UV–Vis Transparent Conductive Film Based on Cross-Linked Ag Nanowire Network: A Design for Photoelectrochemical Device
Abstract
1. Introduction
2. Experimental Section
2.1. Preparation of AgTCFs Based on Interconnected AgNWs
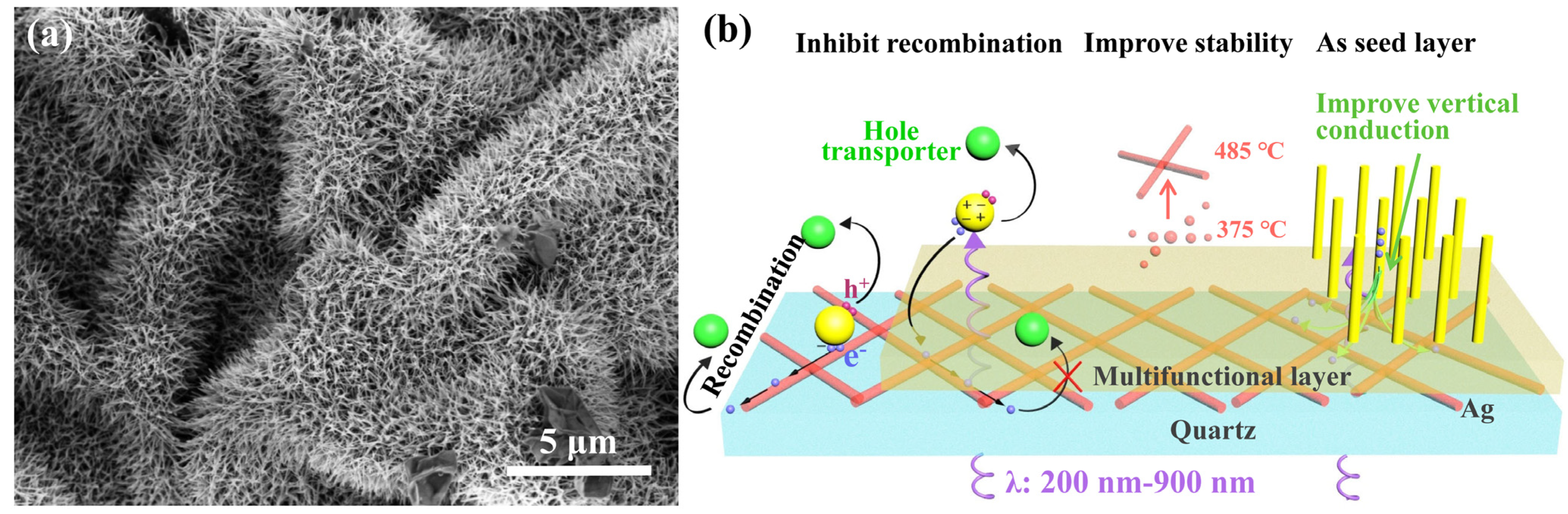
2.2. Preparation of Multifunctional Layer
2.3. Fabrication of TiO2 Nanoarray on the Multifunctional Layer
2.4. Characterization
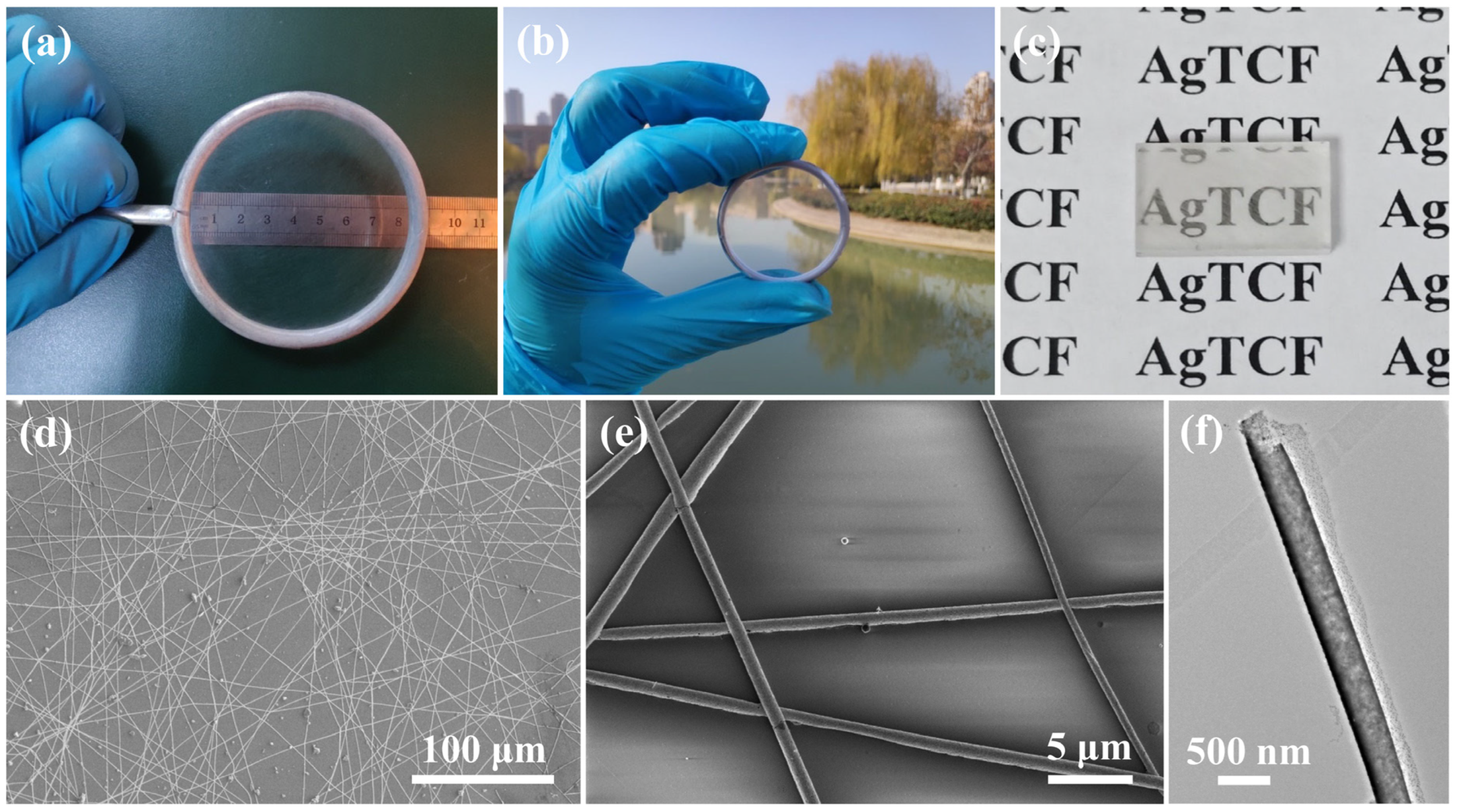
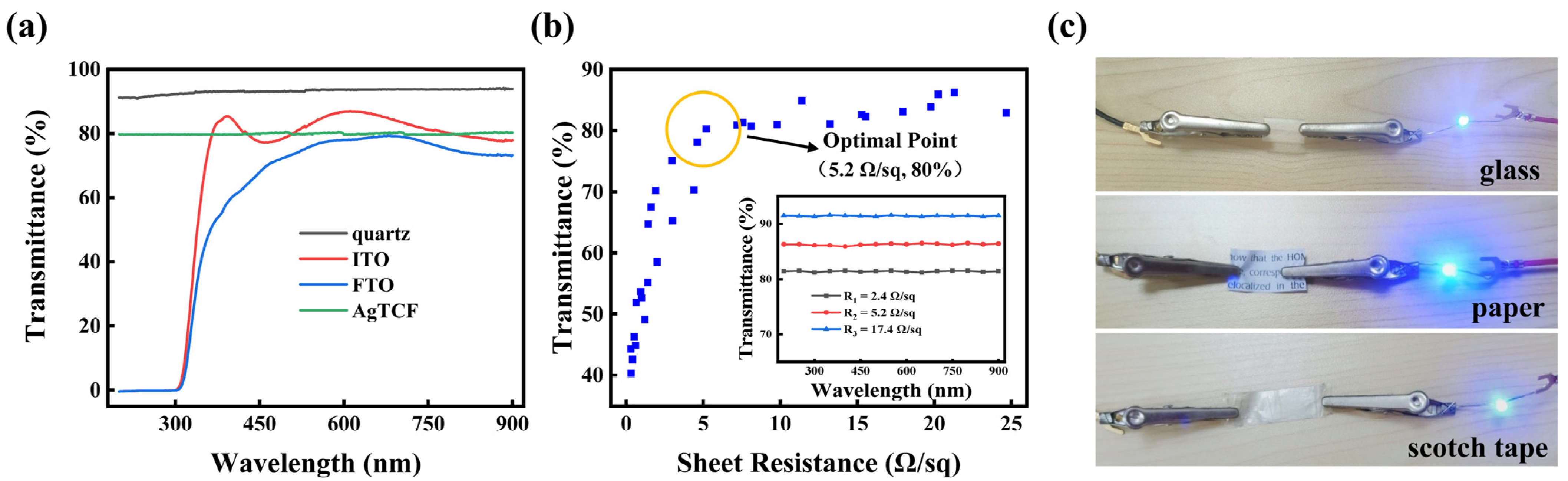
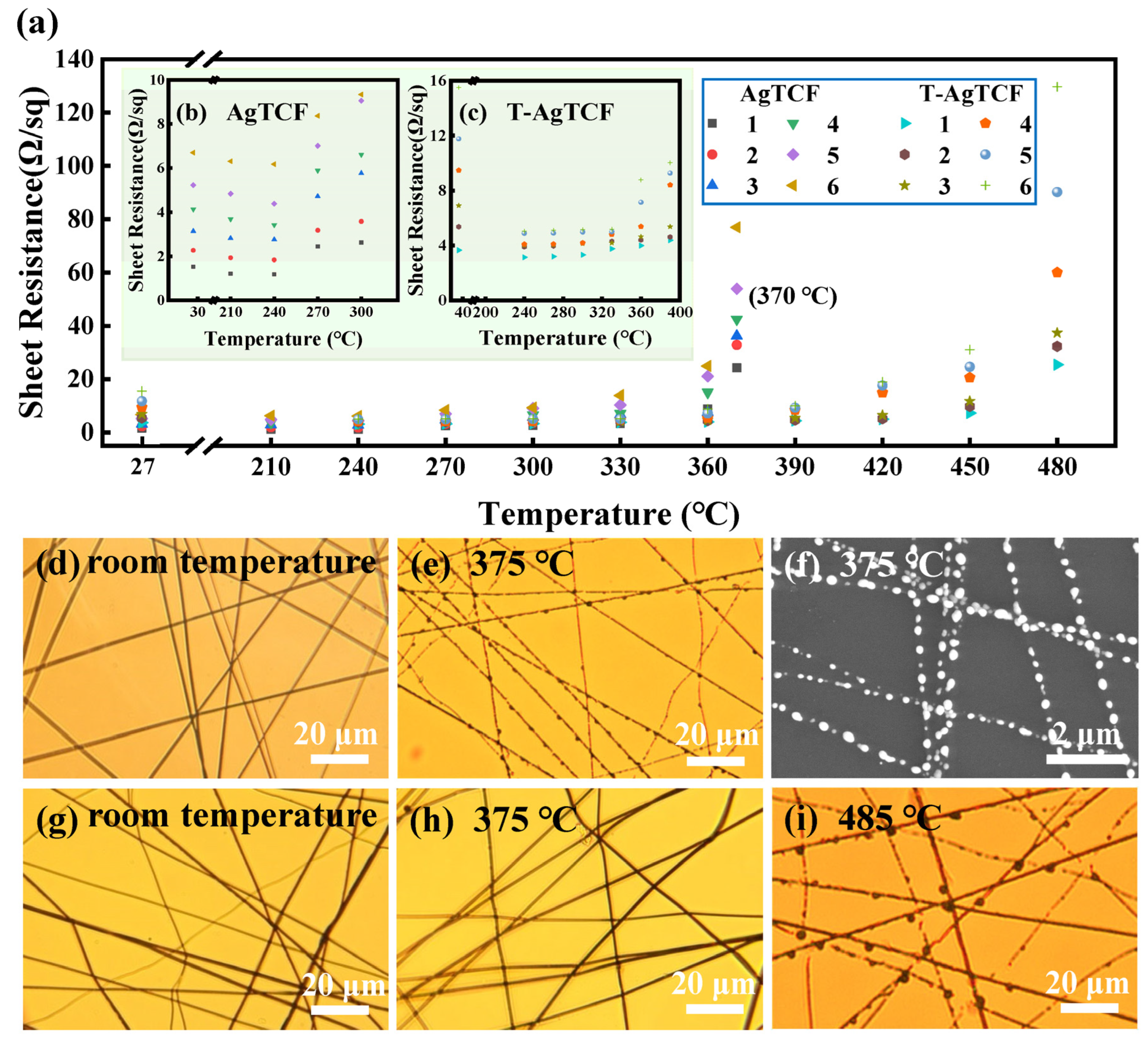
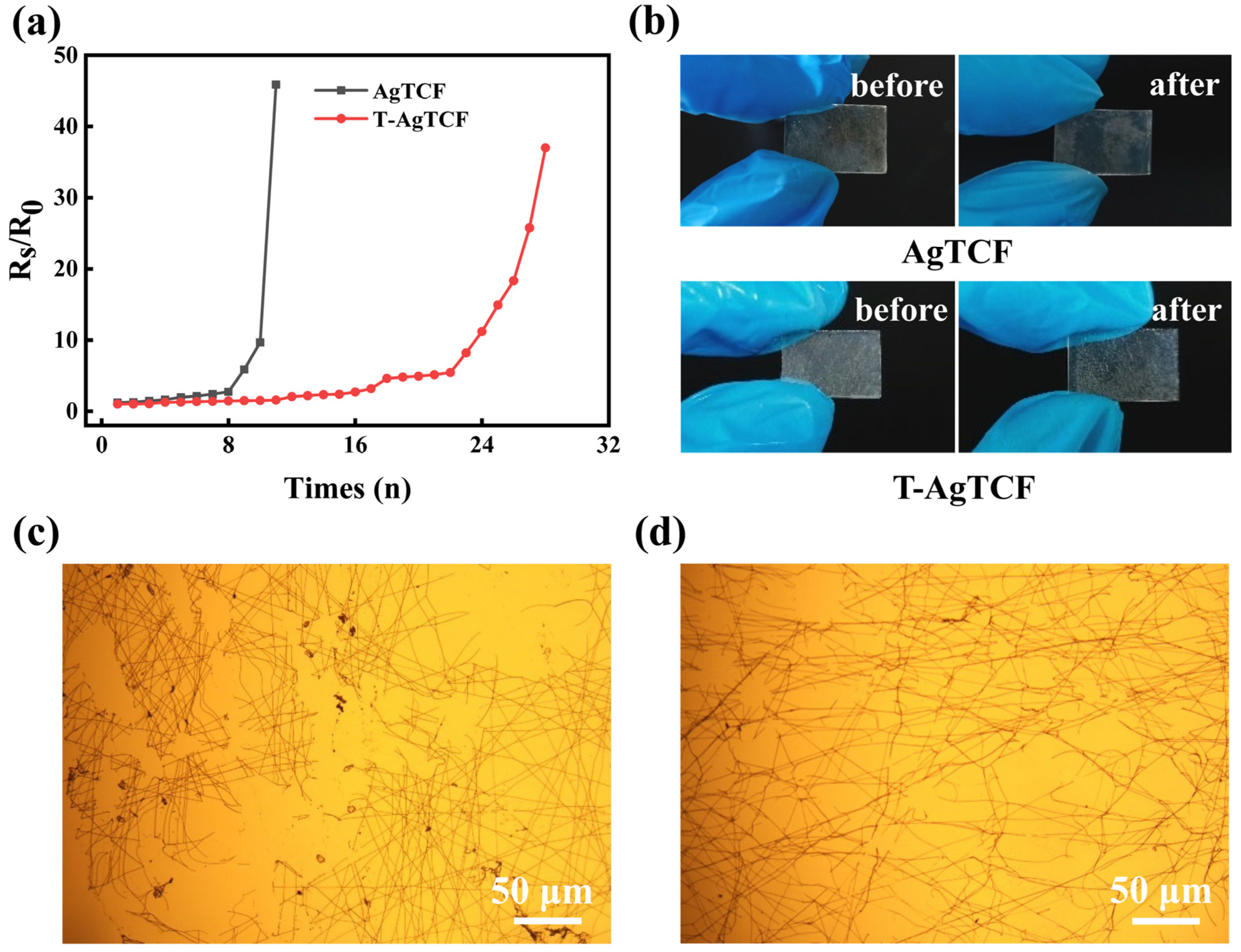
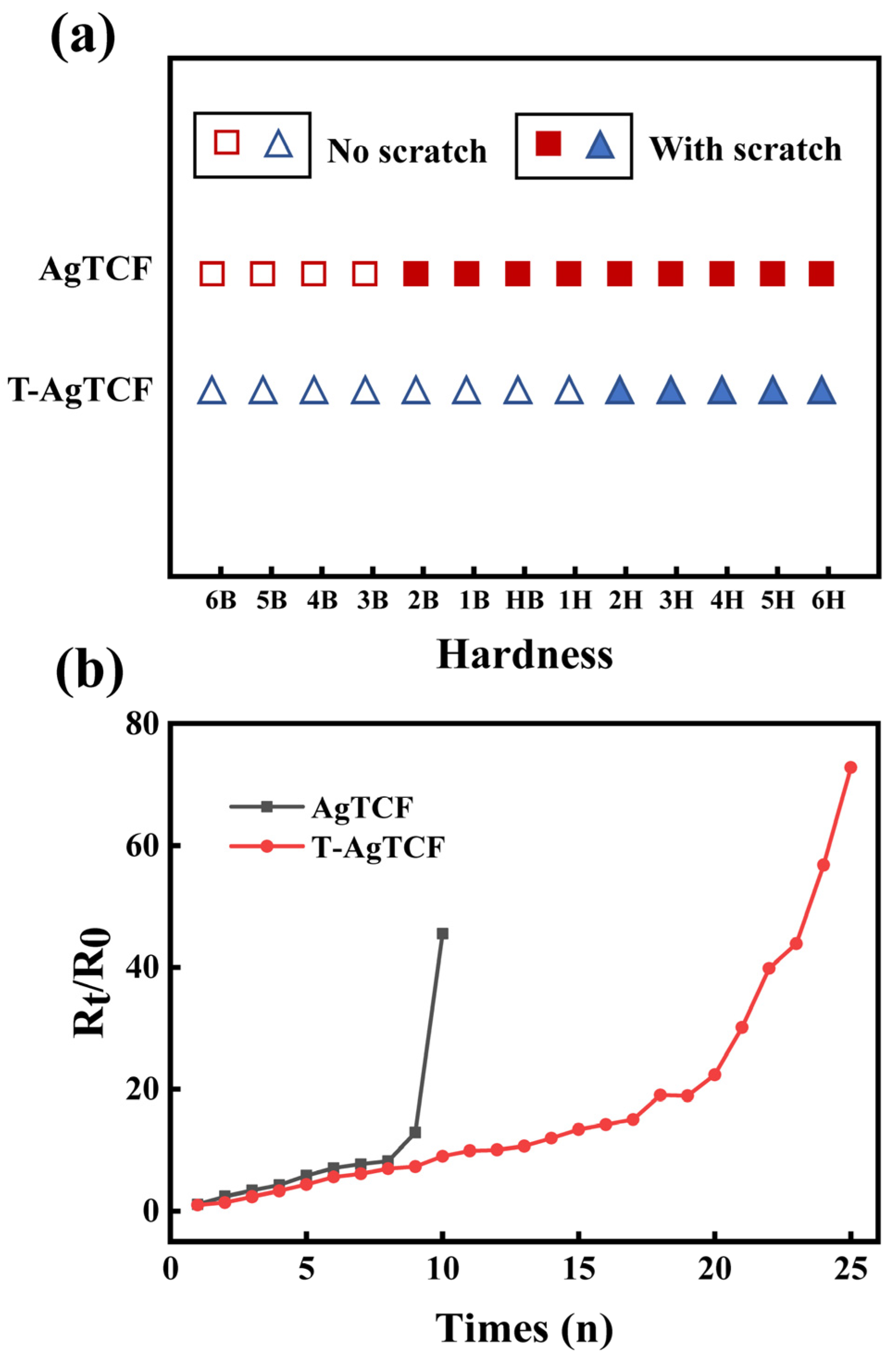
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Guo, W.X.; Xu, Z.J.; Zhang, F.Y.; Xie, S.Y.; Xu, H.Y.; Liu, X.Y. Recent Development of Transparent Conducting Oxide-Free Flexible Thin-Film Solar Cells. Adv. Funct. Mater. 2016, 26, 8855–8884. [Google Scholar] [CrossRef]
- Jo, Y.J.; Kim, C.; Lee, J.H.; Ko, M.S.; Jo, A.; Kim, J.Y.; Jung, W.G.; Lee, N.; Kim, Y.H.; Kim, J.; et al. Development of Patterned 1D Metal Nanowires with Adhesion Layer for Mesh Electrodes of Flexible Transparent Conductive Films for Touch Screen Panels. J. Nanosci. Nanotechnol. 2016, 16, 11586–11590. [Google Scholar] [CrossRef]
- Gu, Z.Z.; Tian, Y.; Geng, H.Z.; Rhen, D.S.; Ethiraj, A.S.; Zhang, X.C.; Jing, L.C.; Wang, T.; Xu, Z.H.; Yuan, X.T. Highly conductive sandwich-structured CNT/PEDOT:PSS/CNT transparent conductive films for OLED electrodes. Appl. Nanosci. 2019, 9, 1971–1979. [Google Scholar] [CrossRef]
- Li, D.; Liu, X.; Chen, X.; Lai, W.Y.; Huang, W. A Simple Strategy towards Highly Conductive Silver-Nanowire Inks for Screen-Printed Flexible Transparent Conductive Films and Wearable Energy-Storage Devices. Adv. Mater. Technol. 2019, 4, 1900196. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Han, W.H.; Zhao, B.; Chen, L.L.; Teng, F.; Li, X.D.; Gao, C.T.; Zhou, J.Y.; Xie, E.Q. Performance optimization of self-powered ultraviolet detectors based on photoelectrochemical reaction by utilizing dendriform titanium dioxide nanowires as photoanode. Sol. Energ. Mater. Sol. C 2015, 140, 376–381. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Chen, L.L.; Wang, Y.Q.; He, Y.M.; Pan, X.J.; Xie, E.Q. An overview on emerging photoelectrochemical self-powered ultraviolet photodetectors. Nanoscale 2016, 8, 50–73. [Google Scholar] [CrossRef]
- Zhang, M.X.; Wang, Y.Q.; Teng, F.; Chen, L.L.; Li, J.; Zhou, J.Y.; Pan, X.J.; Xie, E.Q. A photoelectrochemical type self-powered ultraviolet photodetector based on GaN porous films. Mater. Lett. 2016, 162, 117–120. [Google Scholar] [CrossRef]
- Cao, C.L.; Hu, C.G.; Wang, X.; Wang, S.X.; Tian, Y.S.; Zhang, H.L. UV sensor based on TiO2 nanorod arrays on FTO thin film. Sens. Actuators B Chem. 2011, 156, 114–119. [Google Scholar] [CrossRef]
- Gossen, K.; Ehrmann, A. Influence of FTO glass cleaning on DSSC performance. Optik 2019, 183, 253–256. [Google Scholar] [CrossRef]
- Goak, J.C.; Lee, S.H.; Lee, N. Effect of purification on the electrical properties of transparent conductive films fabricated from single-walled carbon nanotubes. Diamond Relat. Mater. 2020, 106, 107815. [Google Scholar] [CrossRef]
- Ma, Y.J.; Zhi, L.J. Graphene-Based Transparent Conductive Films: Material Systems, Preparation and Applications. Small Methods 2019, 3, 1800199. [Google Scholar] [CrossRef]
- Zhou, B.; Li, Y.H.; Zheng, G.Q.; Dai, K.; Liu, C.T.; Ma, Y.; Zhang, J.X.; Wang, N.; Shen, C.Y.; Guo, Z.H. Continuously fabricated transparent conductive polycarbonate/carbon nanotube nanocomposite films for switchable thermochromic applications. J. Mater. Chem. C 2018, 6, 8360–8371. [Google Scholar] [CrossRef]
- Jin, Y.; Cheng, Y.; Deng, D.; Jiang, C.; Qi, T.; Yang, D.; Xiao, F. Site-selective growth of patterned silver grid networks as flexible transparent conductive film by using poly(dopamine) at room temperature. ACS Appl. Mater. Interfaces 2014, 6, 1447–1453. [Google Scholar] [CrossRef]
- Yang, X.; Hu, X.; Wang, Q.; Xiong, J.; Yang, H.; Meng, X.; Tan, L.; Chen, L.; Chen, Y. Large-Scale Stretchable Semiembedded Copper Nanowire Transparent Conductive Films by an Electrospinning Template. ACS Appl. Mater. Interfaces 2017, 9, 26468–26475. [Google Scholar] [CrossRef]
- Hoeng, F.; Denneulin, A.; Krosnicki, G.; Bras, J. Positive impact of cellulose nanofibrils on silver nanowire coatings for transparent conductive films. J. Mater. Chem. C 2016, 4, 10945–10954. [Google Scholar] [CrossRef]
- Rosli, N.N.; Ibrahim, M.A.; Ludin, N.A.; Teridi, M.A.M.; Sopian, K. A review of graphene based transparent conducting films for use in solar photovoltaic applications. Renew. Sust. Energ. Rev. 2019, 99, 83–99. [Google Scholar] [CrossRef]
- Rathmell, A.R.; Bergin, S.M.; Hua, Y.L.; Li, Z.Y.; Wiley, B.J. The growth mechanism of copper nanowires and their properties in flexible, transparent conducting films. Adv. Mater. 2010, 22, 3558–3563. [Google Scholar] [CrossRef]
- Sanchez-Iglesias, A.; Rivas-Murias, B.; Grzelczak, M.; Perez-Juste, J.; Liz-Marzan, L.M.; Rivadulla, F.; Correa-Duarte, M.A. Highly transparent and conductive films of densely aligned ultrathin Au nanowire monolayers. Nano Lett. 2012, 12, 6066–6070. [Google Scholar] [CrossRef]
- Lee, J.; Lee, P.; Lee, H.; Lee, D.; Lee, S.S.; Ko, S.H. Very long Ag nanowire synthesis and its application in a highly transparent, conductive and flexible metal electrode touch panel. Nanoscale 2012, 4, 6408–6414. [Google Scholar] [CrossRef]
- Azani, M.R.; Hassanpour, A.; Torres, T. Benefits, Problems, and Solutions of Silver Nanowire Transparent Conductive Electrodes in Indium Tin Oxide (ITO)-Free Flexible Solar Cells. Adv. Energy Mater. 2020, 10, 2002536. [Google Scholar] [CrossRef]
- Hu, L.; Kim, H.S.; Lee, J.Y.; Peumans, P.; Cui, Y. Scalable coating and properties of transparent, flexible, silver nanowire electrodes. ACS Nano 2010, 4, 2955–2963. [Google Scholar] [CrossRef]
- Hong, S.J.; Kim, J.W.; Kim, Y.H. Solution-processed silver nanowire/indium-tin-oxide nanoparticle hybrid transparent conductors with high thermal stability. J. Nanosci. Nanotechnol. 2014, 14, 9504–9509. [Google Scholar] [CrossRef]
- Wu, H.; Kong, D.; Ruan, Z.; Hsu, P.C.; Wang, S.; Yu, Z.; Carney, T.J.; Hu, L.; Fan, S.; Cui, Y. A transparent electrode based on a metal nanotrough network. Nat. Nanotechnol. 2013, 8, 421–425. [Google Scholar] [CrossRef]
- Nam, S.; Song, M.; Kim, D.H.; Cho, B.; Lee, H.M.; Kwon, J.D.; Park, S.G.; Nam, K.S.; Jeong, Y.; Kwon, S.H.; et al. Ultrasmooth, extremely deformable and shape recoverable Ag nanowire embedded transparent electrode. Sci. Rep. 2014, 4, 4788. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.; Jeong, U.; Park, J.W. Silver nanowire network embedded in polydimethylsiloxane as stretchable, transparent, and conductive substrates. J. Appl. Polym. Sci. 2016, 133, 43830. [Google Scholar] [CrossRef]
- He, X.; Duan, F.; Liu, J.; Lan, Q.; Wu, J.; Yang, C.; Yang, W.; Zeng, Q.; Wang, H. Transparent Electrode Based on Silver Nanowires and Polyimide for Film Heater and Flexible Solar Cell. Materials 2017, 10, 1362. [Google Scholar] [CrossRef]
- Moon, I.K.; Kim, J.I.; Lee, H.; Hur, K.; Kim, W.C.; Lee, H. 2D Graphene Oxide Nanosheets as an Adhesive Over-Coating Layer for Flexible Transparent Conductive Electrodes. Sci. Rep. 2013, 3, 1112. [Google Scholar] [CrossRef]
- Cai, Y.G.; Piao, X.Q.; Yao, X.J.; Nie, E.; Zhang, Z.J.; Sun, Z. A facile method to prepare silver nanowire transparent conductive film for heaters. Mater. Lett. 2019, 249, 66–69. [Google Scholar] [CrossRef]
- Li, Y.X.; Guo, S.L.; Yang, H.W.; Chao, Y.X.; Jiang, S.Z.; Wang, C. One-step synthesis of ultra-long silver nanowires of over 100 μm and their application in flexible transparent conductive films. RSC Adv. 2018, 8, 8057–8063. [Google Scholar] [CrossRef]
- Bramhankar, T.S.; Pawar, S.S.; Shaikh, J.S.; Gunge, V.C.; Beedri, N.I.; Baviskar, P.K.; Pathan, H.M.; Patil, P.S.; Kambale, R.C.; Pawar, R.S. Effect of Nickel-Zinc Co-doped TiO2 blocking layer on performance of DSSCs. J. Alloys Compd. 2020, 817, 152810. [Google Scholar] [CrossRef]
- Marimuthu, T.; Anandhan, N.; Thangamuthu, R.; Surya, S. Facile growth of ZnO nanowire arrays and nanoneedle arrays with flower structure on ZnO-TiO2 seed layer for DSSC applications. J. Alloys Compd. 2017, 693, 1011–1019. [Google Scholar] [CrossRef]
- Sangiorgi, A.; Bendoni, R.; Sangiorgi, N.; Sanson, A.; Ballarin, B. Optimized TiO2 blocking layer for dye-sensitized solar cells. Ceram. Int. 2014, 40, 10727–10735. [Google Scholar] [CrossRef]
- Teo, W.E.; Ramakrishna, S. A review on electrospinning design and nanofibre assemblies. Nanotechnology 2006, 17, R89–R106. [Google Scholar] [CrossRef]
- Subbiah, T.; Bhat, G.S.; Tock, R.W.; Parameswaran, S.; Ramkumar, S.S. Electrospinning of nanofibers. J. Appl. Polym. Sci. 2005, 96, 557–569. [Google Scholar] [CrossRef]
- Ghosh, D.S.; Martinez, L.; Giurgola, S.; Vergani, P.; Pruneri, V. Widely transparent electrodes based on ultrathin metals. Opt. Lett. 2009, 34, 325–327. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, S.Q.; Zhao, H.J.; Will, G.; Liu, P.R. An efficient and low-cost TiO2 compact layer for performance improvement of dye-sensitized solar cells. Electrochim. Acta 2009, 54, 1319–1324. [Google Scholar] [CrossRef]
- Han, H.S.; Kim, J.S.; Kim, D.H.; Han, G.S.; Jung, H.S.; Noh, J.H.; Hong, K.S. TiO2 nanocrystals shell layer on highly conducting indium tin oxide nanowire for photovoltaic devices. Nanoscale 2013, 5, 3520–3526. [Google Scholar] [CrossRef]
- Kim, H.J.; Jeon, J.D.; Kim, D.Y.; Lee, J.J.; Kwak, S.Y. Improved performance of dye-sensitized solar cells with compact TiO2 blocking layer prepared using low-temperature reactive ICP-assisted DC magnetron sputtering. J. Ind. Eng. Chem. 2012, 18, 1807–1812. [Google Scholar] [CrossRef]
- Horie, Y.; Daizaka, K.; Mukae, H.; Guo, S.R.; Nomiyama, T. Enhancement of photocurrent by columnar Nb-doped TiO2 compact layer in dye sensitized solar cells with low temperature process of dc sputtering. Electrochim. Acta 2016, 187, 348–357. [Google Scholar] [CrossRef]
- Wen, Y.H.; Zhu, Z.Z.; Zhu, R.Z.; Shao, G.F. Size effects on the melting of nickel nanowires: A molecular dynamics study. Physica E 2004, 25, 47–54. [Google Scholar] [CrossRef]
- Guisbiers, G.; Pereira, S. Theoretical investigation of size and shape effects on the melting temperature of ZnO nanostructures. Nanotechnology 2007, 18, 435710. [Google Scholar] [CrossRef]
- Feng, D.L.; Feng, Y.H.; Yuan, S.W.; Zhang, X.X.; Wang, G. Melting behavior of Ag nanoparticles and their clusters. Appl. Therm. Eng. 2017, 111, 1457–1463. [Google Scholar] [CrossRef]
- Zhang, L.; Zhu, Y.F.; He, Y.; Li, W.; Sun, H.B. Preparation and performances of mesoporous TiO2 film photocatalyst supported on stainless steel. Appl. Catal. B Environ. 2003, 40, 287–292. [Google Scholar] [CrossRef]
- Huo, K.; Zhang, X.; Fu, J.; Qian, G.; Xin, Y.; Zhu, B.; Ni, H.; Chu, P.K. Synthesis and field emission properties of rutile TiO2 nanowires arrays grown directly on a Ti metal self-source substrate. J. Nanosci. Nanotechnol. 2009, 9, 3341–3346. [Google Scholar] [CrossRef]
- Lagrange, M.; Sannicolo, T.; Munoz-Rojas, D.; Lohan, B.G.; Khan, A.; Anikin, M.; Jimenez, C.; Bruckert, F.; Brechet, Y.; Bellet, D. Understanding the mechanisms leading to failure in metallic nanowire-based transparent heaters, and solution for stability enhancement. Nanotechnology 2017, 28, 055709. [Google Scholar] [CrossRef]
- Morgenstern, F.S.F.; Kabra, D.; Massip, S.; Brenner, T.J.K.; Lyons, P.E.; Coleman, J.N.; Friend, R.H. Ag-nanowire films coated with ZnO nanoparticles as a transparent electrode for solar cells. Appl. Phys. Lett. 2011, 99, 242. [Google Scholar] [CrossRef]
- Tokuno, T.; Nogi, M.; Karakawa, M.; Jiu, J.T.; Nge, T.T.; Aso, Y.; Suganuma, K. Fabrication of silver nanowire transparent electrodes at room temperature. Nano Res. 2011, 4, 1215–1222. [Google Scholar] [CrossRef]
- Bräuer, G.; Szyszka, B.; Vergöhl, M.; Bandorf, R. Magnetron sputtering-Milestones of 30 years. Vacuum 2010, 84, 1354–1359. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, P.; Wang, Y.; Liu, M.; Zhang, M.; Wu, W.; Wang, H.; Luo, D. UV–Vis Transparent Conductive Film Based on Cross-Linked Ag Nanowire Network: A Design for Photoelectrochemical Device. Inorganics 2022, 10, 164. https://doi.org/10.3390/inorganics10100164
Ren P, Wang Y, Liu M, Zhang M, Wu W, Wang H, Luo D. UV–Vis Transparent Conductive Film Based on Cross-Linked Ag Nanowire Network: A Design for Photoelectrochemical Device. Inorganics. 2022; 10(10):164. https://doi.org/10.3390/inorganics10100164
Chicago/Turabian StyleRen, Peiling, Youqing Wang, Menghan Liu, Miaomiao Zhang, Wenxuan Wu, Hongjun Wang, and Daobin Luo. 2022. "UV–Vis Transparent Conductive Film Based on Cross-Linked Ag Nanowire Network: A Design for Photoelectrochemical Device" Inorganics 10, no. 10: 164. https://doi.org/10.3390/inorganics10100164
APA StyleRen, P., Wang, Y., Liu, M., Zhang, M., Wu, W., Wang, H., & Luo, D. (2022). UV–Vis Transparent Conductive Film Based on Cross-Linked Ag Nanowire Network: A Design for Photoelectrochemical Device. Inorganics, 10(10), 164. https://doi.org/10.3390/inorganics10100164





