Photobiomodulation Therapy through a Novel Flat-Top Hand-Piece Prototype Improves Tissue Regeneration in Amphioxus (Branchiostoma lanceolatum): Proposal of an Ethical Model for Preclinical Screening
Abstract
:1. Introduction
2. Materials and Methods
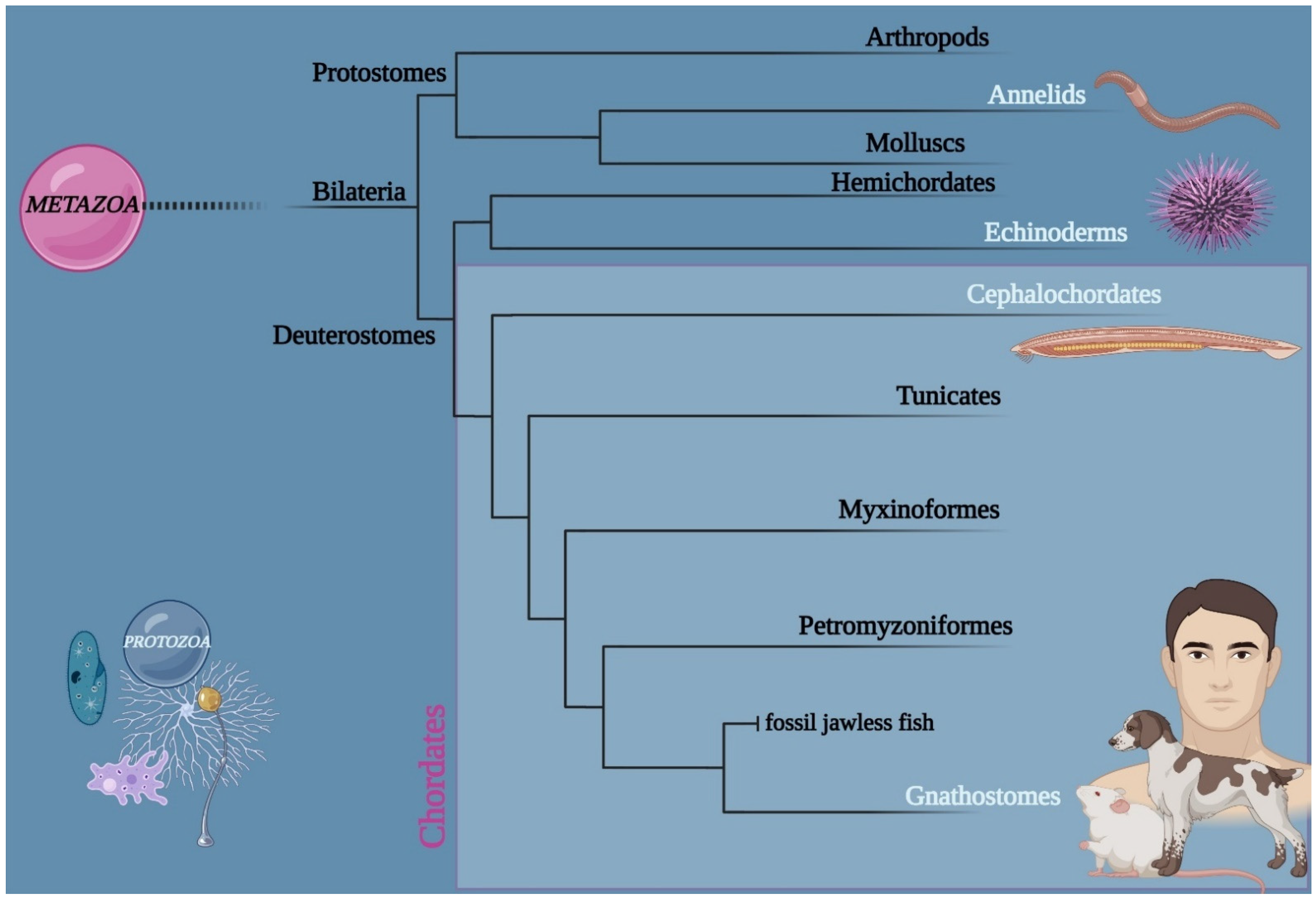
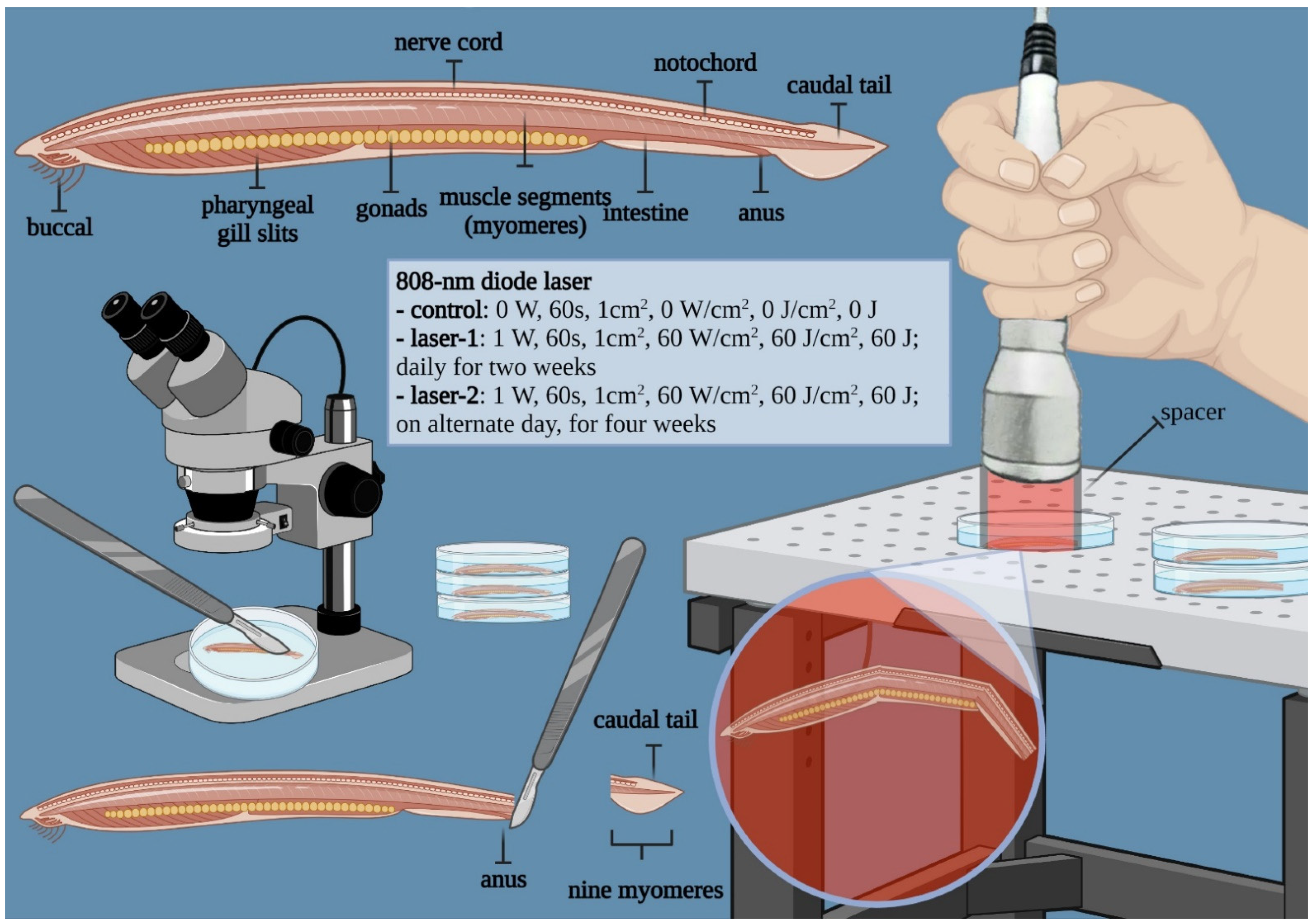
2.1. Animal Model: Branchiostoma lanceolatum
2.2. Device for Irradiation of Photobiomodulation
2.3. Experimental Setup
2.4. Gross Morphology and Histology
3. Results
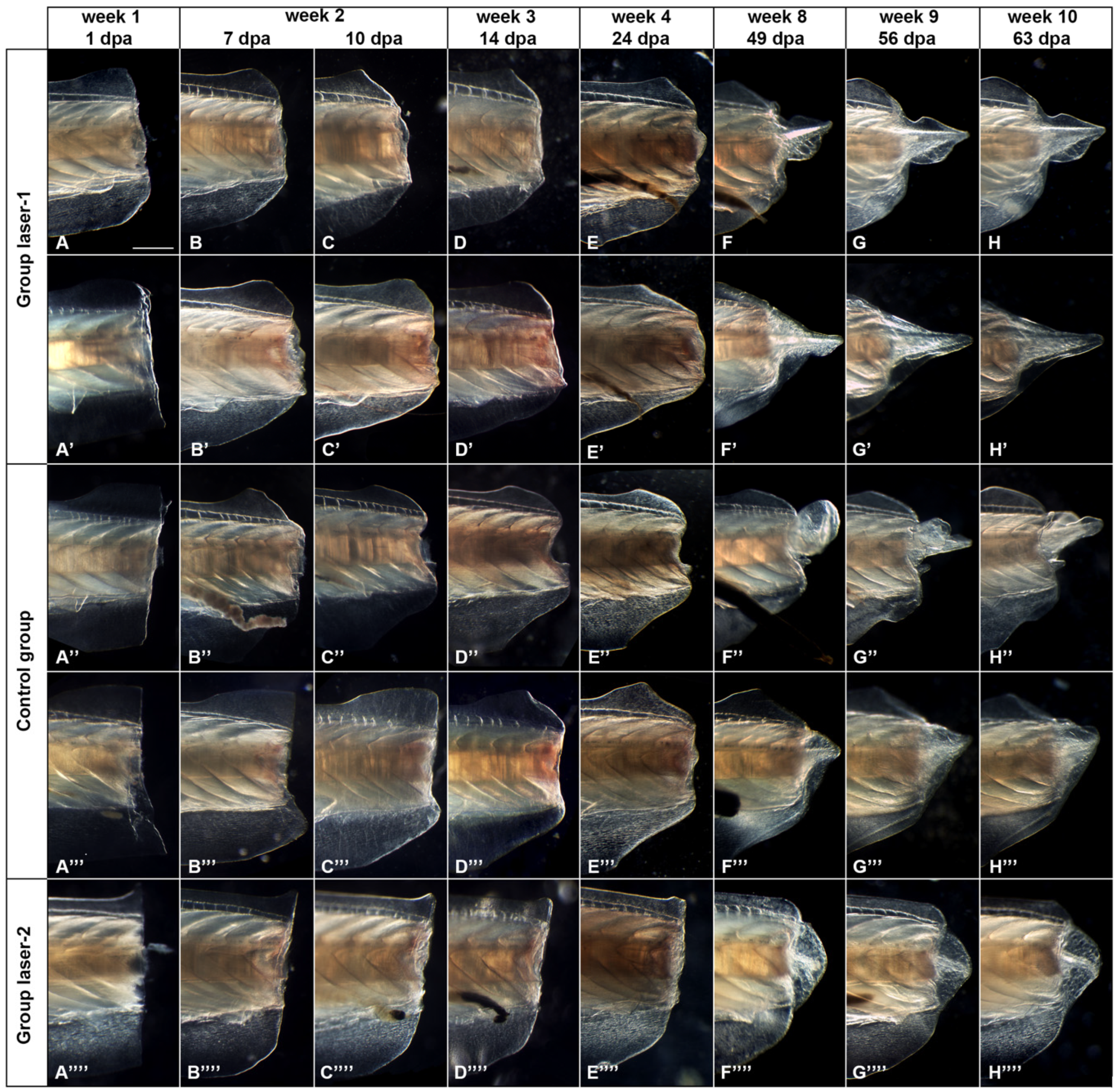
3.1. Morphological Analysis
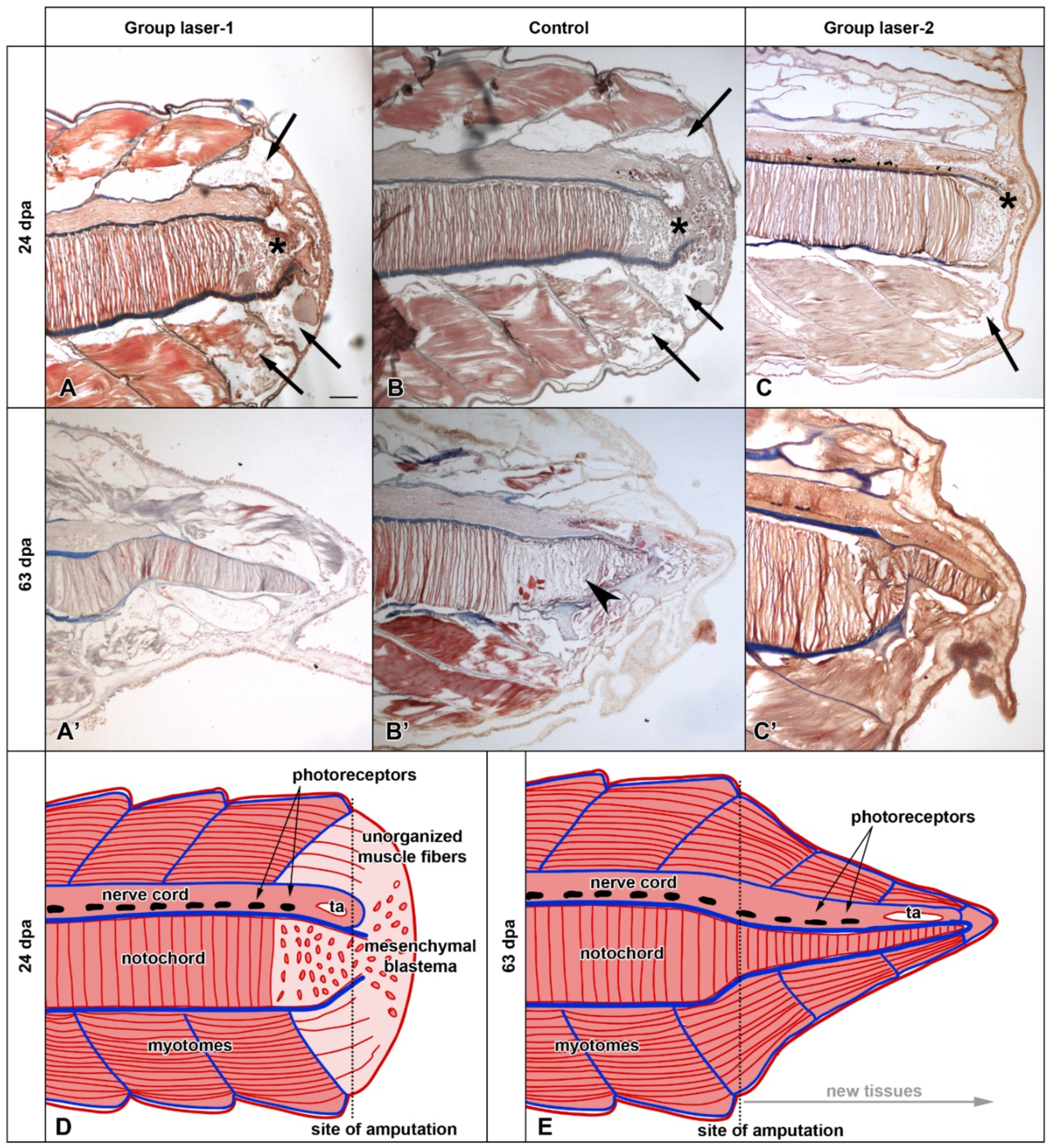
3.2. Histological Analysis
3.3. Thermal Monitoring
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trevors, J.T. Origin of life: Hypothesized roles of high-energy electrical discharges, infrared radiation, thermosynthesis and pre-photosynthesis. Theory Biosci. 2012, 131, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Soo, R.M.; Hemp, J.; Parks, D.H.; Fischer, W.W.; Hugenholtz, P. On the origins of oxygenic photosynthesis and aerobic respiration in Cyanobacteria. Science 2017, 355, 1436–1440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 39, 1724. [Google Scholar] [CrossRef] [PubMed]
- de Freitas, L.F.; Hamblin, M.R. Proposed Mechanisms of Photobiomodulation or Low-Level Light Therapy. IEEE J. Sel. Top. Quantum Electron. 2016, 22, 7000417. [Google Scholar] [CrossRef] [Green Version]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Utyuzh, A.; Benedicenti, S.; Signore, A.; Ravera, S. Photobiomodulation and Oxidative Stress: 980 nm Diode Laser Light Regulates Mitochondrial Activity and Reactive Oxygen Species Production. Oxid. Med. Cell. Longev. 2021, 3, 6626286. [Google Scholar] [CrossRef]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. An 808-nm Diode Laser with a Flat-Top Handpiece Positively Photobiomodulates Mitochondria Activities. Photomed. Laser Surg. 2016, 34, 564–571. [Google Scholar] [CrossRef]
- Ravera, S.; Ferrando, S.; Agas, D.; De Angelis, N.; Raffetto, M.; Sabbieti, M.G.; Signore, A.; Benedicenti, S.; Amaroli, A. 1064 nm Nd:YAG laser light affects transmembrane mitochondria respiratory chain complexes. J. Biophotonics 2019, 12, e201900101. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Benedicenti, S. Photobiomodulation Affects Key Cellular Pathways of all Life-Forms: Considerations on Old and New Laser Light Targets and the Calcium Issue. Photochem. Photobiol. 2019, 95, 455–459. [Google Scholar] [CrossRef] [Green Version]
- Amaroli, A.; Benedicenti, A.; Ferrando, S.; Parker, S.; Selting, W.; Gallus, L.; Benedicenti, S. Photobiomodulation by Infrared Diode Laser: Effects on Intracellular Calcium Concentration and Nitric Oxide Production of Paramecium. Photochem. Photobiol. 2016, 92, 854–862. [Google Scholar] [CrossRef]
- Tsai, S.R.; Hamblin, M.R. Biological effects and medical applications of infrared radiation. J. Photochem. Photobiol. B 2017, 170, 197–207. [Google Scholar] [CrossRef] [Green Version]
- Kujawa, J.; Zavodnik, L.; Zavodnik, I.; Buko, V.; Lapshyna, A.; Bryszewska, M. Effect of low-intensity (3.75–25 J/cm2) near-infrared (810 nm) laser radiation on red blood cell ATPase activities and membrane structure. J. Clin. Laser Med. Surg. 2004, 22, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Arany, P.R. Photobiomodulation-Activated Latent Transforming Growth Factor-β1: A Critical Clinical Therapeutic Pathway and an Endogenous Optogenetic Tool for Discovery. Photobiomodul. Photomed. Laser Surg. 2022, 40, 136–147. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Ravera, S.; Zekiy, A.; Benedicenti, S.; Pasquale, C. A Narrative Review on Oral and Periodontal Bacteria Microbiota Photobiomodulation, through Visible and Near-Infrared Light: From the Origins to Modern Therapies. Int. J. Mol. Sci. 2022, 25, 1372. [Google Scholar] [CrossRef] [PubMed]
- Enengl, J.; Hamblin, M.R.; Dungel, P. Photobiomodulation for Alzheimer’s Disease: Translating Basic Research to Clinical Application. J. Alzheimers Dis. 2020, 75, 1073–1082. [Google Scholar] [CrossRef]
- Amaroli, A.; Marcoli, M.; Venturini, A.; Passalacqua, M.; Agnati, L.F.; Signore, A.; Raffetto, M.; Maura, G.; Benedicenti, S.; Cervetto, C. Near-infrared laser photons induce glutamate release from cerebrocortical nerve terminals. J. Biophotonics 2018, 11, e201800102. [Google Scholar] [CrossRef]
- Hamblin, M.R.; Huang, Y.Y.; Heiskanen, V. Non-mammalian Hosts and Photobiomodulation: Do All Life-forms Respond to Light? Photochem. Photobiol. 2019, 95, 126–139. [Google Scholar] [CrossRef]
- Amaroli, A.; Arany, P.; Pasquale, C.; Benedicenti, S.; Bosco, A.; Ravera, S. Improving Consistency of Photobiomodulation Therapy: A Novel Flat-Top Beam Hand-Piece versus Standard Gaussian Probes on Mitochondrial Activity. Int. J. Mol. Sci. 2021, 21, 7788. [Google Scholar] [CrossRef]
- Hernández-Acosta, M.A.; Martines-Arano, H.; Soto-Ruvalcaba, L.; Martínez-González, C.L.; Martínez-Gutiérrez, H.; Torres-Torres, C. Fractional thermal transport and twisted light induced by an optical two-wave mixing in single-wall carbon nanotubes. Int. J. Therm. Sci. 2020, 147, 106136. [Google Scholar] [CrossRef]
- Amaroli, A.; Benedicenti, S.; Bianco, B.; Bosco, A.; Clemente Vargas, M.R.; Hanna, R.; Kalarickel Ramakrishnan, P.; Raffetto, M.; Ravera, S. Electromagnetic Dosimetry for Isolated Mitochondria Exposed to Near-Infrared Continuous-Wave Illumination in Photobiomodulation Experiments. Bioelectromagnetics 2021, 42, 384–397. [Google Scholar] [CrossRef]
- Bertrand, S.; Escriva, H. Evolutionary crossroads in developmental biology: Amphioxus. Development 2011, 138, 4819–4830. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, J.E.; Lahaye, F.; Schubert, M. Keeping amphioxus in the laboratory: An update on available husbandry methods. Int. J. Dev. Biol. 2017, 61, 773–783. [Google Scholar] [CrossRef] [PubMed]
- Bozzo, M.; Candiani, S.; Schubert, M. Whole mount in situ hybridization and immunohistochemistry for studying retinoic acid signaling in developing amphioxus. Methods Enzymol. 2020, 637, 419–452. [Google Scholar] [PubMed]
- Liang, Y.; Rathnayake, D.; Huang, S.; Pathirana, A.; Xu, Q.; Zhang, S. BMP signaling is required for amphioxus tail regeneration. Development 2019, 15, 166017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Escriva, H. My Favorite Animal, Amphioxus: Unparalleled for Studying Early Vertebrate Evolution. Bioessays 2018, 40, e1800130. [Google Scholar] [CrossRef]
- Somorjai, I.M.; Somorjai, R.L.; Garcia-Fernàndez, J.; Escrivà, H. Vertebrate-like regeneration in the invertebrate chordate amphioxus. Proc. Natl. Acad. Sci. USA 2012, 109, 517–522. [Google Scholar] [CrossRef] [Green Version]
- Zullo, L.; Bozzo, M.; Daya, A.; Di Clemente, A.; Mancini, F.P.; Megighian, A.; Nesher, N.; Röttinger, E.; Shomrat, T.; Tiozzo, S.; et al. The Diversity of Muscles and Their Regenerative Potential across Animals. Cells 2020, 9, 1925. [Google Scholar] [CrossRef]
- Flecknell, P. Replacement, reduction and refinement. ALTEX 2002, 19, 73–78. [Google Scholar]
- Amaroli, A.; Ravera, S.; Parker, S.; Panfoli, I.; Benedicenti, A.; Benedicenti, S. The protozoan, Paramecium primaurelia, as a non-sentient model to test laser light irradiation: The effects of an 808nm infrared laser diode on cellular respiration. Altern. Lab. Anim. 2015, 43, 155–162. [Google Scholar] [CrossRef]
- Ferrando, S.; Agas, D.; Mirata, S.; Signore, A.; De Angelis, N.; Ravera, S.; Utyuzh, A.S.; Parker, S.; Sabbieti, M.G.; Benedicenti, S.; et al. The 808 nm and 980 nm infrared laser irradiation affects spore germination and stored calcium homeostasis: A comparative study using delivery hand-pieces with standard (Gaussian) or flat-top profile. J. Photochem. Photobiol. B 2019, 199, 111627. [Google Scholar] [CrossRef]
- Amaroli, A.; Ferrando, S.; Pozzolini, M.; Gallus, L.; Parker, S.; Benedicenti, S. The earthworm Dendrobaena veneta (Annelida): A new experimental-organism for photobiomodulation and wound healing. Eur. J. Histochem. 2018, 62, 2867. [Google Scholar] [CrossRef]
- Amaroli, A.; Gambardella, C.; Ferrando, S.; Hanna, R.; Benedicenti, A.; Gallus, L.; Faimali, M.; Benedicenti, S. The Effect of Photobiomodulation on the Sea Urchin Paracentrotus lividus (Echinodermata) Using Higher-Fluence on Fertilization, Embryogenesis, and Larval Development: An In Vitro Study. Photomed. Laser Surg. 2017, 35, 127–135. [Google Scholar] [CrossRef] [PubMed]
- Amaroli, A.; Sabbieti, M.G.; Marchetti, L.; Zekiy, A.O.; Utyuzh, A.S.; Marchegiani, A.; Laus, F.; Cuteri, V.; Benedicenti, S.; Agas, D. The effects of 808-nm near-infrared laser light irradiation on actin cytoskeleton reorganization in bone marrow mesenchymal stem cells. Cell Tissue Res. 2021, 383, 1003–1016. [Google Scholar] [CrossRef] [PubMed]
- Pasquale, C.; Utyuzh, A.; Mikhailova, M.V.; Colombo, E.; Amaroli, A. Recovery from Idiopathic Facial Paralysis (Bell’s Palsy) Using Photobiomodulation in Patients Non-Responsive to Standard Treatment: A Case Series Study. Photonics 2021, 8, 341. [Google Scholar] [CrossRef]
- Fuentes, M.; Benito, E.; Bertrand, S.; Paris, M.; Mignardot, A.; Godoy, L.; Jimenez-Delgado, S.; Oliveri, D.; Candiani, S.; Hirsinger, E.; et al. Insights into spawning behavior and development of the European amphioxus (Branchiostoma lanceolatum). J. Exp. Zool. B Mol. Dev. Evol. 2007, 308, 484–493. [Google Scholar] [CrossRef]
- Zhang, H.B.; Menzies, K.J.; Auwerx, J. The role of mitochondria in stem cell fate and aging. Development 2018, 145, 143420. [Google Scholar] [CrossRef] [Green Version]
- Amaroli, A.; Pasquale, C.; Zekiy, A.; Benedicenti, S.; Marchegiani, A.; Sabbieti, M.G.; Agas, D. Review Steering the multipotent mesenchymal cells towards an anti-inflammatory and osteogenic bias via photobiomodulation therapy: How to kill two birds with one stone. J. Tissue Eng. 2022, 5, 20417314221110192. [Google Scholar]
- Amaroli, A.; Agas, D.; Laus, F.; Cuteri, V.; Hanna, R.; Sabbieti, M.G.; Benedicenti, S. The Effects of Photobiomodulation of 808 nm Diode Laser Therapy at Higher Fluence on the In Vitro Osteogenic Differentiation of Bone Marrow Stromal Cells. Front. Physiol. 2018, 9, 123. [Google Scholar] [CrossRef] [Green Version]
- Kaneto, S.; Wada, H. Regeneration of amphioxus oral cirri and its skeletal rods: Implications for the origin of the vertebrate skeleton. J. Exp. Zool. B Mol. Dev. Evol. 2011, 316, 409–417. [Google Scholar] [CrossRef]
- Agas, D.; Hanna, R.; Benedicenti, S.; De Angelis, N.; Sabbieti, M.G.; Amaroli, A. Photobiomodulation by Near-Infrared 980-nm Wavelengths Regulates Pre-Osteoblast Proliferation and Viability through the PI3K/Akt/Bcl-2 Pathway. Int. J. Mol. Sci. 2021, 22, 7586. [Google Scholar] [CrossRef]
- Martins, M.D.; Silveira, F.M.; Martins, M.A.T.; Almeida, L.O.; Bagnato, V.S.; Squarize, C.H.; Castilho, R.M. Photobiomodulation therapy drives massive epigenetic histone modifications, stem cells mobilization and accelerated epithelial healing. J. Biophotonics 2021, 14, e202000274. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bozzo, M.; Pasquale, C.; Cuccaro, F.; Ferrando, S.; Zekiy, A.; Candiani, S.; Amaroli, A. Photobiomodulation Therapy through a Novel Flat-Top Hand-Piece Prototype Improves Tissue Regeneration in Amphioxus (Branchiostoma lanceolatum): Proposal of an Ethical Model for Preclinical Screening. Photonics 2022, 9, 503. https://doi.org/10.3390/photonics9070503
Bozzo M, Pasquale C, Cuccaro F, Ferrando S, Zekiy A, Candiani S, Amaroli A. Photobiomodulation Therapy through a Novel Flat-Top Hand-Piece Prototype Improves Tissue Regeneration in Amphioxus (Branchiostoma lanceolatum): Proposal of an Ethical Model for Preclinical Screening. Photonics. 2022; 9(7):503. https://doi.org/10.3390/photonics9070503
Chicago/Turabian StyleBozzo, Matteo, Claudio Pasquale, Francesco Cuccaro, Sara Ferrando, Angelina Zekiy, Simona Candiani, and Andrea Amaroli. 2022. "Photobiomodulation Therapy through a Novel Flat-Top Hand-Piece Prototype Improves Tissue Regeneration in Amphioxus (Branchiostoma lanceolatum): Proposal of an Ethical Model for Preclinical Screening" Photonics 9, no. 7: 503. https://doi.org/10.3390/photonics9070503
APA StyleBozzo, M., Pasquale, C., Cuccaro, F., Ferrando, S., Zekiy, A., Candiani, S., & Amaroli, A. (2022). Photobiomodulation Therapy through a Novel Flat-Top Hand-Piece Prototype Improves Tissue Regeneration in Amphioxus (Branchiostoma lanceolatum): Proposal of an Ethical Model for Preclinical Screening. Photonics, 9(7), 503. https://doi.org/10.3390/photonics9070503








