Abstract
The chromium-benzenedicarboxylate metal–organic framework (MOF), MIL-101(Cr), is one of the most well-investigated and widely used prototypical MOFs. Regarding its synthesis, the use of a toxic modulator (usually HF) and high reaction temperature (220 °C) are the main factors hindering its further expansion of production and utilization. In fact, high quality MIL-101(Cr) crystals can be prepared at a much lower temperature (160 °C) with spherical morphology via an additive-free approach. Compared to traditional octahedral MIL-101(Cr), the spherical MIL-101(Cr) possesses higher adsorption performance toward dye molecules, including methyl orange (MO) and rhodamine B (RB). The results suggest that toxic additives and high reaction temperatures are not essential in the synthesis of MIL-101(Cr), and the fabrication of spherical MIL-101(Cr) may offer a facile and effective pathway for the large-scale industrial application of MIL-101(Cr).
1. Introduction
Metal–organic frameworks (MOFs) are a kind of three-dimensional porous crystalline material which are formed by metal ions or metal clusters with organic ligands [1,2]. Compared to traditional porous crystalline materials, MOFs have the characteristics of high specific surface area [3], large porosity [4], and good chemical/thermal stability [5]. In addition, most MOFs have the advantages of mild reaction conditions, simple synthesis approaches, and easy availability of raw materials. Therefore, MOFs have broader application prospects than traditional porous materials, such as gas storage/separation [6,7], energy storage/conversion [8,9], drug delivery [10,11], catalysis [12,13], fluorescence [14,15], magnetic [16], clinical diagnosis [17], chemical sensors [18], etc.
MIL-101(Cr) is one of the most representative porous materials among chromium-based MOFs. It has a high pore volume, large specific surface area, and excellent chemical/water stability, with an empirical formula [Cr3(O)X(BDC)3(H2O)2] (where BDC is terephthalic acid and X is OH− or F−) [19]. Its structure is similar to MTN zeolite topology by the coordination of Cr3O ion clusters and terephthalate (BDC2−). MIL-101(Cr) has two types of mesoporous cage cavities with diameters of 29 Å and 34 Å (Figure S1), separately, and the max aperture window can reach 16 Å, with a high specific BET surface area of 4100 m2 g−1. The terminal connected water molecules of MIL-101(Cr) can be removed under high temperature or vacuum conditions, which creates potential Lewis acid sites [20]. Hence, MIL-101(Cr) demonstrates quite good water stability, which makes it suitable for wide applications, especially in the presence of moisture/water conditions, such as catalysis [21,22], adsorption [23,24], separation [25,26], drug delivery [27,28], gas storage [29,30], water pollution treatment [31], and mixed matrix membranes [32,33], among others.
The traditional synthesis of MIL-101(Cr) reports an additive-free route, but also the adding of an equimolar amount of hydrofluoric acid (HF) to Cr3+ and H2BDC, and the products are isostructural [19]. The HF route shows better crystallinity and porosity for the products; thus, most of the other groups follow the HF approach to synthesize MIL-101(Cr) [19,34,35,36,37]. However, both of the above reaction routes occur at 220 °C, the yield are relatively low (~50%), and the added HF is a chemical toxicant, which are not advantageous for large-scale industrial syntheses [38]. Thus, the price of high-quality commercial MIL-101(Cr) products can reach over 570 $/g, which is much higher than other commonly used MOFs (such as ZIFs, UiOs, HKUST-1, etc.). The detailed price list is shown in Table S1.
In the previous study, most of the researchers followed the HF route to prepare MIL-101(Cr) by Férey et al. [19]. Apart from HF, the researchers also tried to use other modulators to replace HF in MIL-101(Cr) synthesis, including acetic acid, nitric acid, hydrochloric acid, sulfuric acid, sodium hydroxide, benzoic acid, sodium acetate, tetramethylammonium hydroxide, etc. [36,39,40,41,42,43,44] (see details in Table S2). Nevertheless, all these procedures still need extra additives, conducted at high temperature, and contain tedious purification processes. Conversely, the study of additive-free synthesis for MIL-101(Cr) is ignored, because of its low crystallinity and low BET surface area in the original report by Férey et al. [19].
Herein, we carefully investigated the fluorine-free route of MIL-101(Cr) synthesis via hydrothermal reaction at various temperatures (220–160 °C). The results showed that high-quality MIL-101(Cr) products also can be prepared at temperatures as low as 160 °C with spherical morphology, which possessed high specific BET surface area (3021 m2 g−1) and quite good yield (>52%). More importantly, among all samples, spherical MIL-101(Cr) exhibited the highest adsorption capacity toward dyes MO and Rhodamine B (RB), with maximum uptakes of 444.3 mg g−1 and 230.3 mg g−1, respectively.
2. Experimental
2.1. Materials and Reagents
Cr(NO3)3·9H2O (99.5%, AR) and terephthalate (H2BDC, 99%, AR), were bought from Aladdin Chemical Reagent Co., Ltd. (Shanghai, China). N,N-Dimethylformamide (DMF, 99.5%, AR), and Ethanol (99.7%, AR) was acquired from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
2.2. Additive-Free Synthesis of MIL-101(Cr)
MIL-101(Cr) was synthesized by hydrothermal method. Typically, Cr(NO3)3·9H2O (0.8 g) and H2BDC (0.332 g) were dissolved in distillated water (10 mL) and stirred for 30 min. Then the mixture was placed in a 25 mL autoclave and heated at different temperatures (220–160 °C) for 8 h. When the reaction was complete, the autoclave was cooled down to room temperature naturally. The green solids were collected and washed with DMF and ethanol twice (8 h) with stirring. The final products were dried under vacuum at 120 °C for 2 h and labeled as 220-MIL-101, 200-MIL-101, 180-MIL-101, and 160-MIL-101 separately (the numbers stand for the reaction temperatures).
2.3. Characterization
The micromorphologies of the samples were characterized using Zeiss Gemini 300 scanning electron microscopy. Transmission electron microscopy (TEM) was performed on an FEI Talos F200X electron microscope. The BET specific surface areas of the samples were measured with N2 adsorption isotherms on a specific surface analyzer (NOVA-4200e, Quantachrome, Boynton Beach, FL, USA). X-ray diffraction (XRD) measurements were performed using a Bruker D8 Advance X-ray diffractometer with CuKα radiation (λ = 1.5418 Å), operating at 30 kV, with a scanning speed of 2 (°)/min and a scanning range of 5–50°. Dye concentrations of the test samples were obtained by UV–vis spectrophotometer (UV-2600, Shimadzu, Kyoto, Japan).
2.4. Dye Adsorption Experiment
The adsorption capacities of the products were assessed by MO and RB. Dye adsorption experiments were conducted by adding 5 mg of adsorbent into 10 mL of different initial concentrations of dye solutions at room temperature (298 K).
The concentrations of remaining MO and RB in the solution were explored using a UV–vis spectrophotometer at wavelengths of 300 and 700 nm, separately. The amount of adsorbent to dye (, mg g−1) was calculated according to Equation (1) [45]
where C0 is the original concentration of the dye (mg L−1), Ce is the remaining concentration of the dye (mg L−1), V is the volume of the dye solution (L), and m (g) is the mass of the adsorbent.
2.5. Adsorption Kinetic Model
To investigate the process of dye adsorption by the adsorbent, pseudo-first-order and pseudo-second-order kinetic models were adopted to fit the adsorption data [46,47], respectively. The linear expressions of the pseudo-first-order kinetic equation and the pseudo-second-order kinetic equation were shown in Equations (2) and (3) [48,49]
where qt is the adsorption capacity at time t (h), and the k1 (min−1) and k2 (g mg−1 min−1) are adsorption rate constant of the pseudo-first-order and pseudo-second-order kinetics models, respectively.
2.6. Adsorption Isotherm Model
The Langmuir isotherm adsorption model assumed that adsorption occurred uniformly at all active sites of the adsorbent and that the dye was adsorbed as a monolayer on its surface [50]. The Freundlich isotherm adsorption model assumed that the surface energy of the adsorbent was not uniform and that the dye was not adsorbed in a single monolayer on the adsorbent surface [51]. The Tempkin adsorption isotherm model assumed a linear decreased heat of adsorption of the dye by the adsorbent [52]. These isotherm adsorption models equations are shown as follows [53,54,55].
where qm (mg g−1) and kL (L mg−1) represent the maximum adsorption capacity of adsorbents and Langmuir constant, separately; the kF [(mg g−1)(L mg−1)1/n] and n are Freundlich constants representing adsorption capacity and adsorption intensity, separately; B = (RT/b) is related to the heat of adsorption, and A (L g−1) is the equilibrium constant.
2.7. Cyclic Adsorption Experiment
After the model dye was adsorbed, 20 mg of adsorbent was washed ultrasonically with 20 mL of ethanol for 30 min and centrifuged 5 times, then dried at 120 °C (under vacuum) for 2 h. The purified adsorbent was then used for the next adsorption process.
3. Results and Discussion
3.1. Characterization of Samples
The TEM and SEM images of the sample are shown in Figure 1. The morphologies of 220-MIL-101, 200-MIL-101, and 180-MIL-101 were regularly octahedral crystals. According to the particle size distribution (Figure S2), it could be seen that 220-MIL-101 possessed the largest particle size about 382 nm, while the particles of 200-MIL-101 (153 nm) and 180-MIL-101 (215 nm) were much smaller. Therefore, 160-MIL-101 revealed a spherical morphology with an average particle size of about 265 nm. Because the crystal formation process was controlled by kinetic factors, that shape is the final morphological structure. Under low-temperature reaction conditions (160 °C), the surface energy of crystal growth was insufficient, the morphology would not be able to form an angled regular octahedron and cause spherical crystals.
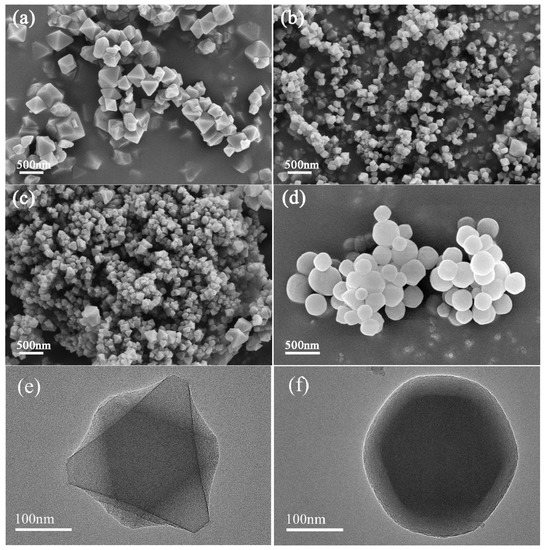
Figure 1.
SEM images of (a) 220-MIL-101, (b) 200-MIL-101, (c) 180-MIL-101, and (d) 160-MIL-101. HRTEM images of (e) 200-MIL-101 and (f) 160-MIL-101.
The XRD patterns of 220-MIL-101, 200-MIL-101, 180-MIL-101, and 160-MIL-101 were consistent with the simulated MIL-101 pattern, which confirmed that all samples were MIL-101(Cr) (Figure 2). The low-angle region of the PXRD patterns also gave an additional confirmation that all the MIL-101(Cr) compounds are practically pure (Figure S3).
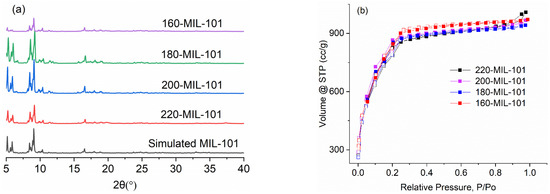
Figure 2.
(a) XRD patterns of the samples, (b) the N2 adsorption–desorption isotherms of the samples.
The N2 adsorption–desorption isotherms of 220-MIL-101, 200-MIL-101, 180-MIL-101, and 160-MIL-101 were presented in Figure 2. 160-MIL-101 showed the highest BET surface area of 3021 m2 g−1, 200-MIL-101 and 180-MIL-101 showed slightly smaller BET surface areas of 2926 and 2909 m2 g−1, respectively. While 220-MIL-101, which followed the initial literature [19], displayed the lowest BET surface area of 2862 m2 g−1 (Table 1). Interestingly, the yields of all samples were over 50%, which was higher than the reported HF route (~50%) [32,33,34]. Most notably, with 200-MIL-101, the yield was above 95%, which might be the highest value ever reported. Hence, the additive-free route for MIL-101(Cr) at lower temperatures could be considered as an alternative for industrial production.

Table 1.
Yield, particle size, surface area, and pore volume of samples.
3.2. Adsorption Capacity of Spherical MIL-101(Cr) for Dyes
Currently, dyes are widely used in daily life—in applications such as food industry, packaging, and printing—and nearly 10–15% of the consumed dyes are directly discharged into the aqueous environment [56,57]. The dumped dyes not only compromise water quality, but they also do great harm to human health [58,59]. Thus, it is important to develop valid technologies for the removal of dyes from wastewater before being discharged into the environment. The adsorption method is considered one of the most promising removal strategies, due to its high efficiency, lower secondary pollution, and low cost [59,60]. MIL-101(Cr), a kind of high water/chemical MOF with high porosity and large pore volume, meets the demands of dye adsorption applications as an adsorbent. For instance, MIL-101(Cr) showed high adsorption efficiency toward MO in an aqueous solution [61]. Yang et al. reported that MIL-101(Cr) can be used as an adsorbent for the removal of Congo red (CR) [62]. Chen et al. found that MIL-101(Cr) exhibited high uptake toward xylenol orange (XO) [63].
Herein, we select methyl orange (MO) and rhodamine B (RB) as the model compounds for the adsorption experiments of MIL-101(Cr)s. Obviously, spherical MIL-101(Cr) (160-MIL-101) exhibited the best adsorption capabilities toward MO and RB among all samples, with maximum uptakes of 444.3 mg g−1 and 230.3 mg g−1, respectively (Figure S4). Especially for the adsorption of MO, the uptake of 160-MIL-101 is significantly higher than that of other adsorbents which have been reported in recent years (Table 2) [64,65,66,67,68,69,70,71,72]. The uptake of 160-MIL-101 is also significantly higher than other MIL-101 and MIL-101 composites from several studies (Table S3) [23,61,73,74,75,76,77,78].

Table 2.
Adsorption capacities (qmax) of MO on several adsorbents.
Figure 3 revealed the maximum adsorption capabilities of 160-MIL-101 toward MO and RB. Absolutely, 160-MIL-101 presented a higher adsorption capability of MO than that of RB. This result implied that the adsorption performance was dye-specific and also closely associated with the MIL-101(Cr) characteristics and the molecular dye structures. The structures of MO and RB were shown in Figure S5, MO was anionic dye, whereas MB was cationic. The RB molecule possessed more aromatic rings than MO, while MO had a smaller molecular size in comparison with RB. The zeta potential of the spherical MIL-101(Cr) crystallite was 19.1 mv (Figure S6), which indicated that the surfaces of 160-MIL-101 were positively charged. Thus, the difference could significantly affect the π-π interactions, electrostatic interactions, and steric hindrance between the MIL-101(Cr)s and the dyes (Figure 4).
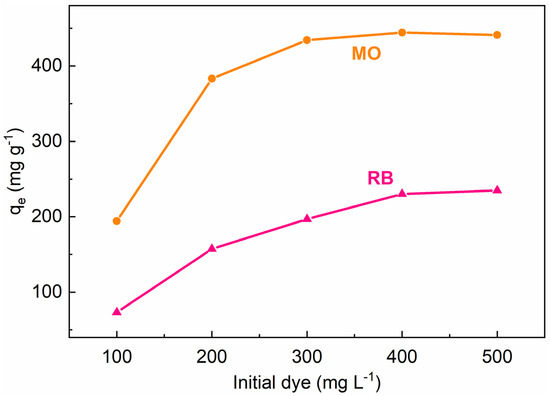
Figure 3.
Adsorption uptakes of 160-MIL-101 toward MO and RB after 24 h, as determined by dispersing 5 mg of 160-MIL-101 in the aqueous dye solution (100 mg L−1 to 500 mg L−1) at ambient temperature (25 °C).
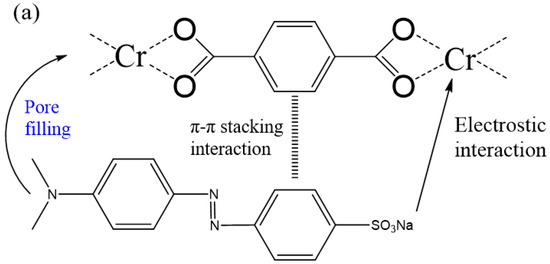
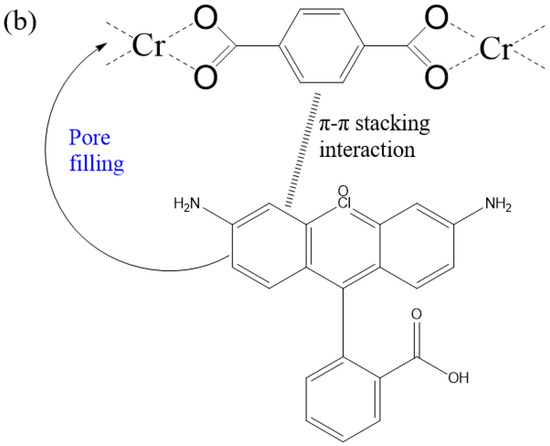
Figure 4.
Possible mechanism of synergetic interplay for (a) MO and (b) RB adsorption on 160-MIL-101.
The possible mechanism of improvement of MO adsorption by spherical MIL-101(Cr) may be attributed to its higher surface area and the positive charge may be more evenly distributed on the surface. The former could significantly increase the adsorption capability of MO, while the latter is also conducive to promoting adsorption of anionic MO. Thus, in our case, the spherical MIL-101(Cr) (160-MIL-101) presented higher adsorption capability of MO than other octahedral MIL-101(Cr).
The time-dependent adsorption capacity of 160-MIL-101 for dyes was investigated, as shown in Figure S7. The adsorption kinetics curves which were fitted with Equations (2) and (3) were displayed in Figure 5, and the corresponding parameters are listed in Table S4. Obviously, the correlation coefficients (i.e., R2 values) of the pseudo-second-order kinetics model were higher than those of the pseudo-first-order model, and the theoretical equilibrium adsorption capacity, theoretical qe, was highly consistent with the experimental data (Table S4). Thus, it is indicated that the adsorption of MO and RB by 160-MIL-101(Cr) was mainly a pseudo-second-order procedure.
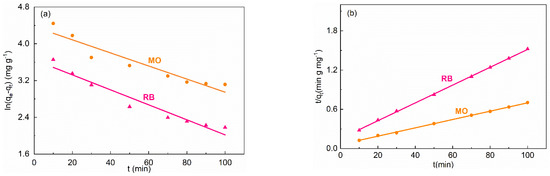
Figure 5.
(a) Pseudo-first-order and (b) pseudo-second-order of the kinetics data.
The Langmuir, Freundlich, and Tempkin isotherm models were employed to fit the experimental data (the models were described in Experimental 2.6). Figure 6 revealed the equilibrium adsorption isotherms for MO and RB, and the related fitting parameters were listed in Table S4. For the adsorption of MO and RB by 160-MIL-101, the experimental adsorption data were in good agreement with the Langmuir isotherm model, especially in the case of MO, the correlation regression coefficient of R2 was over 0.99, which was higher than that of Temkin and Freundlich models (Figure 6, Table S4). Moreover, according to the Langmuir model, the theoretical maximum adsorption capability of 160-MIL-101 toward MO was in line with the experimental data (Table S4).
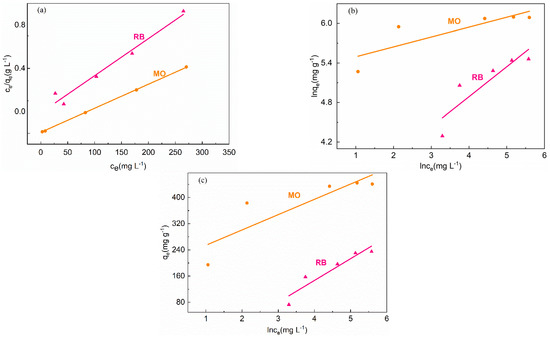
Figure 6.
Experimental data for the adsorption of dyes on160-MIL-101 analyzed using (a) Langmuir, (b) Freundlich, and (c) Temkin models.
The regeneration and reusability of the MIL-101(Cr) adsorbent will be important for the industrial applications. Thus, the regeneration of 160-MIL-101 adsorbent was investigated by using ethanol as an extracting regenerating agent. After five successive adsorption–desorption cycles, the adsorption uptake of 160-MIL-101 toward MO decreased by <10%, indicating that MIL-101(Cr) exhibited excellent reusability toward dye adsorption (Figure S8).
4. Conclusions
In conclusion, we have demonstrated an additive-free strategy for control of the morphology of MIL-101(Cr) by hydrothermal synthesis at relatively lower temperatures. Spherical MIL-101(Cr) could be obtained at a relatively lower temperature at 160 °C. It showed good adsorption capacity toward MO (444.3 mg g−1) and RB (230.3 mg g−1). The analyzed results suggested that a plausible adsorption mechanism was attributed to the synergetic interplay based on porosity, π-π interactions, electrostatic interactions, and steric hindrance influence. Hence, an alternative strategy for the industrial fabrication of MIL-101(Cr) is proposed, with potential for the extension of the application of MIL-101(Cr) in effluent treatment fields, mixed matrix membranes, and other contexts.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/inorganics10030033/s1, Figure S1: Building blocks for MIL-101(Cr); Figure S2: Particle size distribution of samples; Figure S3: The low-angle region of the PXRD patterns of all MIL-101(Cr)s; Figure S4: The adsorption capabilities of all MIL-101(Cr)s toward MO and RB; Figure S5: Dye molecule diagram, (a) MO and (b) RB; Figure S6: Zeta potential of 160-MIL-101 directly dispersed in water; Figure S7: Impact of the time on the adsorption of (a) MO and (b) RB onto 160-MIL-101; Figure S8: Recycle of the removal efficiency of 160-MIL-101 for MO; Table S1: Price parameters of MIL-101(Cr) sold by various manufacturers; Table S2: BET specific surface area, pore volume and yield of MIL-101(Cr) synthesized by various additives; Table S3: Adsorption capacities (qmax) of MO on other MIL-101 and MIL-101 composite; Table S4: Characteristic parameters of the adsorption of dyes on the 160-MIL-101.
Author Contributions
Conceptualization, T.Z.; Methodology, T.Z. and X.L.; Formal Analysis, M.Z., S.T. and M.L.; Investigation, H.Z., M.D. and M.Z.; Resources, T.Z. and X.L.; Writing—Original Draft Preparation, H.Z. and M.D.; Writing—Review & Editing, Supervision and Funding Acquisition, T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
The National Natural Science Foundation of China (51802094, 51927810); the Science and Technology Program of Hunan Province, China (2018RS3084) and the Research Foundation of Education Department of Hunan Province, China (18B288).
Acknowledgments
The work was supported by the National Natural Science Foundation of China (51802094, 51927810); the Science and Technology Program of Hunan Province, China (2018RS3084); the Research Foundation of Education Department of Hunan Province, China (18B288).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen storage in microporous metal-organic frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, H.; Ko, N.; Go, Y.B.; Aratani, N.; Choi, S.B.; Choi, E.; Yazaydin, A.Ö.; Snurr, R.Q.; O’Keeffe, M.; Kim, J.; et al. Ultrahigh porosity in metal-organic frameworks. Science 2010, 329, 424–428. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, H.; Ai, J.; Chen, J.; Du, M. Optimizing strategy for enhancing the stability and 99TcO4− sequestration of poly(ionic liquids)@MOFs composites. ACS Cent. Sci. 2020, 6, 2354–2361. [Google Scholar] [CrossRef]
- Fan, W.; Wang, X.; Xu, B.; Wang, Y.; Liu, D.; Zhang, M.; Shang, Y.; Dai, F.; Zhang, L.; Sun, D. Amino-functionalized MOFs with high physicochemical stability for efficient gas storage/separation, dye adsorption and catalytic performance. J. Mater. Chem. A 2018, 6, 24486–24495. [Google Scholar] [CrossRef]
- Jayaramulu, K.; Geyer, F.; Schneemann, A.; Kment, Š.; Otyepka, M.; Zboril, R.; Vollmer, D.; Fischer, R.A. Hydrophobic metal-organic frameworks. Adv. Mater. 2019, 31, 1900820–1900850. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, A.; Zhong, M.; Zhang, Z.; Zhang, X.; Zhou, Z.; Bu, X.-H. Metal-organic frameworks (MOFs) and MOF-derived materials for energy storage and conversion. Electrochem. Energy Rev. 2018, 2, 29–104. [Google Scholar] [CrossRef]
- Nie, X.; Kulkarni, A.; Sholl, D.S. Computational prediction of metal organic frameworks suitable for molecular infiltration as a route to development of conductive materials. J. Phys. Chem. Lett. 2015, 6, 1586–1591. [Google Scholar] [CrossRef]
- Lin, C.; He, H.; Zhang, Y.; Xu, M.; Tian, F.; Li, L.; Wang, Y. Acetaldehyde-modified-cystine functionalized Zr-MOFs for pH/GSH dual-responsive drug delivery and selective visualization of GSH in living cells. RSC Adv. 2020, 10, 3084–3091. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, H.; Shi, W.; Cheng, P. Synthesis strategies and potential applications of metal-organic frameworks for electrode materials for rechargeable lithium ion batteries. Coord. Chem. Rev. 2019, 388, 293–309. [Google Scholar] [CrossRef]
- Rivera-Torrente, M.; Mandemaker, L.D.B.; Filez, M.; Delen, G.; Seoane, B.; Meirer, F.; Weckhuysen, B.M. Spectroscopy, microscopy, diffraction and scattering of archetypal MOFs: Formation, metal sites in catalysis and thin films. Chem Soc. Rev. 2020, 49, 6694–6732. [Google Scholar] [CrossRef] [PubMed]
- Alzamly, A.; Bakiro, M.; Hussein Ahmed, S.; Alnaqbi, M.A.; Nguyen, H.L. Rare-earth metal-organic frameworks as advanced catalytic platforms for organic synthesis. Coord. Chem. Rev. 2020, 425, 213543–213565. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, X.; Li, P.; Zhang, W.; Wang, H.; Tang, B. Evaluating hyperthyroidism-induced liver injury based on in situ fluorescence imaging of glutathione and phosphate via Nano-MOFs sensor. Anal. Chem. 2020, 92, 8952–8958. [Google Scholar] [CrossRef]
- Ren, K.; Guo, X.; Tang, Y.; Huang, B.; Wang, H. Size-controlled synthesis of metal-organic frameworks and their performance as fluorescence sensors. Analyst 2020, 145, 7349–7356. [Google Scholar] [CrossRef]
- Wu, Z.; Gu, A.; Gao, N.; Cui, H.; Wang, W.; Cui, J. Solvent-dependent assembly and magnetic relaxation behaviors of [Cu4I3] cluster-based lanthanide MOFs: Acting as efficient catalysts for carbon dioxide conversion with propargylic alcohols. Inorg. Chem. 2020, 59, 15111–15119. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Wang, D.; Sheng, N.; Zhang, G.; Yin, L.; Sha, J. Development of a uricase-free colorimetric biosensor for uric acid based on PPy-coated polyoxometalate-encapsulated fourfold helical metal-organic frameworks. ACS Biomater. Sci. Eng. 2020, 6, 1438–1448. [Google Scholar] [CrossRef]
- Yang, F.; Yang, G.; Wu, Y.; Yan, Y.; Liu, J.; Gao, R.; Zhang, W.; Wang, Y. Ln(III)-MOFs (Ln=Tb, Eu, Dy, and Sm) based on triazole carboxylic ligand with carboxylate and nitrogen donors with applications as chemical sensors and magnetic materials. J. Coord. Chem. 2018, 71, 2702–2713. [Google Scholar] [CrossRef]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F. A Chromium Terephthalate-Based Solid with Unusually Large Pore Volumes and Surface Area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef]
- Hong, D.; Hwang, Y.K.; Serre, C.; Férey, G.; Chang, J. Porous Chromium Terephthalate MIL-101 with Coordinatively Unsaturated Sites: Surface Functionalization, Encapsulation, Sorption and Catalysis. Adv. Funct. Mater. 2009, 19, 1537–1552. [Google Scholar] [CrossRef]
- Niknam, E.; Panahi, F.; Khalafi-Nezhad, A. Immobilized Pd on a NHC functionalized metal-organic framework MIL-101(Cr): An efficient heterogeneous catalyst in Suzuki−Miyaura coupling reaction in water. Appl. Organomet. Chem. 2020, 34, 5470. [Google Scholar] [CrossRef]
- Oudi, S.; Oveisi, A.R.; Daliran, S.; Khajeh, M.; Teymoori, E. Brønsted-Lewis dual acid sites in a chromium-based metal-organic framework for cooperative catalysis: Highly efficient synthesis of quinazolin-(4H)-1-one derivatives. J. Colloid Interface Sci. 2020, 561, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yao, T.; Xiang, Y.; Zou, H.; Zhou, Y. Efficient removal of methyl orange by a flower-like TiO2/MIL-101(Cr) composite nanomaterial. Dalton Trans. 2020, 49, 5722–5729. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, J.; Qi, X.; Zhao, Z.; Sun, H. Experimental study on activated carbon-MIL-101(Cr) composites for ethanol vapor adsorption. Materials 2021, 14, 3811. [Google Scholar] [CrossRef]
- Song, N.; Sun, Y.; Xie, X.; Wang, D.; Shao, F.; Yu, L.; Dong, L. Doping MIL-101(Cr)@GO in polyamide nanocomposite membranes with improved water flux. Desalination 2020, 492, 114601–114607. [Google Scholar] [CrossRef]
- Kavun, V.; van der Veen, M.A.; Repo, E. Selective recovery and separation of rare earth elements by organophosphorus modified MIL-101(Cr). Microporous Mesoporous Mater. 2021, 312, 110747. [Google Scholar] [CrossRef]
- Latifi, L.; Sohrabnezhad, S. Drug delivery by micro and meso metal-organic frameworks. Polyhedron 2020, 180, 114321. [Google Scholar] [CrossRef]
- Markopoulou, P.; Panagiotou, N.; Li, A.; Bueno-Perez, R.; Madden, D.; Buchanan, S.; Fairen-Jimenez, D.; Shiels, P.G.; Forgan, R.S. Identifying differing intracellular cargo release mechanisms by monitoring in vitro drug delivery from MOFs in real time. Cell Rep. Phys. Sci. 2020, 1, 100254. [Google Scholar] [CrossRef]
- Hong, W.Y.; Perera, S.P.; Burrows, A.D. Comparison of MIL-101(Cr) metal-organic framework and 13X zeolite monoliths for CO2 capture. Microporous Mesoporous Mater. 2020, 308, 110525. [Google Scholar] [CrossRef]
- Sáenz Cavazos, P.A.; Díaz-Ramírez, M.L.; Hunter-Sellars, E.; McIntyre, S.R.; Lima, E.; Ibarra, I.A.; Williams, D.R. Fluorinated MIL-101 for carbon capture utilisation and storage: Uptake and diffusion studies under relevant industrial conditions. RSC Adv. 2021, 11, 13304–13310. [Google Scholar] [CrossRef]
- Sun, Y.; Liang, J.; Brandt, P.; Spieß, A.; Öztürk, S.; Janiak, C. Cucurbit[6]uril@MIL-101-Cl: Loading polar porous cages in mesoporous stable host for enhanced SO2 adsorption at low pressures. Nanoscale 2021, 13, 15952–15962. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Ferreira, T.J.; Barbosa, A.D.S.; de Castro, B.; Ribeiro, R.P.P.L.; Mota, J.P.B.; Alves, V.D.; Cunha-Silva, L.; Esteves, I.A.A.C.; Neves, L.A. Cr-based MOF/IL composites as fillers in mixed matrix membranes for CO2 separation. Sep. Purif. Technol. 2021, 276, 119303. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, Z.; Zhang, K.; Wang, W.; Pang, J.; Li, Z.; Kang, Z.; Zhao, D. Stable metal-organic frameworks based mixed matrix membranes for Ethylbenzene/N2 separation. Chem. Eng. J. 2021, 416, 129193. [Google Scholar] [CrossRef]
- Sheikh Alivand, M.; Hossein Tehrani, N.H.M.; Shafiei-alavijeh, M.; Rashidi, A.; Kooti, M.; Pourreza, A.; Fakhraie, S. Synthesis of a modified HF-free MIL-101(Cr) nanoadsorbent with enhanced H2S/CH4, CO2/CH4, and CO2/N2 selectivity. J. Environ. Chem. Eng. 2019, 7, 102946. [Google Scholar] [CrossRef]
- Rallapalli, P.B.S.; Raj, M.C.; Senthilkumar, S.; Somani, R.S.; Bajaj, H.C. HF-free synthesis of MIL-101(Cr) and its hydrogen adsorption studies. Environ. Prog. Sustain. Energy 2016, 35, 461–468. [Google Scholar] [CrossRef]
- Zhao, T.; Li, S.; Shen, L.; Wang, Y.; Yang, X.-Y. The sized controlled synthesis of MIL-101(Cr) with enhanced CO2 adsorption property. Inorg. Chem. Commun. 2018, 96, 47–51. [Google Scholar] [CrossRef]
- Noorpoor, Z.; Pakdehi, S.G.; Rashidi, A. High capacity and energy-efficient dehydration of liquid fuel 2-dimethyl amino ethyl azide (DMAZ) over chromium terephthalic (MIL-101) nanoadsorbent. Adsorption 2017, 23, 743–752. [Google Scholar] [CrossRef]
- Vohra, R.; Velez, L.I.; Rivera, W.; Benitez, F.L.; Delaney, K.A. Recurrent life-threatening ventricular dysrhythmias associated with acute hydrofluoric acid ingestion: Observations in one case and implications for mechanism of toxicity. Clin. Toxicol. 2008, 46, 79–84. [Google Scholar] [CrossRef]
- Zhao, T.; Yang, L.; Feng, P.; Gruber, I.; Janiak, C.; Liu, Y. Facile synthesis of nano-sized MIL-101(Cr) with the addition of acetic acid. Inorg. Chim. Acta 2018, 471, 440–445. [Google Scholar] [CrossRef]
- Lammert, M.; Bernt, S.; Vermoortele, F.; De Vos, D.E.; Stock, N. Single-and mixed-linker Cr-MIL-101 derivatives: A high-throughput investigation. Inorg. Chem. 2013, 52, 8521–8528. [Google Scholar] [CrossRef]
- Mortazavi, S.S.; Abbasi, A.; Masteri Farahani, M.; Farzaneh, F. Sulfonic acid functionalized MIL-101(Cr) metal-organic framework for catalytic production of acetals. ChemistrySelect 2019, 4, 7495–7501. [Google Scholar] [CrossRef]
- Khan, N.A.; Kang, I.J.; Seok, H.Y.; Jhung, S.H. Facile synthesis of nano-sized metal-organic frameworks, chromium-benzenedicarboxylate, MIL-101. Chem. Eng. J. 2011, 166, 1152–1157. [Google Scholar] [CrossRef]
- Yang, L.; Zhao, T.; Boldog, I.; Janiak, C.; Yang, X.Y.; Li, Q.; Zhou, Y.J.; Xia, Y.; Lai, D.W.; Liu, Y.J. Benzoic acid as a selector-modulator in the synthesis of MIL-88B(Cr) and Nano-MIL-101(Cr). Dalton Trans. 2019, 48, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Chen, Y.; Jing, Y. Synthesis of metal organic framework MIL-101 with acetate as mineralization agent. Chem. J. Chin. Univeritie 2012, 33, 668–672. [Google Scholar]
- Li, X.; Zhang, W.; Huang, Y.; Wang, Q.; Yang, J. Superior adsorptive removal of azo dyes from aqueous solution by a Ni(II)-doped metal-organic framework. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126549–126556. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.Y.; Bai, Y.; Wang, J.; Pang, H. Anchoring ZIF-67 particles on amidoximerized polyacrylonitrile fibers for radionuclide sequestration in wastewater and seawater. J. Hazard. Mater. 2020, 395, 122692. [Google Scholar] [CrossRef]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Huang, Y.-d. Comments on “Magnetically recoverable Ni@C composites: The synthesis by carbonization and adsorption for Fe3+”. J. Alloys Compd. 2018, 739, 1124. [Google Scholar] [CrossRef]
- Laciste, M.T.; de Luna, M.D.G.; Tolosa, N.C.; Lu, M.-C. Effect of calcination time of a quadruple-element doped titania nanoparticles in the photodegradation of gaseous formaldehyde under blue light irradiation. Chemosphere 2020, 246, 125763. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Onyancha, R.B.; Ukhurebor, K.E.; Obodo, K.O. Removal of fluoride ions using a polypyrrole magnetic nanocomposite influenced by a rotating magnetic field. RSC Adv. 2020, 10, 595–609. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Zhao, J. Adsorption study for removal of Congo red anionic dye using organo-attapulgite. Adsorption 2009, 15, 381–389. [Google Scholar] [CrossRef]
- Sayın, M.; Can, M.; İmamoğlu, M. Adsorption of Pd(II) and Au(III) Ions by commercial tris(2-aminoethyl) amine polystyrene polymer beads. J. Chem. Eng. Data 2021, 66, 1132–1143. [Google Scholar] [CrossRef]
- Tan, S.; Saito, K.; Hearn, M.T.W. Isothermal modelling of protein adsorption to thermo-responsive polymer grafted Sepharose Fast Flow sorbents. J. Sep. Sci. 2021, 44, 1884–1892. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, R. Derivation of Pseudo-First-Order, Pseudo-Second-Order and Modified Pseudo-First-Order rate equations from Langmuir and Freundlich isotherms for adsorption. Chem. Eng. J. 2020, 392, 123705. [Google Scholar] [CrossRef]
- Pooladi, A.; Bazargan-Lari, R. Simultaneous removal of copper and zinc ions by Chitosan/Hydroxyapatite/nano-Magnetite composite. J. Mater. Res. Technol. 2020, 9, 14841–14852. [Google Scholar] [CrossRef]
- Zhao, T.; Zhu, H.; Geng, W.; Zou, M.; Dong, M.; Ying, J. Morphology control synthesis of Cr-benzenedicarboxylate MOFs for the removal of methylene blue. J. Solid State Chem. 2022, 305, 122651. [Google Scholar] [CrossRef]
- Rojas, S.; Horcajada, P. Metal-organic frameworks for the removal of emerging organic contaminants in water. Chem. Rev. 2020, 120, 8378–8415. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of dyes by nanomaterials: Recent developments and adsorption mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Liu, Q.; Yu, H.; Zeng, F.; Li, X.; Sun, J.; Li, C.; Lin, H.; Su, Z. HKUST-1 modified ultrastability cellulose/chitosan composite aerogel for highly efficient removal of methylene blue. Carbohydr. Polym. 2021, 255, 117402. [Google Scholar] [CrossRef]
- Duan, C.; Meng, X.; Liu, C.; Lu, W.; Liu, J.; Dai, L.; Wang, W.; Zhao, W.; Xiong, C.; Ni, Y. Carbohydrates-rich corncobs supported metal-organic frameworks as versatile biosorbents for dye removal and microbial inactivation. Carbohydr. Polym. 2019, 222, 115042. [Google Scholar] [CrossRef]
- Haque, E.; Lee, J.E.; Jang, I.T.; Hwang, Y.K.; Chang, J.-S.; Jegal, J.; Jhung, S.H. Adsorptive removal of methyl orange from aqueous solution with metal-organic frameworks, porous chromium-benzenedicarboxylates. J. Hazard. Mater. 2010, 181, 535–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, R.-Z.; Huang, Y.-Q.; Yang, J.-M. Effect of the synergetic interplay between the electrostatic interactions, size of the dye molecules, and adsorption sites of MIL-101(Cr) on the adsorption of organic dyes from aqueous solutions. Cryst. Growth Des. 2018, 18, 7533–7540. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, M.; Guan, Q.; Li, W. Kinetic and thermodynamic studies on the adsorption of xylenol orange onto MIL-101(Cr). Chem. Eng. J. 2012, 183, 60–67. [Google Scholar] [CrossRef]
- Bekhoukh, A.; Moulefera, I.; Zeggai, F.Z.; Benyoucef, A.; Bachari, K. Anionic methyl orange removal from aqueous solutions by activated carbon reinforced conducting polyaniline as adsorbent: Synthesis, characterization, adsorption behavior, regeneration and kinetics study. J. Polym. Environ. 2021, 30, 886–895. [Google Scholar] [CrossRef]
- Lv, S.; Liu, J.; Ma, H.; Wang, Z.; Li, C.; Zhao, N.; Wang, S. Simultaneous adsorption of methyl orange and methylene blue from aqueous solution using amino functionalized Zr-based MOFs. Microporous Mesoporous Mater. 2019, 282, 179–187. [Google Scholar] [CrossRef]
- Ling, F.; Fang, L.; Lu, Y.; Gao, J.; Wu, F.; Zhou, M.; Hu, B. A novel CoFe layered double hydroxides adsorbent: High adsorption amount for methyl orange dye and fast removal of Cr(VI). Microporous Mesoporous Mater. 2016, 234, 230–238. [Google Scholar] [CrossRef]
- Abo El Naga, A.O.; Shaban, S.A.; El Kady, F.Y.A. Metal organic framework-derived nitrogen-doped nanoporous carbon as an efficient adsorbent for methyl orange removal from aqueous solution. J. Taiwan Inst. Chem. Eng. 2018, 93, 363–373. [Google Scholar] [CrossRef]
- Tella, A.C.; Olawale, M.D.; Neuburger, M.; Obaleye, J.A. Synthesis and crystal structure of Cd-based metal-organic framework for removal of methyl-orange from aqueous solution. J. Solid State Chem. 2017, 255, 157–166. [Google Scholar] [CrossRef]
- Nazir, M.A.; Khan, N.A.; Cheng, C.; Shah, S.S.A.; Najam, T.; Arshad, M.; Sharif, A.; Akhtar, S.; Rehman, A.u. Surface induced growth of ZIF-67 at Co-layered double hydroxide: Removal of methylene blue and methyl orange from water. Appl. Clay Sci. 2020, 190, 105564–105572. [Google Scholar] [CrossRef]
- Huang, L.; He, M.; Chen, B.; Hu, B. Magnetic Zr-MOFs nanocomposites for rapid removal of heavy metal ions and dyes from water. Chemosphere 2018, 199, 435–444. [Google Scholar] [CrossRef]
- KarzarJeddi, M.; Laitinen, O.; Mahkam, M.; Liimatainen, H. Zwitterionic hybrid aerobeads of binary metal organic frameworks and cellulose nanofibers for removal anionic pollutants. Mater. Des. 2020, 196, 109106. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Zhou, L.; Xu, T.; Peng, C.J.; Liu, H.L. Removal of methyl orange from aqueous solutions by a novel Hyper-cross-linked aromatic triazine porous polymer. Acta Phys. Chim. Sin. 2019, 35, 299–306. [Google Scholar] [CrossRef]
- Liu, L.; Ge, J.; Yang, L.; Jiang, X.; Qiu, L. Facile preparation of chitosan enwrapping Fe3O4 nanoparticles and MIL-101(Cr) magnetic composites for enhanced methyl orange adsorption. J. Porous Mater. 2016, 23, 1363–1372. [Google Scholar] [CrossRef]
- Xu, W.; Li, W.; Lu, L.; Zhang, W.; Kang, J.; Li, B. Morphology-control of metal-organic framework crystal for effective removal of dyes from water. J. Solid State Chem. 2019, 279, 120950. [Google Scholar] [CrossRef]
- Zhang, X.; Qian, L.; Yang, S.; Peng, Y.; Xiong, B.; Li, J.; Fang, P.; He, C. Comparative studies of methyl orange adsorption in various metal-organic frameworks by nitrogen adsorption and positron annihilation lifetime spectroscopy. Microporous Mesoporous Mater. 2020, 296, 109993. [Google Scholar] [CrossRef]
- Shen, T.; Luo, J.; Zhang, S.; Luo, X. Hierarchically mesostructured MIL-101 metal-organic frameworks with different mineralizing agents for adsorptive removal of methyl orange and methylene blue from aqueous solution. J. Environ. Chem. Eng. 2015, 3, 1372–1383. [Google Scholar] [CrossRef]
- Hou, P.; Xing, G.; Han, D.; Zhao, Y.; Zhang, G.; Wang, H.; Zhao, C.; Yu, C. MIL-101(Cr)/graphene hybrid aerogel used as a highly effective adsorbent for wastewater purification. J. Porous Mater. 2019, 26, 1607–1618. [Google Scholar] [CrossRef]
- Vo, T.K.; Trinh, T.P.; Nguyen, V.C.; Kim, J. Facile synthesis of graphite oxide/MIL-101(Cr) hybrid composites for enhanced adsorption performance towards industrial toxic dyes. J. Ind. Eng. Chem. 2021, 95, 224–234. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).