Bimetallic Copper-Silver Systems Supported on Natural Clinoptilolite: Long-Term Changes in Nanospecies’ Composition and Stability
Abstract
:1. Introduction
2. Results and Discussion
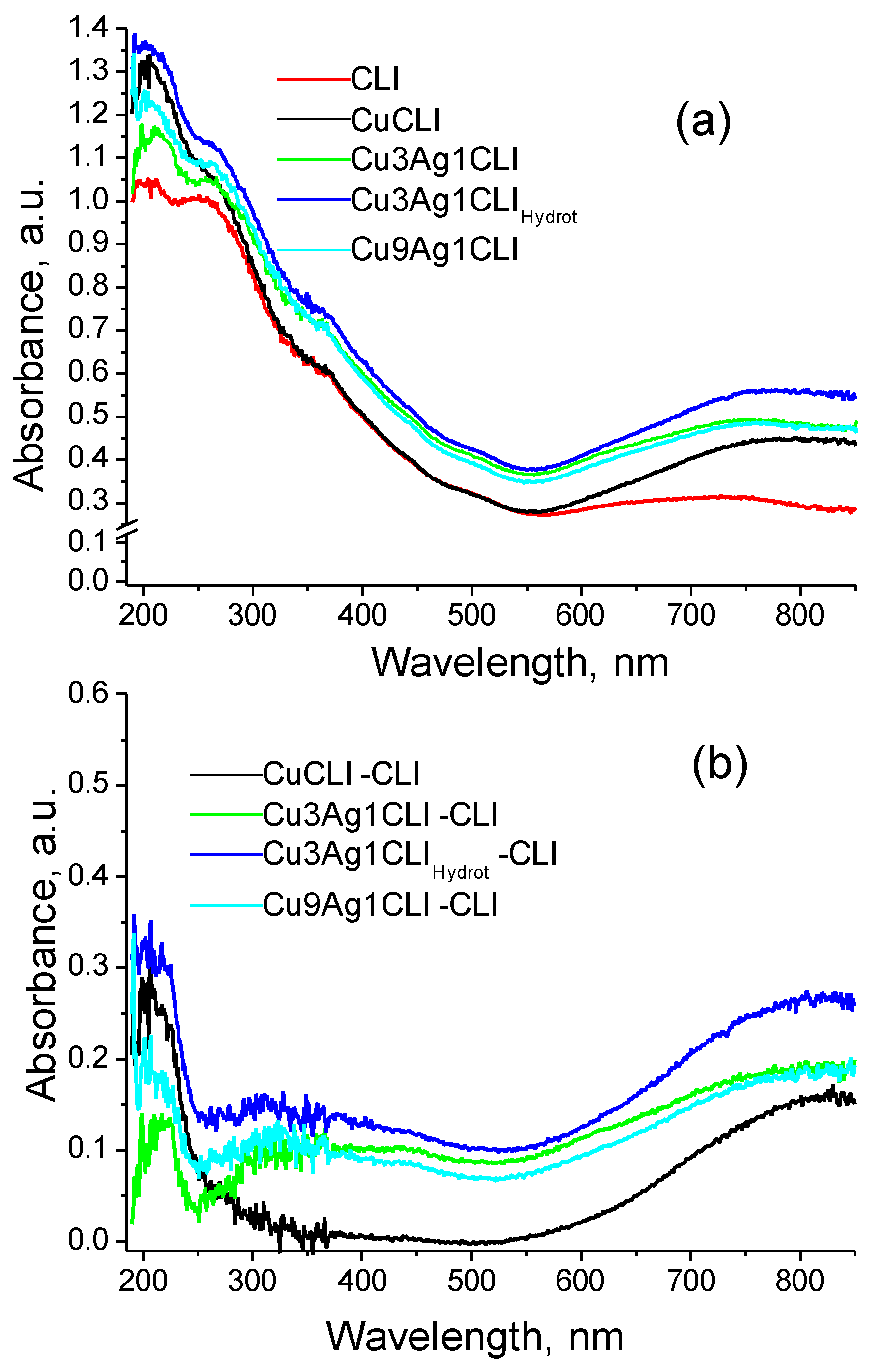
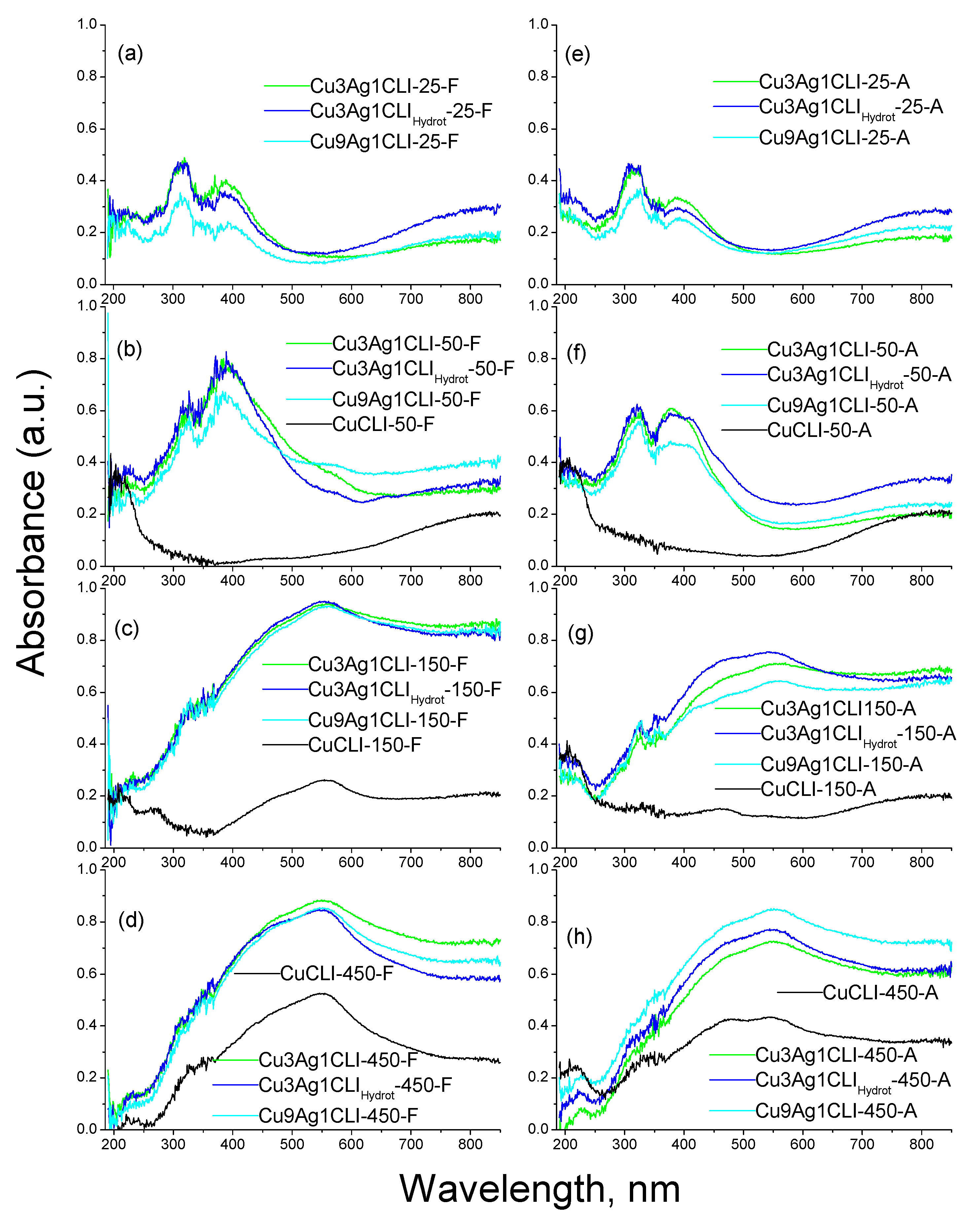
2.1. UV-Vis Diffuse Reflectance Spectroscopy
2.2. X-ray Diffraction
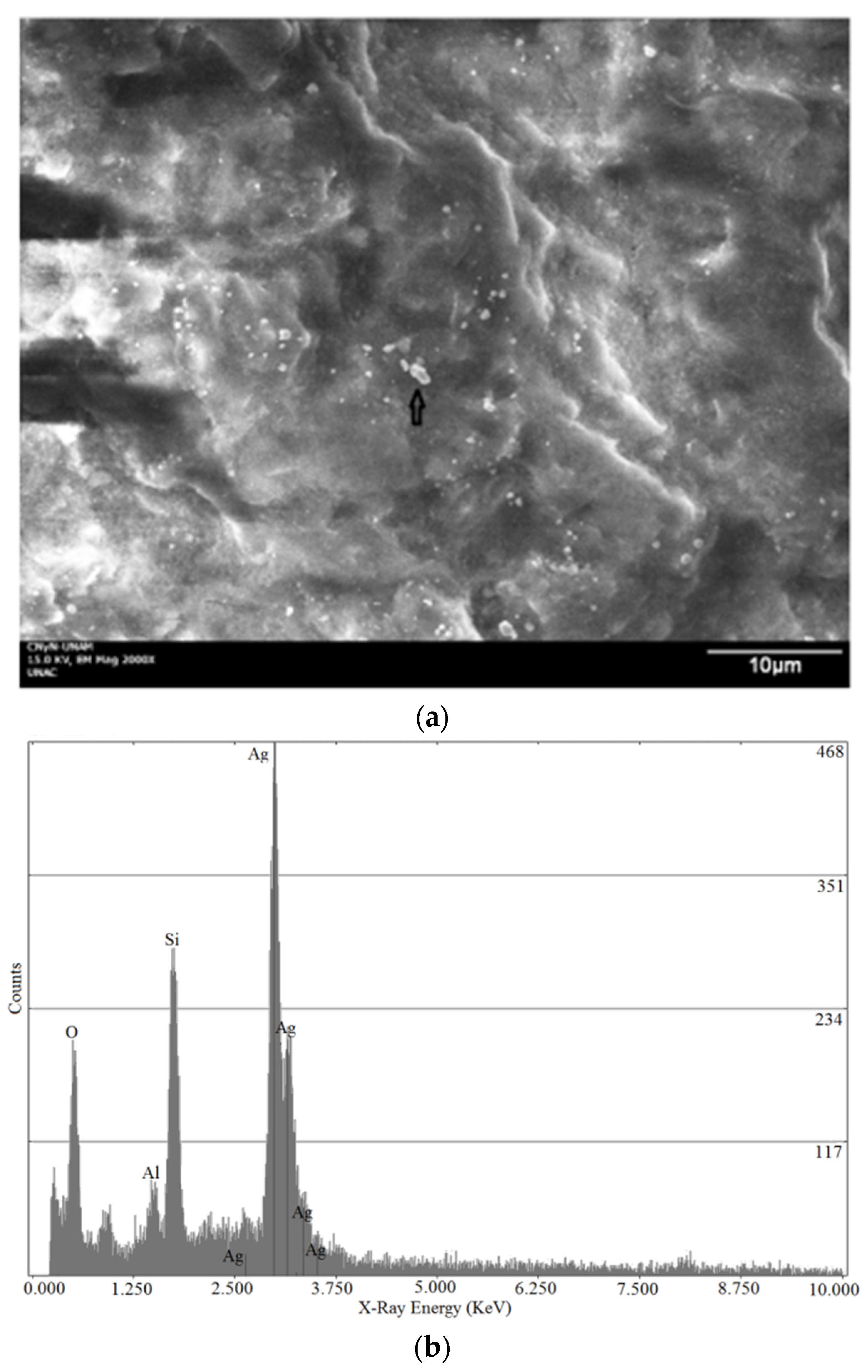
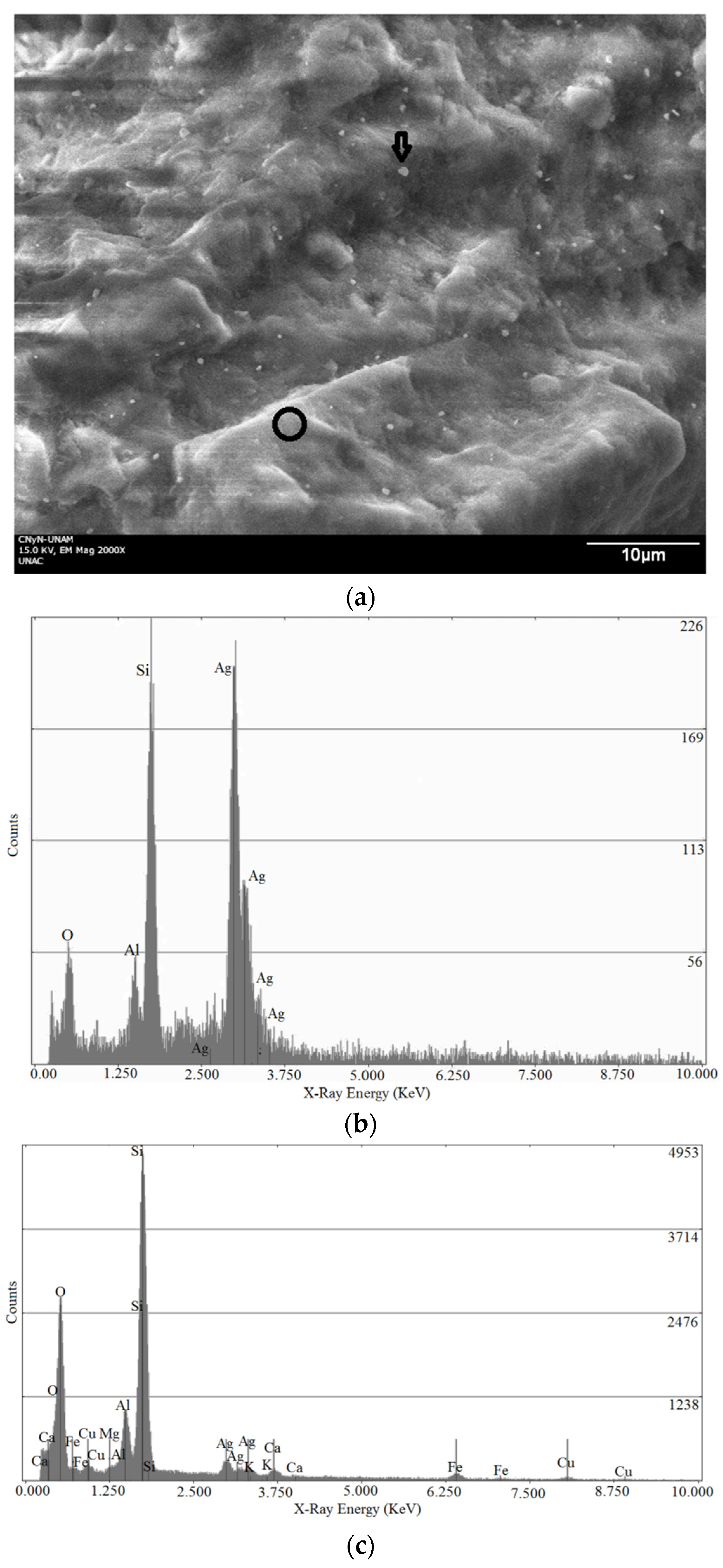
2.3. Scanning Electron Microscopy (SEM)
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jabłnńska, M.; Góra-Marek, K.; Grilc, M.; Bruzzese, P.C.; Poppitz, D.; Pyra, K.; Liebau, M.; Pöppl, A.; Likozar, B.; Gläser, R. Effect of Textural Properties and Presence of Co-Cation on NH3-SCR Activity of Cu-Exchanged ZSM-5. Catalysts 2021, 11, 843. [Google Scholar] [CrossRef]
- Wang, L.; Xu, S.; He, S.; Xiao, F.S. Rational construction of metal nanoparticles fixed in zeolite crystals as highly efficient heterogeneous catalysts. Nano Today 2018, 20, 74–83. [Google Scholar] [CrossRef]
- Farrusseng, D.; Tuel, A. Perspectives on zeolite-encapsulated metal nanoparticles and their applications in catalysis. New J. Chem. 2016, 40, 3933–3949. [Google Scholar] [CrossRef]
- Ramírez-Garza, R.E.; Rodríguez-Iznaga, I.; Simakov, A.; Farías, M.H.; Castillón-Barraza, F.F. Cu-Ag/mordenite catalysts for NO reduction: Effect of silver on catalytic activity and hydrothermal stability. Mater. Res. Bull. 2018, 97, 369–378. [Google Scholar] [CrossRef]
- Liu, J.; Liu, X.; Chen, J.; Li, X.; Ma, T.; Zhong, F. Investigation of ZrMnFe/Sepiolite Catalysts on Toluene Degradation in a One-Stage Plasma-Catalysis System. Catalysts 2021, 11, 828. [Google Scholar] [CrossRef]
- Mori, K.; Yamashita, H. Design of Colloidal and Supported Metal Nanoparticles: Their Synthesis, Characterization, and Catalytic Application. J. Jpn. Petrol. Inst. 2011, 54, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Qian, W.; Ma, H.; Zhang, H.; Ying, W. Silver-Modified Nano Mordenite for Carbonylation of Dimethyl Ether. Catalysts 2021, 11, 197. [Google Scholar] [CrossRef]
- Hessou, E.P.; Bédé, L.A.; Jabraoui, H.; Semmeq, A.; Badawi, M.; Valtchev, V. Adsorption of Toluene and Water over Cationic-Exchanged Y Zeolites: A DFT Exploration. Molecules 2021, 26, 5486. [Google Scholar] [CrossRef] [PubMed]
- Zaimee, M.Z.A.; Sarjadi, M.S.; Rahman, M.L. Heavy Metals Removal from Water by Efficient Adsorbents. Water 2021, 13, 2659. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, V.; Sharma, K.; Kumar, V.; Choudhary, S.; Mankotia, P.; Kumar, B.; Mishra, H.; Moulick, A.; Ekielski, A.; et al. A Review of Adsorbents for Heavy Metal Decontamination: Growing Approach to Wastewater Treatment. Materials 2021, 14, 4702. [Google Scholar] [CrossRef]
- Liu, J.; Yin, Q.; Zhang, H.; Yan, Y.; Yi, Z. Continuous removal of Cr(VI) and Orange II over a novel Fe0-NaA zeolite membrane catalyst. Sep. Purif. Technol. 2019, 209, 734–740. [Google Scholar] [CrossRef]
- AmilUsmani, M.; Khan, I.; Bhat, A.H.; Pillai, R.S.; Ahmad, N.; Haafiz, M.K.M.; Oves, M. Current Trend in the Application of Nanoparticles for Waste Water Treatment and Purification: A Review. Curr. Org. Synth. 2017, 14, 206–226. [Google Scholar]
- Yoshihara, K.; Nagaoka, N.; Umeno, A.; Sonoda, A.; Obika, H.; Yoshida, Y.; Van Meerbeek, B.; Makita, Y. Antibacterial Effect of Amino Acid–Silver Complex Loaded Montmorillonite Incorporated in Dental Acrylic Resin. Materials 2021, 14, 1442. [Google Scholar] [CrossRef] [PubMed]
- Bacakova, L.; Vandrovcova, M.; Kopova, I.; Jirka, I. Applications of zeolites in biotechnology and medicine—A review. Biomater. Sci. 2018, 6, 974–989. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Han, F.; Zhang, S.; Li, P.; Shang, N. Novel Bio-Based Materials and Applications in Antimicrobial Food Packaging: Recent Advances and Future Trends. Int. J. Mol. Sci. 2021, 22, 9663. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Méndez, B.G.; López-Callejas, R.; Olguín, M.T.; Valencia-Alvarado, R.; Mercado-Cabrera, A.; Peña-Eguiluz, R.; Muñoz-Castro, A.E. Growth of Ag particles from Ag-zeolite by pulsed discharges in water and their antibacterial activity. Microporous Mesoporous Mater. 2017, 244, 235–243. [Google Scholar] [CrossRef]
- Yao, G.; Lei, J.; Zhang, W.; Yu, C.; Sun, Z.; Zheng, S.; Komarneni, S. Antimicrobial activity of X zeolite exchanged with Cu2+ and Zn2+ on Escherichia coli and Staphylococcus aureus. Environ. Sci. Pollut. Res. 2019, 26, 2782–2793. [Google Scholar] [CrossRef]
- Paidari, S.; Tahergorabi, R.; Anari, E.S.; Nafchi, A.M.; Zamindar, N.; Goli, M. Migration of Various Nanoparticles into Food Samples: A Review. Foods 2021, 10, 2114. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Carrión-Mero, P.; Espinoza-Santos, N. Cation Exchange of Natural Zeolites: Worldwide Research. Sustainability 2021, 13, 7751. [Google Scholar] [CrossRef]
- Llorens, A.; Lloret, E.; Picouet, P.; Trbojevich, R.; Fernandez, A. Metallic-based micro and nanocomposites in food contact materials and active food packaging. Trends Food Sci. Technol. 2012, 24, 19–29. [Google Scholar] [CrossRef]
- Cheng, X.; Huang, L.; Yang, X.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y. Rational design of a stable peroxidase mimic for colorimetric detection of H2O2 and glucose: A synergistic CeO2/Zeolite Y nanocomposite. J. Coll. Interf. Sci. 2019, 535, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Hovhannisyan, V.; Dong, C.Y.; Chen, S.J. Photodynamic dye adsorption and release performance of natural zeolite. Sci. Rep. 2017, 7, 45503. [Google Scholar] [CrossRef] [Green Version]
- Ahari, H.; Anvar, A.A.; Ataeeand, M.; Naeimabadi, M. Employing Nanosilver, Nanocopper, and Nanoclays in Food Packaging Production: A Systematic Review. Coatings 2021, 11, 509. [Google Scholar] [CrossRef]
- Wang, L.; Gaudet, J.R.; Li, W.; Weng, D. Migration of Cu species in Cu/SAPO-34 during hydrothermal aging. J. Catal. 2013, 306, 68–77. [Google Scholar] [CrossRef]
- Umer, A.; Naveed, S.; Ramzan, N.; Rafique, M.S. Selection of a suitable method for the synthesis of copper nanoparticles. Nano 2012, 7, 1230005–1230023. [Google Scholar] [CrossRef]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Castillón-Barraza, F.; Concepción-Rosabal, B. Copper-Silver Bimetallic System on Natural Clinoptilolite: Thermal Reduction of Cu2+ and Ag+ Exchanged. J. Nanosci. Nanotechnol. 2011, 11, 5580–5586. [Google Scholar] [CrossRef]
- Bajpai, S.K.; Mohan, Y.M.; Bajpai, M.; Tankhiwale, R.; Thomas, V. Synthesis of polymer stabilized silver and gold nanostructures. J. Nanosci. Nanotechnol. 2007, 7, 2994–3010. [Google Scholar] [CrossRef]
- Petranovskii, V.; Gurin, V.; Bogdanchikova, N.; Sugi, Y. Controlling copper reducibility in mordenites by varying the SiO2/Al2O3 molar ratio. Mater. Lett. 2003, 57, 1781–1785. [Google Scholar] [CrossRef]
- Lopez-Bastidas, C.; Smolentseva, E.; Petranovskii, V.; Machorro, R. Plasmon spectra of binary Ag-Cu mixtures supported in Mordenite. In Proceedings of the SPIE Volume 9921, Plasmonics: Design, Materials, Fabrication, Characterization, and Applications XIV, San Diego, CA, USA, 17 September 2016; p. 992130. [Google Scholar]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Castillón, F.F.; Farias, M.H. Effect of the Zn(II) on the reduction of Cu(II) in natural clinoptilolita. Opt. Mater. 2006, 29, 105–109. [Google Scholar] [CrossRef]
- Petranovskii, V.; Bogdanchikova, N. Reduction of binary silver-copper ion mixture in mordenite: An example of synergetic behavior. Stud. Surf. Sci. Catal. 2002, 141, 569–574. [Google Scholar]
- Fiddy, S.; Petranovskii, V.; Ogden, S.; Rodríguez-Iznaga, I. Characterization of Binary Ag-Cu Ion Mixtures in Zeolites: Their Reduction Products and Stability to Air Oxidation. AIP Conf. Proc. 2007, 882, 631–635. [Google Scholar]
- Shelyapina, M.G.; Gurgul, J.; Łątka, K.; Sánchez-López, P.; Bogdanov, D.; Kotolevich, Y.; Petranovskii, V.; Fuentes, S. Mechanism of formation of framework Fe3+ in bimetallic Ag-Fe mordenites—Effective catalytic centers for deNOx reaction. Microporous Mesoporous Mater. 2020, 299, 109841. [Google Scholar] [CrossRef]
- Ozin, G.A.; Ozkart, S. Zeolates: A coordination chemistry view of metal-ligand bonding in zeolite guest host inclusion-compounds. Chem. Mater. 1992, 4, 511–521. [Google Scholar] [CrossRef]
- Kosinov, N.; Liu, C.; Hensen, E.J.M.; Pidko, E.A. Engineering of Transition Metal Catalysts Confined in Zeolites. Chem. Mater. 2018, 30, 3177–3198. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Tuzovskaya, I.; Pestryakov, A.; Susarrey-Arce, A.J. Comparative Study of Formation and Stabilization of Gold and Silver Clusters and Nanoparticles in Mordenites. J. Nanosci. Nanothecnol. 2011, 11, 5476–5482. [Google Scholar] [CrossRef] [PubMed]
- Smolentseva, E.; Bogdanchikova, N.; Simakov, A.; Pestryakov, A.; Avalos, M.; Farias, M.H.; Tompos, A.; Gurin, V. Catalytic activity of gold nanoparticles incorporated into modified zeolites. J. Nanosci. Nanotechnol. 2007, 7, 1882–1886. [Google Scholar] [CrossRef] [PubMed]
- Tuzovskaya, I.; Lima, E.; Bosch, P.; Bogdanchikova, N.; Pestryakov, A.; Froissart, J. Influence of Cation Nature on Stabilization of Gold Nanospecies in Mordenites. J. Nanosci. Nanothecnol. 2011, 11, 5469–5475. [Google Scholar] [CrossRef] [PubMed]
- Mikhailov, M.; Dergachev, A.; Mishin, I.; Kustov, L.; Lapidus, A. The role played by Ga-Pt nanoparticles in the aromatization of lower alkanes on ZSM-5 zeolites. Russ. J. Phys. Chem. A 2008, 82, 612–618. [Google Scholar]
- Mikhailov, M.; Loginova, A.; Kustov, L. The nature of active sites in high-silica zeolites containing platinum and cobalt nanoparticles. Russ. Chem. Bull. 2009, 58, 1598–1601. [Google Scholar] [CrossRef]
- Pârvulescu, V.I.; Grange, P.; Delmon, B. Catalytic removal of NO. Catal. Today 1998, 46, 233–326. [Google Scholar] [CrossRef]
- Rodríguez-Iznaga, I.; Petranovskii, V.; Rodríguez-Fuentes, G.; Mendoza, C.; Benitez-Aguilar, A. Exchange and reduction of Cu2+ ions in clinoptilolita. J. Coll. Interf. Sci. 2007, 316, 877–886. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, M.; Palkovits, R. Copper based catalysts for the selective ammonia oxidation into nitrogen and water vapour—Recent trends and open challenges. Appl. Catal. BEnviron. 2016, 181, 332–351. [Google Scholar] [CrossRef]
- Tan, K.S.; Cheong, K.Y. Advances of Ag, Cu, and Ag-Cu alloy nanoparticles synthesized via chemical reduction route. J. Nanopart. Res. 2013, 15, 1537. [Google Scholar] [CrossRef]
- Zhukov, Y.M.; Efimov, A.Y.; Shelyapina, M.G.; Petranovskii, V.; Zhizhin, E.V.; Burovikhina, A.; Zvereva, I.A. Effect of preparation method on the valence state and encirclement of copper exchange ions in mordenites. Microporous Mesoporous Mater. 2016, 224, 220–228. [Google Scholar] [CrossRef]
- Shelyapina, M.; Zvereva, I.; Yafarova, L.; Bogdanov, D.; Sukharzhevskii, S.; Zhukov, Y.; Petranovskii, V. Thermal analysis and EPR study of copper species in mordenites prepared by conventional and microwave-assisted methods. J. Therm. Anal. Calorim. 2018, 134, 71–79. [Google Scholar] [CrossRef]
- Concepción-Rosabal, B.; Rodríguez-Fuentes, G.; Bogdanchikova, N.; Bosch, P.; Avalos, M.; Lara, V.H. Comparative study of natural and synthetic clinoptilolites containing silver in different states. Microporous Mesoporous Mater. 2005, 86, 249–255. [Google Scholar] [CrossRef]
- Database of Zeolite Structures. Available online: http://www.iza-structure.org/databases/ (accessed on 31 October 2021).
- Baerlocher, C.; McCusker, L.B.; Olson, D.H. Atlas of Zeolite Framework Types, 6th ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 156–157. [Google Scholar]
- Itzel-Hernandez, G.; Hernandez, M.A.; Portillo, R.; Rubio, E.; Petranovskii, V.P.; Pestryakov, A.N. Hierarchical structure of nanoporosity of Mexican natural zeolites of clinoptilolite type. Bull. Tomsk Polytech. Univ. Geo Assets Eng. 2018, 329, 107–117. [Google Scholar]
- Gurin, V.S.; Petranovskii, V.; Hernandez, M.A.; Bogdanchikova, N.E.; Alexeenko, A.A. Silver and copper clusters and small particles stabilized within nanoporous silicate-based materials. Mater. Sci. Engineer. A 2005, 391, 71–76. [Google Scholar] [CrossRef]
- Petranovskii, V.; Gurin, V.; Machorro, R. Spectroscopic observation and ab initio simulation of copper clusters in zeolites. Catal. Today 2005, 107–108, 892–900. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.; Bogdanchikova, N. Metal clusters and nanoparticles assembled in zeolites: An example of stable materials with controllable particle size. Mater. Sci. Engineer. C 2002, 19, 327–331. [Google Scholar] [CrossRef]
- Bogdanchikova, N.; Petranovskii, V.; Machorro, R.; Sugi, Y.; Soto, V.M.; Fuentes, S. Stability of silver clusters in mordenites with different SiO2/Al2O3 molar ratio. Appl. Surf. Sci. 1999, 150, 58–64. [Google Scholar] [CrossRef]
- Gurin, V.S.; Petranovskii, V.P.; Bogdanchikova, N.E. Silver and copper nanostructures within the erionite regular lattice: Interplay between intra- and extra-crystalline locations. Mater. Sci. Engineer. C 2003, 23, 81–85. [Google Scholar] [CrossRef]
- Claridge, S.A.; Castleman, A.W.; Khanna, S.N.; Murray, C.B.; Sen, A.; Weiss, P.S. Cluster-Assembled Materials. ACS NANO 2009, 3, 244–255. [Google Scholar] [CrossRef]
- Cui-Ju, F.; Xiao-yan, Z. Structures and Electronic Properties of Cu-N (N ≤ 13) Clusters. Commun. Theor. Phys. 2009, 52, 675–680. [Google Scholar] [CrossRef]
- Kabir, M.; Mookerjee, A.; Bhattacharya, A.K. Structure and stability of copper clusters: A tight-binding molecular dynamics study. Phys. Rev. A 2004, 69, 043203–043213. [Google Scholar] [CrossRef] [Green Version]
- Koitz, R.; Soini, T.M.; Genest, A.; Trickey, S.B.; Rosch, N. Scalable properties of metal clusters: A comparative study of modern exchange-correlation functional. J. Chem. Phys. 2012, 137, 034102–034111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Ning, X.J. Isomers of copper clusters obtained by a molecular dynamics model. Int. J. Mod. Phys. B 2005, 19, 2359–2364. [Google Scholar] [CrossRef]
- Pestryakov, A.N.; Lunin, V.V.; Devochkin, A.N.; Petrov, L.A.; Bogdanchikova, N.E.; Petranovskii, V. Selective oxidation of alcohols over foam-metal catalysts. Appl. Catal. A 2002, 227, 125–130. [Google Scholar] [CrossRef]
- Valodkar, M.; Modi, S.; Pal, A.; Thakore, S. Synthesis and anti-bacterial activity of Cu, Ag and Cu-Ag alloy nanoparticles: A green approach. Mater. Res. Bull. 2011, 46, 384–389. [Google Scholar] [CrossRef]
- Momin, T.; Bhowmick, A. Nanoscale alloying: A study in free Cu-Ag bimetallic clusters. J. Alloy. Compd. 2013, 559, 24–33. [Google Scholar] [CrossRef]
- Zhang, P.; Xie, Y.; Zhang, W.; Ning, X.; Zhuang, J. Different magic number behaviors in supported metal clusters. J. Nanopart. Res. 2011, 13, 1801–1807. [Google Scholar] [CrossRef]
- Pacioni, N.L.; Borsarelli, C.D.; Rey, V.; Veglia, A.V. Synthetic Routes for the Preparation of Silver Nanoparticles. In Silver Nanoparticle Applications. Engineering Materials; Alarcon, E., Griffith, M., Udekwu, K., Eds.; Springer International Publishing Switzerland: Cham, Switzerland, 2015; pp. 13–46. [Google Scholar]

| Metal | Ag | Cu | ||
|---|---|---|---|---|
| 2θ, [°] | 38.12° | 44.29° | 43.2° | 50.3° |
| Samples | IS/IQ | IS/IQ | ||
| Cu3Ag1CLIHydrot-150-F | 1.360 | 0.573 | 0 | 0 |
| Cu3Ag1CLIHydrot-150-A | 0.608 | 0.400 | 0 | 0 |
| (Ratio Aged/Fresh) | (0.45) | (0.70) | (0) | (0) |
| Cu3Ag1CLI-150-F | 1.270 | 0.395 | 0 | 0 |
| Cu3Ag1CLI-150-A | 0.486 | 0.222 | 0 | 0 |
| (Ratio Aged/Fresh) | (0.38) | (0.56) | (0) | (0) |
| Cu9Ag1CLI-150-F | 1.017 | 0.625 | 0 | 0 |
| Cu9Ag1CLI-150-A | 0.384 | 0.246 | 0 | 0 |
| (Ratio Aged/Fresh) | (0.38) | (0.39) | (0) | (0) |
| Cu3Ag1CLIHydrot-450-F | 3.476 | 1.428 | 0.857 | 0.542 |
| Cu3Ag1CLIHydrot-450-A | 2.680 | 1.085 | 0.744 | 0.382 |
| (Ratio Aged/Fresh) | (0.77) | (0.76) | (0.87) | 0.70 |
| Cu3Ag1CLI-450-F | 3.460 | 1.180 | 0.540 | 0.400 |
| Cu3Ag1CLI-450-A | 1.666 | 0.637 | 0.289 | 0.246 |
| (Ratio Aged/Fresh) | (0.48) | (0.54) | (0.53) | 0.61 |
| Cu9Ag1CLI-450-F | 2.519 | 0.923 | 0.857 | 0.452 |
| Cu9Ag1CLI-450-A | 1.416 | 0.633 | 0.744 | 0.382 |
| (Ratio Aged/Fresh) | (0.56) | (0.68) | (0.87) | (0.84) |
| Samples | CuCLI | Cu3Ag1CLIHydrot | Cu3Ag1CLI | Cu9Ag1CLI |
|---|---|---|---|---|
| Cu (wt%) | 3.40 | 3.16 | 1.44 | 1.50 |
| Ag (wt%) | - | 3.71 | 3.80 | 2.23 |
| Temperature | 100 °C | 100 °C | room | room |
| Solution | Cu(NO3)2 | Cu(NO3)2-AgNO3 | Cu(NO3)2-AgNO3 | Cu(NO3)2-AgNO3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Iznaga, I.; Petranovskii, V.; Chávez-Rivas, F.; Shelyapina, M.G. Bimetallic Copper-Silver Systems Supported on Natural Clinoptilolite: Long-Term Changes in Nanospecies’ Composition and Stability. Inorganics 2022, 10, 34. https://doi.org/10.3390/inorganics10030034
Rodríguez-Iznaga I, Petranovskii V, Chávez-Rivas F, Shelyapina MG. Bimetallic Copper-Silver Systems Supported on Natural Clinoptilolite: Long-Term Changes in Nanospecies’ Composition and Stability. Inorganics. 2022; 10(3):34. https://doi.org/10.3390/inorganics10030034
Chicago/Turabian StyleRodríguez-Iznaga, Inocente, Vitalii Petranovskii, Fernando Chávez-Rivas, and Marina G. Shelyapina. 2022. "Bimetallic Copper-Silver Systems Supported on Natural Clinoptilolite: Long-Term Changes in Nanospecies’ Composition and Stability" Inorganics 10, no. 3: 34. https://doi.org/10.3390/inorganics10030034
APA StyleRodríguez-Iznaga, I., Petranovskii, V., Chávez-Rivas, F., & Shelyapina, M. G. (2022). Bimetallic Copper-Silver Systems Supported on Natural Clinoptilolite: Long-Term Changes in Nanospecies’ Composition and Stability. Inorganics, 10(3), 34. https://doi.org/10.3390/inorganics10030034






