Stabilization of {Ag20(StBu)10} and {Ag19(StBu)10} Toroidal Complexes in DMSO: HPLC-ICP-AES, PL, and Structural Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Structure
2.2. Chromatography Studies
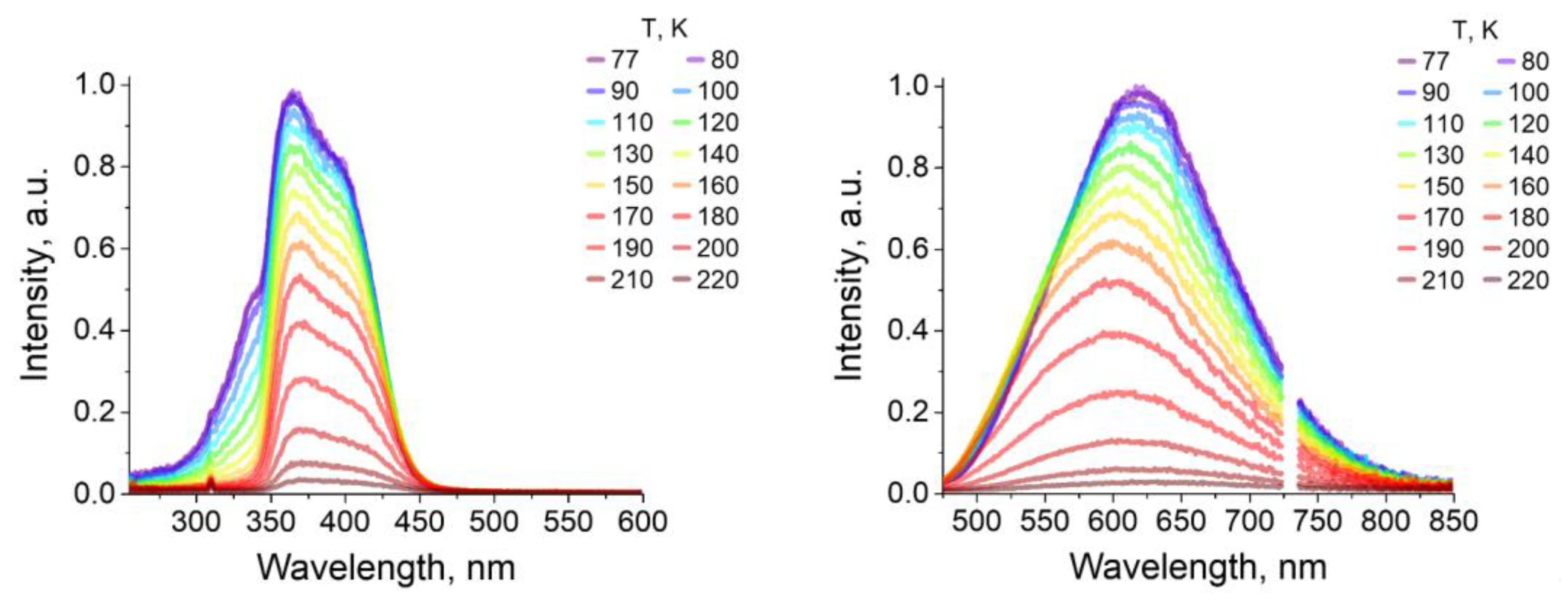
2.3. Photoluminescence
3. Materials and Methods
3.1. General Information
3.2. Synthesis
3.3. PL
3.4. X-ray Diffraction on Single Crystals
3.5. XRPD
3.6. HPLC-ICP-AES
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yang, L.; Wang, X.-Y.; Tang, X.-Y.; Wang, M.-Y.; Ni, C.-Y.; Yu, H.; Song, Y.-L.; Abrahams, B.F.; Lang, J.-P. Temperature-dependent chloride-mediated access to atom-precise silver thiolate nanoclusters. Sci. China Chem. 2022, 65, 1094–1099. [Google Scholar] [CrossRef]
- Cheng, L.-P.; Luo, G.; Zhao, Q.-Q.; Wang, Z.; Wang, X.-P.; Sun, D. Synthesis, structures and luminescence of silver (I) thiolate nanoclusters based on anion templates. Sci. Sin. Chim. 2017, 47, 695–704. [Google Scholar] [CrossRef]
- Pan, Z.-H.; Deng, C.-L.; Wang, Z.; Lin, J.-Q.; Luo, G.-G.; Sun, D. Silver clusters templated by homo- and hetero-anions. CrystEngComm 2020, 22, 3736–3748. [Google Scholar] [CrossRef]
- Wang, Z.; Gupta, R.K.; Luo, G.; Sun, D. Recent Progress in Inorganic Anions Templated Silver Nanoclusters: Synthesis, Structures and Properties. Chem. Rec. 2020, 20, 389–402. [Google Scholar] [CrossRef]
- Sun, D.; Wang, H.; Lu, H.-F.; Feng, S.-Y.; Zhang, Z.-W.; Sun, G.-X.; Sun, D.-F. Two birds with one stone: Anion templated ball-shaped Ag56 and disc-like Ag20 clusters. Dalton Trans. 2013, 42, 6281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Su, H.-F.; Wang, X.-P.; Zhao, Q.-Q.; Tung, C.-H.; Sun, D.; Zheng, L.-S. Johnson Solids: Anion-Templated Silver Thiolate Clusters Capped by Sulfonate. Chem. Eur. J. 2018, 24, 1640–1650. [Google Scholar] [CrossRef]
- Chen, Z.-Y.; Tam, D.Y.S.; Zhang, L.L.-M.; Mak, T.C.W. Silver Thiolate Nano-sized Molecular Clusters and Their Supramolecular Covalent Frameworks: An Approach Toward Pre-templated Synthesis. Chem. Asian J. 2017, 12, 2763–2769. [Google Scholar] [CrossRef]
- Li, S.; Du, X.-S.; Li, B.; Wang, J.-Y.; Li, G.-P.; Gao, G.-G.; Zang, S.-Q. Atom-Precise Modification of Silver(I) Thiolate Cluster by Shell Ligand Substitution: A New Approach to Generation of Cluster Functionality and Chirality. J. Am. Chem. Soc. 2018, 140, 594–597. [Google Scholar] [CrossRef]
- Alhilaly, M.J.; Huang, R.-W.; Naphade, R.; Alamer, B.; Hedhili, M.N.; Emwas, A.-H.; Maity, P.; Yin, J.; Shkurenko, A.; Mohammed, O.F.; et al. Assembly of Atomically Precise Silver Nanoclusters into Nanocluster-Based Frameworks. J. Am. Chem. Soc. 2019, 141, 9585–9592. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, X.-L.; Qin, C.; Wang, H.-N.; Yang, G.-S.; Jiao, Y.-Q.; Huang, P.; Shao, K.-Z.; Su, Z.-M. Serendipitous anion-templated self-assembly of a sandwich-like Ag 20 S 10 macrocycle-based high-nuclearity luminescent nanocluster. Dalton Trans. 2013, 42, 1352–1355. [Google Scholar] [CrossRef]
- Jin, J.-L.; Xie, Y.-P.; Cui, H.; Duan, G.-X.; Lu, X.; Mak, T.C.W. Structure-Directing Role of Phosphonate in the Synthesis of High-Nuclearity Silver(I) Sulfide-Ethynide-Thiolate Clusters. Inorg. Chem. 2017, 56, 10412–10417. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.-H.; Ni, C.-Y.; Li, H.-X.; Ren, Z.-G.; Sun, Z.-R.; Lang, J.-P. [PyH][{TpMo(μ3-S)4Cu3}4(μ12-I)]: A unique tetracubane cluster derived from the S–S bond cleavage and the iodide template effects and its enhanced NLO performances. Chem. Commun. 2013, 49, 4836. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.-H.; Latouche, C.; Li, B.; Kahlal, S.; Saillard, J.-Y.; Liu, C.W. A Twelve-Coordinated Iodide in a Cuboctahedral Silver(I) Skeleton. Inorg. Chem. 2014, 53, 2260–2267. [Google Scholar] [CrossRef]
- Chupina, A.V.; Yanshole, V.V.; Sulyaeva, V.S.; Kokovkin, V.V.; Abramov, P.A.; Sokolov, M.N. Self-assembly patterns of non-metalloid silver thiolates: Structural, HR-ESI-MS and stability studies. Dalton Trans. 2022, 51, 705–714. [Google Scholar] [CrossRef]
- Tang, L.; Yin, Z.; Wang, R.; Wang, B.; Jiang, K.; Ding, M.; Wang, S. Understanding a ligand’s effects on intra-cluster and inter-cluster assembly. Nanoscale 2022, 14, 8842–8848. [Google Scholar] [CrossRef] [PubMed]
- Gruber, F.; Schulz-Dobrick, M.; Jansen, M. Structure-Directing Forces in Intercluster Compounds of Cationic [Ag14(C≡CtBu)12 Cl]+ Building Blocks and Polyoxometalates: Long-Range versus Short-Range Bonding Interactions. Chem. Eur. J. 2010, 16, 1464–1469. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Malola, S.; Hu, C.; Peng, J.; Dittrich, B.; Teo, B.K.; Häkkinen, H.; Zheng, L.; Zheng, N. Co-crystallization of atomically precise metal nanoparticles driven by magic atomic and electronic shells. Nat. Commun. 2018, 9, 3357. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Q.-M. Luminescence Responsive Charge Transfer Intercluster Crystals. Chem. Eur. J. 2012, 18, 11184–11187. [Google Scholar] [CrossRef]
- O’Keefe, M.; Hyde, B.G. Plane nets in crystal chemistry. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Sci. 1980, 295, 553–618. [Google Scholar]
- Aponte, J.C.; Dillon, J.T.; Tarozo, R.; Huang, Y. Separation of unsaturated organic compounds using silver–thiolate chromatographic material. J. Chromatogr. A 2012, 1240, 83–89. [Google Scholar] [CrossRef]
- Liao, S.; Dillon, J.T.; Huang, C.; Santos, E.; Huang, Y. Silver (I)-dimercaptotriazine functionalized silica: A highly selective liquid chromatography stationary phase targeting unsaturated molecules. J. Chromatogr. A 2021, 1645, 462122. [Google Scholar] [CrossRef] [PubMed]
- Dillon, J.T.; Aponte, J.C.; Tarozo, R.; Huang, Y. Efficient liquid chromatographic analysis of mono-, di-, and triglycerols using silver thiolate stationary phase. J. Chromatogr. A 2012, 1240, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Arena, P.; Sciarrone, D.; Dugo, P.; Donato, P.; Mondello, L. Pattern-Type Separation of Triacylglycerols by Silver Thiolate×Non-Aqueous Reversed Phase Comprehensive Liquid Chromatography. Separations 2021, 8, 88. [Google Scholar] [CrossRef]
- Shuvaeva, O.V.; Zhdanov, A.A.; Romanova, T.E.; Abramov, P.A.; Sokolov, M.N. Hyphenated techniques in speciation analysis of polyoxometalates: Identification of individual [PMo12−xVxO40]−3−x (x = 1–3) in the reaction mixtures by high performance liquid chromatography and atomic emission spectrometry with inductively coupled. Dalton Trans. 2017, 46, 3541–3546. [Google Scholar] [CrossRef]
- AbdulHalim, L.G.; Kothalawala, N.; Sinatra, L.; Dass, A.; Bakr, O.M. Neat and Complete: Thiolate-Ligand Exchange on a Silver Molecular Nanoparticle. J. Am. Chem. Soc. 2014, 136, 15865–15868. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Kang, X.; Wang, X.; Zhang, X.; Yuan, X.; Wang, S. Dynamic Metal Exchange between a Metalloid Silver Cluster and Silver(I) Thiolate. Inorg. Chem. 2021, 60, 3037–3045. [Google Scholar] [CrossRef]
- Yuan, X.; Setyawati, M.I.; Tan, A.S.; Ong, C.N.; Leong, D.T.; Xie, J. Highly luminescent silver nanoclusters with tunable emissions: Cyclic reduction–decomposition synthesis and antimicrobial properties. NPG Asia Mater. 2013, 5, e39. [Google Scholar] [CrossRef]
- Veselska, O.; Dessal, C.; Melizi, S.; Guillou, N.; Podbevšek, D.; Ledoux, G.; Elkaim, E.; Fateeva, A.; Demessence, A. New Lamellar Silver Thiolate Coordination Polymers with Tunable Photoluminescence Energies by Metal Substitution. Inorg. Chem. 2019, 58, 99–105. [Google Scholar] [CrossRef]
- Shafikov, M.Z.; Czerwieniec, R.; Yersin, H. Ag(I) complex design affording intense phosphorescence with a landmark lifetime of over 100 milliseconds. Dalton Trans. 2019, 48, 2802–2806. [Google Scholar] [CrossRef]
- Shmakova, A.A.; Berezin, A.S.; Abramov, P.A.; Sokolov, M.N. Self-Assembly of Ag+/[PW11NbO40]4− Complexes in Nonaqueous Solutions. Inorg. Chem. 2020, 59, 1853–1862. [Google Scholar] [CrossRef]
- Weerawardene, K.L.D.M.; Aikens, C.M. Theoretical Insights into the Origin of Photoluminescence of Au25(SR)18− Nanoparticles. J. Am. Chem. Soc. 2016, 138, 11202–11210. [Google Scholar] [CrossRef] [PubMed]
- Aikens, C.M. Electronic and Geometric Structure, Optical Properties, and Excited State Behavior in Atomically Precise Thiolate-Stabilized Noble Metal Nanoclusters. Acc. Chem. Res. 2018, 51, 3065–3073. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhou, M.; So, W.Y.; Huang, J.; Li, M.; Kauffman, D.R.; Cotlet, M.; Higaki, T.; Peteanu, L.A.; Shao, Z.; et al. A Mono-cuboctahedral Series of Gold Nanoclusters: Photoluminescence Origin, Large Enhancement, Wide Tunability, and Structure–Property Correlation. J. Am. Chem. Soc. 2019, 141, 5314–5325. [Google Scholar] [CrossRef]
- Feng, Y.-H.; Lin, Z.-S.; Liu, S.-Q.; Shi, J.-F.; Zhou, K.; Ji, J.-Y.; Bi, Y.-F. A stably discrete 31-nuclearity silver(I) thiolate nanocluster luminescent thermometer supported by DMF auxiliary ligands. New J. Chem. 2020, 44, 663–667. [Google Scholar] [CrossRef]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d 8 and d 10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, A.; Accorsi, G.; Armaroli, N. Luminescent complexes beyond the platinum group: The d10 avenue. Chem. Commun. 2008, 2185. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Lei, Z.; Wang, Q.-M. Luminescent Molecular Ag−S Nanocluster [Ag62S13(SBut)32](BF4)4. J. Am. Chem. Soc. 2010, 132, 17678–17679. [Google Scholar] [CrossRef]
- Yang, T.-Q.; Peng, B.; Shan, B.-Q.; Zong, Y.-X.; Jiang, J.-G.; Wu, P.; Zhang, K. Origin of the Photoluminescence of Metal Nanoclusters: From Metal-Centered Emission to Ligand-Centered Emission. Nanomaterials 2020, 10, 261. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, T.; Pan, H.; Yuan, Y.; Chen, L.; Liu, M.; Zhang, K.; Zhang, S.; Wu, P.; Xu, J. Photoemission Mechanism of Water-Soluble Silver Nanoclusters: Ligand-to-Metal–Metal Charge Transfer vs Strong Coupling between Surface Plasmon and Emitters. J. Am. Chem. Soc. 2014, 136, 1686–1689. [Google Scholar] [CrossRef]
- You, M.-H.; Li, M.-H.; Di, Y.-M.; Zhang, S.-Q.; Lin, M.-J. Photochromic Polyoxometalate/Perylenediimide Donor–Acceptor Hybrid Crystals with Interesting Luminescent Properties. Inorg. Chem. 2022, 61, 105–112. [Google Scholar] [CrossRef]
- Xu, C.; Gan, J.; Mei, X.; Zhou, Y.; Duanmu, J.; Zhu, G.; Zhang, H.; Han, X.; Wang, Y.; Liu, S.-B. Highly Active Silver ion-Exchanged Silicotungstic Acid Catalysts for Selective Esterification of Glycerol with Lauric Acid. Catal. Letters 2020, 150, 3584–3597. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Hübschle, C.B.; Sheldrick, G.M.; Dittrich, B. ShelXle: A Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. [Google Scholar] [CrossRef] [PubMed]










| Maxima | Solution | 1 | 2 |
|---|---|---|---|
| λ1 | 1.74 | 1.73 | |
| λ2 | 1.83 | 1.98 | |
| λ3 | 2.05 | 2.23 | 2.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volchek, V.V.; Berezin, A.S.; Sokolov, M.N.; Abramov, P.A. Stabilization of {Ag20(StBu)10} and {Ag19(StBu)10} Toroidal Complexes in DMSO: HPLC-ICP-AES, PL, and Structural Studies. Inorganics 2022, 10, 225. https://doi.org/10.3390/inorganics10120225
Volchek VV, Berezin AS, Sokolov MN, Abramov PA. Stabilization of {Ag20(StBu)10} and {Ag19(StBu)10} Toroidal Complexes in DMSO: HPLC-ICP-AES, PL, and Structural Studies. Inorganics. 2022; 10(12):225. https://doi.org/10.3390/inorganics10120225
Chicago/Turabian StyleVolchek, Victoria V., Alexey S. Berezin, Maxim N. Sokolov, and Pavel A. Abramov. 2022. "Stabilization of {Ag20(StBu)10} and {Ag19(StBu)10} Toroidal Complexes in DMSO: HPLC-ICP-AES, PL, and Structural Studies" Inorganics 10, no. 12: 225. https://doi.org/10.3390/inorganics10120225
APA StyleVolchek, V. V., Berezin, A. S., Sokolov, M. N., & Abramov, P. A. (2022). Stabilization of {Ag20(StBu)10} and {Ag19(StBu)10} Toroidal Complexes in DMSO: HPLC-ICP-AES, PL, and Structural Studies. Inorganics, 10(12), 225. https://doi.org/10.3390/inorganics10120225









