Abstract
Malignant tumors rank as a leading cause of death worldwide. Accurate diagnosis and advanced treatment options are crucial to win battle against tumors. In recent years, Cherenkov luminescence (CL) has shown its technical advantages and clinical transformation potential in many important fields, particularly in tumor diagnosis and treatment, such as tumor detection in vivo, surgical navigation, radiotherapy, photodynamic therapy, and the evaluation of therapeutic effect. In this review, we summarize the advances in CL for tumor diagnosis and treatment. We first describe the physical principles of CL and discuss the imaging techniques used in tumor diagnosis, including CL imaging, CL endoscope, and CL tomography. Then we present a broad overview of the current status of surgical resection, radiotherapy, photodynamic therapy, and tumor microenvironment monitoring using CL. Finally, we shed light on the challenges and possible solutions for tumor diagnosis and therapy using CL.
1. Introduction
Human cancer is the second leading cause of deaths worldwide with nearly 19.3 million new cancer cases and nearly 10 million cancer deaths globally in 2020 [1]. Facing various challenges in tumor diagnosis and treatment, scientists and health experts around the world have made unremitting explorations [2,3]. However, the complexity and diversity of malignant tumors at the molecular level makes it difficult to effectively grasp its pathogenesis and progression and to improve therapeutic effect using traditional detection strategies and treatment options [4,5].
The development of molecular imaging has emerged as a new powerful weapon for human beings to fight against malignant tumors [6,7,8,9,10]. Molecular imaging was first proposed by Weissleder in 1999 [11]. It offers the ability to conduct non-invasive real-time imaging of the internal physiological or pathological processes at the molecular level to observe the diverse biological processes, such as tumor growth and metastasis, disease occurrence and development, and gene expression and reaction in organisms, thereby leading to the improvement of available treatment options [12,13,14]. At present, a variety of molecular imaging modalities have been employed for medical imaging, including magnetic resonance imaging, radionuclide imaging, optical imaging, and ultrasonic molecular imaging, etc. [15,16,17]. Among them, optical molecular imaging technology is a newly emerging technology and growing rapidly because of its high sensitivity, sturdy specificity, high cost-effectiveness, and swift imaging speed [18,19,20,21].
Currently, FDA-approved fluorescent molecular probes for clinical use are limited [22]. The toxicity of optical molecular probes and their multiple labeling have affected their specificity, affinity, and stability with target molecules, which limits the development of molecular imaging technology based on optical molecular imaging [23]. Alternatively, Cherenkov luminescence (CL) has opened a new avenue for optical imaging in molecular imaging [24,25]. CL was observed first time about one century ago and characterized by Cherenkov in 1934, and then further studies by Frank and Tamm showed that the CL was produced by charged particles moving in the dielectric medium with velocity greater than the phase velocity of light in that medium [26]. Since the discovery of CL, this unique physical effect has played an important role in many research fields, such as detection of labelled biomolecules, medical imaging of radionuclide, nuclear reactors, astrophysics experiments, and particle physics experiments [27,28,29,30,31]. Cherenkov, Frank, and Tamm were awarded the Nobel Prize in Physics for the discovery and interpretation of the Cherenkov effect in 1958.
Currently, CL is applicable both with radionuclides (e.g., 18F, 64Cu, 89Zr, 131I, 177Lu, and 90Y) and medical linear accelerators in both the tumor diagnosis and treatment setting [32,33,34,35,36]. By integrating the advantages of optical molecular imaging and nuclear imaging, recent evidence has proved that CL is an effective and highly promising technique for precise tumor imaging. Further development has upgraded CL from two-dimensional imaging to three-dimensional imaging. CL is a by-product of tumor radiotherapy. Besides tumor imaging, its potential applications in the field of tumor therapy have also been explored, including dose delivery verification [37,38,39,40,41,42,43], daily position tracking [44,45], quality assurance of radiotherapy equipment [46,47,48,49,50], photodynamic therapy [51,52,53,54,55,56,57], and physiological changes assessment [58,59,60,61,62,63,64,65].
This review paper aims to describe the research and clinical applications of CL in accurate tumor diagnosis and treatment in recent years. The structure of this review paper is as follows: the first section is a general introduction. The second section outlines the generation and characteristics of CL in a simple way, instead of using complicated physical equations as others have done [66,67]. The third section summarizes imaging technologies using CL. It provides an overview of the published literature in this field so far. The fourth section highlights the clinical applications and technical progress of CL in tumor treatment. The last section is the summary of this paper.
2. The Generation and Characteristics of Cherenkov Luminescence
The charged particles produced by radionuclides or medical linear accelerators transfer part of the energy to the surrounding dielectric molecules along their trajectory, thus polarizing these molecules. As the charged particles continue to move forward, the dielectric molecules behind them gradually releases the energy which they absorbed and return to the ground state. If the charged particles travel through a dielectric medium with a speed greater than light speed in that medium, the dielectric molecules polarized by charged particles forms a cone-like light signal at a certain angle and emits near infrared and visible light with a continuous spectrum, named Cherenkov radiation [68], also known as CL (Figure 1).
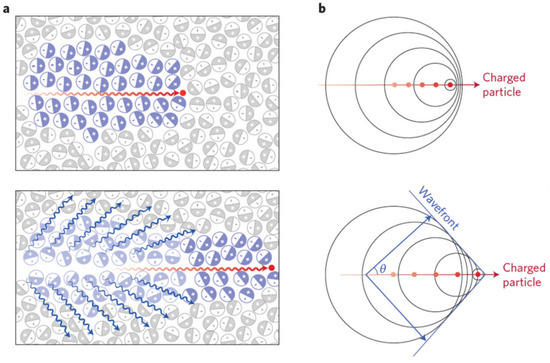
Figure 1.
The generation of Cherenkov luminescence (CL). (a) Top: A charged particle (red dot) travelling faster than light in a medium polarizes the medium. Bottom: As the medium returns to the ground state, CL (blue wavy lines) is emitted in a forward direction. (b) Top: Analogous to a sonic boom, coherent waves are produced through the Cherenkov mechanism, thereby leading to a photonic wavefront. Bottom: As the particle travels forward, the photonic wavefront propagates at a forward angle θ with light being emitted in the direction of travel. Adapted with permission from Ref. [69] 2017 Springer Nature.
According to the Frank–Tamm theory, CL exhibits the following characteristics. (1) CL intensity is proportional to the moving speed and number of charged particles. β particles emitted by radionuclides are commonly used in biomedical Cherenkov applications along with particle energy and Cherenkov photons per disintegration can be found in reference [69]. The amount of Cherenkov photons per unit radiotherapy dose can be increased by elevating photon beam energy and applying specialized beam hardening filtration [70]. (2) CL spectrum is continuous, and the relative intensity per unit frequency is proportional to its frequency. These unique features make it a strong candidate for optical molecular imaging [71,72,73,74].
3. Imaging Technology Using Cherenkov Luminescence
3.1. Cherenkov Luminescence Imaging
Radioactive agents are traditionally studied by PET, SPECT, or photon cameras, which are expensive, hard to maintain, and not widely available to many researchers. A commercially available optical imaging instrument can be used for studying radioactive probes. The concept of Cherenkov luminescence imaging (CLI) was first time proposed in 2009 [75]. Then, the analytical expression of CL spectrum was theoretically deduced and two methods for measuring the CL depth in vivo based on multispectral analysis were verified [76,77]. Not all radionuclides produce CL. The research on CLI imaging conditions provided a foundation for the synthesis and screening of radionuclide probes [78,79]. In 2012, Spinelli et al. used CLI to image the thyroid gland in patients with hyperthyroidism during radiotherapy (Figure 2a). This was the first time the CLI method was used to image the treatment of human diseases, further paving the way for the development of CLI [80].
CLI combines the advantages of optical molecular imaging and radionuclide imaging. The broadband spectrum of CL provides potential to excite almost all the optical molecular probes with absorption in this spectral region in theory. So, it can also be used for studying bioluminescence and fluorescence probes. Time-gated acquisition and spectrally resolved detection can unmix the Cherenkov emission and the secondary optical emission signal [81,82]. CL has the advantages of fast imaging, strong sensitivity, and high cost-effectiveness. However, with advanced research developments, the limitations of CL have become prominent, which are mainly due to the blue emissions of CL highly absorbed in tissues, thereby leading to relatively short mean pathlengths. Numerous studies have been carried out to overcome these limitations.
Red-shifted emissions penetrate biological tissues more readily than that of blue emissions. CL can be spectrally coupled by energy transfer to high Stokes-shift quantum fluorescent particles to produce highly red-shifted photonic emissions. This technology was termed Cherenkov radiation energy transfer (CRET). Both the in vitro and in vivo studies showed that the CRET ratio (the normalized quotient of light detected within a spectral window centered on the fluorophore emission divided by light detected within a spectral window of the Cherenkov radiation emission) could be 8.8 and 3.5 by using Qtracker705 nanoparticles [83]. CL has served as a source to excite various fluorescent particles that act as fluorescence emitters of red-shifted emissions, such as quantum dots (QDs) [84,85,86], metallic nanoparticles [87,88], fluorophores [89,90], and lanthanides [91,92].
Part of CL energy can be transferred to the most transparent near-infrared spectral region in biological tissue through double CL induction. Secondary Cherenkov-induced fluorescence imaging (SCIFI) is an imaging strategy that utilizes targeted nanoparticles to achieve activatable imaging through fluorescent conversion of CL. This approach reduced background signal compared with conventional fluorescence imaging, and helped in overcoming issues of target specificity, tracer clearance, and quantitation [93]. In the mode of Cherenkov excited fluorescence scanning imaging (CELSI), CL was used to excite the molecules or cells labeled by the fluorescent probe in the organism to produce fluorescence with a longer wavelength than CL [59,94]. Both methods provide a novel application idea for CL detection of deep lesion tissue.
Radiopharmaceutical-excited fluorescence imaging (REFI) is a method similar to radioluminescence. It utilized nanoparticles or a scintillator to convert γ-radiation and CL into fluorescence. A series of imaging studies demonstrated that this approach provides strong optical signals with high signal-to-background ratios, an ideal tissue penetration spectrum and activatable imaging ability [95,96].
CRET, SCIFI, CELSI, and REFI methods proposed in recent years have effectively overcome the CL defects of low photon yield, short wavelength, easy scattering, and poor penetration and improved the performance of CLI. It also popularized CL in more diverse biomedical application scenarios.
3.2. Cherenkov Luminescence Endoscope
CL is often located deep in biological tissues like the signals generated by other fluorescent probes, which brings great difficulties regarding the transmission of optical signals to the outside tissues. Various absorption and scattering effects in tissues further affect the final collected fluorescent signals. Weak intensity and poor penetration of CL limit its applications in clinical practice. Cherenkov luminescence endoscope (CLE) was developed to extend the applications in gastrointestinal tract imaging [97,98,99]. The CLE integrated traditional CLI technology with an optical fiber-based endoscope by using an optical adaptor. Through the combination with an optical fiber, the CL signal can be transferred from a deep or remote location to the detecting area (Figure 2b).
The detection sensitivity of the CLE system is relatively low due to a small aperture of endoscope and transmission attenuation of optical fibers. Cao et al. constructed an imaging system using clinically available gastroscopes and tested the performance of the system for in vivo tumor detection from different perspectives. Through a detailed comparative experiment, it was demonstrated that the flexible endoscope clinically used at present, such as the gastroscope, may cause more than 93% energy loss in the process of transmitting CLI signals [100]. Carter et al. used 90Y as an imaging probe in CLE research and found that the utilization of β-radiation radionuclide probe further improved the sensitivity of the CLE imaging system in living tumor detection and avoid the noise interference of γ-radiation radionuclide [101]. The lanthanides based radioluminescence nanoparticles (RLNPs) can emit luminescence under the irradiation of high energy rays. The sensitivity of CLE system can be improved 50-fold by using lanthanide luminescent particles RLNPs mixed with radiotracers [102].
After verifying feasibility of CLE in living tumor detection, the effect of combining CLI imaging with clinical equipment has received great attention. Song et al. established a CLE system that used a clinically approved laparoscope. The system performance was tested on nude mice injected with human colon carcinoma cells HCT-8. The tumor lesions were successfully removed under the guidance of CLE image, which showed the clinical transformation potential of the system [103]. By integrating an electron-multiplying charge coupled device with a flexible fiber endoscope, a CLE system can provide both the luminescent and photographic images. Results from phantom experiments and animal studies demonstrated that there is a good linear quantitative relation between the CL signals and the radiotracer activity. A human pilot trial was conducted using this system, and it confirmed the feasibility and potential of the CLE system in a clinical setting [104].
Together, these novel findings demonstrate that CL possesses great scope for development in the field of endoscopic imaging and also provide a guided roadmap to further solve the problem of limited imaging depth of CLI in clinical application.
3.3. Cherenkov Luminescence Tomography
CLI is a two-dimensional optical imaging, which cannot obtain tumor information from a three-dimensional (3D) space. Cherenkov luminescence tomography (CLT) is a 3D tomography method. It uses CL signals measured on the surface to reconstruct the spatial position and quantitative distribution information of radionuclide probes in vivo by constructing an appropriate biological tissue light transmission model and internal target reconstruction algorithm. CLT obtains the 3D spatial distribution of the radioactive source in biological tissues (Figure 2c).
Li et al. proposed the concept of CLT for the first time in 2010 [105]. They ensured that the illumination intensity at each finite element node was the same and used the diffusion approximation strategy to describe the in vivo transmission process of CL. The 3D distribution of the CL source was reconstructed iteratively with a preconditioned conjugate gradient method. Then an in vivo 3D CLT method, based on a heterogeneous mouse model, was conducted. The reconstruction result based on a heterogeneous mouse model was more accurate in localization than using the homogeneous one [106]. Since the traditional diffusion approximation model not suitable for the short wavelength of CL spectrum, a higher-order spherical harmonic approximation model was adopted to approximate the radiative transfer equation with a higher-dimensional function space, so as to achieve the goal of improving the reconstruction accuracy of CLT [107]. In 2012, Hu et al. proposed a single photon emission computed tomography (SPECT) guided CLT reconstruction method, which incorporated the a priori information of the permissible source region from SPECT imaging results to reduce the morbid nature of the inverse reconstruction problem [108]. Inspired by the depth compensation algorithm in diffuse optical tomography, Wang et al. developed a prior compensation algorithm for CLT reconstruction based on depth calibration strategy. The depth calibration matrix has the same dimensions as the CLT system matrix and was composed of different compensation weights. With the help of this strategy, the reconstruction accuracy from single-view image of CLT was improved [109].
Besides the algorithms mentioned above, several other studies have been carried out to reduce the insufficiency of CL inverse reconstruction. The broad spectrum of the CL makes it ideal for multispectral 3D imaging. Several research groups are working on multispectral methods to improve the sensitivity, accuracy, and imaging quality of CLT. These include multispectral diffuse CLT [71], hybrid spectral CLT [72], multispectral hybrid CLT based on the finite element SPn method [73], and weight multispectral CLT [74]. Recently, the artificial intelligence-based methods have been proposed to provide a different tool to CLT reconstruction [110,111,112]. Artificial intelligence reconstruction method directly analyzes the complex relationship between the internal luminescence source and the surface optical signal, effectively avoids the calculation error in the process of optical transmission modeling and solutions and provides a new perspective for the breakthrough of CLT tumor detection accuracy. These strategies make CLT imaging more reliable and operable and expand the application scope of CLT imaging in the field of biomedical research.
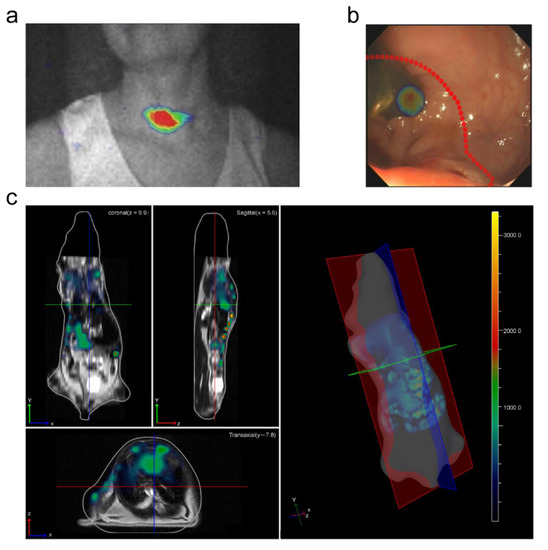
Figure 2.
Examples of imaging technology using Cherenkov luminescence. (a) First Cherenkov luminescence image (CLI) of a thyroid patient. Adapted with permission from Ref. [80] 2013 SPIE. (b) CLE images overlaid with white-light photographs. Adapted with permission from Ref. [104] 2015 Springer Nature. (c) Whole body CLT coregistered with magnetic resonance imaging. Adapted with permission from Ref. [71] 2011 Optica Publishing Group.
4. Cherenkov Luminescence Applications in Tumor Treatment
4.1. Surgical Resection
Surgical resection is a common approach in tumor treatment. However, surgery leads to various types of serious problems due to the infiltration of surrounding tissues in the process of tumor diffusion. Among them, the major problem is tumors with vague boundaries. The images obtained from CL reflect molecular biological information. In vivo CLI is used to visualize the biological distribution of radiopharmaceuticals [66,113]. Studies showed that the tumor cells can be precisely located by injecting different kinds of radionuclide probes and analyzing the changes of the CL signal [75,103,114,115,116]. The REFI imaging combined with nanoparticles released in vivo allowed the detection of small tumor lesions of 2 mm, which significantly improves the sensitivity of single-mode imaging [95].
Surgery could benefit from the translation of optical molecular imaging techniques to visualize deep tumor lesions or metastatic involvement intraoperatively and thereby provide real-time information to guide surgical resection (Figure 3). Intraoperative tumor imaging with CL has the ability to guide better surgical resection [22,117]. In 2011, the positron-emitting radiotracer 89Zr-DFO-trastuzumab was used for target-specific, quantitative imaging of HER2/neu-positive tumors in vivo. By observing the changes of CLI optical signals before and after tumor resection, CLI was shown to have an advantage in guiding and monitoring tumor operation [118]. Whether there is lymph node metastasis after tumor resection needs to be considered in surgery. Thorek et al. found that CLI can visualize lymph nodes even before the exposure of surgical field of vision and can guide lymph node resection through CLI [119]. In the open resection of a breast tumor, the distribution of CL effectively revealed the boundary information of breast tumor and showed a high consistency with the results of intraoperative radiography. CLI provided a quick reference for the judgement of the margin of breast tumor surgery [120]. As can be seen from the above research, in the surgical resection of meningiomas, the CLI obtained from isolated tumor tissue during operation can accurately reflect the boundary of tumor tissue, so as to assist surgeons to improve the quality of surgical resection.

Figure 3.
Schematic overview of the in vivo Cherenkov luminescence image-guided surgery. Adapted with permission from Ref. [116] 2019 John Wiley and Sons.
4.2. Radiotherapy
Radiotherapy is a standard treatment approach to treat many types of tumors with radiation. It was estimated that about half of the patients received radiotherapy at some time after diagnosis of cancer, with indications ranging from treatment to symptom relief [121]. In the course of radiotherapy, patient CLI has been proved to be a non-contact and a real-time visualization method of dose delivery (Figure 4a) [39,122].
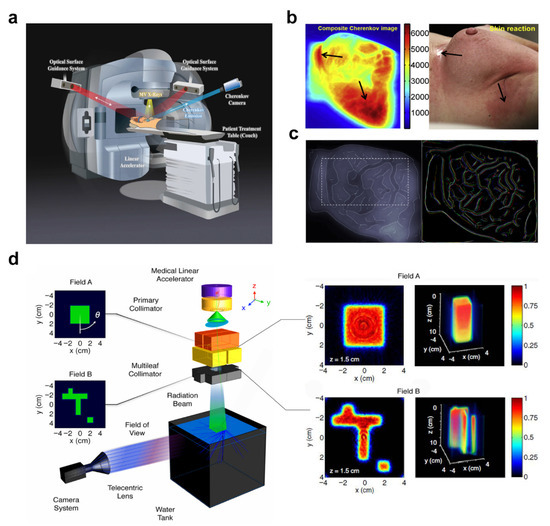
Figure 4.
Schematic diagram of Cherenkov luminescence (CL) used in radiotherapy. (a) The patient setup during radiotherapy. The Cherenkov camera is synced to the pulses of the medical linear accelerator to remove ambient light. Adapted with permission from Ref. [38] 2020 Springer Nature. (b) Left: CL examined for imaging superficial doses. Right: Skin reaction of the treatment region at the end of radiation. High superficial doses in areas typical for higher-grade radiation skin reactions. Arrow pointing to inframammary fold indicates skin desquamation. Adapted with permission from Ref. [122] 2014 Elsevier. (c) Left: The beam edge enhanced CL image with a chosen internal region (indicated by dotted lines) within the treatment beam field. Right: Detected edges in CL images for patient, false colored for different imaged treatment fractions. Adapted with permission from Ref. [44] 2015 IPO Publishing. Ltd. (d) Left: Experimental schematic of a Cherenkov dosimetry imaging system. Right: The reconstructed cross section. Adapted with permission from Ref. [46] 2013 Optica Publishing Group.
The CLI has become a valuable tool for dose verification (Figure 4b). Although CLI is obtained only on the surface, CL changes in surface can be used to infer changes in dose deep in tissue [123]. The calibration of CL intensity to absolute dose is the first step to visualize and verify radiotherapy dose delivery. Theory and simulation showed that the CL photon number is proportional to the absorbed dose of tissue under specific conditions [37,42,43,124,125]. However, the varying factors, such as patient tissue differences, radiation beam characteristics, tissue thickness, entrance/exit geometry, and curved surface effect, affect the relationship between surface-escaped CL and the absolute superficial dose [126,127]. Monte Carlo simulation can be used to study these influencing factors [40]. The establishment of the correction factor between CL and CT number provided an important step for the direct quantitative radiation dose imaging of patients [38]. A CLI system with high temporal and spatial resolution was constructed in 2016 to quantitatively monitor the distribution of radiation dose received by tumor patients throughout the body during treatment [128]. Then a new type of time-controlled three channel camera was developed in 2021 [129]. Combining spatially resolved color information from Cherenkov images with reflectance and/or tissue density allows a more accurate CL to dose conversion model for in vivo surface dosimetry [41].
The CL features also contribute to more accurate tracking of daily position (Figure 4c). In a phase 1 clinical trial, CLI was obtained with a time-gated intensified charge-coupled device (ICCD), which was synchronized to the medical linear accelerator (LINAC) pulse output during fractionated radiotherapy. The results showed that real-time CLI can simultaneously monitor the treatment delivery, patient motion, and alignment of the beam edge to the treatment region [44]. If CLI is recognized as a more advanced lighting verification method, a series of image processing steps must be established to maximize accurate recovery of beam edges. The processing algorithm was developed in reference [45].
Traditional quality assurance (QA) of radiotherapy equipment is time-consuming and involves the use of several different measurement tools. Glaser et al. presented a novel method to recover volumetric dose distributions in pure water by tomographically capturing optical projection images of the induced CL from a megavoltage X-ray photon LINAC beam using an ICCD (Figure 4d) [46,47]. It provides a faster method for clinical QA. The ICCD device is expensive. If the phosphor is added to the water phantom, more photons will be radiated into the water phantom because the phosphor is excited by CL, and cheaper CMOS detectors can also be used for fast imaging. Yogo et al. developed a simple QA method for HDR brachytherapy based on CLI and evaluated its performance, and this method allowed the measurement of dose distribution, source intensity, and source position simultaneously with a single image [48].
4.3. Photodynamic Therapy
Photodynamic therapy (PDT) is a new emerging tumor treatment approach mediated by reactive oxygen species (ROS) produced by a light source and photosensitizer to destroy target cells and tissues [130,131]. PDT appeared in the late 1970s and has developed rapidly in recent years [57,132,133,134]. Considering that the absorption of photons with wavelengths longer than 800 nm does not provide enough energy to excite oxygen to its singlet state and to form a substantial yield of reactive oxygen species, the wavelength of light commonly used in PDT is less than 800 nm [135]. The penetration ability of light in this wavelength range in tissues is limited, the penetration depth is generally of the order of millimeter, and it has been reported that PDT with wavelength of 652 nm and 690 nm can eradicate large (8–10 mm) mouse tumors in a consistent way [136]. The poor penetration of light in living tissues constitutes the bottleneck of PDT. Overcoming this limitation would undoubtedly improve PDT. The CL-induced PDT (CLPDT) is a new technology that was proposed in 2015 [137]. It uses CL generated by ionizing radiation to overcome the conventional PDT limitation on deep-seated targets. Conventional PDT is more advantageous for superficial tumors or tumors with easy access to light sources, Photosensitizers and ionizing radiation in one nanostructure or photosensitizers activated by high energy external X-ray beams could be used as a choice option for deep-seated tumors with difficulty in obtaining light sources [138]. The use of CL-induced PDT as a complementary of conventional PDT extents the treatment applicability of PDT. A schematic illustration of CLPDT principle is shown in Figure 5a.
It has been reported that CL produced by radionuclides 18F, 64Cu, or 68Ga can activate titanium dioxide nanoparticles and photocatalyst, generate hydroxyl and superoxide radicals, and destroy tumor cells [53,137]. However, the relatively weak interaction of CL and the low tumor accumulation of therapeutic ROS reduce the therapeutic effect of CLPDT, which leaves uncertainty on the clinical efficacy [139,140]. High intensity CL is beneficial to providing enough ROS, which leads to cell damage. The CL of different radionuclides varies greatly. When radionuclides with higher energy were used, their CL output and related ROS production were greatly amplified and the CLPDT were enhanced [51,69]. Colocalization is a feasible approach to enhance targeting ability. Many radiopharmaceuticals and photosensitizers can be selectively accumulated in tumors [57,141]. After the two components are rendered to be tumor targeting, the PDT activation in tumor cells is much higher than that in normal tissues.
A PDT system can be constructed in one nanostructure. The photosensitizer Ce6 was activated by CL of 89Zr to produce ROS. In vivo studies showed that the subcutaneous injection of 89Zr HMSN-Ce6 inhibits tumor growth in mice (Figure 5b) [52]. Many photosensitizing agents concentrate in many normal organs of the host, e.g., liver, spleen, and kidney. In Figure 5b, the ‘L’ shape in the middle of the mice shows the accumulation of 89Zr HMSN-Ce6 agents in liver. In the scenario where photosensitizers and radionuclides are found in one nanostructure, there could be phototoxic side effects in normal tissues if photosensitizers are administered systemically. Therefore, a smart choice of nanostructure with high targeting ability and rigorous phototoxic evaluations should be carried out before use. Ni et al. synthesized magnetic nanoparticles with 89Zr radiolabeling and porphyrin molecules surface modification (Figure 5c). Both the magnetic field-targeted and non-targeted tumors showed the time-dependent uptake of 89Zr-MNP-PEG, thereby indicating the passive enhanced permeability and retention effect. Importantly, the magnetic field-targeted tumors evidently exhibited the higher accumulation of 89Zr-MNP-PEG, especially at the outer side of the tumor which was attached to the magnetic field [130].
High-energy X-rays commonly used in radiotherapy have strong penetrating power in tissues and the number of CL photons generated is proportional to the radiation dose. The combination of PDT and radiotherapy is another application scenario of PDT. Radiotherapy enhanced with Cherenkov photon activation (RECA) is a process of using CL produced from the megavoltage photon beam to photo-activate light-sensitive drugs. In vitro studies of B16 melanoma and 4T1 murine breast tumor cells demonstrated that RECA increased the cytotoxicity of tumor cells [142]. Sun et al. designed an aggregation-induced emission heterogeneous photosensitizer-conjugated gold clustoluminogen (AIE-Au) for efficient low-dose X-ray induced photodynamic therapy, and the results show that under low-dose X-ray radiation, AIE-Au strongly absorbed X-rays and enhanced the radiotherapy effect. Additionally, AIE-Au converted X-rays to optical luminescence, which excited the conjugated photosensitizers for PDT [143]. They all have the potential to extend the scope of light-sensitive drugs treatments from superficial to deep seated lesions.
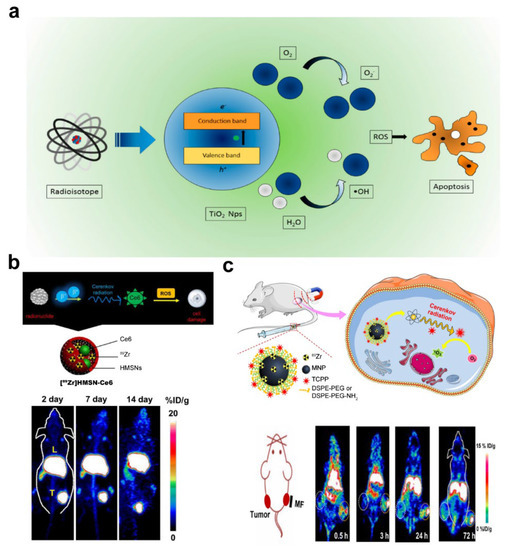
Figure 5.
Schematic illustration for Cherenkov luminescence (CL) photodynamic therapy (PDT) and its effects. (a) Principle of PDT based on CL in TiO2 nanoparticles. Adapted with permission from Ref. [144] 2020 MDPI. (b) Top: Diagrams of 89Zr HMSN-Ce6 structure. Bottom: Maximum intensity projection positron emission tomography (PET) images of 4T1 tumor-bearing mice taken at various time points post injection of 89Zr HMSN-Ce6. Adapted with permission from Ref. [52] 2016 American Chemical Society. (c) Top: Diagrams of magnetism-enhanced CL induced PDT. Bottom: representative coronal PET imaging slices of bilateral 4T1 tumor-bearing mice after the intravenous injection of 89Zr-MNP-PEG at different time points. Adapted with permission from Ref. [130] 2018 American Chemical Society. NPs: nanoparticles; ROS: reactive oxygen species; HMSN: hollow mesoporous silica nanoparticle; MNP: magnetic nanoparticle; MF: magnetic field.
4.4. Tumor Microenvironment Monitoring in Treatment
In the era of personalized medicine, there is an urgent need for in vivo techniques able to sensitively detect and quantify molecular activities. Real time monitoring the physiological changes of tumor tissue during treatment can predict and improve the therapeutic outcome. CLI enables quantitative, repeatable, highly sensitive and specific imaging of target macromolecules, target regions, and physiological and pathological activities in living organisms in a non-invasive way [58].
Huge progress has been made in monitoring the biological changes in the process of tumor treatment with CL. Pogue et al. realized in vivo mapping of tumor oxidation at a depth of several millimeters through time-gated CCD, the signal of which was synchronized with an X-ray pulse, with submillimeter resolution and nanomolar sensitivity [59]. Rickard et al. observed the CL emission of six pet dogs with naturally occurring superficial soft tissue tumors. The results showed that there were significant subscore differences in the CL intensity of normal tissue, whole tumor tissue, tumor marginal tissue, and tumor central tissue [60]. Soter et al. used megavolt X-ray radiation beams to track the diffusion of ultraviolet-sensitive phosphorescent tattoo ink that was injected into tumors (Figure 6a), and the results indicated that the diffusive spreading of the injected ink was related to radiation-induced necrosis [61].
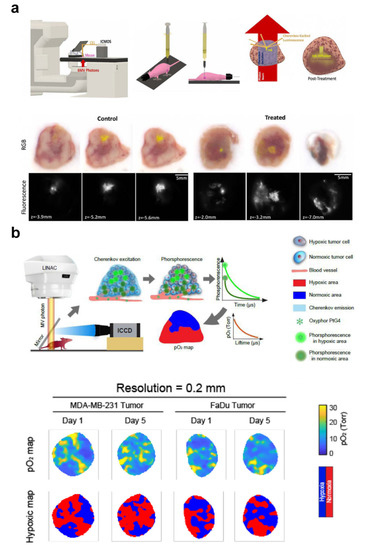
Figure 6.
Tumor microenvironment monitoring with Cherenkov luminescence image. (a) Top: Workflow diagram of tracking tumor radiotherapy response in vivo with Cherenkov excited luminescence ink imaging. Bottom: Examples of the results. Adapted with permission from Ref. [61] 2020 IPO Publishing. Ltd. (b) Top: Workflow diagram of in vivo pO2 imaging based on Cherenkov excited luminescence imaging. Bottom: Examples of the results. Adapted with permission from Ref. [35] 2021 Elsevier.
Tissue oxygenation plays an important role in killing a tumor in radiotherapy. Hypoxic tumors usually lead to poor prognosis in cancer patients, thus tumor oxygenation can be regarded as a prognostic factor for survival after radiotherapy [145]. Many clinical trials have been conducted to improve the therapeutic outcomes by changing tumor oxygenation [146,147]. However, due to the complexity of oxygen measurement technology, it is still difficult to realize in vivo measurement in routine use [148]. CL is a by-product of radiation in tissue. Studies on animal tumors have confirmed the correlation between CL spectrum and optical measurement of blood oxygen saturation based on the tissue absorption coefficient [58,62,63]. PtG4 is an oxygen-quenched dendrimer. The phosphorescence of PtG4 excited by CL can be used to quantify pO2 in tissues (Figure 6b) [35,64,65].
5. Conclusions and Perspectives
Accurate tumor diagnosis and effective treatment strategies can provide new hope of defeating tumors for cancer patients. To this end, CL has shown its technical advantages and clinical transformation potential in many important fields, including tumor detection in vivo, surgical navigation, radiotherapy, PDT, and therapeutic outcomes evaluation. However, CL is generated by charged particles, any source of ionizing radiation has potentially carcinogenic potential. Much attention should be paid to investigating all the processes involved ionizing radiation since the physiopathology of toxicity from ionizing radiation is complex [149]. In order to reduce damage to normal tissues caused by radiation, further research is needed to provide an understanding of the detailed molecular and pathophysiological mechanisms of radiation carcinogenesis.
In the exploration process, CL has shown some limitations, including weak signal intensity, being easily interfered with by external ambient light, being easily attenuated by biological tissue, the lack of a photosensitizer corresponding to the spectrum. In recent years, various new methods, such as CRET, SCIFI, CELSI, REFI, and photosensitizer surface modification combined with nanotechnology, have improved light propagation model-based reconstruction algorithms, and machine learning-based reconstruction algorithms have expanded the CL capability of tumor diagnosis and treatment to a certain extent and enlarged the application scenario. Despite the impressive progress outlined above, the existing research on CL is insufficient.
Weak CL signals are an inherent challenge for imaging applications, which leads to a long exposure time to obtain high-quality images. In a clinical setting, organs could be displaced and deformed due to the influence of respiration and peristalsis during the process of image data collection. This affects the image accuracy if it is acquired over a long time. Therefore, the temporal resolution of CL images needs further improvement. The amount of Cherenkov photons can be increased by radionuclides with higher energy [69]. It has been suggested that various nanoparticles respond well to the CL spectrum with red-shift, thus improving imaging sensitivity and time resolution [69,88,95]. At this stage, most CL investigations are preclinical, with only a few trials having been carried out on humans, which in part is due to the limited tissue penetration depth of CL. Thus, advances in camera sensitivity for the detection in deeper tissue and combination with other imaging or biomedical techniques may be appropriate as future avenues for accelerating the clinical translation of CL.
PDT is as an attractive option for cancer treatment. However, conventional PDT is activated by light that has poor tissue penetration, which limits its clinical application. CL has emerged as an attractive internal light source to address the issue of outer light penetration [138], but the biosafety of CLPDT should be investigated for potential clinical application in the future.
Author Contributions
Conceptualization, Y.Y.; methodology, X.W., L.L., J.L. (Jie Li), P.W. and J.L. (Jinyi Lang); writing—original draft preparation, X.W., L.L., J.L. (Jie Li) and P.W.; writing—review and editing, Y.Y. and J.L. (Jinyi Lang); funding acquisition, Y.Y., P.W., X.W. and J.L. (Jie Li) All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Medical Engineering Innovation Fund for Cancer (ZYGX2021YGCX002), Sichuan Science and Technology Program (2022YFG0194, 2021YFG0320).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, M.J.; Jain, R.K.; Langer, R. Engineering and physical sciences in oncology: Challenges and opportunities. Nat. Rev. Cancer 2017, 17, 659–675. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.Y.; Chen, T.Y.; Williamson, D.F.K.; Zhao, M.; Shady, M.; Lipkova, J.; Mahmood, F. AI-based pathology predicts origins for cancers of unknown primary. Nature 2021, 594, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Navin, N.E. Tumor Evolution in Response to Chemotherapy: Phenotype versus Genotype. Cell Rep. 2014, 6, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Blaszczak, W.; Swietach, P. What do cellular responses to acidity tell us about cancer? Cancer Metastasis Rev. 2021, 40, 1159–1176. [Google Scholar] [CrossRef]
- Weissleder, R. Molecular imaging in cancer. Science 2006, 312, 1168–1171. [Google Scholar] [CrossRef]
- Gambhir, S.S. Molecular imaging of cancer with positron emission tomography. Nat. Rev. Cancer 2002, 2, 683–693. [Google Scholar] [CrossRef]
- Han, Z.; Ke, M.; Liu, X.; Wang, J.; Guan, Z.; Qiao, L.; Wu, Z.; Sun, Y.; Sun, X. Molecular Imaging, How Close to Clinical Precision Medicine in Lung, Brain, Prostate and Breast Cancers. Mol. Imaging Biol. 2021, 24, 8–22. [Google Scholar] [CrossRef]
- Zhong, Y.; Ma, Z.; Wang, F.; Wang, X.; Yang, Y.; Liu, Y.; Zhao, X.; Li, J.; Du, H.; Zhang, M.; et al. In vivo molecular imaging for immunotherapy using ultra-bright near-infrared-IIb rare-earth nanoparticles. Nat. Biotechnol. 2019, 37, 1322–1331. [Google Scholar] [CrossRef]
- Li, Y.; Lu, G.; Zhou, Q.; Chen, Z. Advances in Endoscopic Photoacoustic Imaging. Photonics 2021, 8, 281. [Google Scholar] [CrossRef]
- Weissleder, R. Molecular imaging: Exploring the next frontier. Radiology 1999, 212, 609–614. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Farwell, M.D.; Clark, A.S.; Pryma, D.A. Making Molecular Imaging a Clinical Tool for Precision Oncology: A Review. JAMA Oncol. 2017, 3, 695–701. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Sun, Y.; Wu, S.; Zhou, M.; Zhang, X.; Zhou, R.; Zhang, T.; Gao, Y.; Chen, T.; Chen, Y.; et al. Systematic imaging in medicine: A comprehensive review. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1736–1758. [Google Scholar] [CrossRef] [PubMed]
- Mankoff, D.A. A definition of molecular imaging. J. Nucl. Med. 2007, 48, 18N–21N. [Google Scholar] [PubMed]
- James, M.L.; Gambhir, S.S. A molecular imaging primer: Modalities, imaging agents, and applications. Physiol. Rev. 2012, 92, 897–965. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Shu, J. Multimodal molecular imaging: Current status and future directions. Contrast Media Mol. Imaging 2018, 2018, 1382183. [Google Scholar] [CrossRef]
- De Vries, E.G.E.; Kist de Ruijter, L.; Lub-de Hooge, M.N.; Dierckx, R.A.; Elias, S.G.; Oosting, S.F. Integrating molecular nuclear imaging in clinical research to improve anticancer therapy. Nat. Rev. Clin. Oncol. 2019, 16, 241–255. [Google Scholar] [CrossRef]
- Koch, M.; Symvoulidis, P.; Ntziachristos, V. Tackling standardization in fluorescence molecular imaging. Nat. Photonics 2018, 12, 505–515. [Google Scholar] [CrossRef]
- Garland, M.; Yim, J.J.; Bogyo, M. A Bright Future for Precision Medicine: Advances in Fluorescent Chemical Probe Design and Their Clinical Application. Cell Chem. Biol. 2016, 23, 122–136. [Google Scholar] [CrossRef]
- Berrones-Reyes, J.C.; Vidyasagar, C.C.; Muñoz Flores, B.M.; Jiménez-Pérez, V.M. Luminescent molecules of main group elements: Recent advances on synthesis, properties and their application on fluorescent bioimaging (FBI). J. Lumin. 2018, 195, 290–313. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, D.; Wang, Z.; Xie, L.; Ying, Y. Spatial-Frequency Domain Imaging: An Emerging Depth-Varying and Wide-Field Technique for Optical Property Measurement of Biological Tissues. Photonics 2021, 8, 162. [Google Scholar] [CrossRef]
- Grootendorst, M.R.; Cariati, M.; Kothari, A.; Tuch, D.S.; Purushotham, A. Cerenkov luminescence imaging (CLI) for image-guided cancer surgery. Clin. Transl. Imaging 2016, 4, 353–366. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yu, F.; Lv, C.; Choo, J.; Chen, L. Fluorescent chemical probes for accurate tumor diagnosis and targeting therapy. Chem. Soc. Rev. 2017, 46, 2237–2271. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, T.M.; Drain, C.M.; Grimm, J. Optical Imaging of Ionizing Radiation from Clinical Sources. J. Nucl. Med. 2016, 57, 1661–1666. [Google Scholar] [CrossRef] [PubMed]
- Pogue, B.W.; Zhang, R.; Cao, X.; Jia, J.M.; Petusseau, A.; Bruza, P.; Vinogradov, S.A. Review of in vivo optical molecular imaging and sensing from X-ray excitation. J. Biomed. Opt. 2021, 26, 010902. [Google Scholar] [CrossRef]
- Collins, G.B.; Reiling, V.G. Cerenkov radiation. Phys. Rev. 1938, 54, 499. [Google Scholar] [CrossRef]
- Mc Larney, B.; Skubal, M.; Grimm, J. A Review of Recent and Emerging Approaches for the Clinical Application of Cerenkov Luminescence Imaging. Front. Phys. 2021, 9, 419. [Google Scholar] [CrossRef]
- Branger, E.; Grape, S.; Jansson, P.; Svärd, S.J. On the inclusion of light transport in prediction tools for Cherenkov light intensity assessment of irradiated nuclear fuel assemblies. J. Instrum. 2019, 14, T01010. [Google Scholar] [CrossRef]
- Tamura, R.; Pratt, E.C.; Grimm, J. Innovations in nuclear imaging instrumentation: Cerenkov imaging. In Seminars in Nuclear Medicine; WB Saunders: Philadelphia, PA, USA, 2018; pp. 359–366. [Google Scholar]
- Hu, H.; Lin, X.; Wong, L.J.; Yang, Q.; Liu, D.; Zhang, B.; Luo, Y. Surface Dyakonov–Cherenkov radiation. eLight 2022, 2, 2. [Google Scholar] [CrossRef]
- Katori, T.; Yanez, J.P.; Yuan, T. Neutrino interaction physics in neutrino telescopes. Eur. Phys. J. Spec. Top. 2021, 230, 4293–4308. [Google Scholar] [CrossRef]
- Mitchell, G.S.; Lloyd, P.N.T.; Cherry, S.R. Cerenkov luminescence and PET imaging of (90)Y: Capabilities and limitations in small animal applications. Phys. Med. Biol. 2020, 65, 065006. [Google Scholar] [CrossRef]
- Bhatt, N.B.; Pandya, D.N.; Dezarn, W.A.; Marini, F.C.; Zhao, D.; Gmeiner, W.H.; Triozzi, P.L.; Wadas, T.J. Practical Guidelines for Cerenkov Luminescence Imaging with Clinically Relevant Isotopes. Methods Mol. Biol. 2018, 1790, 197–208. [Google Scholar] [CrossRef]
- Pétusseau, A.F.; Bruza, P.; Pogue, B.W. Survey of X-ray induced Cherenkov excited fluorophores with potential for human use. J. Radiat. Res. 2021, 62, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Allu, S.R.; Jiang, S.; Gunn Bs, J.R.; Yao Ph, D.C.; Xin Ph, D.J.; Bruza Ph, D.P.; Gladstone Sc, D.D.; Jarvis Md Ph, D.L.; Tian Ph, D.J.; et al. High-Resolution pO(2) Imaging Improves Quantification of the Hypoxic Fraction in Tumors During Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2021, 109, 603–613. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Ren, G.; Miao, Z.; Zhang, X.; Tang, X.; Han, P.; Gambhir, S.S.; Cheng, Z. Molecular optical imaging with radioactive probes. PLoS ONE 2010, 5, e9470. [Google Scholar] [CrossRef] [PubMed]
- Glaser, A.K.; Zhang, R.; Gladstone, D.J.; Pogue, B.W. Optical dosimetry of radiotherapy beams using Cherenkov radiation: The relationship between light emission and dose. Phys. Med. Biol. 2014, 59, 3789–3811. [Google Scholar] [CrossRef]
- Hachadorian, R.L.; Bruza, P.; Jermyn, M.; Gladstone, D.J.; Pogue, B.W.; Jarvis, L.A. Imaging radiation dose in breast radiotherapy by X-ray CT calibration of Cherenkov light. Nat. Commun. 2020, 11, 2298. [Google Scholar] [CrossRef] [PubMed]
- Glaser, A.K.; Andreozzi, J.M.; Davis, S.C.; Zhang, R.; Pogue, B.W.; Fox, C.J.; Gladstone, D.J. Video-rate optical dosimetry and dynamic visualization of IMRT and VMAT treatment plans in water using Cherenkov radiation. Med. Phys. 2014, 41, 062102. [Google Scholar] [CrossRef]
- Zhang, R.; Glaser, A.K.; Andreozzi, J.; Jiang, S.; Jarvis, L.A.; Gladstone, D.J.; Pogue, B.W. Beam and tissue factors affecting Cherenkov image intensity for quantitative entrance and exit dosimetry on human tissue. J. Biophotonics 2017, 10, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Hachadorian, R.; Bruza, P.; Jermyn, M.; Mazhar, A.; Cuccia, D.; Jarvis, L.; Gladstone, D.; Pogue, B. Correcting Cherenkov light attenuation in tissue using spatial frequency domain imaging for quantitative surface dosimetry during whole breast radiation therapy. J. Biomed. Opt. 2018, 24, 071609. [Google Scholar] [CrossRef]
- Zlateva, Y.; Muir, B.R.; El Naqa, I.; Seuntjens, J.P. Cherenkov emission-based external radiotherapy dosimetry: I. Formalism and feasibility. Med. Phys. 2019, 46, 2370–2382. [Google Scholar] [CrossRef] [PubMed]
- Zlateva, Y.; Muir, B.R.; Seuntjens, J.P.; El Naqa, I. Cherenkov emission-based external radiotherapy dosimetry: II. Electron beam quality specification and uncertainties. Med. Phys. 2019, 46, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Andreozzi, J.M.; Gladstone, D.J.; Hitchcock, W.L.; Glaser, A.K.; Jiang, S.; Pogue, B.W.; Jarvis, L.A. Cherenkoscopy based patient positioning validation and movement tracking during post-lumpectomy whole breast radiation therapy. Phys. Med. Biol. 2015, 60, L1–L14. [Google Scholar] [CrossRef]
- Snyder, C.; Pogue, B.W.; Jermyn, M.; Tendler, I.; Andreozzi, J.M.; Bruza, P.; Krishnaswamy, V.; Gladstone, D.J.; Jarvis, L.A. Algorithm development for intrafraction radiotherapy beam edge verification from Cherenkov imaging. J. Med. Imaging (Bellingham) 2018, 5, 015001. [Google Scholar] [CrossRef] [PubMed]
- Glaser, A.K.; Voigt, W.; Davis, S.C.; Zhang, R.; Gladstone, D.J.; Pogue, B.W. Three-dimensional erenkov tomography of energy deposition from ionizing radiation beams. Opt. Lett. 2013, 38, 634–636. [Google Scholar] [CrossRef]
- Glaser, A.K.; Davis, S.C.; McClatchy, D.M.; Zhang, R.; Pogue, B.W.; Gladstone, D.J. Projection imaging of photon beams by the Čerenkov effect. Med. Phys. 2013, 40, 012101. [Google Scholar] [CrossRef] [PubMed]
- Yogo, K.; Matsushita, A.; Tatsuno, Y.; Shimo, T.; Hirota, S.; Nozawa, M.; Ozawa, S.; Ishiyama, H.; Yasuda, H.; Nagata, Y.; et al. Imaging Cherenkov emission for quality assurance of high-dose-rate brachytherapy. Sci. Rep. 2020, 10, 3572. [Google Scholar] [CrossRef] [PubMed]
- Yogo, K.; Noguchi, Y.; Okudaira, K.; Nozawa, M.; Ishiyama, H.; Okamoto, H.; Yasuda, H.; Oguchi, H.; Yamamoto, S. Source position measurement by Cherenkov emission imaging from applicators for high-dose-rate brachytherapy. Med. Phys. 2021, 48, 488–499. [Google Scholar] [CrossRef]
- Alexander, D.A.; Bruza, P.; Rassias, A.G.; Andreozzi, J.M.; Pogue, B.W.; Zhang, R.; Gladstone, D.J. Visual Isocenter Position Enhanced Review (VIPER): A Cherenkov imaging-based solution for MR-linac daily QA. Med. Phys. 2021, 48, 2750–2759. [Google Scholar] [CrossRef]
- Hartl, B.; Hirschberg, H.; Marcu, L.; Cherry, S.R. Activating Photodynamic Therapy in vitro with Cerenkov Radiation Generated from Yttrium-90. J. Environ. Pathol. Toxicol. Oncol. 2016, 35, 185–192. [Google Scholar] [CrossRef]
- Kamkaew, A.; Cheng, L.; Goel, S.; Valdovinos, H.F.; Barnhart, T.E.; Liu, Z.; Cai, W. Cerenkov Radiation Induced Photodynamic Therapy Using Chlorin e6-Loaded Hollow Mesoporous Silica Nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 26630–26637. [Google Scholar] [CrossRef]
- Duan, D.; Liu, H.; Xu, Y.; Han, Y.; Xu, M.; Zhang, Z.; Liu, Z. Activating TiO(2) Nanoparticles: Gallium-68 Serves as a High-Yield Photon Emitter for Cerenkov-Induced Photodynamic Therapy. ACS Appl. Mater. Interfaces 2018, 10, 5278–5286. [Google Scholar] [CrossRef]
- Blum, N.T.; Zhang, Y.; Qu, J.; Lin, J.; Huang, P. Recent Advances in Self-Exciting Photodynamic Therapy. Front. Bioeng. Biotechnol. 2020, 8, 594491. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hao, Y.; Chen, S.; Xu, M. Photodynamic Therapy of Cancers With Internal Light Sources: Chemiluminescence, Bioluminescence, and Cerenkov Radiation. Front. Chem. 2020, 8, 770. [Google Scholar] [CrossRef] [PubMed]
- Bessière, A.; Durand, J.-O.; Noûs, C. Persistent luminescence materials for deep photodynamic therapy. Nanophotonics 2021, 10, 2999–3029. [Google Scholar] [CrossRef]
- Pham, T.C.; Nguyen, V.N.; Choi, Y.; Lee, S.; Yoon, J. Recent Strategies to Develop Innovative Photosensitizers for Enhanced Photodynamic Therapy. Chem. Rev. 2021, 121, 13454–13619. [Google Scholar] [CrossRef]
- Pogue, B.W.; Cao, X.; Swartz, H.M.; Vinogradov, S.A. Review of Tissue Oxygenation Sensing During Radiotherapy Based Upon Cherenkov-Excited Luminescence Imaging. Appl. Magn. Reson. 2021, 52, 1521–1536. [Google Scholar] [CrossRef]
- Pogue, B.W.; Feng, J.; LaRochelle, E.P.; Bruža, P.; Lin, H.; Zhang, R.; Shell, J.R.; Dehghani, H.; Davis, S.C.; Vinogradov, S.A.; et al. Maps of in vivo oxygen pressure with submillimetre resolution and nanomolar sensitivity enabled by Cherenkov-excited luminescence scanned imaging. Nat. Biomed. Eng. 2018, 2, 254–264. [Google Scholar] [CrossRef]
- Rickard, A.G.; Yoshikawa, H.; Palmer, G.M.; Liu, H.Q.; Dewhirst, M.W.; Nolan, M.W.; Zhang, X. Cherenkov emissions for studying tumor changes during radiation therapy: An exploratory study in domesticated dogs with naturally-occurring cancer. PLoS ONE 2020, 15, e0238106. [Google Scholar] [CrossRef]
- Soter, J.A.; LaRochelle, E.P.M.; Byrd, B.K.; Tendler, I.I.; Gunn, J.R.; Meng, B.; Strawbridge, R.R.; Wirth, D.J.; Davis, S.C.; Gladstone, D.J.; et al. Tracking tumor radiotherapy response in vivo with Cherenkov-excited luminescence ink imaging. Phys. Med. Biol. 2020, 65, 095004. [Google Scholar] [CrossRef]
- Zhang, X.; Lam, S.K.; Palmer, G.; Das, S.; Oldham, M.; Dewhirst, M. Noninvasive measurement of tissue blood oxygenation with Cerenkov imaging during therapeutic radiation delivery. Opt. Lett. 2017, 42, 3101–3104. [Google Scholar] [CrossRef]
- Axelsson, J.; Glaser, A.K.; Gladstone, D.J.; Pogue, B.W. Quantitative Cherenkov emission spectroscopy for tissue oxygenation assessment. Optics Express 2012, 20, 5133–5142. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Gunn, J.R.; Allu, S.R.; Bruza, P.; Jiang, S.; Vinogradov, S.A.; Pogue, B.W. Implantable sensor for local Cherenkov-excited luminescence imaging of tumor pO2 during radiotherapy. J. Biomed. Opt. 2020, 25, 112704. [Google Scholar] [CrossRef]
- Cao, X.; Rao Allu, S.; Jiang, S.; Jia, M.; Gunn, J.R.; Yao, C.; LaRochelle, E.P.; Shell, J.R.; Bruza, P.; Gladstone, D.J.; et al. Tissue pO(2) distributions in xenograft tumors dynamically imaged by Cherenkov-excited phosphorescence during fractionated radiation therapy. Nat. Commun. 2020, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.S.; Gill, R.K.; Boucher, D.L.; Li, C.; Cherry, S.R. In vivo Cerenkov luminescence imaging: A new tool for molecular imaging. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 4605–4619. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, J.; Cheng, Z. Cerenkov radiation: A multi-functional approach for biological sciences. Front. Phys. 2014, 2, 4. [Google Scholar] [CrossRef]
- Jelley, J.V. Cerenkov radiation and its applications. Br. J. Appl. Phys. 1955, 6, 227–232. [Google Scholar] [CrossRef]
- Shaffer, T.M.; Pratt, E.C.; Grimm, J. Utilizing the power of Cerenkov light with nanotechnology. Nat. Nanotechnol. 2017, 12, 106. [Google Scholar] [CrossRef]
- Shrock, Z.; Yoon, S.W.; Gunasingha, R.; Oldham, M.; Adamson, J. Technical Note: On maximizing Cherenkov emissions from medical linear accelerators. Med. Phys. 2018, 45, 3315–3320. [Google Scholar] [CrossRef]
- Spinelli, A.E.; Kuo, C.; Rice, B.W.; Calandrino, R.; Marzola, P.; Sbarbati, A.; Boschi, F. Multispectral Cerenkov luminescence tomography for small animal optical imaging. Opt. Express 2011, 19, 12605–12618. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, X.; Qu, X.; Yang, W.; Liang, J.; Wang, J.; Tian, J. Three-dimensional noninvasive monitoring iodine-131 uptake in the thyroid using a modified Cerenkov luminescence tomography approach. PLoS ONE 2012, 7, e37623. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, X.; Song, T.; Bao, C.; Shi, L.; Hu, Z.; Wang, K.; Tian, J. Multispectral hybrid Cerenkov luminescence tomography based on the finite element SPn method. J. Biomed. Opt. 2015, 20, 86007. [Google Scholar] [CrossRef] [PubMed]
- Hongbo, G.; Xiaowei, H.; Muhan, L.; Zeyu, Z.; Zhenhua, H.; Jie, T. Weight Multispectral Reconstruction Strategy for Enhanced Reconstruction Accuracy and Stability With Cerenkov Luminescence Tomography. IEEE Trans. Med. Imaging 2017, 36, 1337–1346. [Google Scholar] [CrossRef]
- Robertson, R.; Germanos, M.S.; Li, C.; Mitchell, G.S.; Cherry, S.R.; Silva, M.D. Optical imaging of Cerenkov light generation from positron-emitting radiotracers. Phys. Med. Biol. 2009, 54, N355–N365. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.E.; D’Ambrosio, D.; Calderan, L.; Marengo, M.; Sbarbati, A.; Boschi, F. Cerenkov radiation allows in vivo optical imaging of positron emitting radiotracers. Phys. Med. Biol. 2010, 55, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.S.; Gill, R.K.; Cherry, S.R. Comments on ‘Cerenkov radiation allows in vivo optical imaging of positron emitting radiotracers’. Phys. Med. Biol. 2010, 55, L43–L44; author reply L45–L49. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, H.; Cheng, Z. Harnessing the power of radionuclides for optical imaging: Cerenkov luminescence imaging. J. Nucl. Med. 2011, 52, 2009–2018. [Google Scholar] [CrossRef]
- Ruggiero, A.; Holland, J.P.; Lewis, J.S.; Grimm, J. Cerenkov luminescence imaging of medical isotopes. J. Nucl. Med. 2010, 51, 1123–1130. [Google Scholar] [CrossRef]
- Spinelli, A.E.; Ferdeghini, M.; Cavedon, C.; Zivelonghi, E.; Calandrino, R.; Fenzi, A.; Sbarbati, A.; Boschi, F. First human Cerenkography. J. Biomed. Opt. 2013, 18, 20502. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, R.; Gunn, J.R.; Esipova, T.V.; Vinogradov, S.; Gladstone, D.J.; Jarvis, L.A.; Pogue, B.W. Comparison of Cherenkov excited fluorescence and phosphorescence molecular sensing from tissue with external beam irradiation. Phys. Med. Biol. 2016, 61, 3955–3968. [Google Scholar] [CrossRef]
- Glaser, A.K.; Zhang, R.; Davis, S.C.; Gladstone, D.J.; Pogue, B.W. Time-gated Cherenkov emission spectroscopy from linear accelerator irradiation of tissue phantoms. Opt. Lett. 2012, 37, 1193–1195. [Google Scholar] [CrossRef] [PubMed]
- Dothager, R.S.; Goiffon, R.J.; Jackson, E.; Harpstrite, S.; Piwnica-Worms, D. Cerenkov radiation energy transfer (CRET) imaging: A novel method for optical imaging of PET isotopes in biological systems. PLoS ONE 2010, 5, e13300. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Dobrucki, L.W.; Marjanovic, M.; Chaney, E.J.; Suslick, K.S.; Boppart, S.A. Enhancement and wavelength-shifted emission of Cerenkov luminescence using multifunctional microspheres. Phys. Med. Biol. 2015, 60, 727–739. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Xing, B.; Han, P.; Gambhir, S.S.; Cheng, Z. Radiation-luminescence-excited quantum dots for in vivo multiplexed optical imaging. Small 2010, 6, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, B.; Zheng, Z.; Liu, T. Semiconductor quantum dots in tumor research. J. Lumin. 2019, 209, 61–68. [Google Scholar] [CrossRef]
- Zhou, C.; Hao, G.; Thomas, P.; Liu, J.; Yu, M.; Sun, S.; Öz, P.K.; Sun, P.; Zheng, P. Near-Infrared Emitting Radioactive Gold Nanoparticles with Molecular Pharmacokinetics. Angew. Chem. Int. Ed. 2012, 51, 10118–10122. [Google Scholar] [CrossRef]
- Volotskova, O.; Sun, C.; Stafford, J.H.; Koh, A.L.; Ma, X.; Cheng, Z.; Cui, B.; Pratx, G.; Xing, L. Efficient Radioisotope Energy Transfer by Gold Nanoclusters for Molecular Imaging. Small 2015, 11, 4002–4008. [Google Scholar] [CrossRef] [PubMed]
- Bernhard, Y.; Collin, B.; Decréau, R.A. Inter/intramolecular Cherenkov radiation energy transfer (CRET) from a fluorophore with a built-in radionuclide. Chem. Commun. 2014, 50, 6711–6713. [Google Scholar] [CrossRef]
- Lioret, V.; Bellaye, P.S.; Arnould, C.; Collin, B.; Decreau, R.A. Dual Cherenkov Radiation-Induced Near-Infrared Luminescence Imaging and Photodynamic Therapy toward Tumor Resection. J. Med. Chem. 2020, 63, 9446–9456. [Google Scholar] [CrossRef]
- Cao, X.; Chen, X.; Kang, F.; Zhan, Y.; Cao, X.; Wang, J.; Liang, J.; Tian, J. Intensity Enhanced Cerenkov Luminescence Imaging Using Terbium-Doped Gd2O2S Microparticles. ACS Appl. Mater. Interfaces 2015, 7, 11775–11782. [Google Scholar] [CrossRef]
- Ma, X.; Kang, F.; Xu, F.; Feng, A.; Zhao, Y.; Lu, T.; Yang, W.; Wang, Z.; Lin, M.; Wang, J. Enhancement of Cerenkov luminescence imaging by dual excitation of Er(3+),Yb(3+)-doped rare-earth microparticles. PLoS ONE 2013, 8, e77926. [Google Scholar] [CrossRef][Green Version]
- Thorek, D.L.; Ogirala, A.; Beattie, B.J.; Grimm, J. Quantitative imaging of disease signatures through radioactive decay signal conversion. Nat. Med. 2013, 19, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; D’Souza, A.V.; Gunn, J.R.; Esipova, T.V.; Vinogradov, S.A.; Glaser, A.K.; Jarvis, L.A.; Gladstone, D.J.; Pogue, B.W. Cherenkov-excited luminescence scanned imaging. Opt. Lett. 2015, 40, 827–830. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Qu, Y.; Wang, K.; Zhang, X.; Zha, J.; Song, T.; Bao, C.; Liu, H.; Wang, Z.; Wang, J.; et al. In vivo nanoparticle-mediated radiopharmaceutical-excited fluorescence molecular imaging. Nat. Commun. 2015, 6, 7560. [Google Scholar] [CrossRef]
- Shimamoto, M.; Gotoh, K.; Hasegawa, K.; Kojima, A. Hybrid Light Imaging Using Cerenkov Luminescence and Liquid Scintillation for Preclinical Optical Imaging In Vivo. Mol. Imaging Biol. 2016, 18, 500–509. [Google Scholar] [CrossRef]
- Liu, H.; Carpenter, C.M.; Jiang, H.; Pratx, G.; Sun, C.; Buchin, M.P.; Gambhir, S.S.; Xing, L.; Cheng, Z. Intraoperative imaging of tumors using Cerenkov luminescence endoscopy: A feasibility experimental study. J. Nucl. Med. 2012, 53, 1579–1584. [Google Scholar] [CrossRef]
- Kothapalli, S.R.; Liu, H.; Liao, J.C.; Cheng, Z.; Gambhir, S.S. Endoscopic imaging of Cerenkov luminescence. Biomed. Opt. Express 2012, 3, 1215–1225. [Google Scholar] [CrossRef]
- Cao, X.; Zhan, Y.; Cao, X.; Liang, J.; Chen, X. Harnessing the Power of Cerenkov Luminescence Imaging for Gastroenterology: Cerenkov Luminescence Endoscopy. Curr. Med. Imaging 2017, 13, 50–57. [Google Scholar] [CrossRef]
- Cao, X.; Chen, X.; Kang, F.; Lin, Y.; Liu, M.; Hu, H.; Nie, Y.; Wu, K.; Wang, J.; Liang, J.; et al. Performance evaluation of endoscopic Cerenkov luminescence imaging system: In vitro and pseudotumor studies. Biomed. Opt. Express 2014, 5, 3660–3670. [Google Scholar] [CrossRef]
- Carpenter, C.M.; Ma, X.; Liu, H.; Sun, C.; Pratx, G.; Wang, J.; Gambhir, S.S.; Xing, L.; Cheng, Z. Cerenkov luminescence endoscopy: Improved molecular sensitivity with β--emitting radiotracers. J. Nucl. Med. 2014, 55, 1905–1909. [Google Scholar] [CrossRef]
- Cao, X.; Chen, X.; Cao, X.; Zhan, Y.; Liang, J. Sensitivity improvement of Cerenkov luminescence endoscope with terbium doped Gd2O2S nanoparticles. Appl. Phys. Lett. 2015, 106, 213702. [Google Scholar] [CrossRef]
- Song, T.; Liu, X.; Qu, Y.; Liu, H.; Bao, C.; Leng, C.; Hu, Z.; Wang, K.; Tian, J. A Novel Endoscopic Cerenkov Luminescence Imaging System for Intraoperative Surgical Navigation. Mol. Imaging 2015, 14, 443–449. [Google Scholar] [CrossRef]
- Hu, H.; Cao, X.; Kang, F.; Wang, M.; Lin, Y.; Liu, M.; Li, S.; Yao, L.; Liang, J.; Liang, J.; et al. Feasibility study of novel endoscopic Cerenkov luminescence imaging system in detecting and quantifying gastrointestinal disease: First human results. Eur. Radiol. 2015, 25, 1814–1822. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Mitchell, G.S.; Cherry, S.R. Cerenkov luminescence tomography for small-animal imaging. Opt. Lett. 2010, 35, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Liang, J.; Yang, W.; Fan, W.; Li, C.; Ma, X.; Chen, X.; Ma, X.; Li, X.; Qu, X.; et al. Experimental Cerenkov luminescence tomography of the mouse model with SPECT imaging validation. Opt. Express 2010, 18, 24441–24450. [Google Scholar] [CrossRef]
- Zhong, J.; Tian, J.; Yang, X.; Qin, C. Whole-body Cerenkov luminescence tomography with the finite element SP(3) method. Ann. Biomed. Eng. 2011, 39, 1728–1735. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, X.; Liang, J.; Qu, X.; Chen, D.; Yang, W.; Wang, J.; Cao, F.; Tian, J. Single photon emission computed tomography-guided Cerenkov luminescence tomography. J. Appl. Phys. 2012, 112, 227. [Google Scholar] [CrossRef]
- Wang, L.; He, X.; Yu, J. Prior Compensation Algorithm for Cerenkov Luminescence Tomography From Single-View Measurements. Front. Oncol. 2021, 11, 749889. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, K.; An, Y.; Jiang, S.; Meng, H.; Tian, J. Nonmodel-based bioluminescence tomography using a machine-learning reconstruction strategy. Optica 2018, 5, 1451–1454. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, M.; Gao, Y.; Shi, X.; Zhang, X.; Hu, Z.; Tian, J. A novel Cerenkov luminescence tomography approach using multilayer fully connected neural network. Phys. Med. Biol. 2019, 64, 245010. [Google Scholar] [CrossRef]
- Cai, M.; Zhang, Z.; Shi, X.; Yang, J.; Tian, J. Non-negative Iterative Convex Refinement Approach for Accurate and Robust Reconstruction in Cerenkov Luminescence Tomography. IEEE Trans. Med. Imaging 2020, 39, 3207–3217. [Google Scholar] [CrossRef]
- Thorek, D.; Robertson, R.; Bacchus, W.A.; Hahn, J.; Rothberg, J.; Beattie, B.J.; Grimm, J. Cerenkov imaging—A new modality for molecular imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 163–173. [Google Scholar] [PubMed]
- Tanha, K.; Pashazadeh, A.M.; Pogue, B.W. Review of biomedical Cerenkov luminescence imaging applications. Biomed. Opt. Express 2015, 6, 3053–3065. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.Y.; Mair, R.; Wright, A.; Allinson, K.; Lyons, S.K.; Booth, T.; Jones, J.; Bielik, R.; Soloviev, D.; Brindle, K.M. [(18)F]fluoroethyltyrosine-induced Cerenkov Luminescence Improves Image-Guided Surgical Resection of Glioma. Theranostics 2018, 8, 3991–4002. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qu, Y.; Cao, Y.; Shi, X.; Guo, H.; Zhang, X.; Zheng, S.; Liu, H.; Hu, Z.; Tian, J. A novel in vivo Cerenkov luminescence image-guided surgery on primary and metastatic colorectal cancer. J. Biophotonics 2020, 13, e201960152. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, A.E.; Schiariti, M.P.; Grana, C.M.; Ferrari, M.; Cremonesi, M.; Boschi, F. Cerenkov and radioluminescence imaging of brain tumor specimens during neurosurgery. J. Biomed. Opt. 2016, 21, 50502. [Google Scholar] [CrossRef]
- Holland, J.P.; Normand, G.; Ruggiero, A.; Lewis, J.S.; Grimm, J. Intraoperative imaging of positron emission tomographic radiotracers using Cerenkov luminescence emissions. Mol. Imaging 2011, 10, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Thorek, D.L.; Abou, D.S.; Beattie, B.J.; Bartlett, R.M.; Huang, R.; Zanzonico, P.B.; Grimm, J. Positron lymphography: Multimodal, high-resolution, dynamic mapping and resection of lymph nodes after intradermal injection of 18F-FDG. J. Nucl. Med. 2012, 53, 1438–1445. [Google Scholar] [CrossRef]
- Grootendorst, M.R.; Cariati, M.; Pinder, S.E.; Kothari, A.; Douek, M.; Kovacs, T.; Hamed, H.; Pawa, A.; Nimmo, F.; Owen, J.; et al. Intraoperative Assessment of Tumor Resection Margins in Breast-Conserving Surgery Using (18)F-FDG Cerenkov Luminescence Imaging: A First-in-Human Feasibility Study. J. Nucl. Med. 2017, 58, 891–898. [Google Scholar] [CrossRef]
- Chandra, R.A.; Keane, F.K.; Voncken, F.E.M.; Thomas, C.R., Jr. Contemporary radiotherapy: Present and future. Lancet 2021, 398, 171–184. [Google Scholar] [CrossRef]
- Jarvis, L.A.; Zhang, R.; Gladstone, D.J.; Jiang, S.; Hitchcock, W.; Friedman, O.D.; Glaser, A.K.; Jermyn, M.; Pogue, B.W. Cherenkov video imaging allows for the first visualization of radiation therapy in real time. Int. J. Radiat. Oncol. Biol. Phys. 2014, 89, 615–622. [Google Scholar] [CrossRef]
- Alexander, D.A.; Tendler, I.I.; Bruza, P.; Cao, X.; Schaner, P.E.; Marshall, B.S.; Jarvis, L.A.; Gladstone, D.J.; Pogue, B.W. Assessment of imaging Cherenkov and scintillation signals in head and neck radiotherapy. Phys. Med. Biol. 2019, 64, 145021. [Google Scholar] [CrossRef] [PubMed]
- Glaser, A.K.; Zhang, R.; Andreozzi, J.M.; Gladstone, D.J.; Pogue, B.W. Cherenkov radiation fluence estimates in tissue for molecular imaging and therapy applications. Phys. Med. Biol. 2015, 60, 6701–6718. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, M.R.; Rahman, M.; Zhang, R.; Cao, X.; Williams, B.B.; Hoopes, P.J.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Technical Note: Single-pulse beam characterization for FLASH-RT using optical imaging in a water tank. Med. Phys. 2021, 48, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Van de Ven, S.M.; Elias, S.G.; van den Bosch, M.A.; Luijten, P.; Mali, W.P. Optical imaging of the breast. Cancer Imaging 2008, 8, 206–215. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pogue, B.W.; Leblond, F.; Krishnaswamy, V.; Paulsen, K.D. Radiologic and near-infrared/optical spectroscopic imaging: Where is the synergy? AJR Am. J. Roentgenol. 2010, 195, 321–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andreozzi, J.M.; Zhang, R.; Gladstone, D.J.; Williams, B.B.; Glaser, A.K.; Pogue, B.W.; Jarvis, L.A. Cherenkov imaging method for rapid optimization of clinical treatment geometry in total skin electron beam therapy. Med. Phys. 2016, 43, 993–1002. [Google Scholar] [CrossRef]
- Alexander, D.A.; Nomezine, A.; Jarvis, L.A.; Gladstone, D.J.; Pogue, B.W.; Bruza, P. Color Cherenkov imaging of clinical radiation therapy. Light Sci. Appl. 2021, 10, 226. [Google Scholar] [CrossRef]
- Ni, D.; Ferreira, C.A.; Barnhart, T.E.; Quach, V.; Yu, B.; Jiang, D.; Wei, W.; Liu, H.; Engle, J.W.; Hu, P.; et al. Magnetic Targeting of Nanotheranostics Enhances Cerenkov Radiation-Induced Photodynamic Therapy. J. Am. Chem. Soc. 2018, 140, 14971–14979. [Google Scholar] [CrossRef]
- Yu, G.; Yu, S.; Saha, M.L.; Zhou, J.; Cook, T.R.; Yung, B.C.; Chen, J.; Mao, Z.; Zhang, F.; Zhou, Z.; et al. A discrete organoplatinum(II) metallacage as a multimodality theranostic platform for cancer photochemotherapy. Nat. Commun. 2018, 9, 4335. [Google Scholar] [CrossRef]
- Agostinis, P.; Berg, K.; Cengel, K.A.; Foster, T.H.; Girotti, A.W.; Gollnick, S.O.; Hahn, S.M.; Hamblin, M.R.; Juzeniene, A.; Kessel, D.; et al. Photodynamic therapy of cancer: An update. CA Cancer J. Clin. 2011, 61, 250–281. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lovell, J.F.; Yoon, J.; Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 2020, 17, 657–674. [Google Scholar] [CrossRef] [PubMed]
- Hopper, C. Photodynamic therapy: A clinical reality in the treatment of cancer. Lancet Oncol. 2000, 1, 212–219. [Google Scholar] [CrossRef]
- Juzeniene, A.; Nielsen, K.P.; Moan, J. Biophysical aspects of photodynamic therapy. J. Environ. Pathol. Toxicol. Oncol. 2006, 25, 7–28. [Google Scholar] [CrossRef] [PubMed]
- Cincotta, L.; Szeto, D.; Lampros, E.; Hasan, T.; Cincotta, A.H. Benzophenothiazine and Benzoporphyrin Derivative Combination Phototherapy Effectively Eradicates Large Murine Sarcomas. Photochem. Photobiol. 1996, 63, 229–237. [Google Scholar] [CrossRef]
- Kotagiri, N.; Sudlow, G.P.; Akers, W.J.; Achilefu, S. Breaking the depth dependency of phototherapy with Cerenkov radiation and low-radiance-responsive nanophotosensitizers. Nat. Nanotechnol. 2015, 10, 370–379. [Google Scholar] [CrossRef]
- Cline, B.; Delahunty, I.; Xie, J. Nanoparticles to mediate X-ray-induced photodynamic therapy and Cherenkov radiation photodynamic therapy. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1541. [Google Scholar] [CrossRef]
- Kotagiri, N.; Laforest, R.; Achilefu, S. Reply to ‘Is Cherenkov luminescence bright enough for photodynamic therapy?’. Nat. Nanotechnol. 2018, 13, 354–355. [Google Scholar] [CrossRef]
- Pratx, G.; Kapp, D.S. Is Cherenkov luminescence bright enough for photodynamic therapy? Nat. Nanotechnol. 2018, 13, 354. [Google Scholar] [CrossRef]
- Packer, S. Tumor detection with radiopharmaceuticals. Semin. Nucl. Med. 1984, 14, 21–30. [Google Scholar] [CrossRef]
- Yoon, S.W.; Tsvankin, V.; Shrock, Z.; Meng, B.; Zhang, X.; Dewhirst, M.; Fecci, P.; Adamson, J.; Oldham, M. Enhancing Radiation Therapy Through Cherenkov Light-Activated Phototherapy. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Luo, L.; Feng, Y.; Cai, Y.; Zhuang, Y.; Xie, R.J.; Chen, X.; Chen, H. Aggregation-Induced Emission Gold Clustoluminogens for Enhanced Low-Dose X-ray-Induced Photodynamic Therapy. Angew. Chem. Int. Ed. Engl. 2020, 59, 9914–9921. [Google Scholar] [CrossRef] [PubMed]
- Boschi, F.; Spinelli, A.E. Nanoparticles for Cerenkov and Radioluminescent Light Enhancement for Imaging and Radiotherapy. Nanomaterials 2020, 10, 1771. [Google Scholar] [CrossRef] [PubMed]
- Hompland, T.; Fjeldbo, C.S.; Lyng, H. Tumor Hypoxia as a Barrier in Cancer Therapy: Why Levels Matter. Cancers 2021, 13, 499. [Google Scholar] [CrossRef] [PubMed]
- Bettegowda, C.; Dang, L.H.; Abrams, R.; Huso, D.L.; Dillehay, L.; Cheong, I.; Agrawal, N.; Borzillary, S.; McCaffery, J.M.; Watson, E.L.; et al. Overcoming the hypoxic barrier to radiation therapy with anaerobic bacteria. Proc. Natl. Acad. Sci. USA 2003, 100, 15083–15088. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, J.G.; Hendrickson, K.R.; Spence, A.M.; Muzi, M.; Krohn, K.A.; Mankoff, D.A. Hypoxia imaging-directed radiation treatment planning. Eur. J. Nucl. Med. Mol. Imaging 2006, 33 (Suppl. S1), 44–53. [Google Scholar] [CrossRef]
- Rickard, A.G.; Palmer, G.M.; Dewhirst, M.W. Clinical and Pre-clinical Methods for Quantifying Tumor Hypoxia. Adv. Exp. Med. Biol. 2019, 1136, 19–41. [Google Scholar] [CrossRef]
- De Ruysscher, D.; Niedermann, G.; Burnet, N.G.; Siva, S.; Lee, A.W.M.; Hegi-Johnson, F. Radiotherapy toxicity. Nat. Rev. Dis. Primers 2019, 5, 13. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).