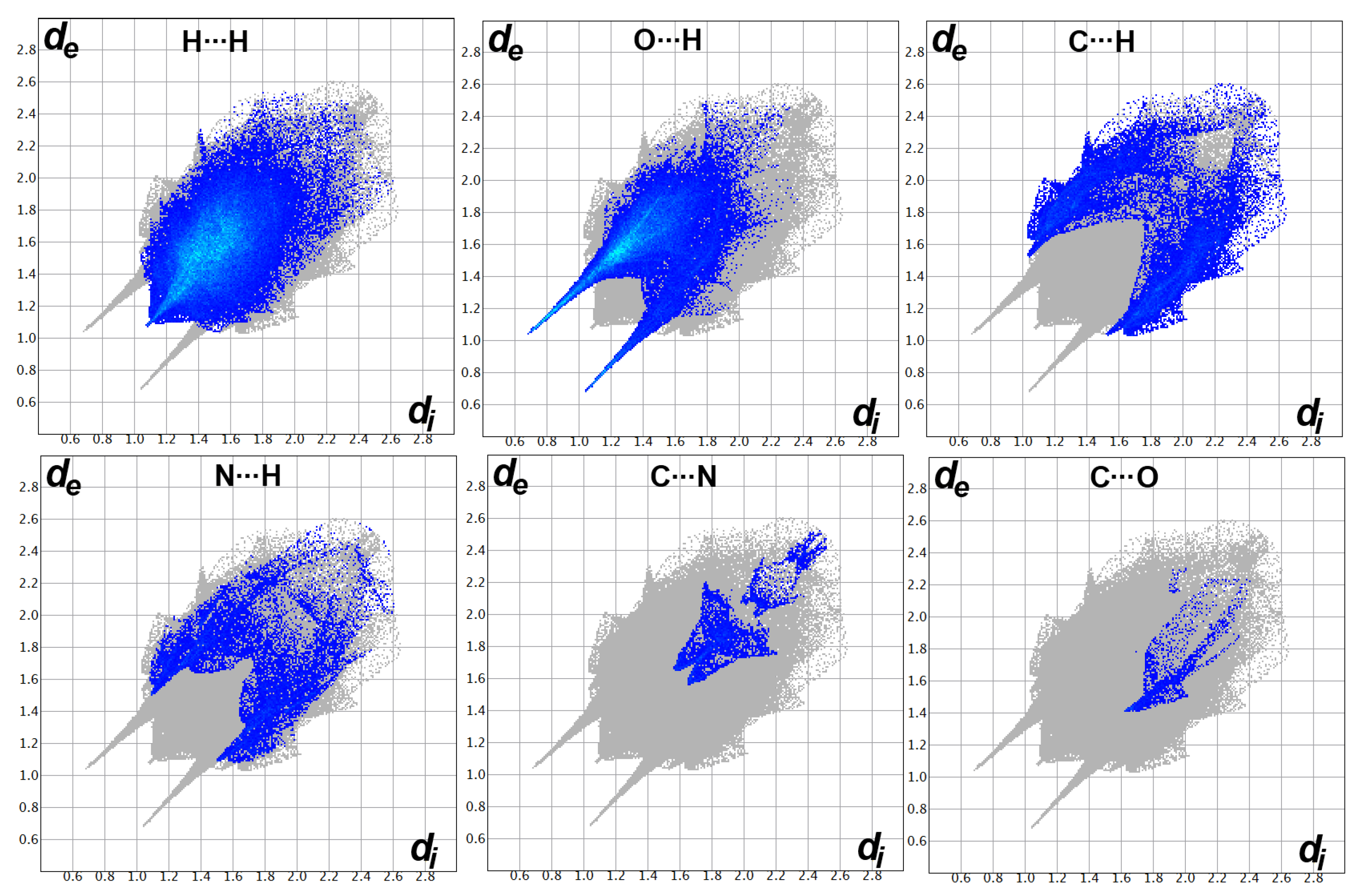
3.5. Hirshfeld Analysis
3.6. Antimicrobial Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, H.; Chen, S.; Zhong, A.; Sun, R.; Jin, J.; Yang, J.; Liu, D.; Niu, J.; Lu, S. Tuning Photophysical Properties via Positional Isomerization of the Pyridine Ring in Donor–Acceptor-Structured Aggregation-Induced Emission Luminogens Based on Phenylmethylene Pyridineacetonitrile Derivatives. Molecules 2023, 28, 3282. [Google Scholar] [CrossRef]
- Ye, D.; Liu, L.; Peng, Q.; Qiu, J.; Gong, H.; Zhong, A.; Liu, S. Effect of Controlling Thiophene Rings on D-A Polymer Photocatalysts Accessed via Direct Arylation for Hydrogen Production. Molecules 2023, 28, 4507. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; He, X.; Rong, C.; Zhong, A.; Liu, S.; Zhao, D. On the origin and nature of internal methyl rotation barriers: An information-theoretic approach study. Theor. Chem. Acc. 2022, 141, 68. [Google Scholar] [CrossRef]
- Zhang, W.; Ye, G.; Liao, D.; Chen, X.; Lu, C.; Ejhieh, A.N.; Khan, M.S.; Liu, J.; Pan, Y.; Dai, Z. Recent Advances of Silver-Based Coordination Polymers on Antibacterial Applications. Molecules 2022, 27, 7166. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Z.; Ma, J.; Nezamzadeh-Ejhieh, A.; Lu, C.; Pan, Y.; Liu, J.; Bai, Z. Current status and prospects of MOFs in controlled delivery of Pt anticancer drugs. Dalton Trans. 2023, 52, 6226–6238. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Sheyi, R.; de la Torre, B.G.; El-Faham, A.; Albericio, F. s-Triazine: A Privileged Structure for Drug Discovery and Bioconjugation. Molecules 2021, 26, 864. [Google Scholar] [CrossRef]
- Maliszewski, D.; Drozdowska, D. Recent Advances in the Biological Activity of s-Triazine Core Compounds. Pharmaceuticals 2022, 15, 221. [Google Scholar] [CrossRef]
- Dai, Q.; Sun, Q.; Ouyang, X.; Liu, J.; Jin, L.; Liu, A.; He, B.; Fan, T.; Jiang, Y. Antitumor Activity of s-Triazine Derivatives: A Systematic Review. Molecules 2023, 28, 4278. [Google Scholar] [CrossRef]
- Barakat, A.; El-Faham, A.; Haukka, M.; Al-Majid, A.M.; Soliman, S.M. s-Triazine pincer ligands: Synthesis of their metal complexes, coordination behavior, and applications. Appl. Organomet. Chem. 2021, 35, e6317. [Google Scholar] [CrossRef]
- Majeed Ganai, A.; Khan Pathan, T.; Hampannavar, G.A.; Pawar, C.; Obakachi, V.A.; Kushwaha, B.; Deshwar Kushwaha, N.; Karpoormath, R. Recent Advances on the s-Triazine Scaffold with Emphasis on Synthesis, Structure-Activity and Pharmacological Aspects: A Concise Review. ChemistrySelect 2021, 6, 1616–1660. [Google Scholar] [CrossRef]
- Shawish, I.; Barakat, A.; Aldalbahi, A.; Malebari, A.M.; Nafie, M.S.; Bekhit, A.A.; Albohy, A.; Khan, A.; Ul-Haq, Z.; Haukka, M.; et al. Synthesis and Antiproliferative Activity of a New Series of Mono- and Bis(dimethylpyrazolyl)-s-triazine Derivatives Targeting EGFR/PI3K/AKT/mTOR Signaling Cascades. ACS Omega 2022, 7, 24858–24870. [Google Scholar] [CrossRef]
- Shawish, I.; Barakat, A.; Aldalbahi, A.; Alshaer, W.; Daoud, F.; Alqudah, D.A.; Al Zoubi, M.; Hatmal, M.M.; Nafie, M.S.; Haukka, M.; et al. Acetic Acid Mediated for One-Pot Synthesis of Novel Pyrazolyl s-Triazine Derivatives for the Targeted Therapy of Triple-Negative Breast Tumor Cells (MDA-MB-231) via EGFR/PI3K/AKT/mTOR Signaling Cascades. Pharmaceutics 2022, 14, 1558. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Long, S.; Rakesh, K.P.; Zha, G.F. Structure-activity relationships (SAR) of triazine derivatives: Promising antimicrobial agents. Eur. J. Med. Chem. 2020, 185, 111804. [Google Scholar] [CrossRef] [PubMed]
- Krečmerová, M.; Dračínský, M.; Snoeck, R.; Balzarini, J.; Pomeisl, K.; Andrei, G. New prodrugs of two pyrimidine acyclic nucleoside phosphonates: Synthesis and antiviral activity. Bioorg. Med. Chem. 2017, 25, 4637–4648. [Google Scholar] [CrossRef] [PubMed]
- Venkatraj, M.; Salado, I.G.; Heeres, J.; Joossens, J.; Lewi, P.J.; Caljon, G.; Maes, L.; Van der Veken, P.; Augustyns, K. Novel triazine dimers with potent antitrypanosomal activity. Eur. J. Med. Chem. 2018, 143, 306–319. [Google Scholar] [CrossRef]
- Singla, P.; Luxami, V.; Paul, K. Synthesis, in Vitro Antitumor Activity, Dihydrofolate Reductase Inhibition, DNA Intercalation and Structure—Activity Relationship Studies of 1,3,5-Triazine Analogues. Bioorg. Med. Chem. Lett. 2016, 26, 518–523. [Google Scholar] [CrossRef]
- Singla, P.; Luxami, V.; Paul, K. Triazine–Benzimidazole Hybrids: Anticancer Activity, DNA Interaction and Dihydrofolate Reductase Inhibitors. Bioorg. Med. Chem. 2015, 23, 1691–1700. [Google Scholar] [CrossRef]
- Kumar, G.J.; Kumar, S.N.; Thummuri, D.; Adari, L.B.S.; Naidu, V.G.M.; Srinivas, K.; Rao, V.J. Synthesis and Characterization of New s-Triazine Bearing Benzimidazole and Benzothiazole Derivatives as Anticancer Agents. Med. Chem. Res. 2015, 24, 3991–4001. [Google Scholar] [CrossRef]
- Singla, P.; Luxami, V.; Paul, K. Synthesis and in Vitro Evaluation of Novel Triazine Analogues as Anticancer Agents and Their Interaction Studies with Bovine Serum Albumin. Eur. J. Med. Chem. 2016, 117, 59–69. [Google Scholar] [CrossRef]
- Refaat, H.M.; Alotaibi, A.A.M.; Dege, N.; El-Faham, A.; Soliman, S.M. Co (II) Complexes Based on the Bis-Pyrazol-s-Triazine Pincer Ligand: Synthesis, X-ray Structure Studies, and Cytotoxic Evaluation. Crystals 2022, 12, 741. [Google Scholar] [CrossRef]
- Aouled, I.M.; Uysal, S. The synthesis and characterization of s-triazine bridged calixarene based a Schiff base: Investigation of its [MSalen/Salophen](M = Cr3+, Fe3+ or Co3+) capped complexes. J. Mol. Struct. 2022, 1250, 131872. [Google Scholar] [CrossRef]
- Aouled, I.M.; Uysal, S. Investigation of thermal and magnetic properties of [MSalen/Saloph](M = Cr3+, Fe3+ or Co3+) capped dinuclear complexes of two novel tetraoxocalix[2]arene[2]triazine ligands. J. Mol. Struct. 2023, 1280, 135084. [Google Scholar] [CrossRef]
- Cahiez, G.R.; Moyeux, A. Cobalt-catalyzed cross-coupling reactions. Chem. Rev. 2010, 110, 1435–1462. [Google Scholar] [CrossRef] [PubMed]
- Hebrard, F.; Kalck, P. Cobalt-catalyzed hydroformylation of alkenes: Generation and recycling of the carbonyl species, and catalytic cycle. Chem. Rev. 2009, 109, 4272–4282. [Google Scholar] [CrossRef]
- Junge, K.; Papa, V.; Beller, M. Cobalt–pincer complexes in catalysis. Chem. Eur. J. 2019, 25, 122–143. [Google Scholar] [CrossRef] [PubMed]
- Pototschnig, G.; Maulide, N.; Schnürch, M. Direct functionalization of C−H bonds by iron, nickel, and cobalt catalysis. Chem. Eur. J. 2017, 23, 9206–9232. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Matsunaga, S. Cobalt-catalyzed C(sp3)−H functionalization reactions. Asian J. Org. Chem. 2018, 7, 1193–1205. [Google Scholar] [CrossRef]
- Tilly, D.; Dayaker, G.; Bachu, P. Cobalt mediated C–H bond functionalization: Emerging tools for organic synthesis. Catal. Sci. Technol. 2014, 4, 2756–2777. [Google Scholar] [CrossRef]
- Asgharpour, Z.; Farzaneh, F.; Abbasi, A. Synthesis, characterization and immobilization of a new cobalt(ii) complex on modified magnetic nanoparticles as catalyst for epoxidation of alkenes and oxidation of activated alkanes. RSC Adv. 2016, 6, 95729–95739. [Google Scholar] [CrossRef]
- Shao, D.; Shi, L.; Wei, H.Y.; Wang, X.Y. Field-induced single-ion magnet behavior in two new cobalt(II) coordination polymers with 2,4,6-tris(4-pyridyl)-1,3,5-triazine. Inorganics 2017, 5, 90. [Google Scholar] [CrossRef]
- Osman, A.H. Synthesis and characterization of cobalt(II) and nickel(II) complexes of some Schiff bases derived from 3-hydrazino-6-methyl [1,2,4] triazin-5(4H)one. Transit. Met. Chem. 2006, 31, 35–41. [Google Scholar] [CrossRef]
- Menati, S.; Rudbari, H.A.; Askari, B.; Farsani, M.R.; Jalilian, F.; Dini, G. Synthesis and characterization of insoluble cobalt(II), nickel(II), zinc(II) and palladium(II) Schiff base complexes: Heterogeneous catalysts for oxidation of sulfides with hydrogen peroxide. Compets Rendus Chim. 2016, 19, 347–356. [Google Scholar] [CrossRef]
- Marandi, F.; Moeini, K.; Arkak, A.; Mardani, Z.; Krautscheid, H. Docking studies to evaluate the biological activities of the Co(II) and Ni(II) complexes containing the triazine unit: Supported by structural, spectral, and theoretical studies. J. Coord. Chem. 2018, 71, 3893–3911. [Google Scholar] [CrossRef]
- Soliman, S.M.; Elsilk, S.E.; El-Faham, A. Syntheses, structure, Hirshfeld analysis and antimicrobial activity of four new Co (II) complexes with s-triazine-based pincer ligand. Inorg. Chim. Acta 2020, 510, 119753. [Google Scholar] [CrossRef]
- Soliman, S.M.; Massoud, R.A.; Al-Rasheed, H.H.; El-Faham, A. Syntheses and Structural Investigations of Penta-Coordinated Co(II) Complexes with Bis-Pyrazolo-s-Triazine Pincer Ligands, and Evaluation of Their Antimicrobial and Antioxidant Activities. Molecules 2021, 26, 3633. [Google Scholar] [CrossRef]
- Fathalla, E.M.; Abu-Youssef, M.A.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Badr, A.M.A.; Soliman, S.M.; Slawin, A.M.Z.; Woollins, J.D. Synthesis, Characterizations, Antitumor and Antimicrobial Evaluations of Novel Mn(II) and Cu(II) Complexes with NNN-tridentate s-Triazine-Schiff base Ligand. Inorg. Chim. Acta 2023, 555, 121586. [Google Scholar] [CrossRef]
- Fathalla, E.M.; Abu-Youssef, M.A.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Haukka, M.; Soliman, S.M. Synthesis, X-ray Structure of Two Hexa-Coordinated Ni(II) Complexes with s-Triazine Hydrazine Schiff Base Ligand. Inorganics 2023, 11, 222. [Google Scholar] [CrossRef]
- Fathalla, E.M.; Abu-Youssef, M.A.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Haukka, M.; Soliman, S.M. Supramolecular Structure and Antimicrobial Activity of Ni(II) Complexes with s-Triazine/Hydrazine Type Ligand. Inorganics 2023, 11, 253. [Google Scholar] [CrossRef]
- Matsuno, T.; Karo, M.; Sasahara, H.; Watanabe, T.; Inaba, M.; Takahashi, M.; Yaguchi, S.I.; Yoshioka, K.; Sakato, M.; Kawashima, S. Synthesis and antitumor activity of benzimidazolyl-1,3,5-triazine and benzimidazolylpyrimidine derivatives. Chem. Pharm. Bull. 2000, 48, 1778–1781. [Google Scholar] [CrossRef]
- Sherif, O.E.; Abdel-Kader, N.S. DFT calculations, spectroscopic studies, thermal analysis and biological activity of supramolecular Schiff base complexes. Arab. J. Chem. 2018, 11, 700–713. [Google Scholar] [CrossRef]
- Rikagu Oxford Diffraction. CrysAlisPro; Rikagu Oxford Diffraction Inc.: Oxford, UK, 2020. [Google Scholar]
- Sheldrick, G.M. Shelxt—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. C Struct. Chem. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Lu, P.-L.; Liu, Y.-C.; Toh, H.-S.; Lee, Y.-L.; Liu, Y.-M.; Ho, C.-M.; Huang, C.-C.; Liu, C.-E.; Ko, W.-C.; Wang, J.-H. Epidemiology and antimicrobial susceptibility profiles of Gram-negative bacteria causing urinary tract infections in the Asia-Pacific region: 2009–2010 results from the Study for Monitoring Antimicrobial Resistance Trends (SMART). Int. J. Antimicrob. Agents. 2012, 40, S37–S43. [Google Scholar] [CrossRef]


| Bond | Distance | Bond | Distance |
|---|---|---|---|
| Co(1)-O(3) | 2.0817(12) | Co(1)-N(8) | 2.1267(13) |
| Co(1)-O(1) | 2.0878(12) | Co(1)-N(7) | 2.1436(13) |
| Co(1)-O(2) | 2.1160(11) | Co(1)-O(4) | 2.1831(12) |
| Bonds | Angle | Bonds | Angle |
| O(3)-Co(1)-O(1) | 173.42(5) | O(2)-Co(1)-N(7) | 85.59(5) |
| O(3)-Co(1)-O(2) | 90.88(5) | N(8)-Co(1)-N(7) | 173.36(5) |
| O(1)-Co(1)-O(2) | 95.65(5) | O(3)-Co(1)-O(4) | 87.45(5) |
| O(3)-Co(1)-N(8) | 93.49(5) | O(1)-Co(1)-O(4) | 85.99(5) |
| O(1)-Co(1)-N(8) | 86.56(5) | O(2)-Co(1)-O(4) | 176.91(5) |
| O(2)-Co(1)-N(8) | 96.50(5) | N(8)-Co(1)-O(4) | 86.21(5) |
| O(3)-Co(1)-N(7) | 92.77(5) | N(7)-Co(1)-O(4) | 91.88(5) |
| O(1)-Co(1)-N(7) | 86.96(5) |
| D-H…A | D-H | H···A | D···A | D-H···A |
|---|---|---|---|---|
| C7-H7A···O3 #1 | 0.98 | 2.4 | 3.292(6) | 150.6 |
| O(1)-H(1A)···O(12) | 0.78(2) | 1.98(2) | 2.7418(19) | 168(2) |
| O(1)-H(1B)···O(8) #1 | 0.85(3) | 1.93(3) | 2.7533(18) | 165(3) |
| O(2)-H(2A)···O(5) #2 | 0.82(3) | 2.06(3) | 2.8337(16) | 159(2) |
| O(2)-H(2B)···O(6) #3 | 0.79(3) | 1.91(3) | 2.7011(16) | 174(3) |
| O(3)-H(3A)···O(7) #4 | 0.82(3) | 1.92(3) | 2.7209(17) | 166(3) |
| O(3)-H(3B)···O(17) | 0.86(3) | 1.89(3) | 2.7436(18) | 174(3) |
| O(4)-H(4A)···O(14) #5 | 0.92(4) | 2.18(4) | 3.082(3) | 168(3) |
| O(4)-H(4B)···O(15) #1 | 0.87(3) | 2.01(3) | 2.806(4) | 153(3) |
| C(25)-H(25)···O(17) | 0.95 | 2.43 | 3.286(2) | 150 |
| O(17)-H(17B)···O(13) | 0.93 | 2.03 | 2.914(4) | 157.4 |
| O(17)-H(17A)···O(15) #1 | 0.94(4) | 1.98(5) | 2.797(5) | 143(4) |
| Contact | Distance | Contact | Distance |
|---|---|---|---|
| O5···H2A | 1.902 | C8···H2B | 2.653 |
| O7···H3A | 1.760 | C9···H2B | 2.696 |
| O6···H2B | 1.722 | C17···H8A | 2.709 |
| O15···H4B | 1.904 | C14···H4C | 2.713 |
| O16···H15 | 2.588 | C14···H21 | 2.566 |
| O11···H1C | 2.557 | C20···H3D | 2.761 |
| O10···H2C | 2.445 | C14···H21 | 2.566 |
| O12···H1A | 1.777 | C35···H1B | 2.665 |
| O8···H1B | 1.795 | C25···H28A | 2.651 |
| O15···H17A | 1.956 | C19···H29A | 2.683 |
| O14···H4A | 2.116 | C20···H29A | 2.680 |
| O14···H35A | 2.448 | C29···H3A | 2.647 |
| O15···H17B | 2.481 | C30···H3A | 2.711 |
| O13···H17B | 1.985 | C2···H2A | 2.768 |
| O13···H28B | 2.574 | C6···N2 | 3.222 |
| O12···H7A | 2.560 | C5···N3 | 3.247 |
| O13···H28B | 2.574 | C25···O14 | 3.040 |
| N12···H15 | 2.595 | C32···O16 | 3.079 |
| H7B···H34B | 2.175 |
| Microorganism | BMBIT | [Co(BMBIT)2(H2O)4](ClO4)2*H2O | Control |
|---|---|---|---|
| A. fumigatus | NA b (ND) c | 13(312) | 17(156) d |
| C. albicans | NA b (ND) c | 15(156) | 20(312) d |
| S. aureus | NA b (ND) c | 16(156) | 24(9.7) e |
| B. subtilis | 13 (312) | 13(312) | 26(4.8) e |
| E. coli | NA b (ND) c | 15(156) c | 30(4.8) e |
| P. vulgaris | 13 (ND) c | 17(78) c | 25(4.8) e |
| CCDC | 2266666 |
| empirical formula | C36H52Cl2CoN14O17 |
| fw | 1082.74 |
| temp (K) | 120(2) |
| λ(Å) | 1.54184 |
| cryst syst | Monoclinic |
| space group | P21/c |
| a (Å) | 22.21971(11) |
| b (Å) | 8.86743(4) |
| c (Å) | 24.38673(12) |
| β (deg) | 113.4401(6) |
| V (Å3) | 4408.44(4) |
| Z | 4 |
| ρcalc (Mg/m3) | 1.631 |
| μ(Mo Kα) (mm−1) | 4.967 |
| No. reflns. | 125499 |
| Unique reflns. | 9260 |
| Completeness to θ = 67.684° | 99.9% |
| GOOF (F2) | 1.036 |
| Rint | 0.0300 |
| R1a (I ≥ 2σ) | 0.0314 |
| wR2b (I ≥ 2σ) | 0.0841 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soliman, S.M.; Fathalla, E.M.; Sharaf, M.M.; El-Faham, A.; Barakat, A.; Haukka, M.; Slawin, A.M.Z.; Woollins, J.D.; Abu-Youssef, M.A.M. Synthesis, Structure and Antimicrobial Activity of New Co(II) Complex with bis-Morpholino/Benzoimidazole-s-Triazine Ligand. Inorganics 2023, 11, 278. https://doi.org/10.3390/inorganics11070278
Soliman SM, Fathalla EM, Sharaf MM, El-Faham A, Barakat A, Haukka M, Slawin AMZ, Woollins JD, Abu-Youssef MAM. Synthesis, Structure and Antimicrobial Activity of New Co(II) Complex with bis-Morpholino/Benzoimidazole-s-Triazine Ligand. Inorganics. 2023; 11(7):278. https://doi.org/10.3390/inorganics11070278
Chicago/Turabian StyleSoliman, Saied M., Eman M. Fathalla, Mona M. Sharaf, Ayman El-Faham, Assem Barakat, Matti Haukka, Alexandra M. Z. Slawin, John Derek Woollins, and Morsy A. M. Abu-Youssef. 2023. "Synthesis, Structure and Antimicrobial Activity of New Co(II) Complex with bis-Morpholino/Benzoimidazole-s-Triazine Ligand" Inorganics 11, no. 7: 278. https://doi.org/10.3390/inorganics11070278
APA StyleSoliman, S. M., Fathalla, E. M., Sharaf, M. M., El-Faham, A., Barakat, A., Haukka, M., Slawin, A. M. Z., Woollins, J. D., & Abu-Youssef, M. A. M. (2023). Synthesis, Structure and Antimicrobial Activity of New Co(II) Complex with bis-Morpholino/Benzoimidazole-s-Triazine Ligand. Inorganics, 11(7), 278. https://doi.org/10.3390/inorganics11070278











