Modern Trends in Bio-Organometallic Ferrocene Chemistry
Abstract
1. Introduction
2. A Bit of History
3. Antimalarial Analogs
4. Anticoronavirus Activity
5. Antitumor Research
5.1. Chemical Transformations
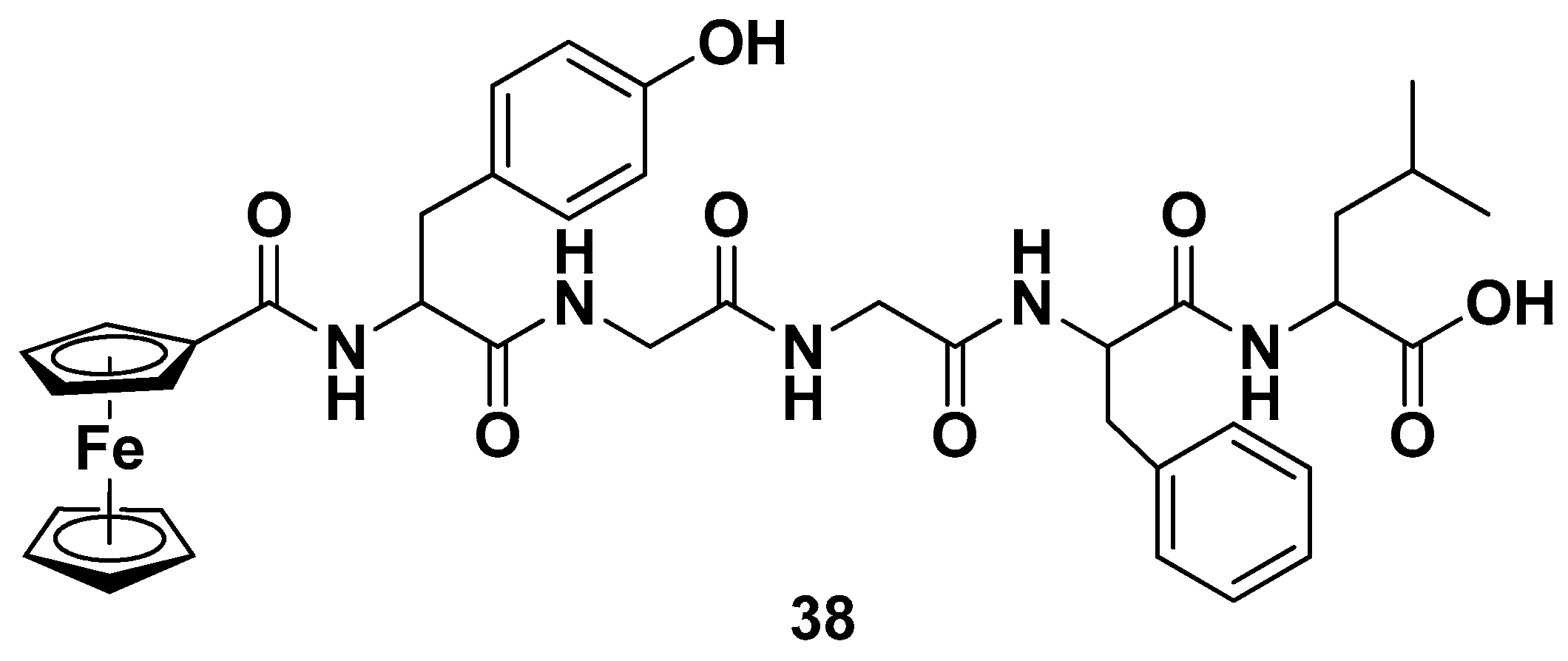
5.1.1. Ferrocene–Steroid Conjugates
5.1.2. Ferrocene-Containing Alkaloids
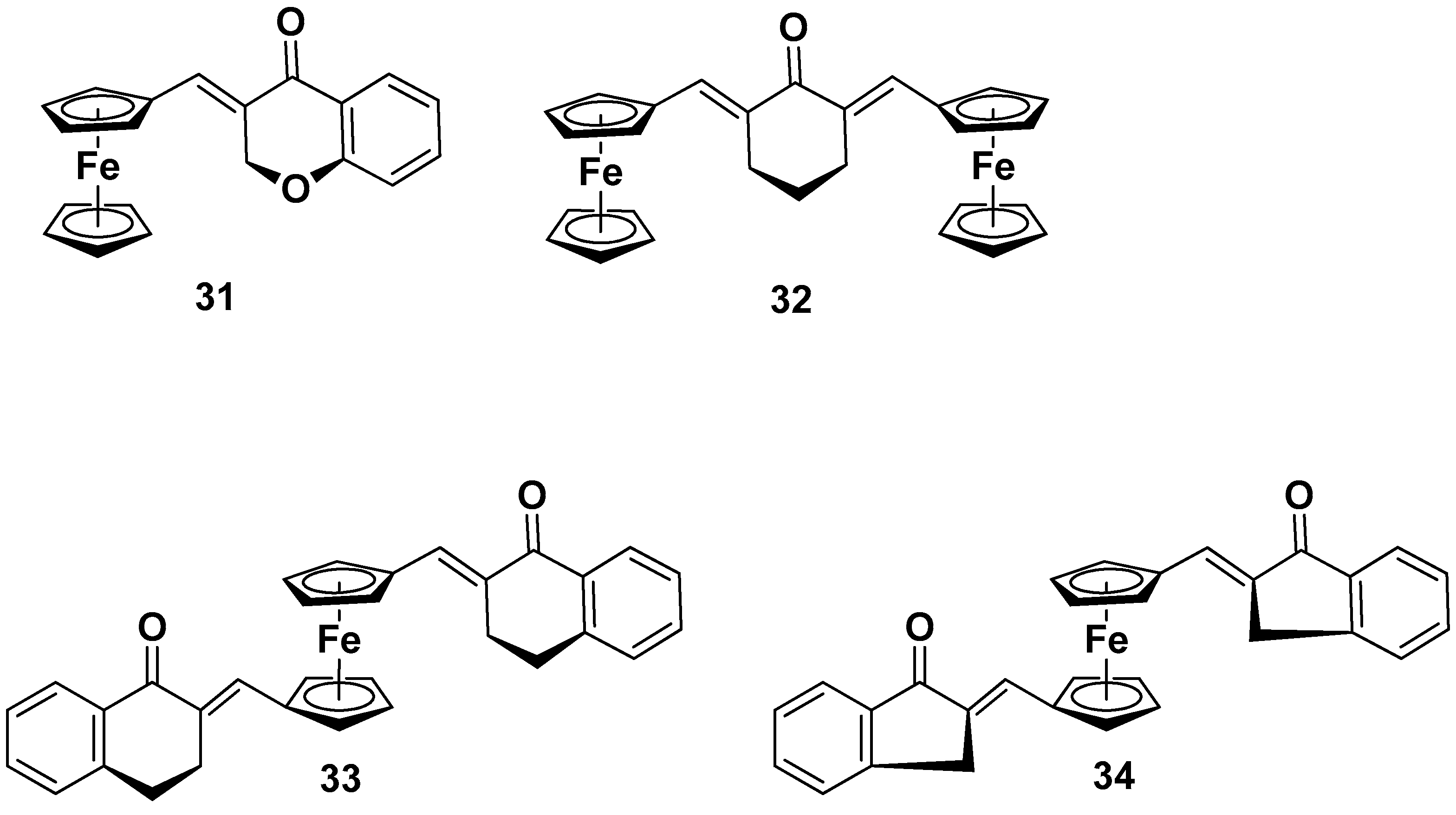
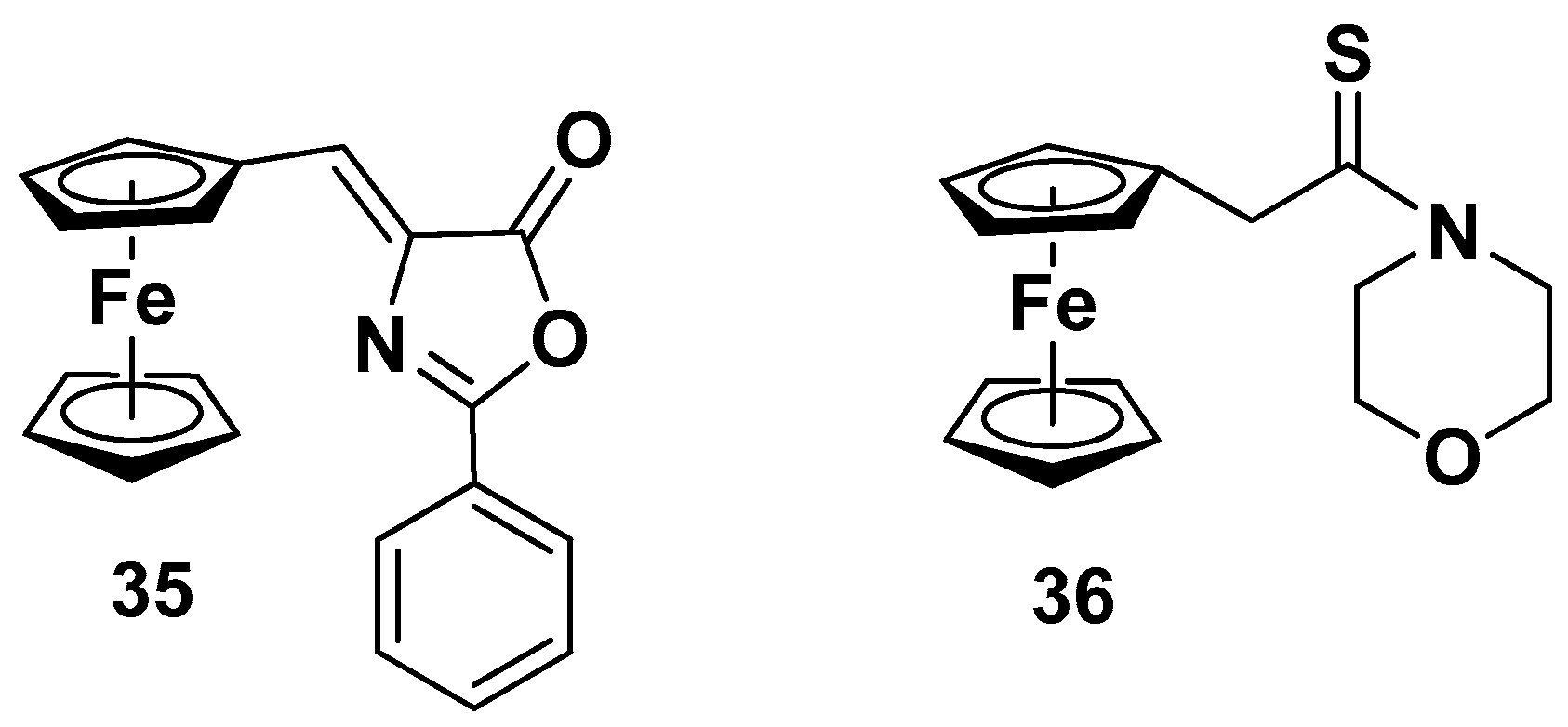
5.1.3. Polymetallocomplexes
5.2. Apoptosis Induction
5.3. DFTB Calculations of Ferrocenes’ Interaction with DNA
5.4. Inhibitors of DNA Topoisomerase I and II Activity
6. Antimycotic Activity
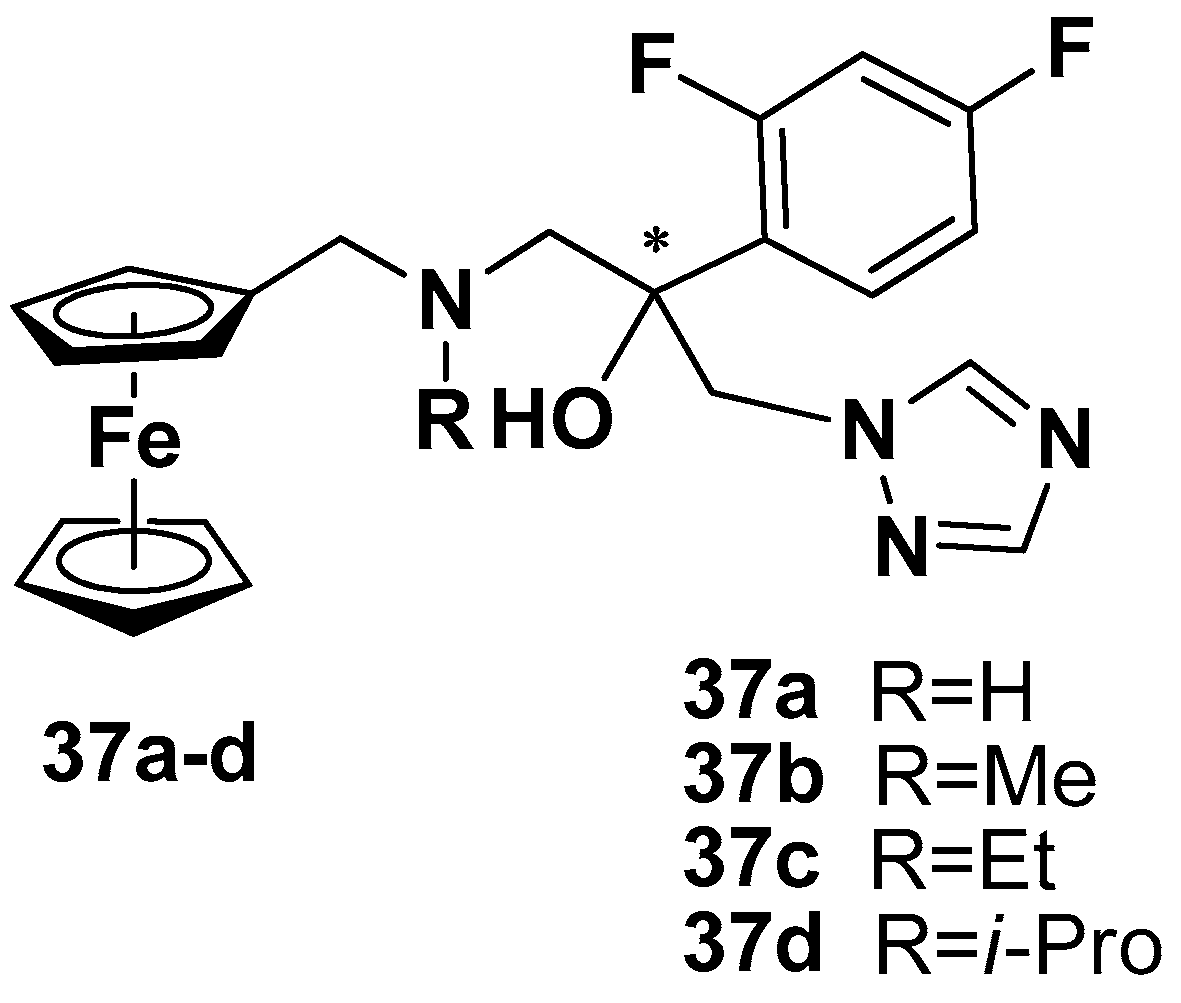
7. Brain Diseases
7.1. Crossing of the Blood–Brain Barrier
7.2. A Model of Regional Brain Iron Overload
7.3. Electrophysiological Investigations
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Sharma, B.; Kumar, V. Has ferrocene really delivered its role in accentuating the bioactivity of organic scaffolds? J. Med. Chem. 2021, 64, 16865–16921. [Google Scholar] [CrossRef]
- Pedotti, S.; Ussia, M.; Patti, A.; Musso, N.; Barresi, V.; Condorelli, D.F. Synthesis of the ferrocenyl analogue of clotrimazole drug. J. Organomet. Chem. 2017, 830, 56–61. [Google Scholar] [CrossRef]
- Tsypysheva, I.P.; Koval’skaya, A.V.; Petrova, P.R.; Lobov, A.N.; Erastov, A.S.; Zileeva, Z.R.; Vakhitov, V.A.; Vakhitova, Y.V. Synthesis of conjugates of (−)-cytisine derivatives with ferrocene-1-carbaldehyde and their cytotoxicity against HEK293, Jurkat, A549, MCF-7 and SH-SY5Y cells. Tetrahedron 2020, 76, 130902. [Google Scholar] [CrossRef]
- Karagöz, A.Ç.; Leidenberger, M.; Hahn, F.; Hamoel, F.; Friedrich, O.; Marschall, M.; Kappes, B.; Tsogoeva, S.B. Synthesis of new betulinic acid/betulin-derived dimers and hybrids with potent antimalarial and antiviral activities. Bioorg. Med. Chem. 2019, 27, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, L.; Wang, Y.; Nottingham, C.; Pagsulingan, J.; Jaouen, G.; McGlinchey, M.J.; Guiry, P.J. Enantioselective Synthesis of Planar Chiral Ferrocifens that Show Chiral Discrimination in Antiproliferative Activity on Breast Cancer Cells. ChemBioChem 2020, 21, 2974–2981. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, B.S.; Correia, J.D.G.; Kühn, F.E. Ferrocene Derivatives as Anti-Infective Agents. Coord. Chem. Rev. 2019, 396, 22–48. [Google Scholar] [CrossRef]
- Larik, F.A.; Saeed, A.; Fattah, T.A.; Muqadar, U.; Channar, P.A. Recent advances in the synthesis, biological activities and various applications of ferrocene derivatives. Appl. Organomet. Chem. 2017, 31, e3664. [Google Scholar] [CrossRef]
- Maguene, G.M.; Jakhlal, J.; Ladyman, M.; Vallin, A.; Ralambomanana, D.A.; Bousquet, T.; Maugein, J.; Lebibi, J.; Pélinski, L. Synthesis and antimycobacterial activity of a series of ferrocenyl derivatives. Eur. J. Med. Chem. 2011, 46, 31–38. [Google Scholar] [CrossRef]
- Kulikov, V.N.; Nikulin, R.S.; Rodionov, A.N.; Babusenko, E.S.; Babin, V.N.; Kovalenko, L.V.; Belousov, Y.A. Synthesis and antimycobacterial activity of N-isonicotinoyl-N’-alkylideneferrocenecarbohydrazides. Russ. Chem. Bull. Int. Ed. 2017, 66, 1122–1126. [Google Scholar] [CrossRef]
- Asghar, F.; Munir, S.; Fatima, S.; Murtaza, B.; Patujo, J.; Badshah, A.; Butler, I.S.; Taj, M.B.; Tahir, M.N. Ferrocene-functionalized anilines as potent anticancer and antidiabetic agents: Synthesis, spectroscopic elucidation, and DFT calculations. J. Mol. Struct. 2022, 1249, 131632. [Google Scholar] [CrossRef]
- Glushkov, V.A.; Anikina, L.V.; Gorbunova, L.V.; Nedugov, A.N.; Slepukhin, P.A. Synthesis of ferrocenyl-substituted triterpenoids. Russ. J. Org. Chem. 2013, 49, 1226–1230. [Google Scholar] [CrossRef]
- Ismail, M.K.; Khan, Z.; Rana, M.; Horswell, S.L.; Male, L.; Nguyen, H.V.; Perotti, A.; Romero-Canelón, I.; Wilkinson, E.A.; Hodges, N.J.; et al. Effect of Regiochemistry and Methylation on the Anticancer Activity of a Ferrocene-Containing Organometallic Nucleoside Analogue. ChemBioChem 2020, 21, 2487–2494. [Google Scholar] [CrossRef]
- Jadhav, J.; Das, R.; Kamble, S.; Chowdhury, M.G.; Kapoor, S.; Gupta, A.; Vyas, H.; Shard, A. Ferrocene-based modulators of cancer-associated tumor pyruvate kinase M2. J. Organomet. Chem. 2022, 968–969, 122338. [Google Scholar] [CrossRef]
- Snegur, L.V.; Lyapunova, M.V.; Verina, D.D.; Kachala, V.V.; Korlyukov, A.A.; Ilyin, M.M., Jr.; Davankov, V.A.; Ostrovskaya, L.A.; Bluchterova, N.V.; Fomina, M.M.; et al. Nitro-imidazoles in ferrocenyl alkylation reaction. Synthesis, enantiomeric resolution and in vitro and in vivo bioeffects. J. Organomet. Chem. 2018, 871, 10–20. [Google Scholar] [CrossRef]
- Abbas, G.; Irfan, A.; Ahmed, I.; Al-Zeidaneen, F.K.; Muthu, S.; Fuhr, O.; Thomas, R. Synthesis and investigation of anti-COVID19 ability of ferrocene Schiff base derivatives by quantum chemical and molecular docking. J. Mol. Struct. 2022, 1253, 132242. [Google Scholar] [CrossRef] [PubMed]
- Snegur, L.V.; Simenel, A.A.; Rodionov, A.N.; Boev, V.I. Ferrocene modification of organic compounds for medicinal applications. Russ. Chem. Bull. Int. Ed. 2014, 63, 26–36. [Google Scholar] [CrossRef]
- Kowalski, K. Ferrocenyl-nucleobase complexes: Synthesis, chemistry and applications. Coord. Chem. Rev. 2016, 317, 132–156. [Google Scholar] [CrossRef]
- Astruc, D. Why is Ferrocene so Exceptional? Eur. J. Inorg. Chem. 2017, 6–29. [Google Scholar] [CrossRef]
- Zhang, P.; Sadler, P.J. Advances in the design of organometallic anticancer complexes. J. Organomet. Chem. 2017, 839, 5–14. [Google Scholar] [CrossRef]
- Shevaldina, E.V.; Moiseev, S.K. Ferrocenylalkylation reactions under acid-free conditions. INEOS OPEN 2021, 4, 41–52. [Google Scholar] [CrossRef]
- Babin, V.N.; Belousov, Y.A.; Borisov, V.I.; Gumenyuk, V.V.; Nekrasov, Y.S.; Ostrovskaya, L.A.; Sviridova, I.K.; Sergeeva, N.S.; Simenel, A.A.; Snegur, L.V. Ferrocenes as potential anticancer drugs. Facts and hypotheses. Russ. Chem. Bull. 2014, 63, 2405–2422. [Google Scholar] [CrossRef]
- Wang, R.; Chen, H.; Yan, W.; Zheng, M.; Zhang, T.; Zhang, Y. Ferrocene-containing hybrids as potential anticancer agents: Current developments, mechanisms of action and structure-activity relationships. Eur. J. Med. Chem. 2020, 190, 112109. [Google Scholar] [CrossRef] [PubMed]
- Idlas, P.; Lepeltier, E.; Jaouen, G.; Passirani, C. Ferrocifen Loaded Lipid Nanocapsules: A Promising Anticancer Medication against Multidrug Resistant Tumors. Cancers 2021, 13, 2291. [Google Scholar] [CrossRef]
- Vessières, A.; Wang, Y.; McGlinchey, M.J.; Jaouen, G. Multifaceted Chemical Behaviour of Metallocene (M = Fe, Os) Quinone Methides. Their Contribution to Biology. Coord. Chem. Rev. 2021, 430, 213658. [Google Scholar] [CrossRef]
- Shoukat, H.; Altaf, A.A.; Badshah, A. Ferrocene-Based Metallodrugs. In Advances in Metallodrugs: Preparation and Applications in Medicinal Chemistry; Shahid-ul-Islam, Hashmi, A.A., Khan, S.A., Eds.; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 115–136. [Google Scholar] [CrossRef]
- Chaudhary, A.; Poonia, K. The redox mechanism of ferrocene and its phytochemical and biochemical compounds in anticancer therapy: A mini review. Inorg. Chem. Commun. 2021, 134, 109044. [Google Scholar] [CrossRef]
- Guk, D.A.; Krasnovskaya, O.O.; Beloglazkina, E.K. Coordination compounds of biogenic metals as cytotoxic agents in cancer therapy. Russ. Chem. Rev. 2021, 90, 1566–1623. [Google Scholar] [CrossRef]
- Sansook, S.; Hassell-Hart, S.; Ocasio, C.; Spencer, J. Ferrocenes in medicinal chemistry; a personal perspective. J. Organomet. Chem. 2020, 905, 121017. [Google Scholar] [CrossRef]
- Zhang, S.; Yi, C.; Li, W.-W.; Luo, Y.; Wu, Y.-Z.; Ling, H.-B. The current scenario on anticancer activity of artemisinin metal complexes, hybrids, and dimers. Arch. Pharm. 2022, 355, e2200086. [Google Scholar] [CrossRef]
- Pauson, P.L. Ferrocene–how it all began. J. Organomet. Chem. 2001, 637, 3–6. [Google Scholar] [CrossRef]
- Nesmeyanov, A.N.; Bogomolova, L.G.; Andrianova, I.G.; Vilchevskaya, V.D.; Kochetkova, N.S. New Preparation (Ferroceron) for Treating Iron-deficient Anemia. Farm. Chem. J. 1972, 6, 269–270. [Google Scholar]
- Nesmeyanov, A.N.; Bogomolova, L.G.; Viltcheskaya, V.; Palitsyne, N.; Andrianova, I.; Belozerova, O. Medicinal preparation for treating parodontosis and method of treating parodontosis. U.S. Patent 119 356, 7 December 1971. [Google Scholar]
- Biot, C.; Glorian, G.; Maciejewski, L.A.; Brocard, J.S.; Domarle, O.; Blampain, G.; Millet, P.; Georges, A.J.; Abessolo, H.; Dive, D.; et al. Synthesis and Antimalarial Activity in Vitro and in Vivo of a New Ferrocene−Chloroquine Analogue. J. Med. Chem. 1997, 40, 3715–3718. [Google Scholar] [CrossRef] [PubMed]
- Dive, D.; Biot, C. Ferrocene conjugates of chloroquine and other antimalarials: The development of ferroquine, a new antimalarial. ChemMedChem 2008, 3, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, G.; Top, S.; Vessières, A.; Leclercq, G.; McGlinchey, M.J. The First Organometallic Selective Estrogen Receptor Modulators (SERMs) and Their Relevance to Breast Cancer. Curr. Med. Chem. 2004, 11, 2505–2517. [Google Scholar] [CrossRef] [PubMed]
- Vessières, A.; Top, S.; Beck, W.; Hillard, E.; Jaouen, G. Metal complex SERMs (selective oestrogen receptor modulators). The influence of different metal units on breast cancer cell antiproliferative effects. Dalton Trans. 2006, 529–541. [Google Scholar] [CrossRef]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anticancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef]
- Delhaes, L.; Biot, C.; Berry, L.; Delcourt, P.; Maciejewski, L.A.; Camus, D.; Brocard, J.S.; Dive, D. Synthesis of ferroquine enantiomers: First investigation of effects of metallocenic chirality upon antimalarial activity and cytotoxicity. ChemBioChem 2002, 3, 418–423. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, Z.; Kong, F.; Gao, F. Current scenario of ferrocene-containing hybrids for antimalarial activity. Eur. J. Med. Chem. 2020, 185, 111791. [Google Scholar] [CrossRef]
- Delhaes, L.; Biot, C.; Berry, L.; Maciejewski, L.A.; Camus, D.; Brocard, J.S.; Dive, D. Novel Ferrocenic Artemisinin Derivatives: Synthesis, In Vitro Antimalarial Activity and Affinity of Binding with Ferroprotoporphyrin IX. Bioorg. Med. Chem. 2000, 8, 2739–2745. [Google Scholar] [CrossRef]
- de Lange, C.; Coertzen, D.; Smit, F.J.; Wentzel, J.F.; Wong, H.N.; Birkholtz, L.-M.; Haynes, R.K.; N’Da, D.D. Synthesis, in vitro antimalarial activities and cytotoxicities of amino-artemisinin-ferrocene derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 289–292. [Google Scholar] [CrossRef]
- de Lange, C.; Coertzen, D.; Smit, F.J.; Wentzel, J.F.; Wong, H.N.; Birkholtz, L.-M.; Haynes, R.K.; N’Da, D.D. Synthesis, antimalarial activities and cytotoxicities of amino-artemisinin-1,2-disubstituted ferrocene hybrids. Bioorg. Med. Chem. Lett. 2018, 28, 3161–3163. [Google Scholar] [CrossRef] [PubMed]
- Reiter, C.; Frohlich, T.; Zeino, M.; Marschall, M.; Bahsi, H.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Hampel, F.; Efferth, T.; et al. New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1,2,4-trioxane-ferrocene hybrids. Eur. J. Med. Chem. 2015, 97, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Vessières, A.; Quissac, E.; Lemaire, N.; Alentorn, A.; Domeracka, P.; Pigeon, P.; Sanson, M.; Idbaih, A.; Verreault, M. Heterogeneity of Response to Iron-Based Metallodrugs in Glioblastoma Is Associated with Differences in Chemical Structures and Driven by FAS Expression Dynamics and Transcriptomic Subtypes. Int. J. Mol. Sci. 2021, 22, 10404. [Google Scholar] [CrossRef] [PubMed]
- Pigeon, P.; Gaschard, M.; Othman, M.; Salmain, M.; Jaouen, G. α-Hydroxylactams as Efficient Entries to Diversely Functionalized Ferrociphenols: Synthesis and Antiproliferative Activity Studies. Molecules 2022, 27, 4549. [Google Scholar] [CrossRef] [PubMed]
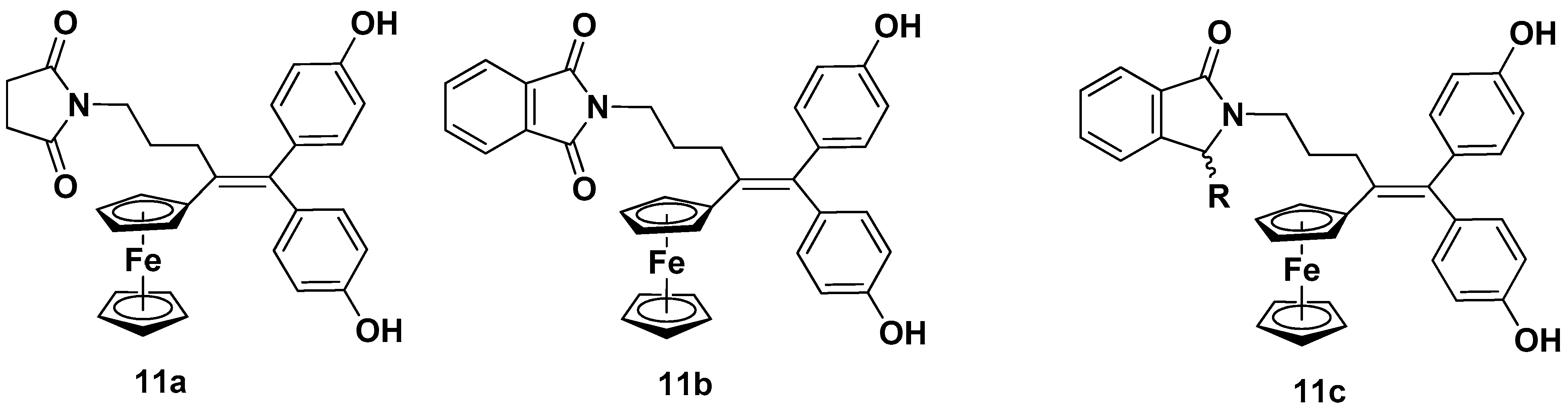
- Pigeon, P.; Wang, Y.; Top, S.; Najlaoui, F.; Alvarez, M.C.G.; Bignon, J.; McGlinchey, M.J.; Jaouen, G. A New Series of Succinimido-ferrociphenols and Related Heterocyclic Species Induce Strong Antiproliferative Effects, Especially against Ovarian Cancer Cells Resistant to Cisplatin. J. Med. Chem. 2017, 60, 8358–8368. [Google Scholar] [CrossRef]
- Wang, Y.; Pigeon, P.; Li, W.; Yan, J.; Dansette, P.M.; Othman, M.; McGlinchey, M.J.; Jaouen, G. Diversity-oriented synthesis and bioactivity evaluation of N-substituted ferrocifen compounds as novel antiproliferative agents against TNBC cancer cells. Eur. J. Med. Chem. 2022, 234, 114202. [Google Scholar] [CrossRef]
- Garcia, J.S.; Gaschard, M.; Navizet, I.; Sahihi, M.; Top, S.; Wang, Y.; Pigeon, P.; Vessières, A.; Salmain, M.; Jaouen, G. Inhibition of Cathepsin B by Ferrocenyl Indenes Highlights a new Pharmacological Facet of Ferrocifens. Eur. J. Inorg. Chem. 2022, e202101075. [Google Scholar] [CrossRef]
- Raičević, V.; Radulović, N.; Sakač, M. Toward Selective Anticancer Agents: Ferrocene-Steroid Conjugates. Eur. J. Inorg. Chem. 2022, 2022, e202100951. [Google Scholar] [CrossRef]
- Carmona, J.A.; Santana-Negrón, A.; Rheingold, A.L.; Melendez, E. Synthesis, structure, docking and cytotoxic studies of ferrocene–hormone conjugates for hormone-dependent breast cancer application. Dalton Trans. 2019, 48, 5952–5964. [Google Scholar] [CrossRef]
- Raičević, V.; Radulović, N.; Jovanović, L.; Rodić, M.; Kuzminac, I.; Jakimov, D.; Wrodnigg, T.; Knedel, T.-O.; Janiak, C.; Sakač, M. Ferrocenylmethylation of estrone and estradiol: Structure, electrochemistry, and antiproliferative activity of new ferrocene–steroid conjugates. Appl. Organomet. Chem. 2020, 34, e5889. [Google Scholar] [CrossRef]
- Baartzes, N.; Szabo, C.; Cenariu, M.; Imre-Lucaci, F.; Dorneanu, S.A.; Fischer-Fodor, E.; Smith, G.S. In vitro antitumour activity of two ferrocenyl metallodendrimers in a colon cancer cell line. Inorg. Chem. Commun. 2018, 98, 75–79. [Google Scholar] [CrossRef]
- Gadre, S.; Manikandan, M.; Duari, P.; Chhatar, S.; Sharma, A.; Khatri, S.; Kode, J.; Barkume, M.; Kasinathan, N.K.; Nagare, M.; et al. A Rationally Designed Bimetallic Platinum (II)-Ferrocene Antitumor Agent Induces Non-Apoptotic Cell Death and Exerts in Vivo Efficacy. Chem. Eur. J. 2022, 2022, e2020201259. [Google Scholar] [CrossRef] [PubMed]
- Skoupilova, H.; Bartosik, M.; Sommerova, L.; Pinkas, J.; Vaculovic, T.; Kanicky, V.; Karban, J.; Hrstka, R. Ferrocenes as new anticancer drug candidates: Determination of the mechanism of action. Eur. J. Pharmacol. 2020, 867, 172825. [Google Scholar] [CrossRef]
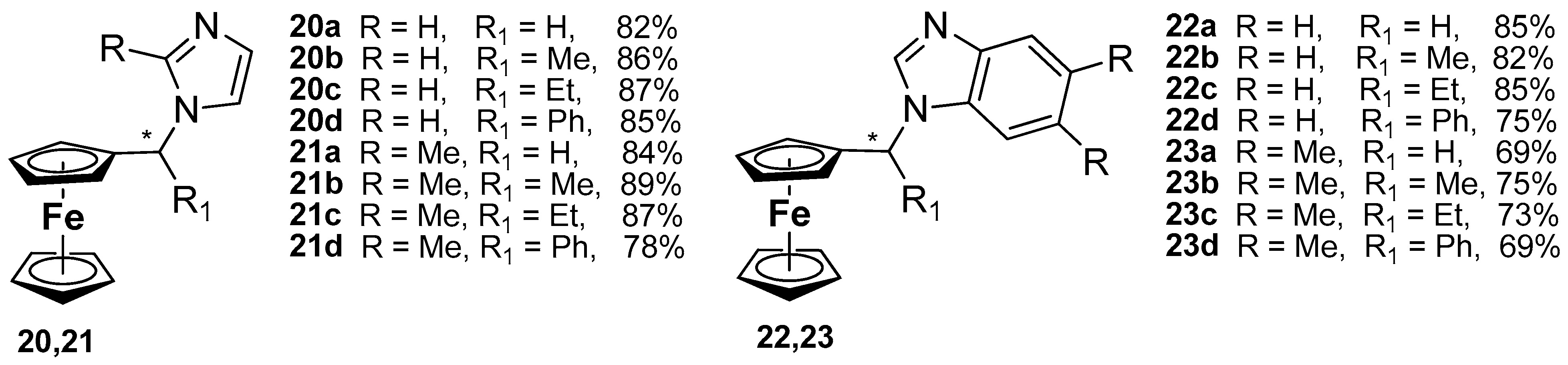
- Snegur, L.V. Synthesis of Biologically Active Ferrocenyl Alkyl Azoles. Ph.D. Thesis, A.N. Nesmeyanov Institute of Organoelement Compounds, Moscow, Russia, 2002. [Google Scholar]
- Snegur, L.V.; Rodionov, A.N.; Ostrovskaya, L.A.; Ilyin, M.M.; Simenel, A.A. Ferrocene-modified imidazoles: One-pot oxalyl chloride-assisted synthesis, HPLC enantiomeric resolution, and in vivo antitumor effects. Appl. Organomet. Chem. 2022, 36, e6681. [Google Scholar] [CrossRef]
- Khennoufa, A.; Bechki, L.; Lanez, T.; Lanez, E.; Zegheb, N. Spectrophotometric, voltammetric and molecular docking studies of binding interaction of N-ferrocenylmethylnitroanilines with bovine serum albumin. J. Mol. Struct. 2021, 1224, 129052. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Mahmoud, N.F.; Mohamed, G.G. Synthesis, physicochemical characterization, geometric structure and molecular docking of new biologically active ferrocene based Schiff base ligand with transition metal ions. Appl. Organomet. Chem. 2017, 31, e3858. [Google Scholar] [CrossRef]
- Mahmoud, W.H.; Deghadi, R.G.; Mohamed, G.G. Metal complexes of ferrocenyl-substituted Schiff base: Preparation, characterization, molecular structure, molecular docking studies, and biological investigation. J. Organomet. Chem. 2020, 917, 121113. [Google Scholar] [CrossRef]
- Lanez, T.; Lanez, E. A molecular docking study of N-ferrocenylmethylnitroanilines as potential anticancer drugs. Int. J. Pharmacol. Phytochem. Ethnomed. 2016, 2, 5–12. [Google Scholar] [CrossRef]
- Rodionov, A.N.; Korlyukov, A.A.; Simenel, A.A. Synthesis, structure of 5,7-dimethyl-3-ferrocenyl-2,3-dihydro-1H-pyrazolo-[1,2-a]-pyrazol-4-ium tetrafluoroborate. DFTB calculations of interaction with DNA. J. Mol. Struct. 2022, 1251, 132070. [Google Scholar] [CrossRef]
- Pešić, M.; Bugarinović, J.; Minić, A.; Novaković, S.B.; Bogdanović, G.A.; Todosijević, A.; Stevanović, D.; Damljanović, I. Electrochemical characterization and estimation of DNA-binding capacity of a series of novel ferrocene derivatives. Bioelectrochemistry 2020, 132, 107412. [Google Scholar] [CrossRef]
- Gopal, Y.N.V.; Jayaraju, D.; Kondapi, A.K. Topoisomerase II Poisoning and Antineoplastic Action by DNA-Nonbinding Diacetyl and Dicarboxaldoxime Derivatives of Ferrocene. Arch. Biochem. Biophys. 2000, 376, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Konkolová, E.; Janočková, J.; Perjési, P.; Vašková, J.; Kožurková, M. Selected ferrocenyl chalcones as DNA/BSA-interacting agents and inhibitors of DNA topoisomerase I and II activity. J. Organomet. Chem. 2018, 861, 1–9. [Google Scholar] [CrossRef]
- Krishna, A.D.S.; Panda, G.; Kondapi, A.K. Mechanism of action of ferrocene derivatives on the catalytic activity of topoisomerase IIα and β. Distinct mode of action of two derivatives. Arch. Biochem. Biophys. 2005, 438, 206–216. [Google Scholar] [CrossRef]
- Rubbiani, R.; Weil, T.; Tocci, N.; Mastrobuoni, L.; Jeger, S.; Moretto, M.; Ng, J.; Lin, Y.; Hess, J.; Ferrari, S.; et al. In vivo active organometallic-containing antimycotic agents. RSC Chem. Biol. 2021, 2, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Hoffmans, U.; Ott, V.; Fricker, G.; Metzler-Nolte, N. Modification with Organometallic Compounds Improves Crossing of the Blood–Brain Barrier of [Leu5]-Enkephalin Derivatives in an In Vitro Model System. ChemBioChem 2009, 10, 1852–1860. [Google Scholar] [CrossRef]
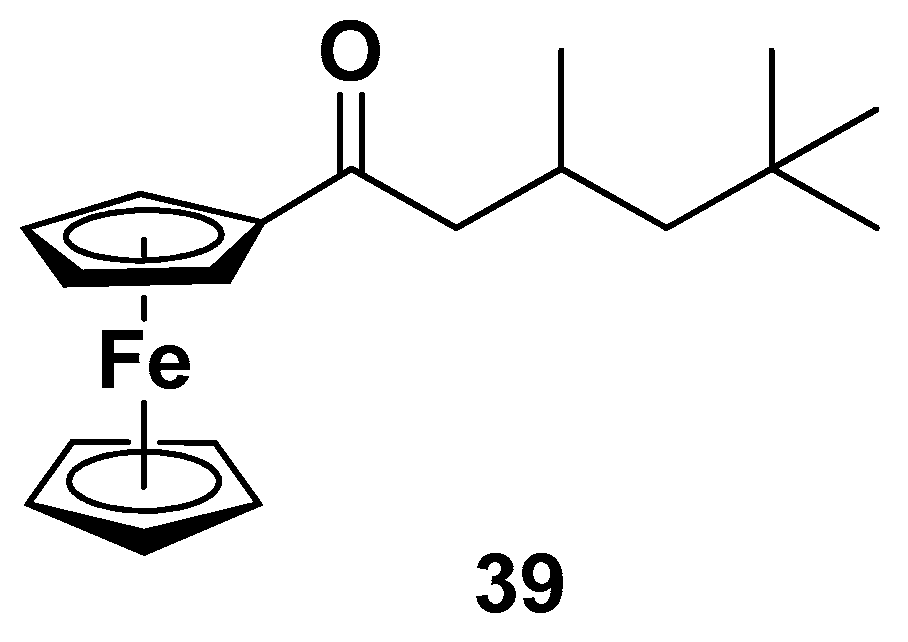
- Malecki, E.A.; Cable, E.E.; Isom, H.C.; Connor, G.R. The lipophilic iron compound TMH-ferrocene [(3,5,5-trimethylhexanoyl)ferrocene] increases iron concentrations, neuronal l-ferritin, and hemeoxygenase in brains of BALB/c mice. Biol. Trace Elem. Res. 2002, 86, 73–84. [Google Scholar] [CrossRef]
- Peters, D.G.; Purnell, C.J.; Haaf, M.P.; Yang, Q.X.; Connor, J.R.; Meadowcroft, M.D. Dietary lipophilic iron accelerates regional brain iron-load in C57BL6 mice. Brain Struct. Funct. 2018, 223, 1519–1536. [Google Scholar] [CrossRef]
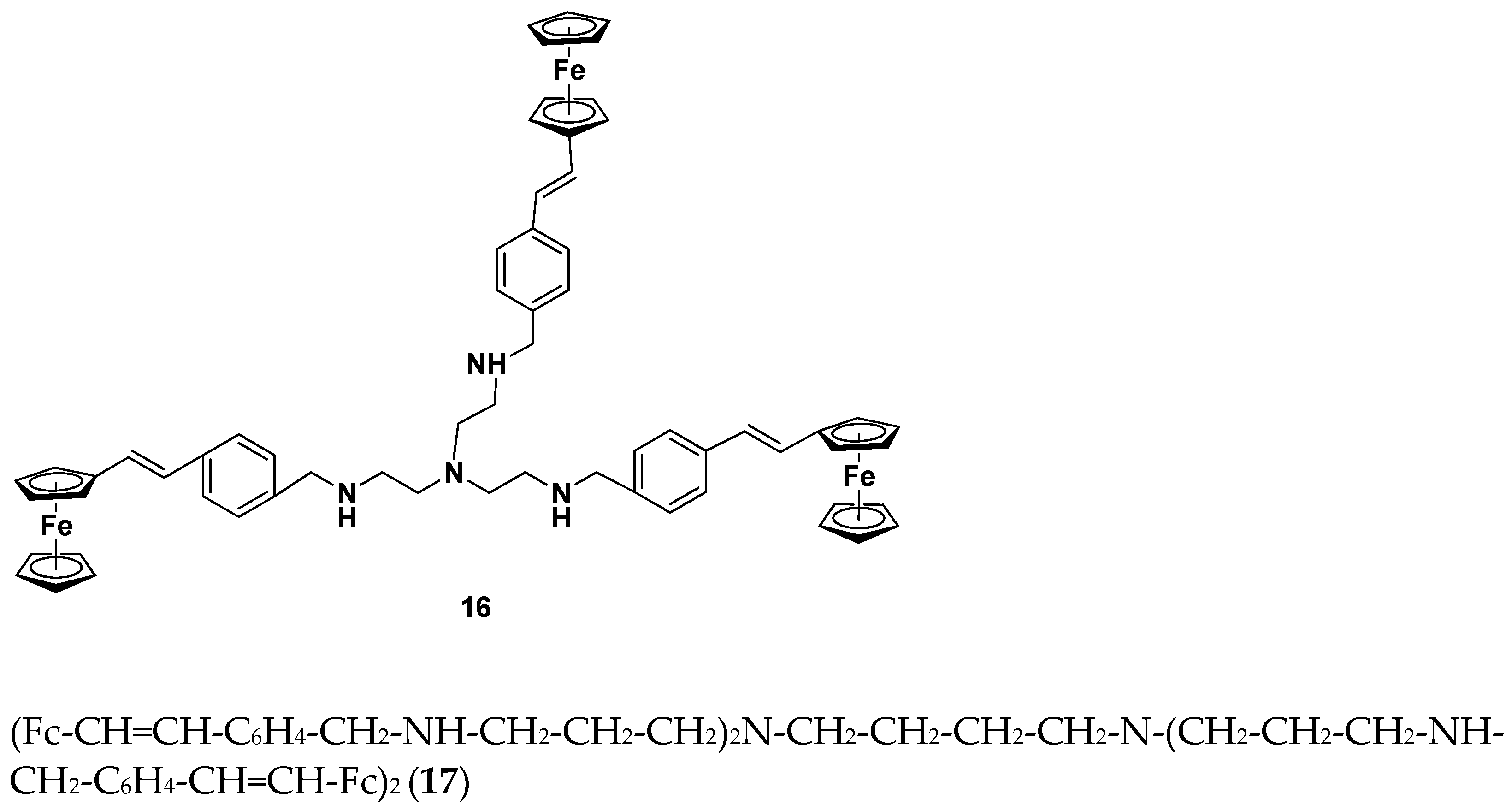
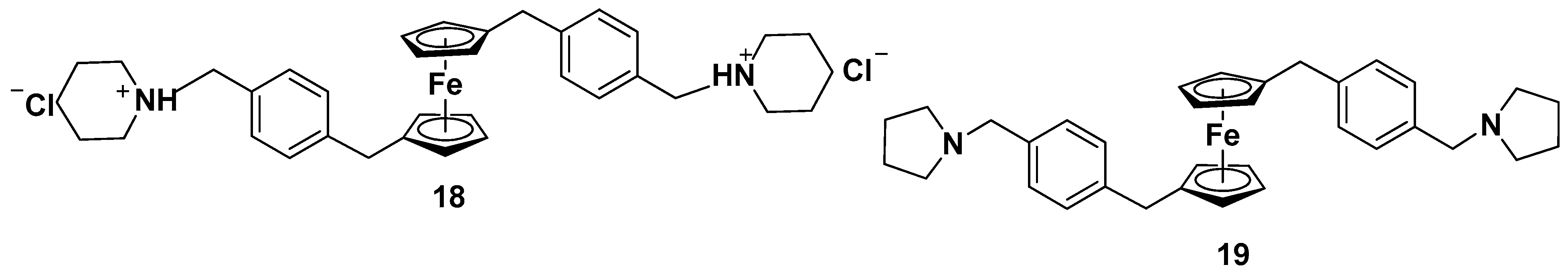
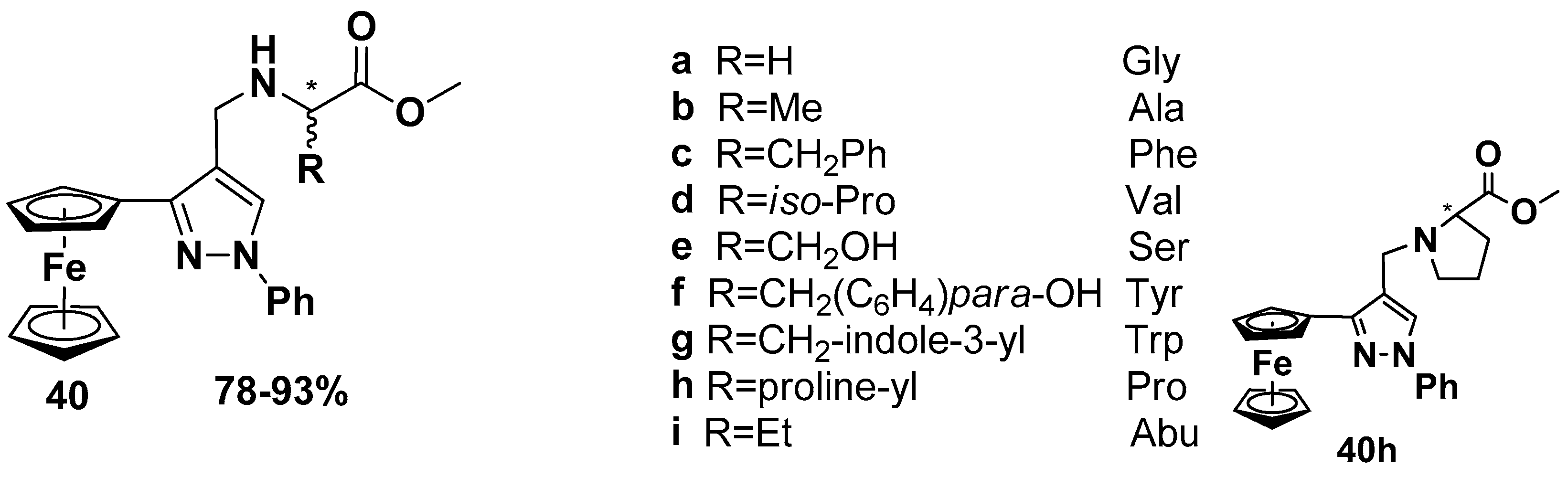
- Rodionov, A.N.; Snegur, L.V.; Simenel, A.A.; Dobryakova, Y.V.; Markevich, V.A. Ferrocene-modification of Amino Acids: Synthesis and in vivo Bioeffects on Hippocampus. Russ. Chem. Bull. Int. Ed. 2017, 66, 136–142. [Google Scholar] [CrossRef]
- Rodionov, A.N.; Snegur, L.V.; Dobryakova, Y.V.; Ilyin, M.M.; Markevich, V.A.; Simenel, A.A. Administration of ferrocene-modified amino acids induces changes in synaptic transmission in the CA1 area of the hippocampus. Appl. Organomet. Chem. 2020, 34, e5276. [Google Scholar] [CrossRef]
- Pejović, A.; Denić, M.S.; Stevanović, D.; Damljanović, I.; Vukićević, M.; Kostova, K.; Tavlinova-Kirilova, M.; Randjelović, P.; Stojanović, N.M.; Bogdanović, G.A.; et al. Discovery of anxiolytic 2-ferrocenyl-1,3-thiazolidin-4-ones exerting GABAA receptor interaction via the benzodiazepine-binding site. Eur. J. Med. Chem. 2014, 83, 57–73. [Google Scholar] [CrossRef]

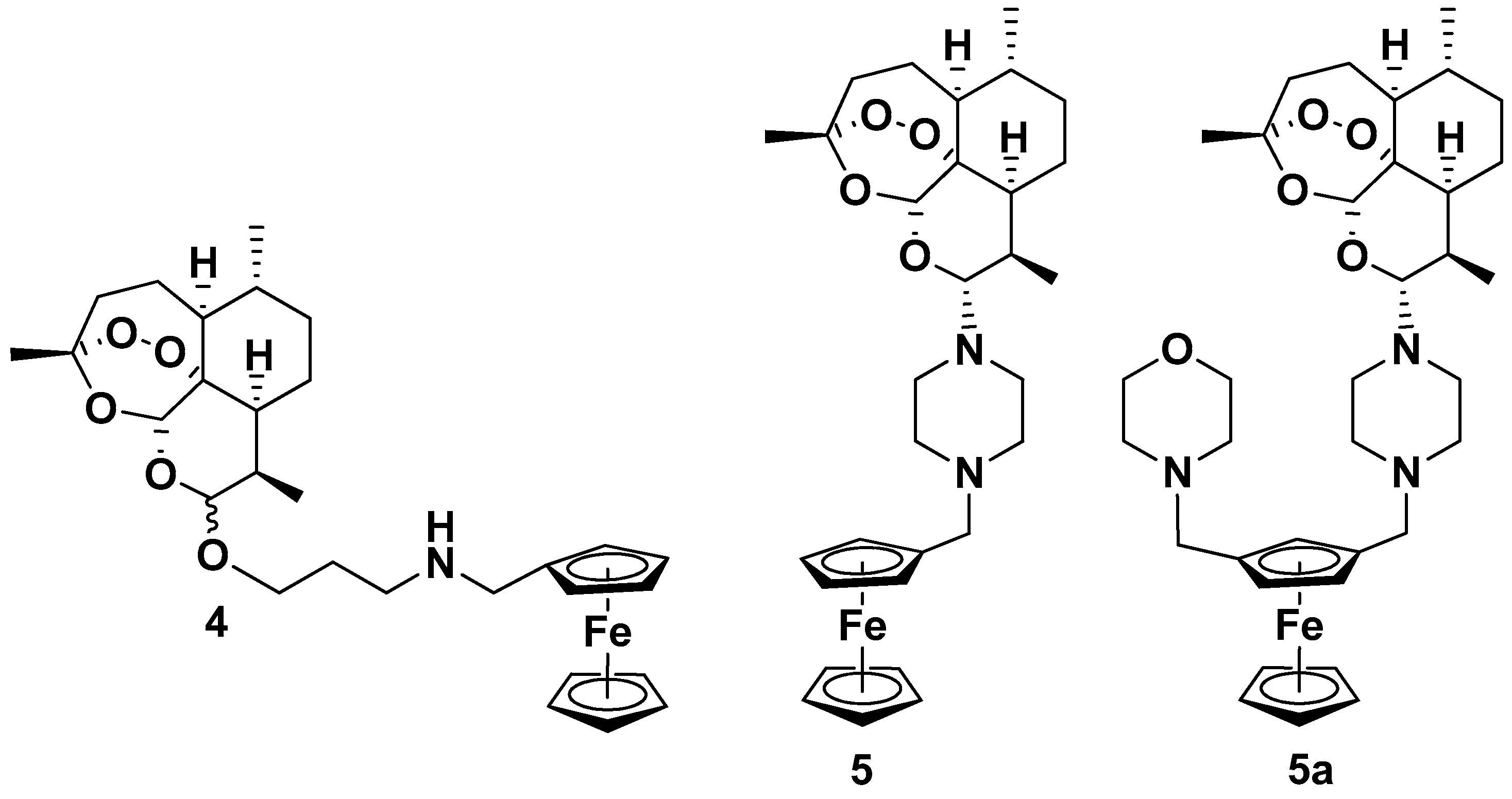
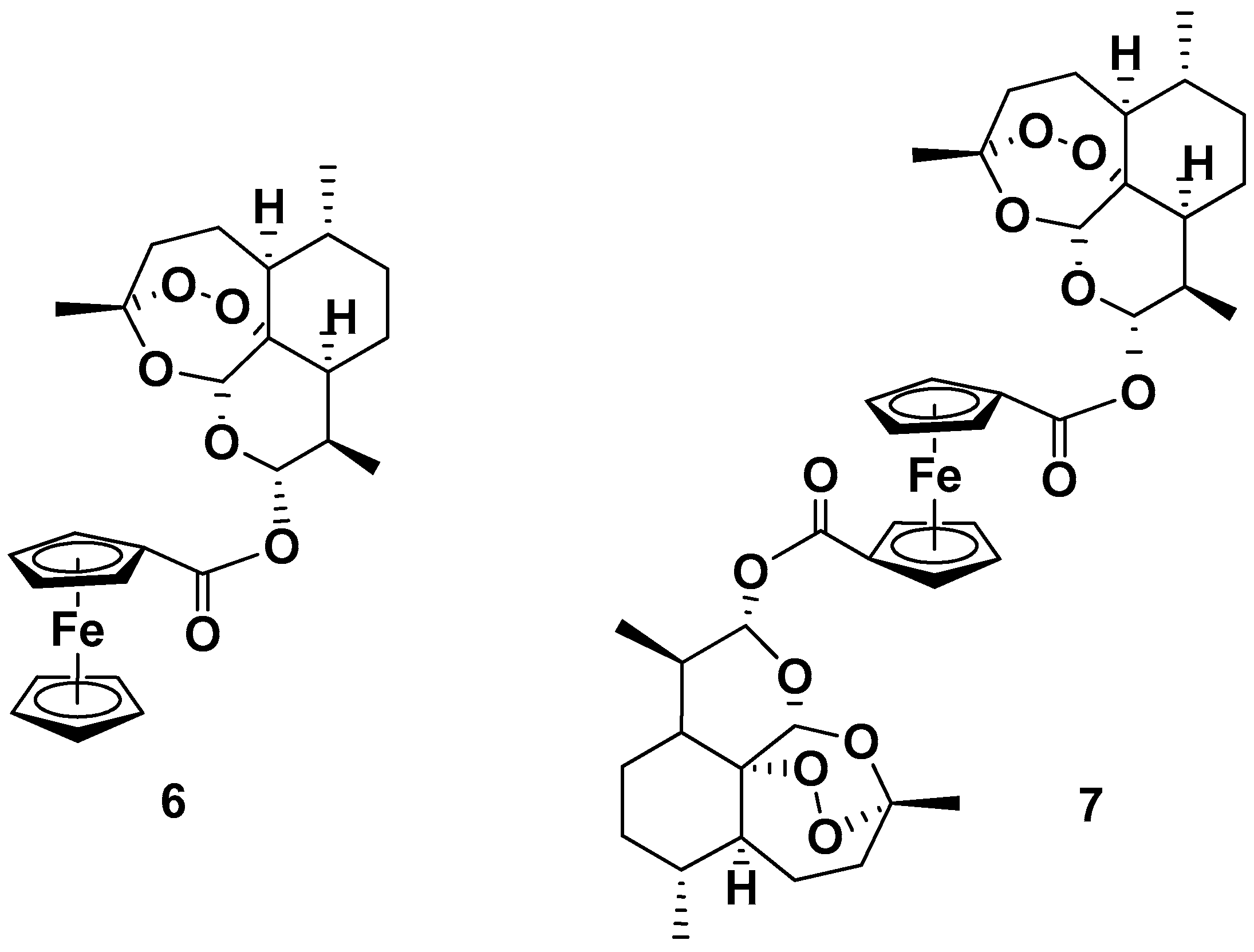

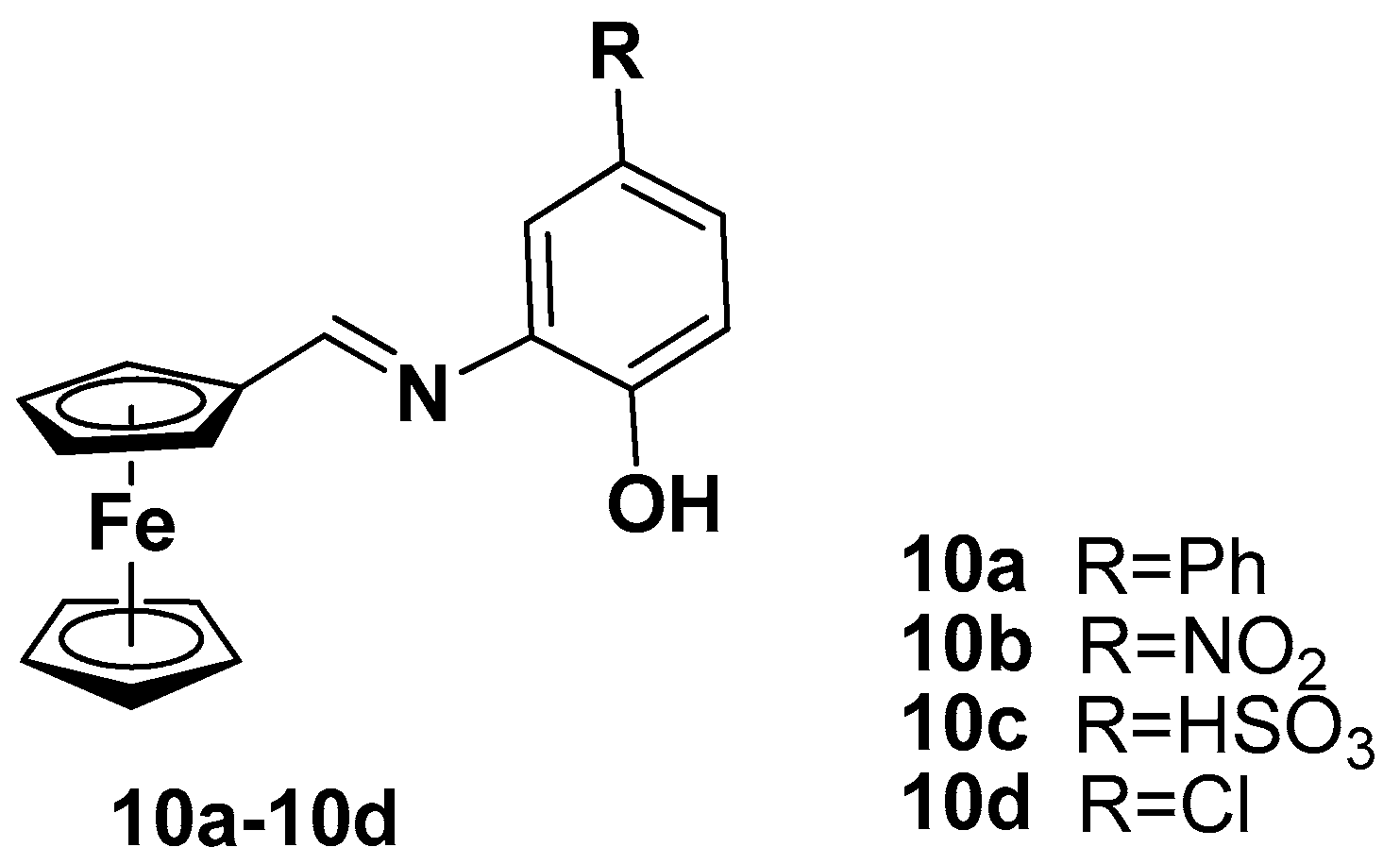


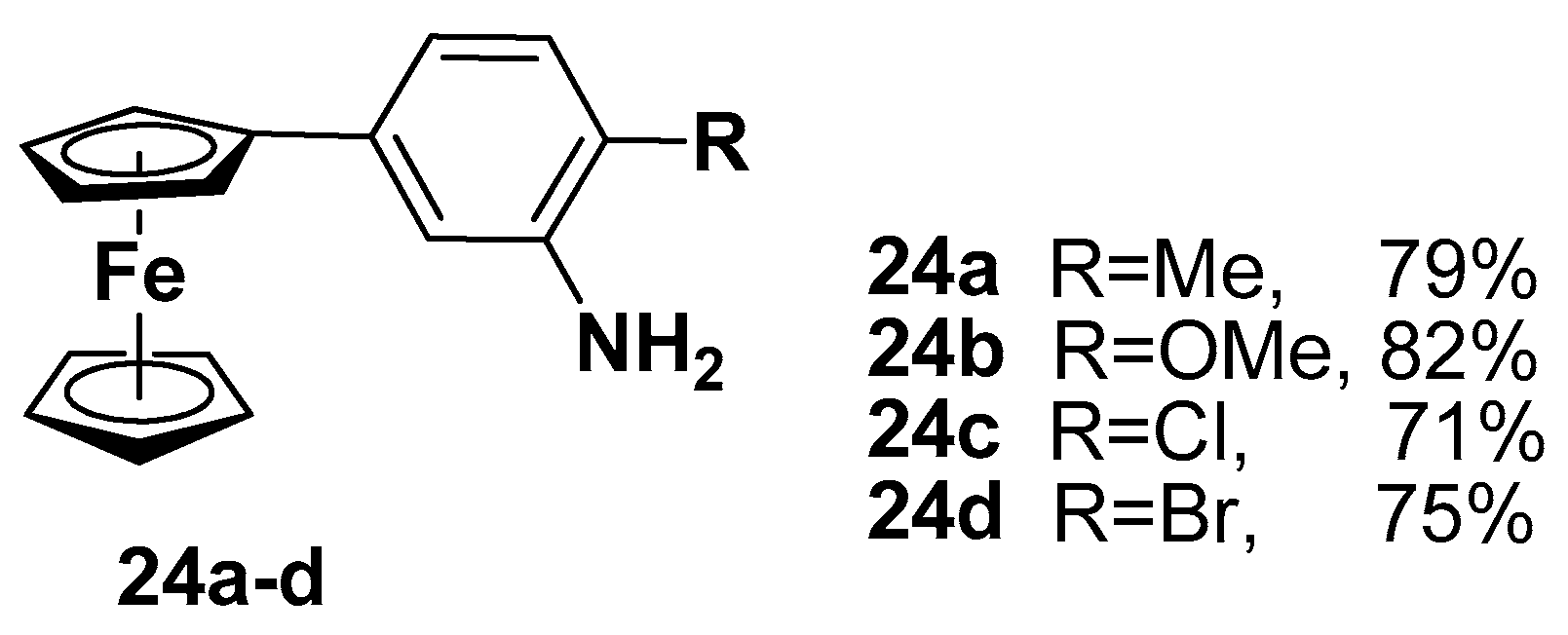
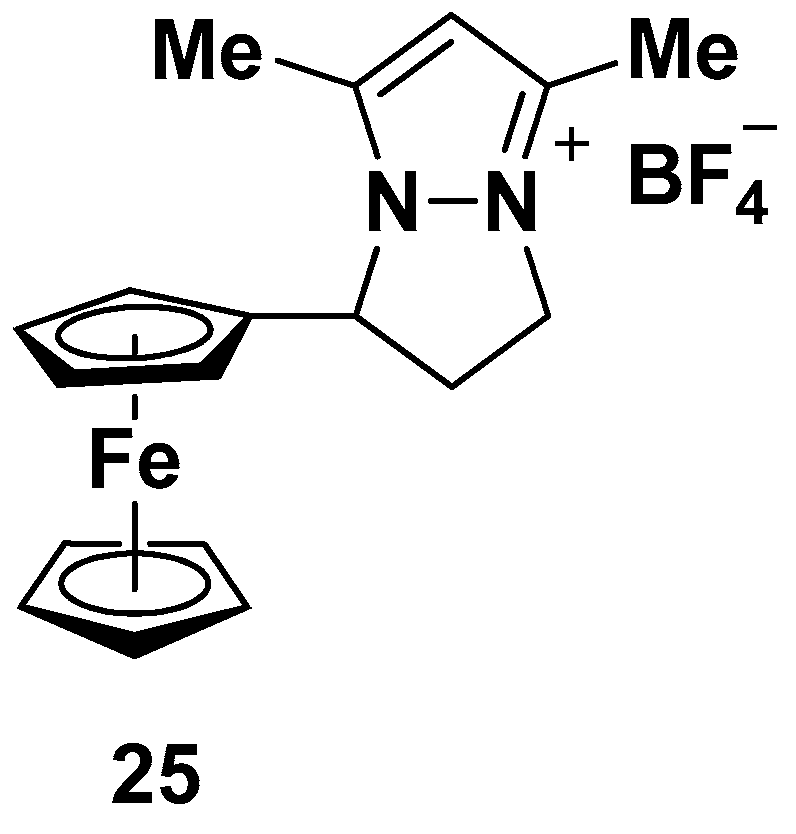
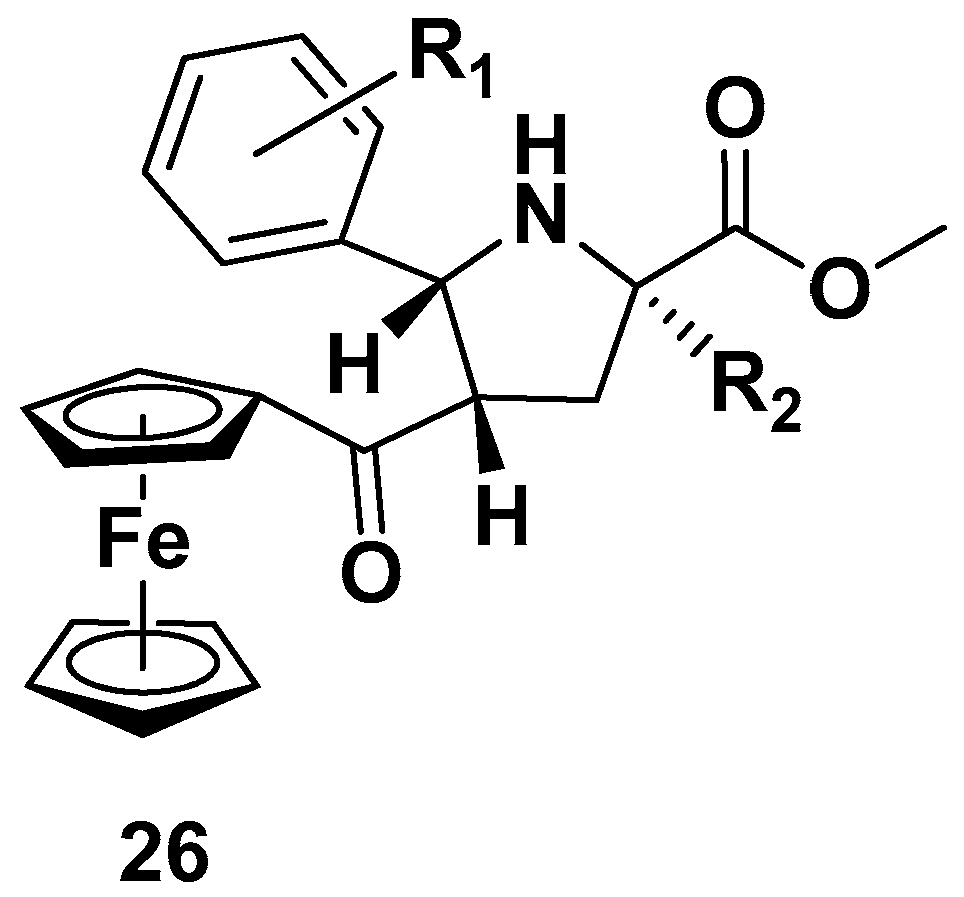
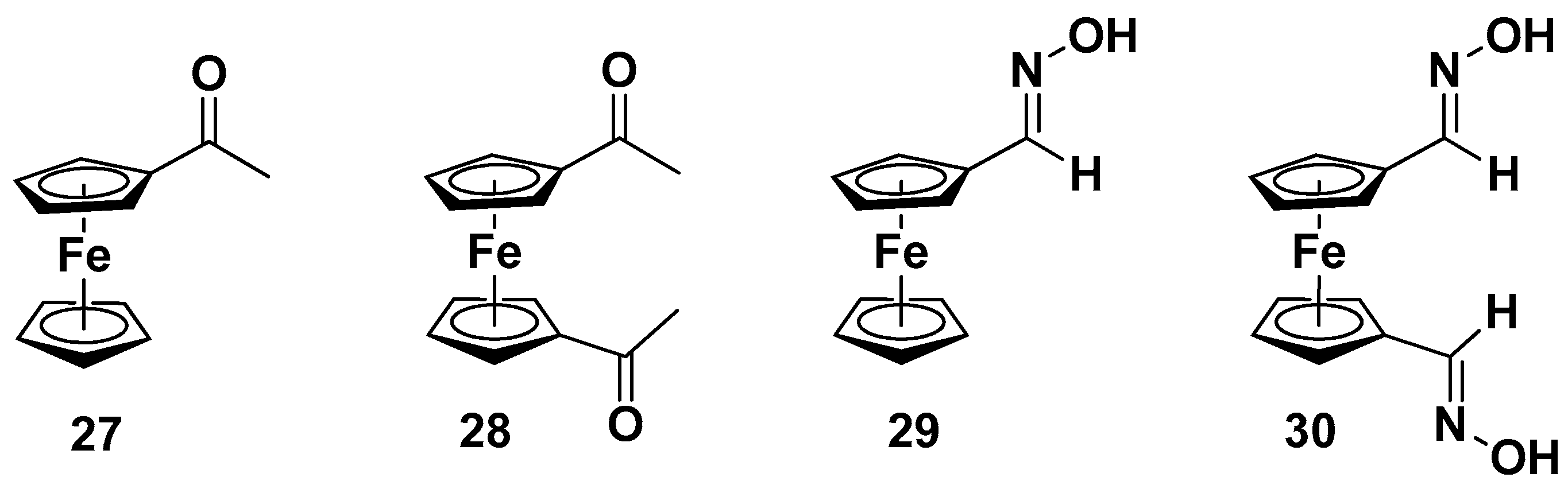
| Compound | P. falciparum, IC50 (nM) | ||
|---|---|---|---|
| HB3 | SGE2 | Dd2 | |
| Compound 4 | 12 ± 2 | 11 ± 5 | 14 ± 2 |
| QHS | 7 ± 1 | 9 ± 4 | 13 ± 3 |
| DQHS | 5 ± 1 | 9 ± 1 | 5 ± 2 |
| Compound | P. falciparum |
|---|---|
| EC50 (μM) | |
| Betulin | 3.938 |
| Betulinic acid | 1.419 |
| 8 | 27.854 |
| 9 | 11.752 |
| FcCOOH | 91 |
| Compound | BE, kcal/mol |
|---|---|
| 10a | −6.00 |
| 10b | −5.20 |
| 10c | −4.64 |
| 10d | −5.01 |
| Dexamethasone | −6.69 |
| Hydroxychloroquine | −5.07 |
| Favipiravir | −3.77 |
| Remdesivire | −1.41 |
| Fc–Hormone Conjugates | MCF-7 (µM) | T-47D (µM) | MDA-MB-231 (µM) |
|---|---|---|---|
| 12 | 15 (1) | 8 (2) | 41 (1) |
| 13 | 22 (4) | 27 (3) | 29 (1) |
| 14 | 32 (3) | 34 (3) | 34 (2) |
| Compound | HEK293 | Jurkat | A549 | MCF-7 | SH-SY-5Y |
|---|---|---|---|---|---|
| 15a | >100 | >100 | >100 | >100 | >100 |
| 15b | 37.52 ± 11.0 | 59.3 ± 8.40 | 33.11 ± 5.14 | 81.02 ± 8.13 | 22.31 ± 3.05 |
| 15c | 22.76 ± 1.38 | 13.85 ± 5.47 | 42.10 ± 12.21 | 32.09 ± 1.93 | 28.94 ± 3.88 |
| 15d | 55.90 ± 2.36 | 15.29 ± 4.09 | >100 | >100 | 16.06 ± 5.46 |
| 15e | 49.29 ± 10.01 | 72.18 ± 3.38 | >100 | 63.34 ± 3.11 | 64.60 ± 5.33 |
| 15f | >100 | 48.39 ± 9.19 | >100 | >100 | >100 |
| Fluorouracil | 7.43 ± 0.83 | 0.70 ± 0.10 | 0.32 ±0.02 | 1.20 ± 0.09 | 1.97 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Snegur, L.V. Modern Trends in Bio-Organometallic Ferrocene Chemistry. Inorganics 2022, 10, 226. https://doi.org/10.3390/inorganics10120226
Snegur LV. Modern Trends in Bio-Organometallic Ferrocene Chemistry. Inorganics. 2022; 10(12):226. https://doi.org/10.3390/inorganics10120226
Chicago/Turabian StyleSnegur, Lubov V. 2022. "Modern Trends in Bio-Organometallic Ferrocene Chemistry" Inorganics 10, no. 12: 226. https://doi.org/10.3390/inorganics10120226
APA StyleSnegur, L. V. (2022). Modern Trends in Bio-Organometallic Ferrocene Chemistry. Inorganics, 10(12), 226. https://doi.org/10.3390/inorganics10120226