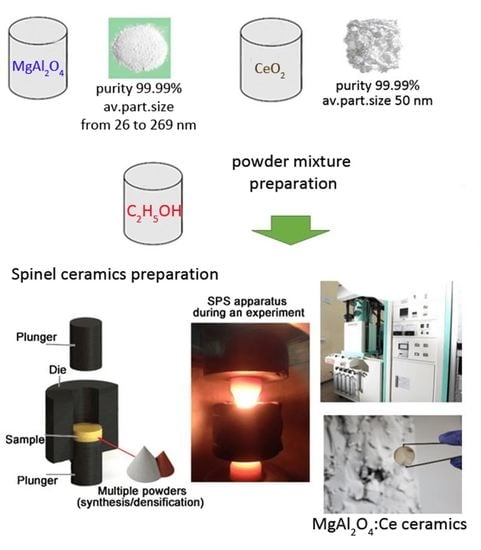
Structural and Luminescent Peculiarities of Spark Plasma Sintered Transparent MgAl2O4 Spinel Ceramics Doped with Cerium Ions
Abstract
:1. Introduction
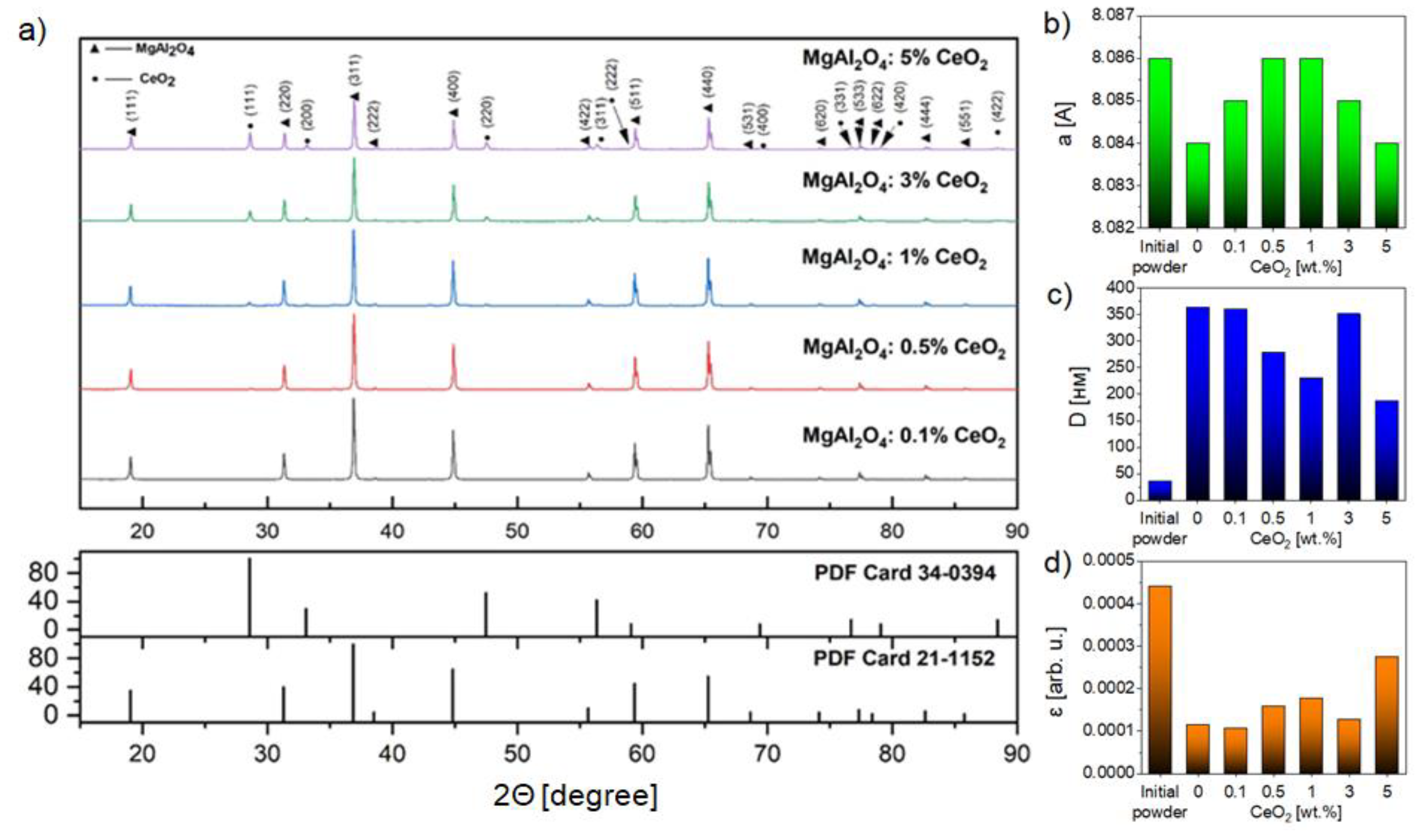
2. Results and Discussion
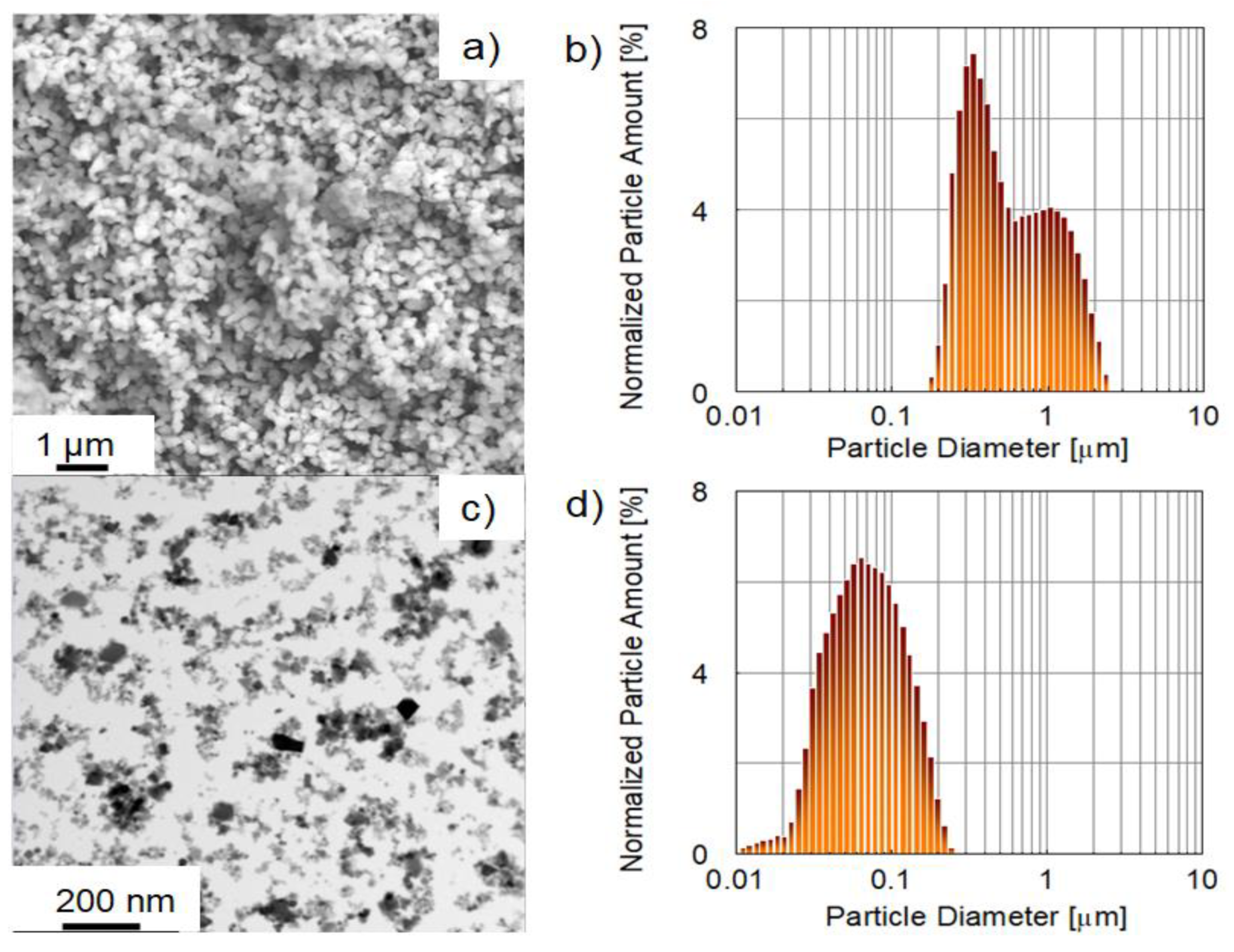
2.1. Powders Characterization
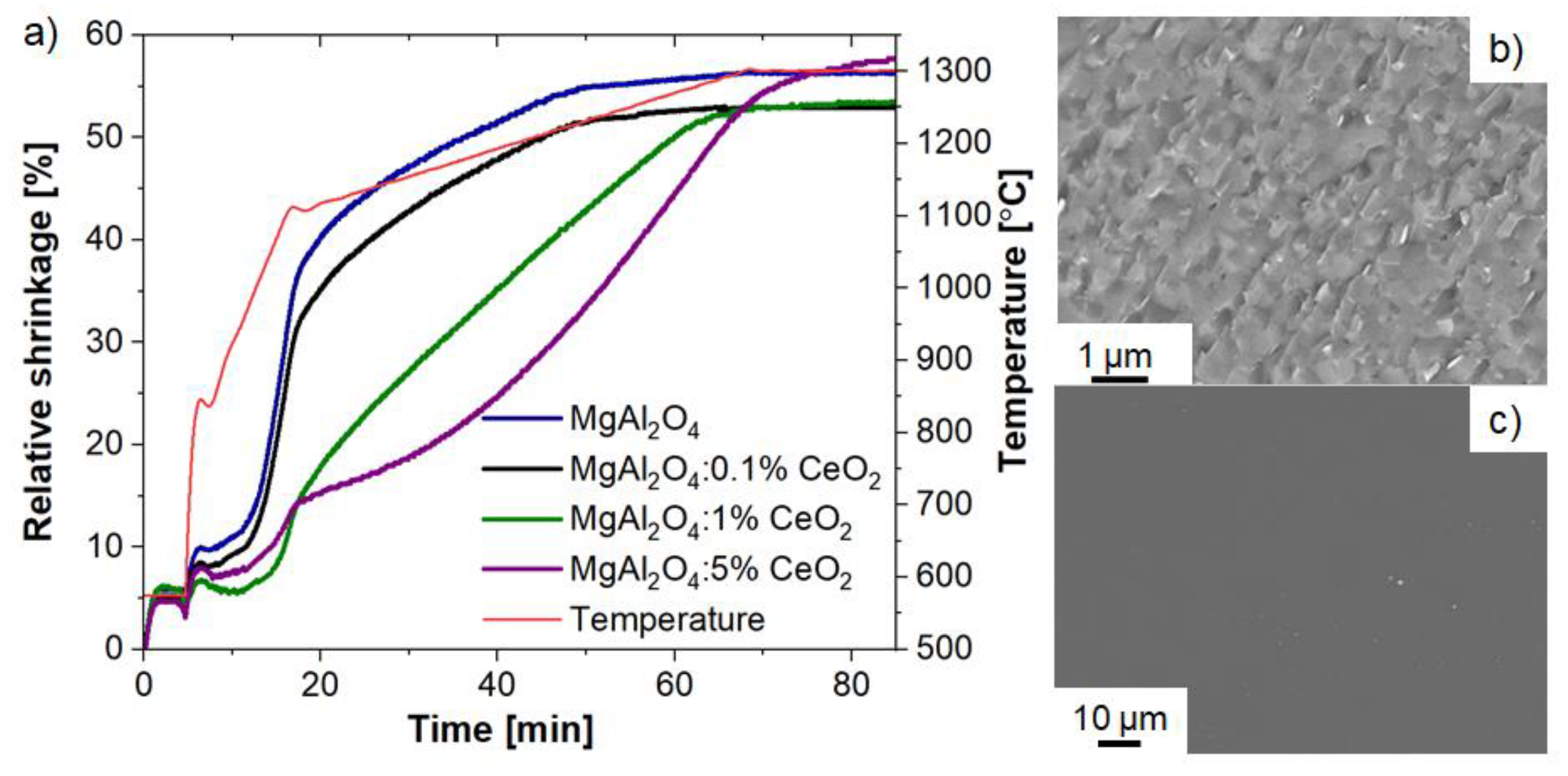
2.2. Ceramics Characterization
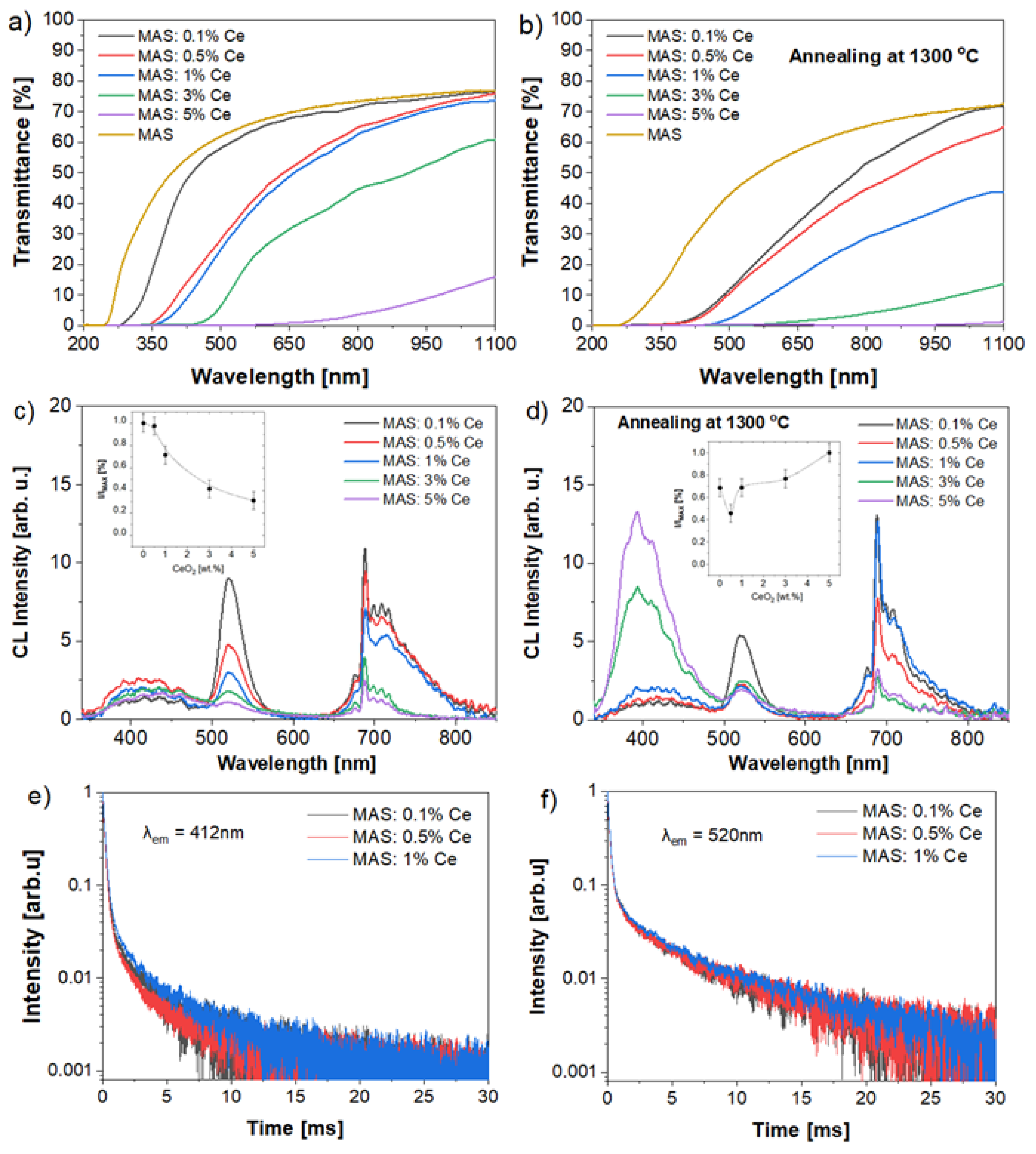
2.3. Optical and Luminescent Properties
3. Materials and Methods
3.1. MgAl2O4 Ceramics Preparation
3.2. Experimental
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rubat du Merac, M.; Kleebe, H.-J.; Müller, M.M.; Reimanis, I.E. Fifty years of research and development coming to fruition; unraveling the complex interactions during processing of transparent magnesium aluminate (MgAl2O4) spinel. J. Am. Ceram. Soc. 2013, 96, 3341–3365. [Google Scholar] [CrossRef]
- Xiao, Z.; Yu, S.; Li, Y.; Ruan, S.; Kong, L.B.; Huang, Q.; Huang, Z.; Zhou, K.; Su, H.; Yao, Z.; et al. Materials development and potential applications of transparent ceramics: A review. Mater. Sci. Eng. R Rep. 2020, 139, 100518. [Google Scholar] [CrossRef]
- Li, J.G.; Ikegami, T.; Lee, J.-H. Fabrication of translucent magnesium aluminum spinel ceramics. J. Am. Ceram. Soc. 2000, 83, 2866–2868. [Google Scholar] [CrossRef]
- Klym, H.; Karbovnyk, I.; Piskunov, S.; Popov, A. Positron Annihilation Lifetime Spectroscopy Insight on Free Volume Conversion of Nanostructured MgAl2O4 Ceramics. Nanomaterials 2021, 11, 3373. [Google Scholar] [CrossRef] [PubMed]
- Tokariev, O.; Steinbrech, R.W.; Schnetter, L.; Malzbender, J. Micro-and macro-mechanical testing of transparent MgAl2O4 spinel. J. Mater. Sci. 2012, 47, 4821–4826. [Google Scholar] [CrossRef]
- Shi, Z.; Zhao, Q.; Guo, B.; Ji, T.; Wang, H. A review on processing polycrystalline magnesium aluminate spinel (MgAl2O4): Sintering techniques, material properties and machinability. Mater. Design. 2020, 193, 108858. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, T.; Chang, X.; Wei, N.; Xu, W. Related mechanism of transparency in MgAl2O4 nano-ceramics prepared by sintering under high pressure and low temperature. J. Phys. D Appl. Phys. 2009, 42, 052002. [Google Scholar] [CrossRef]
- Wiglusz, R.J.; Boulon, G.; Guyot, Y.; Guzik, M.; Hreniak, D.; Strek, W. Structural and spectroscopic properties of Yb3+-doped MgAl2O4 nanocrystalline spinel. Dalton Trans. 2014, 43, 7752–7759. [Google Scholar] [CrossRef]
- Kato, T.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Thermally-stimulated luminescence and optical properties of Eu-doped MgAl2O4 transparent ceramics. J. Lumin. 2022, 251, 119136. [Google Scholar] [CrossRef]
- Katsumata, T.; Minowa, S.; Sakuma, T.; Yoshida, A.; Komuro, S.; Aizawa, H. X-ray Excited Optical Luminescence from Mn Doped Spinel Crystals. ECS Solid State Lett. 2014, 3, R23–R25. [Google Scholar] [CrossRef]
- Ramisetty, M.; Sastri, S.; Kashalikar, U.; Goldman, L.M.; Nag, N. Transparent polycrystalline cubic spinels protect and defend. Am. Ceram. Soc. Bull. 2013, 92, 20–25. [Google Scholar]
- Goldstein, A. Correlation between MgAl2O4-spinel structure, processing factors and functional properties of transparent parts (progress review). J. Eur. Ceram. Soc. 2012, 32, 2869–2886. [Google Scholar] [CrossRef]
- Alekseev, M.K.; Kulikova, G.I.; Rusin, M.Y.; Savanina, N.N.; Balabanov, S.S.; Belyaev, A.V.; Gavrishchuk, E.M.; Ivanov, A.V.; Rizakhanov, R.N. Transparent ceramics prepared from ultrapure magnesium aluminate spinel nanopowders by spark plasma sintering. Inorg. Mater. 2016, 52, 324–330. [Google Scholar] [CrossRef]
- Ganesh, I. A review on magnesium aluminate (MgAl2O4) spinel: Synthesis, processing and applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Zou, Y.; He, D.; Wei, X.; Yu, R.; Lu, T.; Chang, X.; Wang, S.; Lei, L. Nanosintering mechanism of MgAl2O4 transparent ceramics under high pressure. Mater. Chem. Phys. 2010, 123, 529–533. [Google Scholar] [CrossRef]
- Chen, Q.; Meng, C.; Lu, T.; Chang, X.; Ji, G.; Zhang, L.; Zhao, F. Enhancement of sintering ability of magnesium aluminate spinel (MgAl2O4) ceramic nanopowders by shock compression. Powder Technol. 2010, 200, 91–95. [Google Scholar] [CrossRef]
- Sutorik, A.C.; Gilde, G.; Swab, J.J.; Cooper, C.; Gamble, R.; Shanholtz, E. Transparent solid solution magnesium aluminate spinel polycrystalline ceramic with the alumina-rich composition MgO·1.2Al2O3. J. Am. Ceram. Soc. 2012, 95, 636–643. [Google Scholar] [CrossRef]
- Dericioglu, A.F.; Boccaccini, A.R.; Dlouhy, I.; Kagawa, Y. Effect of Chemical Composition on the Optical Properties and Fracture Toughness of Transparent Magnesium Aluminate Spinel Ceramics. Mater. Trans. 2005, 46, 996–1003. [Google Scholar] [CrossRef]
- Chaim, R.; Marder, R.; Estournes, C. Optically transparent ceramics by spark plasma sintering of oxide nanoparticles. Scr. Mater. 2010, 63, 211–214. [Google Scholar] [CrossRef]
- Jiang, D.T.; Hulbert, D.M.; Kuntz, J.D.; Anselmi-Tamburini, U.; Mukherjee, A.K. Spark plasma sintering: A high strain rate low temperature forming tool for ceramics. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Processing 2007, 463, 89–93. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Z. Transparent MgAl2O4 ceramic produced by spark plasma sintering. Scr. Mater. 2009, 61, 193–196. [Google Scholar] [CrossRef]
- Frage, N.; Hulbert, D.M.; Kuntz, J.D.; Anselmi-Tamburini, U.; Mukherjee, A.K. Spark plasma sintering (SPS) of transparent magnesium-aluminate spinel. J. Mater. Sci. 2007, 42, 3273–3275. [Google Scholar] [CrossRef]
- Lemanski, K.; Deren, P.J.; Walerczyk, W.; Strek, W.; Boulesteix, R.; Epherre, R.; Maˆtre, A. Spectroscopic and structural properties of MgAl2O4:Nd3 nanopowders and ceramics. J. Rare Earths 2014, 32, 265–268. [Google Scholar] [CrossRef]
- Kato, T.; Nakauchi, D.; Kawaguchi, N.; Yanagida, T. Optical, scintillation, and dosimetric properties of Dy-doped MgAl2O4 transparent ceramics. Optik 2020, 207, 164433. [Google Scholar] [CrossRef]
- Raja, E.A.; Menon, S.; Dhabekar, B.; Rawat, N.S.; Gundu Rao, T.K. Investigation of defect centres responsible for TL/OSL in MgAl2O4: Tb3+. J. Lumin. 2009, 129, 829–835. [Google Scholar] [CrossRef]
- Wiglusz, R.J.; Grzyb, T.; Lukowiak, A.; Głuchowski, P.; Lis, S.; Strek, W. Comparative studies on structural and luminescent properties of Eu3+: MgAl2O4 and Eu3+/Na+: MgAl2O4 nanopowders and nanoceramics. Opt. Mat. 2012, 35, 130–135. [Google Scholar] [CrossRef]
- He, L.; Fan, G.; Lei, M.; Lou, Z.; Zheng, S.; Su, C.; Zhang, T. Preparation of MgAl2O4/Ce: YAG transparent ceramics by hot-pressed sintering and its microstructure. Rare Met. Mater. Eng. 2013, 42, 463–466. [Google Scholar]
- Harris, D.C.; Johnson, L.F.; Seaver, R.; Lewis, T.; Turri, G.; Bass, M.A.; Zelmon, D.E.; Haynes, N. Optical and thermal properties of spinel with revised (increased) absorption at 4 top 5 μm wavelengths and comparison with sapphire. Opt. Eng. 2013, 52, 87113. [Google Scholar]
- Maia, A.S.; Stefani, R.; Kodaira, C.A.; Felinto, M.C.F.C.; Teotonio, E.E.S.; Brito, H.F. Luminescent nanoparticles of MgAl2O4:Eu, Dy prepared by citrate sol-gel method. Opt. Mater. 2008, 31, 440–444. [Google Scholar] [CrossRef]
- Perkins, J.M.; West, G.D.; Lewis, M.H. Analysis and spectroscopy of rare earth doped magnesium aluminate spinel. Adv. Appl. Ceram. 2005, 104, 131–134. [Google Scholar] [CrossRef]
- Sokol, M.; Halabi, M.; Kalabukhov, S.; Frage, N. Nano-structured MgAl2O 4 spinel consolidated by high pressure spark plasma sintering (HPSPS). J. Eur. Ceram. Soc. 2017, 37, 755–762. [Google Scholar] [CrossRef]
- Bonnefont, G.; Fantozzi, G.; Trombert, S.; Bonneau, L. Fine-grained transparent MgAl2O4 spinel obtained by spark plasma sintering of commercially available nanopowders. Ceram. Int. 2012, 38, 131–140. [Google Scholar] [CrossRef]
- Wang, Z.; Jiao, S.; Xu, Y.; Zhang, Q.; Chen YPang, G.; Feng, S. Effects of heat-treatment on photoluminescence properties of MgAl2O4:Eu3+ red phosphors synthesized by a solution combustion method. J. Lumin. 2019, 211, 108–113. [Google Scholar] [CrossRef]
- Singh, V.; Kumar Rai, V.; Watanabe, S.; Gundu Rao, T.K.; Badie, L.; Ledoux-Rak, I. Synthesis, characterization, optical absorption, luminescence and defect centers in Er3+ and Yb3+ co-doped MgAl2O4 phosphors. Appl. Phys. B 2012, 108, 437–446. [Google Scholar] [CrossRef]
- Wiglusz, R.; Grzyb, T. The effect of Tb3+ doping on the structure and spectroscopic properties of MgAl2O4 nanopowders. Opt. Mater. 2011, 33, 1506–1513. [Google Scholar] [CrossRef]
- Singh, V.; Sivaramaiah, G.; Rao, J.L.; Kim, S.H. Luminescence and electron paramagnetic resonance investigation on ultraviolet emitting Gd doped MgAl2O4 phosphors. J. Lumin. 2013, 143, 162–168. [Google Scholar] [CrossRef]
- Golyeva, E.; Kolesnikov, I.; Lähderanta, E.; Kurochkin, A.; Mikhailov, M. Effect of synthesis conditions on structural, morphological and luminescence properties of MgAl2O4:Eu3+ nanopowders. J. Lumin. 2018, 194, 387–393. [Google Scholar] [CrossRef]
- Dereń, P.; Maleszka-Bagińska, K.; Głuchowski, P.; Małecka, M. Spectroscopic properties of Nd3+ in MgAl2O4 spinel nanocrystals. J. Alloy. Compd. 2012, 525, 39–43. [Google Scholar] [CrossRef]
- Golyeva, E.; Vaishlia, E.; Kurochkin, M.; Kolesnikov, E.Y.; Lähderanta, E.; Semencha, A.; Kolesnikov, I. Nd3+ concentration effect on luminescent properties of MgAl2O4 nanopowders synthesized by modified Pechini method. J. Solid State Chem. 2020, 289, 121486. [Google Scholar] [CrossRef]
- Balabanov, S.S.; Belyaev, A.V.; Gavrishchuk, E.M.; Mukhin, B.I.; Novikova, A.V.; Palashov, O.V.; Permin, D.A.; Snetkov, I.L. Fabrication and measurement of optical and spectral properties of the transparent Yb: MgAl2O4 ceramics. Opt. Mat. 2016, 71, 17–22. [Google Scholar] [CrossRef]
- Chen, C.-F.; Doty, F.P.; Houk, R.J.T.; Loutfy, R.O.; Volz, H.; Yang, P. Characterizations of a Hot-Pressed Polycrystalline Spinel:Ce Scintillator. J. Am. Ceram. Soc. 2010, 93, 2399–2402. [Google Scholar] [CrossRef]
- Valiev, D.; Khasanov, O.; Dvilis, E.; Stepanov, S.; Polisadova, E.; Paygin, V. Luminescent properties of MgAl2O4 ceramics doped with rare earth ions fabricated by spark plasma sintering technique. Ceram. Int. 2018, 44, 20768–20773. [Google Scholar] [CrossRef]
- Valiev, D.; Khasanov, O.; Stepanov, S.; Dvilis, E.; Paygin, V. MgAl2O4 ceramics doped with rare earth ions: Synthesis and luminescent properties. AIP Conf. Proc. 2019, 2174, 020262. [Google Scholar] [CrossRef]
- Valiev, D.; Khasanov, O.; Stepanov, S.; Dvilis, E.; Paygin, V. Synthesis and optical properties of Tb3+ or Dy3+-doped MgAl2O4 transparent ceramics. Opt. Mat. 2019, 91, 396–400. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valiev, D.; Stepanov, S.; Paygin, V.; Khasanov, O.; Dvilis, E.; Chaolu, L. Structural and Luminescent Peculiarities of Spark Plasma Sintered Transparent MgAl2O4 Spinel Ceramics Doped with Cerium Ions. Inorganics 2022, 10, 153. https://doi.org/10.3390/inorganics10100153
Valiev D, Stepanov S, Paygin V, Khasanov O, Dvilis E, Chaolu L. Structural and Luminescent Peculiarities of Spark Plasma Sintered Transparent MgAl2O4 Spinel Ceramics Doped with Cerium Ions. Inorganics. 2022; 10(10):153. https://doi.org/10.3390/inorganics10100153
Chicago/Turabian StyleValiev, Damir, Sergey Stepanov, Vladimir Paygin, Oleg Khasanov, Edgar Dvilis, and Lin Chaolu. 2022. "Structural and Luminescent Peculiarities of Spark Plasma Sintered Transparent MgAl2O4 Spinel Ceramics Doped with Cerium Ions" Inorganics 10, no. 10: 153. https://doi.org/10.3390/inorganics10100153
APA StyleValiev, D., Stepanov, S., Paygin, V., Khasanov, O., Dvilis, E., & Chaolu, L. (2022). Structural and Luminescent Peculiarities of Spark Plasma Sintered Transparent MgAl2O4 Spinel Ceramics Doped with Cerium Ions. Inorganics, 10(10), 153. https://doi.org/10.3390/inorganics10100153