Analysis of Optical and Near-Infrared Luminescence of Er3+ and Er3+/Yb3+ Co-Doped Heavy Metal Borate Glasses for Optical Amplifier Applications
Abstract
:1. Introduction
2. Experimental and Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, X.; Zhang, M.; Guo, R.; Liu, X.; Pan, X.; Chen, K. Deng, W. Optical properties and luminescence of Er3+/Yb3+ co-doped La2O3–Nb2O5–Ga2O3 glasses prepared by aerodynamic levitation method. Opt. Mater. 2020, 109, 110288. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, Y.; Huang, M.; Chen, R.; Luo, Z. Spectroscopic properties of Er3+ ions in bismuth borate glasses. Opt. Mater. 2004, 25, 271–278. [Google Scholar] [CrossRef]
- Pal, I.; Sanghi, S.; Agarwal, A.; Aggarwal, M.P. Spectroscopic and structural investigations of Er3+ doped zinc bismuth borate glasses. Mater. Chem. Phys. 2012, 133, 151–158. [Google Scholar] [CrossRef]
- Jose, A.; Gopi, S.; Krishnapriya, T.; Jose, T.A.; Joseph, C.; Unnikrishnan, N.V.; Biju, P.R. Spectroscopic investigations on 1.53 μm NIR emission of Er3+ doped multicomponent borosilicate glasses for telecommunication and lasing applications. Mater. Chem. Phys. 2021, 261, 124223. [Google Scholar] [CrossRef]
- Devarajulu, G.; Ravi, O.; Reddy, C.M.; Ahamed, S.Z.A.; Raju, B.D.P. Spectroscopic properties and upconversion studies of Er3+-doped SiO2-Al2O3-Na2CO3-SrF2-CaF2 oxyfluoride glasses for optical amplifier applications. J. Lumin. 2018, 194, 499–506. [Google Scholar] [CrossRef]
- Ravi, O.; Dhoble, S.J.; Ramesh, B.; Devarajulu, G.; Reddy, C.M.; Linganna, K.; Reddy, G.R.; Raju, B.D.P. NIR fluorescence spectroscopic investigations of Er3+-ions doped borate based tellurium calcium zinc niobium oxide glasses. J. Lumin. 2015, 164, 154–159. [Google Scholar] [CrossRef]
- Gao, G.; Wei, J.; Shen, Y.; Peng, M.; Wondraczek, L. Heavily Eu2O3-doped yttria-aluminoborate glasses for red photoconversion with a high quantum yield: Luminescence quenching and statistics of cluster formation. J. Mater. Chem. C 2014, 28, 8678–8682. [Google Scholar] [CrossRef] [Green Version]
- Bomfim, F.A.; Martinelli, J.R.; Kassab, L.R.P.; Wetter, N.U.; Neto, J.J. Effect of the ytterbium concentration on the upconversion luminescence of Yb3+/Er3+ co-doped PbO–GeO2–Ga2O3 glasses. Non-Cryst. Solids 2008, 354, 4755–4759. [Google Scholar] [CrossRef]
- Feng, L.; Wang, J.; Tang, Q.; Hu, H.; Liang, H.; Su, Q. Solids Optical properties of Er3+-singly doped and Er3+/Yb3+-codoped novel oxyfluoride glasses. J. Non-Cryst. 2006, 352, 2090–2095. [Google Scholar] [CrossRef]
- Qi, C.; Hu, L.; Dai, S.; Jiang, Y.; Liu, Z. Spectra and lasing properties of Er3+, Yb3+:phosphate glasses. Chin. Opt. Lett. 2003, 1, 37–40. [Google Scholar]
- Ji, Y.; Xiao, Y.; Huang, S.; Wang, W. Optical properties of Er3+ and Yb3+/Er3+-doped NaF-Na2SO4-Al(PO3)3 fluoro-sulfo-phosphate glasses. Am. Ceram. Soc. 2020, 103, 5664–5677. [Google Scholar] [CrossRef]
- Pisarski, W.A.; Grobelny, Ł.; Pisarska, J.; Lisiecki, R.; Ryba-Romanowski, W. Spectroscopic properties of Yb3+ and Er3+ ions in heavy metal glasses. J. Alloys Compd. 2011, 509, 8088–8092. [Google Scholar] [CrossRef]
- Shang, Z.; Ren, G.; Yang, Q.; Xu, C.; Liu, Y.; Zhang, Y.; Wu, Q. Spectroscopic properties of Er3+-doped and Er3+/Yb3+-codoped PbF2–MOx (M = Te, Ge, B) oxyfluoride glasses. J. Alloys Compd. 2008, 460, 539–543. [Google Scholar] [CrossRef]
- Deopa, N.; Rao, A.S.; Gupta, M.; Prakash, G.V. Spectroscopic investigations of Nd3+ doped Lithium Lead Alumino Borate glasses for 1.06 μm laser applications. Opt. Mater. 2018, 75, 127–134. [Google Scholar] [CrossRef]
- Hegde, V.; Wagh, A.; Hegde, H.; Vishwanath, C.S.D. Spectroscopic investigation on europium doped heavy metal borate glasses for red luminescent application. Appl. Phys. A 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Jadach, R.; Zmojda, J.; Kochanowicz, M.; Miluski, P.; Pisarska, J.; Pisarski, W.A.; Sołtys, M.; Lesniak, M.; Sitarz, M.; Dorosz, D. Investigation of the aluminum oxide content on structural and optical properties of germanium glasses doped with RE ions. Spectrochi. Acta Part A Mol. Biomol. Spectrosc. 2018, 201, 143–152. [Google Scholar] [CrossRef]
- Dias, J.D.M.; Melo, G.H.A.; Lodi, T.A.; Carvalho, J.O.; Filho, P.F.F.; Barboza, M.J.; Steimacher, A.; Pedrochi, F. Thermal and structural properties of Nd2O3-doped calcium boroaluminate glasses. J. Rare Earths 2016, 34, 521–528. [Google Scholar] [CrossRef]
- Rani, S.; Sanghi, S.; Ahlawat, N.; Agarwal, A. Influence of Bi2O3 on thermal, structural and dielectric properties of lithium zinc bismuth borate glasses. J. Alloys Compd. 2014, 597, 110–118. [Google Scholar] [CrossRef]
- Doweidar, H.; Saddeek, Y.B. FTIR and ultrasonic investigations on modified bismuth borate glasses. J. Non-Cryst. Solids 2009, 355, 348–354. [Google Scholar] [CrossRef]
- Pascuta, P.; Pop, L.; Rada, S.; Bosca, M.; Culea, E. The local structure of bismuth borate glasses doped with europium ions evidenced by FT-IR spectroscopy. J. Mater. Sci. Mater. Electron. 2008, 19, 424–428. [Google Scholar] [CrossRef]
- Carnall, W.T.; Fields, P.R.; Rajnak, K. Electronic Energy Levels in the Trivalent Lanthanide Aquo Ions. I. Pr3+, Nd3+, Pm3+, Sm3+, Dy3+, Ho3+, Er3+, and Tm3+. J. Chem. Phys. 1968, 49, 4424–4442. [Google Scholar] [CrossRef]
- Linganna, K.; Rathaiah, M.; Vijaya, N.; Basavapoornima, C.; Jayasankar, C.K.; Ju, S.; Han, W.T.; Venkatramu, V. 1.53 µm luminescence properties of Er3+-doped K–Sr–Al phosphate glasses. Ceram. Int. 2015, 41, 5765–5771. [Google Scholar] [CrossRef]
- Burtan-Gwizdala, B.; Reben, M.; Cisowski, J.; Szpil, S.; Yousef, E.S.; Lisiecki, R.; Grelowska, I. Thermal and spectroscopic properties of Er3+-doped fluorotellurite glasses modified with TiO2 and BaO. Opt. Mater. 2020, 107, 109968. [Google Scholar] [CrossRef]
- Swetha, B.N.; Devarajulu, G.; Keshavamurthy, K.; Jagannath, G.; Deepa, H.R. Enhanced 1.53 µm emission of Er3+ in nano-Ag embedded sodium-boro-lanthanate glasses. J. Alloys Compd. 2021, 856, 158212. [Google Scholar] [CrossRef]
- Wu, L.; Zhou, Y.; Zhou, Z.; Cheng, P.; Huang, B.; Yang, F.; Li, J. Effect of silver nanoparticles on the 1.53 μm fluorescence in Er3+/Yb3+ codoped tellurite glasses. J. Opt. Mater. 2016, 57, 185–192. [Google Scholar] [CrossRef]
- Judd, B.R. Optical absorption intensities of rare-earth ions. Phys. Rev. 1962, 127, 750–761. [Google Scholar] [CrossRef]
- Ofelt, G.S. Intensities of crystal spectra of rare-earth ions. J. Chem. Phys. 1962, 37, 511–520. [Google Scholar] [CrossRef]
- Li, Y.; Dou, B.; Xiao, Z.; Li, B.; Huang, F.; Li, Y.; Xu, S. Visible-infrared luminescence of Er3+-doped fluorotellurite glasses. Opt. Mater. 2020, 105, 109900. [Google Scholar] [CrossRef]
- Mariyappan, M.; Arunkumar, S.; Marimuthu, K. Judd-Ofelt analysis and NIR luminescence investigations on Er3+ ions doped B2O3–Bi2O3–Li2O–K2O glasses for photonic applications. Phys. B Condens. Matter. 2019, 572, 27–35. [Google Scholar] [CrossRef]
- Luewarasirikul, N.; Chanthima, N.; Tariwong, Y.; Kaewkhao, J. Erbium-doped calcium barium phosphate glasses for 1.54 µm broadband optical amplifier. Mater. Today Proc. 2018, 5, 14009–14016. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Z.; Lei, H.; Zeng, L.; Tang, J. Er3+/Yb3+ co-doped tellurite glasses for optical fiber thermometry upon UV and NIR excitations. J. Lumin. 2019, 212, 61–68. [Google Scholar] [CrossRef]
- Basavapoornima, C.; Linganna, K.; Kesavulu, C.R.; Ju, S.; Kim, B.H.; Han, W.T.; Jayasankar, C.K. Spectroscopic and pump power dependent upconversion studies of Er3+-doped lead phosphate glasses for photonic applications. J. Alloys Compd. 2017, 699, 959–968. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, B.; Gong, Y.; Ren, Y.; Huo, M.; Wang, Y. Concentration effect of Yb3+ ions on the spectroscopic properties of high-concentration Er3+/Yb3+ co-doped phosphate glasses. J. Mol. Struct. 2020, 1216, 128322. [Google Scholar] [CrossRef]
- Linganna, K.; Agawane, G.L.; In, J.H.; Park, J.; Choi, J.H. Spectroscopic properties of Er3+/Yb3+ co-doped fluorophosphate glasses for NIR luminescence and optical temperature sensor applications. J. Ind. Eng. Chem. 2018, 67, 236–243. [Google Scholar] [CrossRef]
- Linganna, K.; Narro-García, R.; Manasa, P.; Desirena, H.; de la Rosa, E.; Jayasankar, C.K. Effect of BaF2 addition on luminescence properties of Er3+/Yb3+ co-doped phosphate glasses. J. Rare Earths 2018, 36, 58–63. [Google Scholar] [CrossRef]
- Langar, A.; Bouzidi, C.; Elhouichet, H.; Férid, M. Er–Yb codoped phosphate glasses with improved gain characteristics for an efficient 1.55 µm broadband optical amplifiers. J. Lumin. 2014, 148, 249–255. [Google Scholar] [CrossRef]
- Basavapoornima, C.; Maheswari, T.; Depuru, S.R.; Jayasankar, C.K. Sensitizing effect of Yb3+ ions on photoluminescence properties of Er3+ ions in lead phosphate glasses: Optical fiber amplifiers. Opt. Mater. 2018, 86, 256–269. [Google Scholar] [CrossRef]
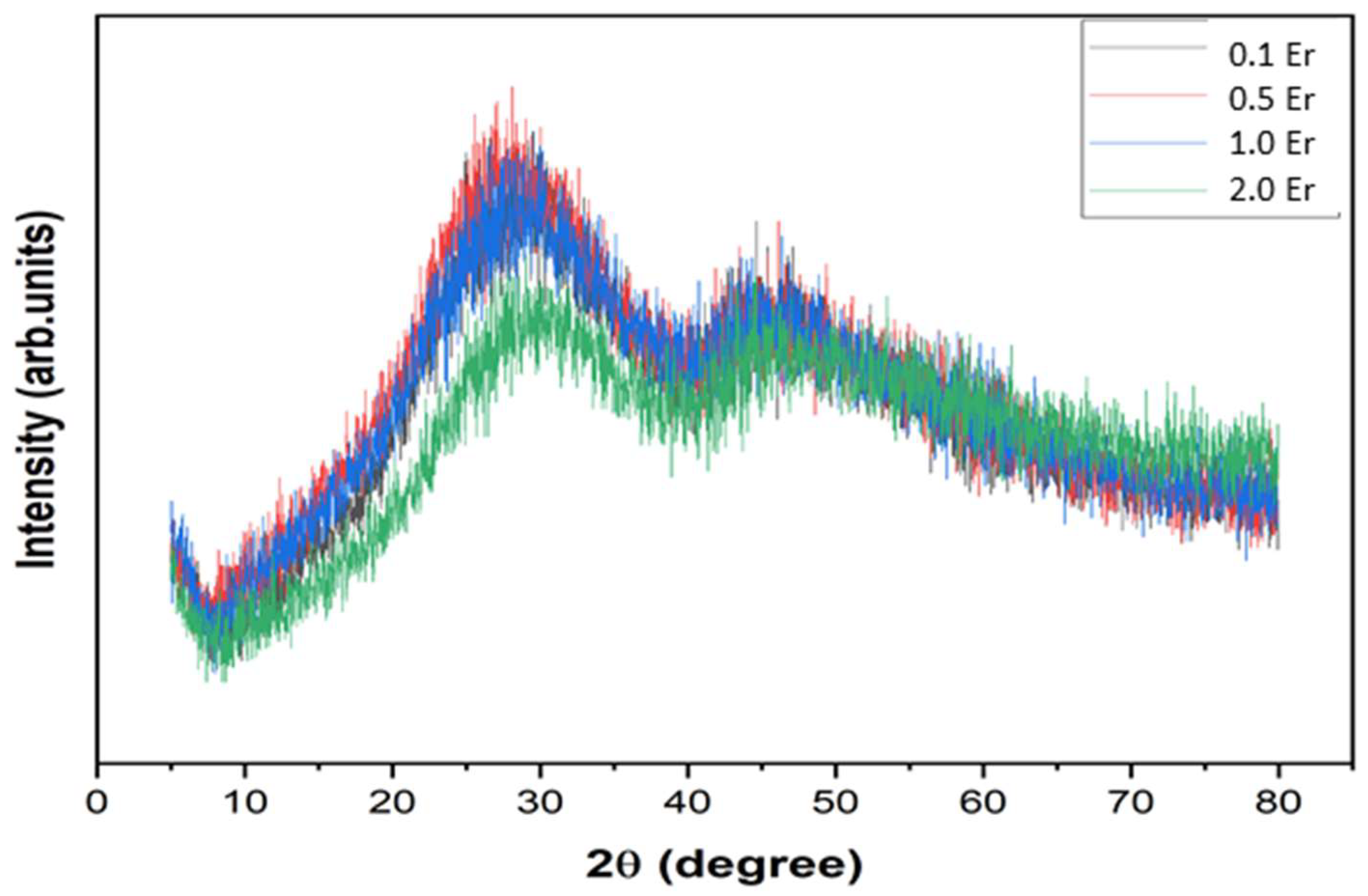
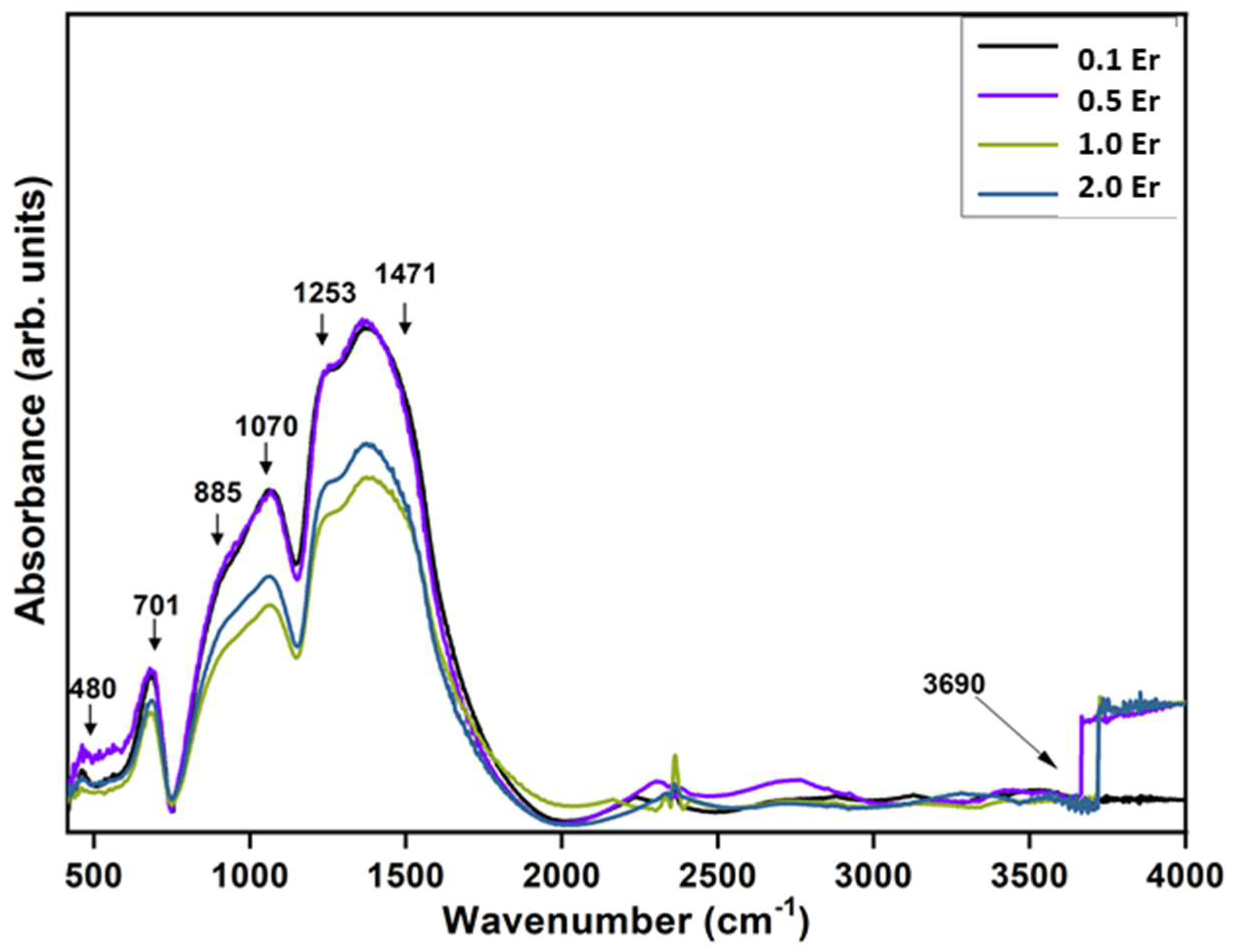
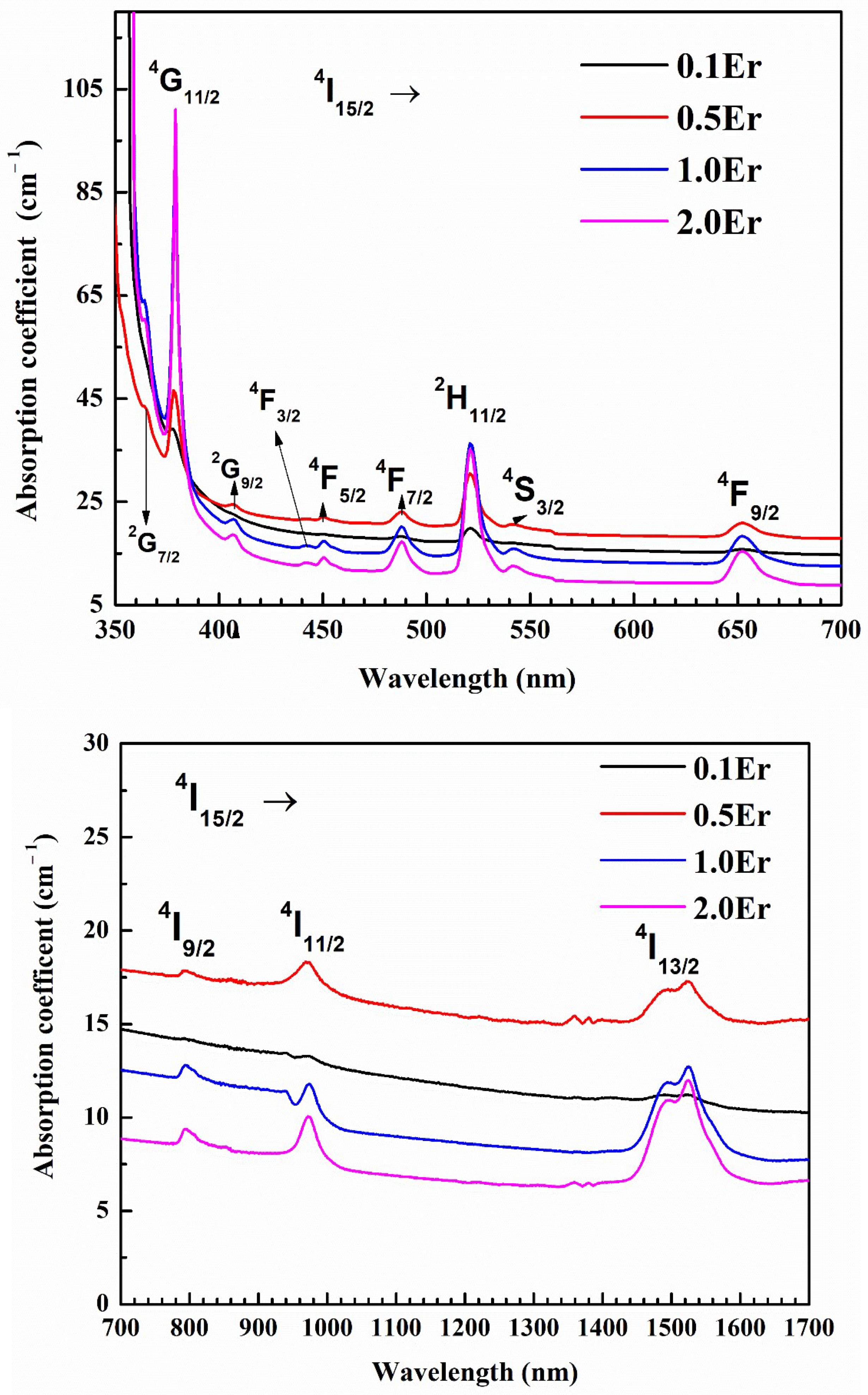
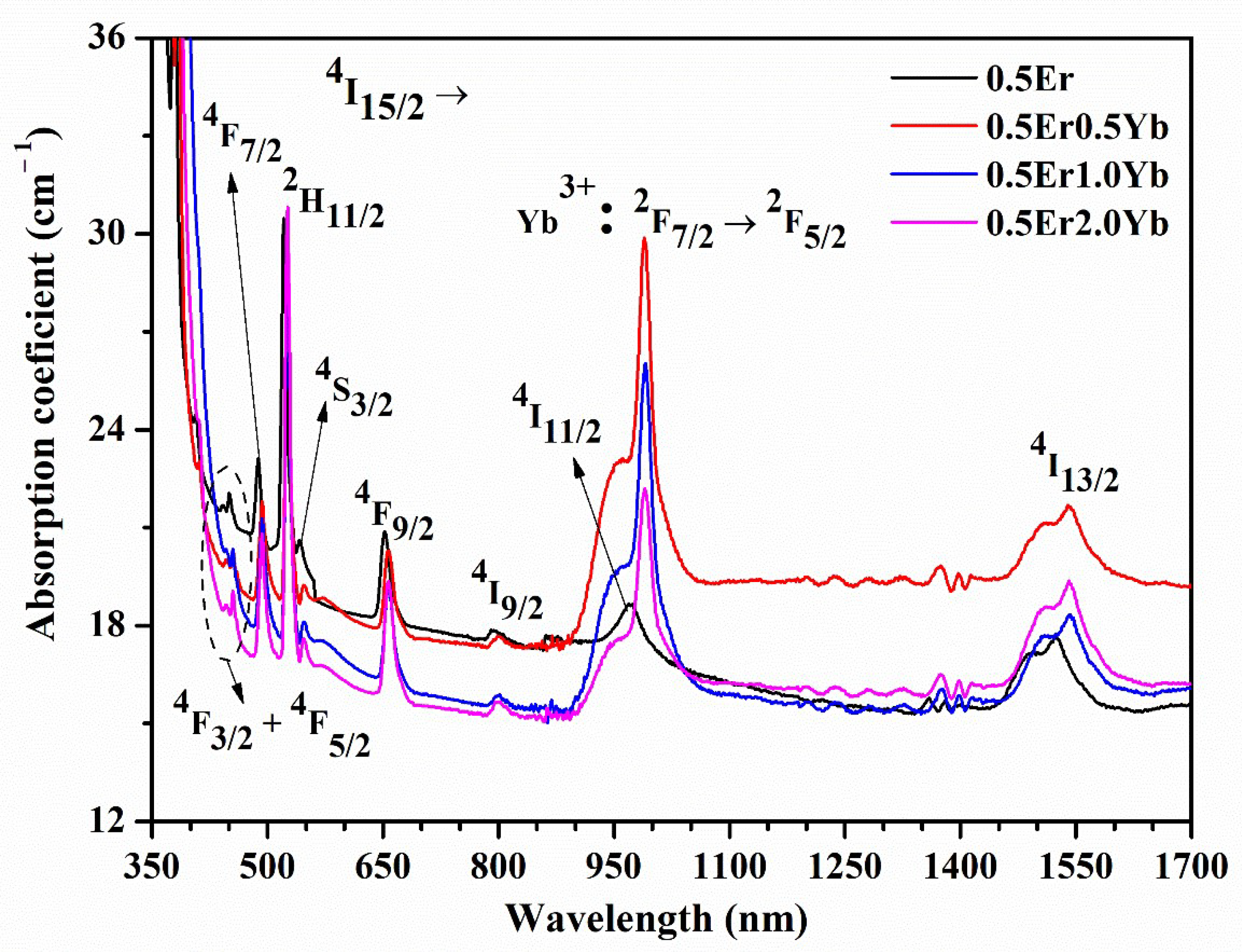
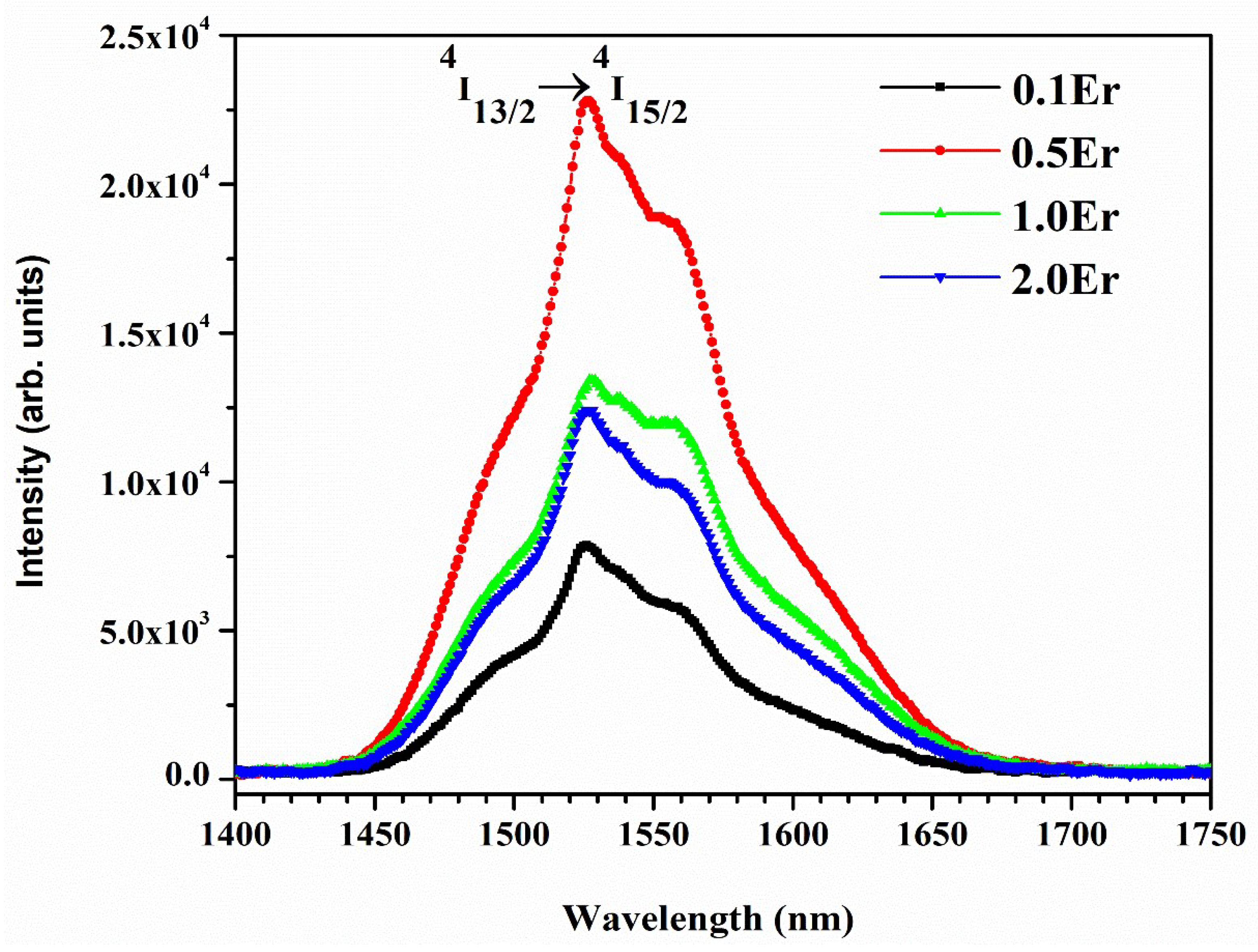
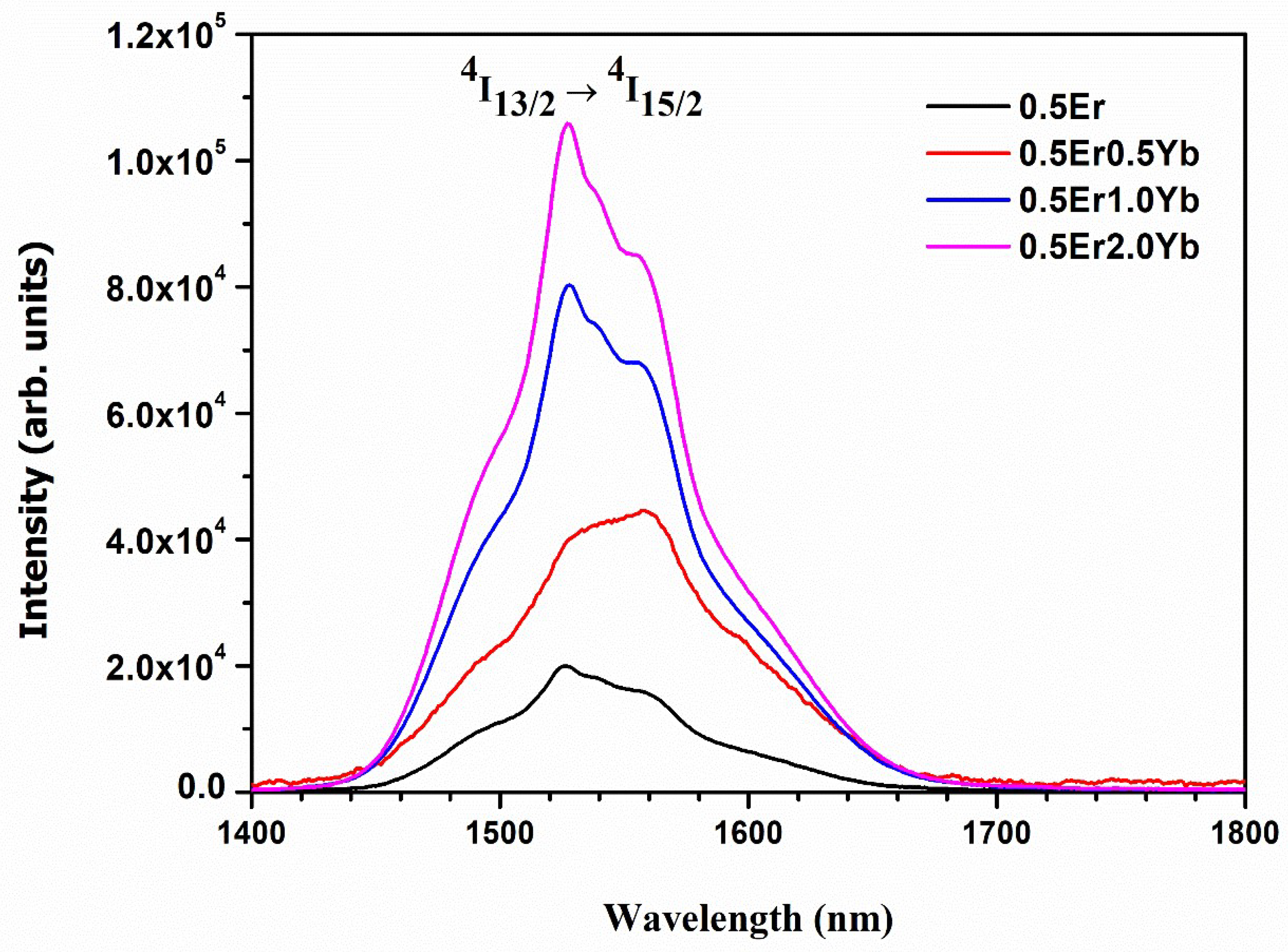

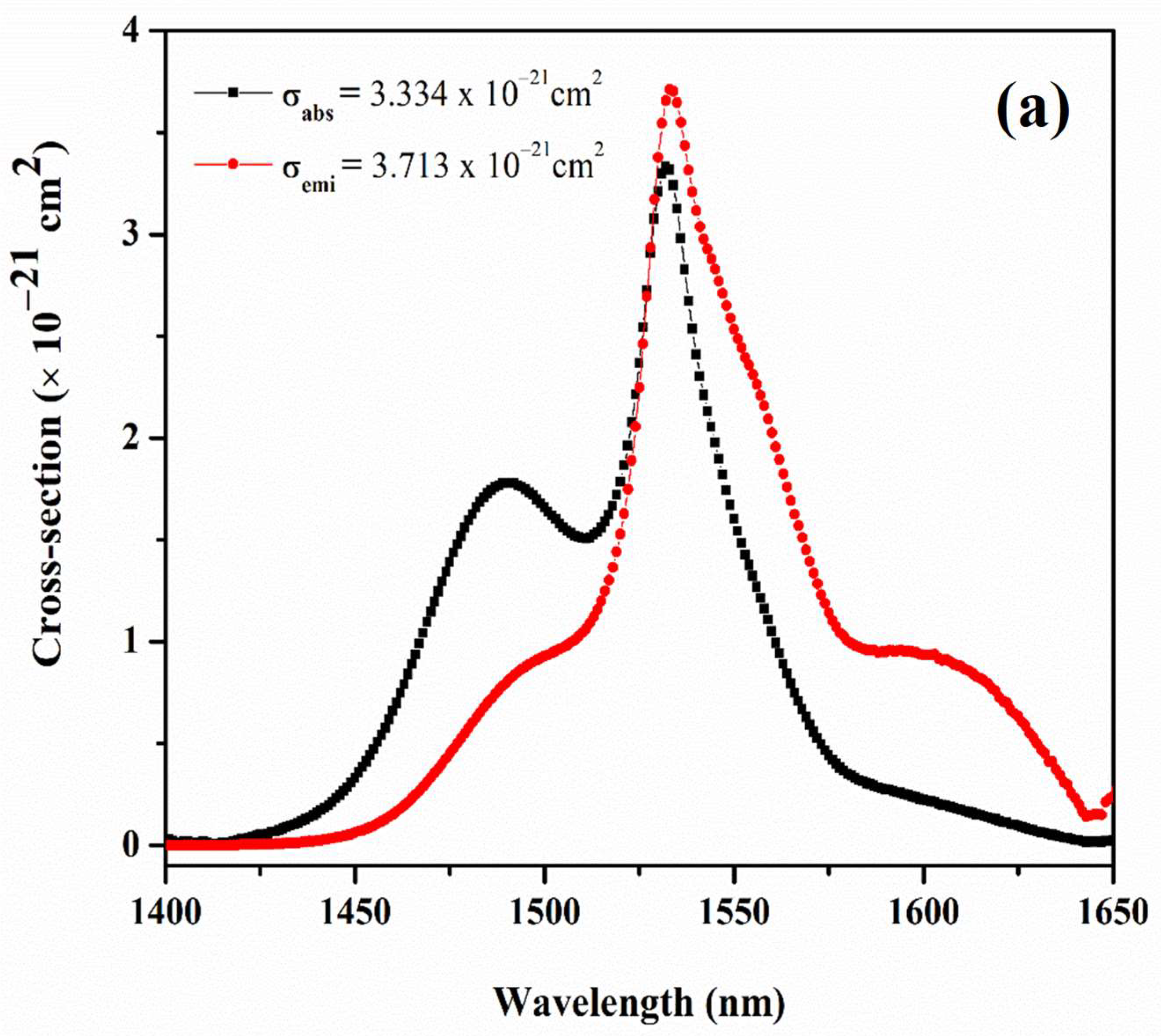
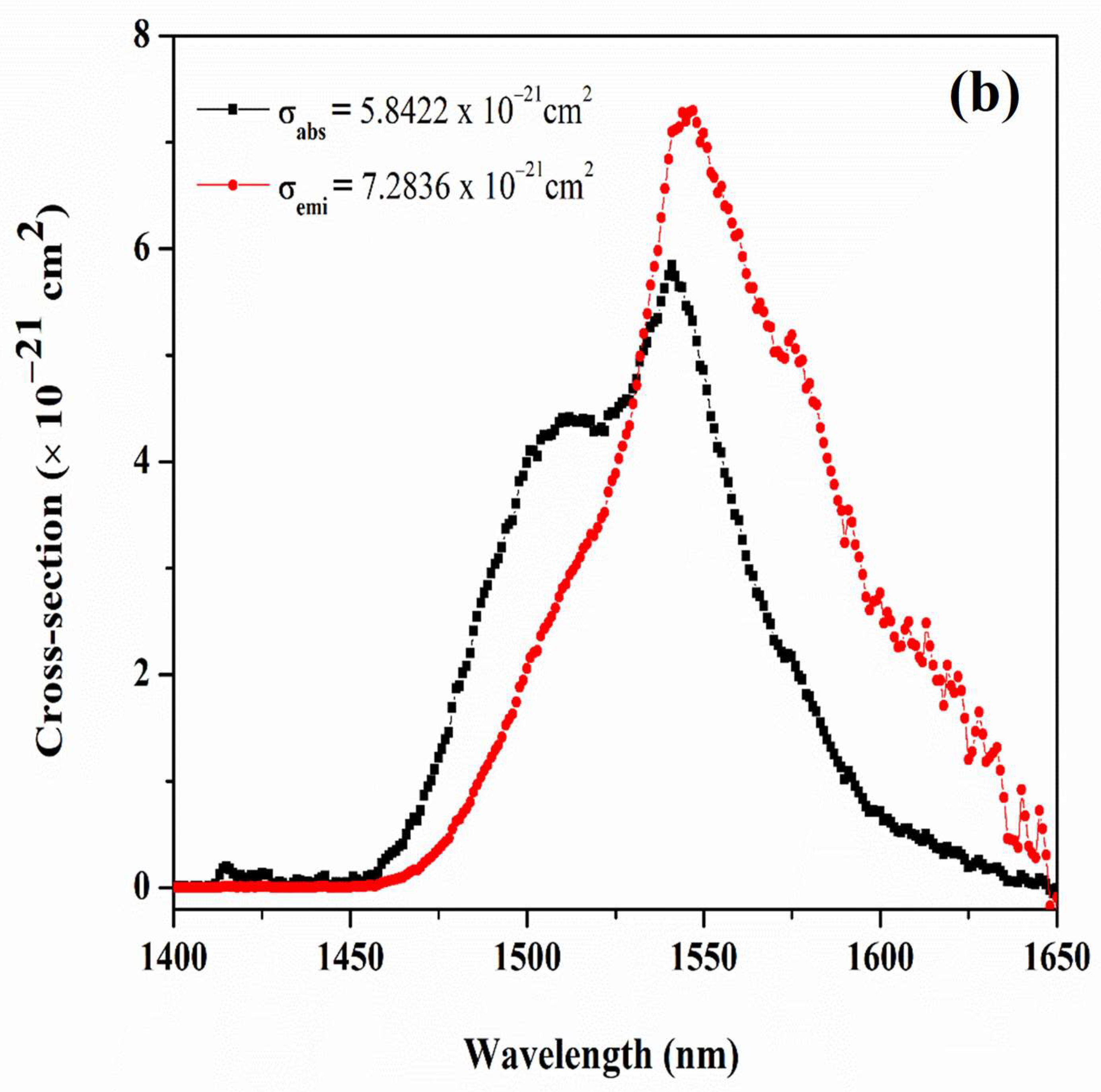
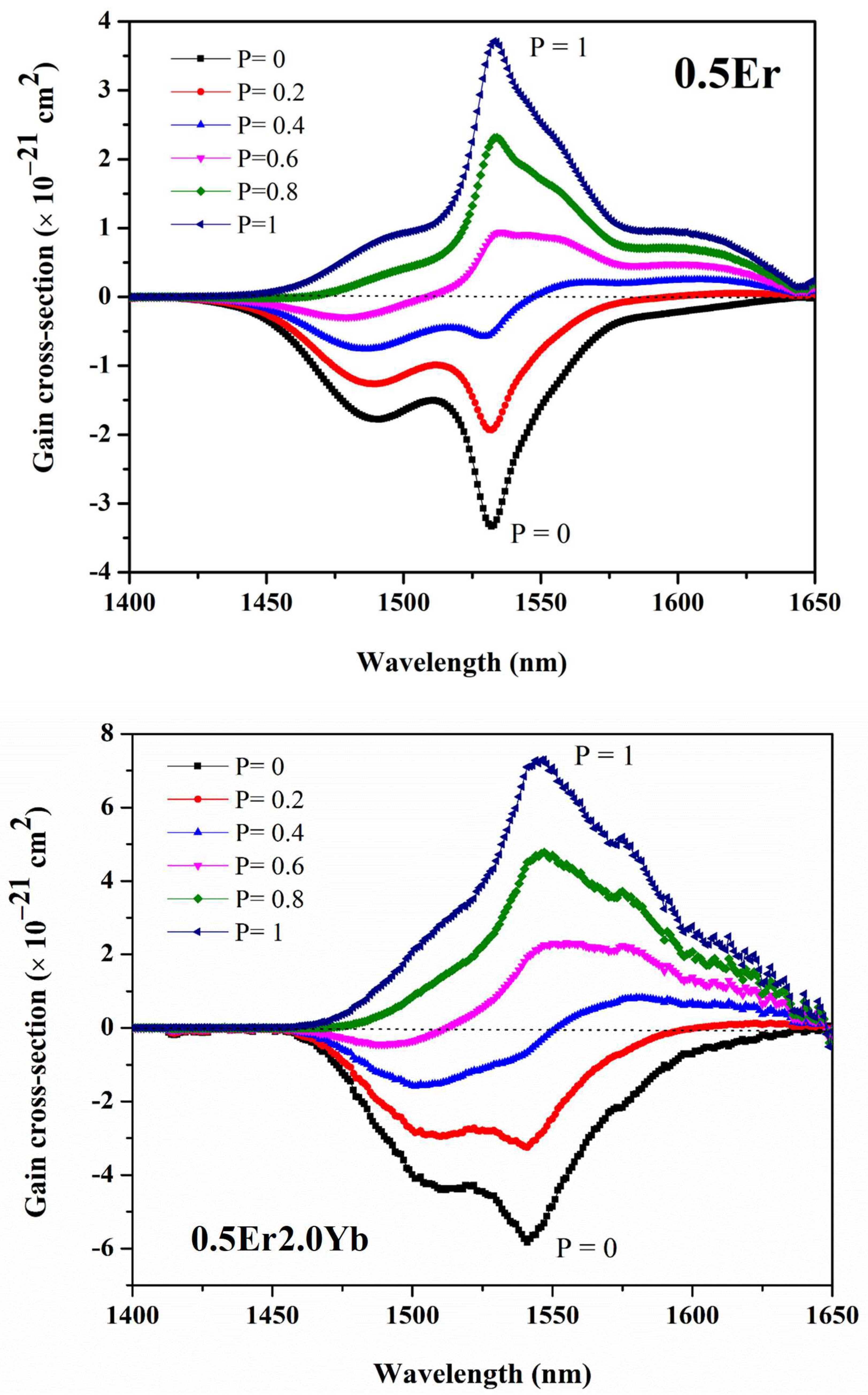
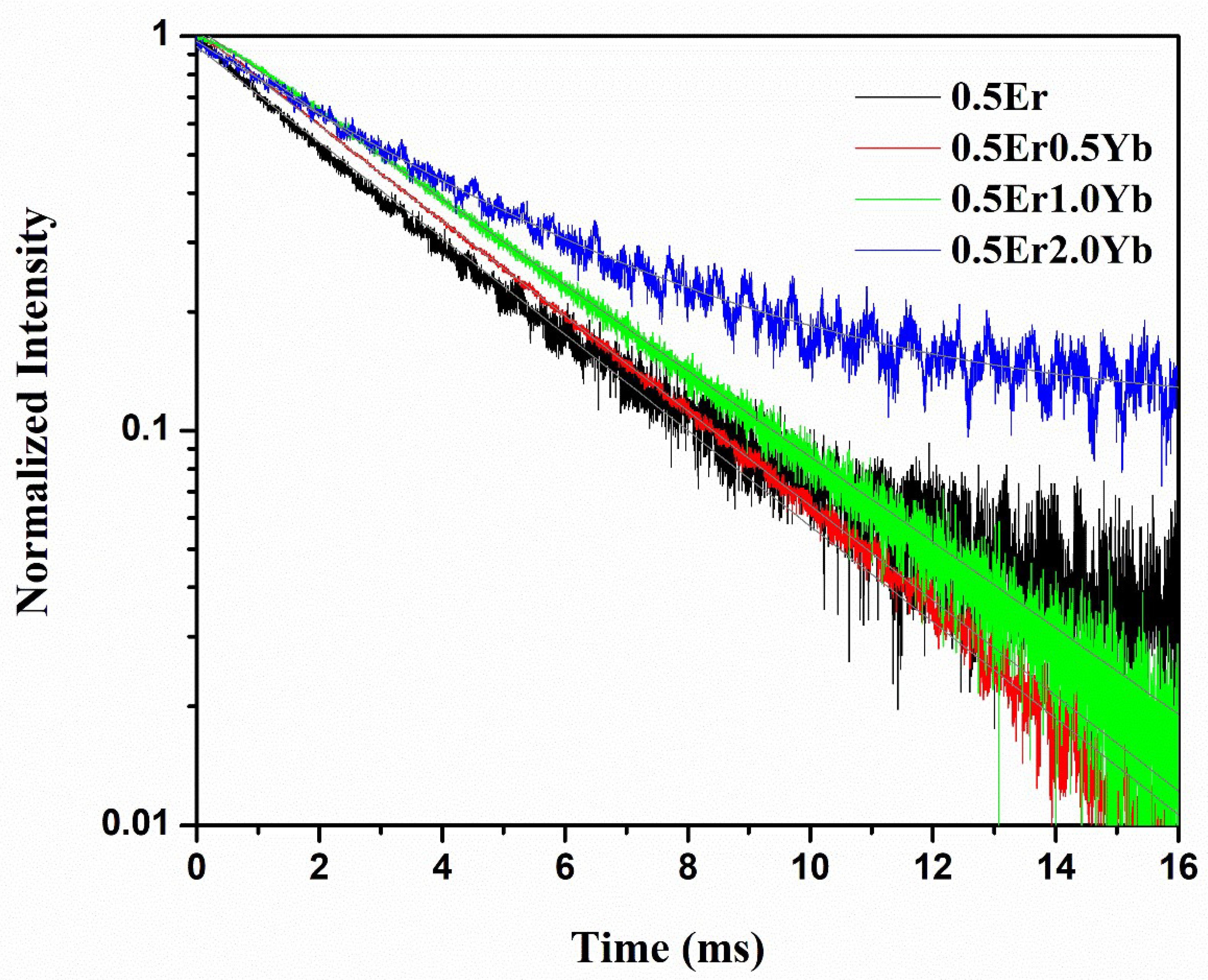
| Physical Parameters | 0.1Er | 0.5Er | 1.0Er | 2.0Er |
|---|---|---|---|---|
| Density (g/cm3) | 3.31 | 3.45 | 3.59 | 3.70 |
| Thickness (cm) | 0.152 | 0.154 | 0.153 | 0.151 |
| Refractive index (n) | 1.621 | 1.625 | 1.628 | 1.632 |
| Concentration N (Ions/c.c ×1020) | 0.156 | 0.903 | 1.963 | 3.938 |
| Transition | Energy (cm−1) | 0.1Er | 0.5Er | 1Er | 2Er | ||||
|---|---|---|---|---|---|---|---|---|---|
| fexp | fcal | fexp | fcal | fexp | fcal | fexp | fcal | ||
| 4I13/2 | 6527 | 5.41 | 4.67 | 2.33 | 2.67 | 3.22 | 3.52 | 2.93 | 1.05 |
| 4I11/2 | 10,256 | 2.46 | 2.72 | 1.56 | 1.23 | 1.17 | 1.61 | 1.33 | 7.17 |
| 4I9/2 | 12,516 | - | - | 2.66 | 2.70 | 1.07 | 0.92 | 1.30 | 1.72 |
| 4F9/2 | 15,361 | 5.65 | 5.89 | 2.91 | 1.11 | 5.39 | 5.71 | 3.53 | 0.84 |
| 4S3/2 | 18,416 | 2.25 | 3.67 | - | - | 2.65 | 1.35 | 1.83 | 1.45 |
| 2H11/2 | 19,231 | 13.47 | 14.20 | 8.33 | 7.36 | 16.67 | 16.37 | 4.47 | 4.37 |
| 4F7/2 | 20,492 | 7.72 | 8.13 | 4.74 | 3.78 | 7.03 | 5.49 | 3.45 | 4.06 |
| 4F5/2 | 22,173 | 2.73 | 4.04 | 1.86 | 1.35 | 2.05 | 1.64 | 1.36 | 1.19 |
| 4F3/2 | 22,573 | - | - | 1.28 | 0.78 | 2.11 | 0.95 | 1.13 | 3.73 |
| 2G9/2 | 24,631 | 1.58 | 2.49 | 2.15 | 1.56 | 3.08 | 2.03 | 2.97 | 0.47 |
| 4G11/2 | 26,525 | 3.16 | 2.85 | 12.02 | 13.05 | 28.77 | 29.02 | 6.73 | 1.23 |
| 2G7/2 | 28,090 | - | - | - | - | 3.34 | 1.28 | 2.08 | 2.90 |
| σ(N)a | ±0.26 × 10−6 | ±0.85 × 10−6 | ±0.45 × 10−6 | ±0.69 × 10−6 | |||||
| Glass Composition | Ω2 | Ω4 | Ω6 |
|---|---|---|---|
| 0.1Er (Present work) | 7.28 | 2.76 | 5.09 |
| 0.5Er (Present work) | 9.15 | 4.07 | 3.31 |
| 1.0Er (Present work) | 4.49 | 2.94 | 2.73 |
| 2.0Er (Present work) | 1.42 | 1.99 | 2.91 |
| SANSCEr10 [5] | 7.04 | 1.71 | 1.39 |
| PKSAEr10 [22] | 6.14 | 0.61 | 1.40 |
| Florotellurite [23] | 3.60 | 1.26 | 0.77 |
| Sodium-boro-Lanthanate (ELB-1) [24] | 5.96 | 1.96 | 2.04 |
| TBA1 [25] | 5.99 | 2.69 | 1.05 |
| TZN [28] | 5.96 | 2.42 | 0.53 |
| BLK1.0Er [29] | 3.19 | 1.38 | 2.16 |
| CaBaPEr20 [30] | 7.44 | 2.52 | 4.34 |
| TBL [31] | 6.17 | 1.50 | 1.10 |
| Lead-Phosphate [32] | 4.79 | 0.79 | 1.22 |
| Glass Sample | AR(s−1) | Δλeff (nm) | (βR) | σemi (×10−21 cm2) | τRad (ms) | (σemi × Δλeff) (×10−28 cm3) | (σemi × τRad) (×10−24 cm2s) |
|---|---|---|---|---|---|---|---|
| 0.1Er (Present work) | 281.1 | 88.98 | 1 | 15.200 | 3.557 | 1352.50 | 54.07 |
| 0.5Er (Present work) | 488.14 | 86.30 | 1 | 11.412 | 3.284 | 984.86 | 37.48 |
| 1.0Er (Present work) | 357.76 | 101.28 | 1 | 8.246 | 2.795 | 835.15 | 23.05 |
| 2.0Er (Present work) | 304.43 | 95.75 | 1 | 8.016 | 2.058 | 767.53 | 16.50 |
| SANSCEr10 [5] | 193 | 53 | 1 | 9.8 | 5.18 | 519.4 | 50.76 |
| PKSAEr10 [22] | 134 | 34 | 1 | 6.03 | 7.430 | 204 | 44.02 |
| BLK1.0Er [30] | 141.89 | 81.21 | 1 | 8.215 | 7.047 | 667.14 | 57.89 |
| CaBaPEr20 [31] | 183.23 | 64 | 1 | 7.99 | 2.728 | 511 | 21.80 |
| PKAPbNEr10 [32] | 144 | 46 | 1 | 6.73 | 6.910 | 310 | 13.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hegde, V.; Devarajulu, G.; Pramod, A.G.; Kolavekar, S.B.; Aloraini, D.A.; Almuqrin, A.H.; Sayyed, M.I.; Jagannath, G. Analysis of Optical and Near-Infrared Luminescence of Er3+ and Er3+/Yb3+ Co-Doped Heavy Metal Borate Glasses for Optical Amplifier Applications. Photonics 2022, 9, 355. https://doi.org/10.3390/photonics9050355
Hegde V, Devarajulu G, Pramod AG, Kolavekar SB, Aloraini DA, Almuqrin AH, Sayyed MI, Jagannath G. Analysis of Optical and Near-Infrared Luminescence of Er3+ and Er3+/Yb3+ Co-Doped Heavy Metal Borate Glasses for Optical Amplifier Applications. Photonics. 2022; 9(5):355. https://doi.org/10.3390/photonics9050355
Chicago/Turabian StyleHegde, Vinod, G. Devarajulu, A. G. Pramod, Sangeeta B. Kolavekar, Dalal Abdullah Aloraini, Aljawhara H. Almuqrin, M. I. Sayyed, and G. Jagannath. 2022. "Analysis of Optical and Near-Infrared Luminescence of Er3+ and Er3+/Yb3+ Co-Doped Heavy Metal Borate Glasses for Optical Amplifier Applications" Photonics 9, no. 5: 355. https://doi.org/10.3390/photonics9050355
APA StyleHegde, V., Devarajulu, G., Pramod, A. G., Kolavekar, S. B., Aloraini, D. A., Almuqrin, A. H., Sayyed, M. I., & Jagannath, G. (2022). Analysis of Optical and Near-Infrared Luminescence of Er3+ and Er3+/Yb3+ Co-Doped Heavy Metal Borate Glasses for Optical Amplifier Applications. Photonics, 9(5), 355. https://doi.org/10.3390/photonics9050355





