Optical Coherence Tomography Angiography and Attenuation Imaging for Label-Free Observation of Functional Changes in the Intestine after Sympathectomy: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
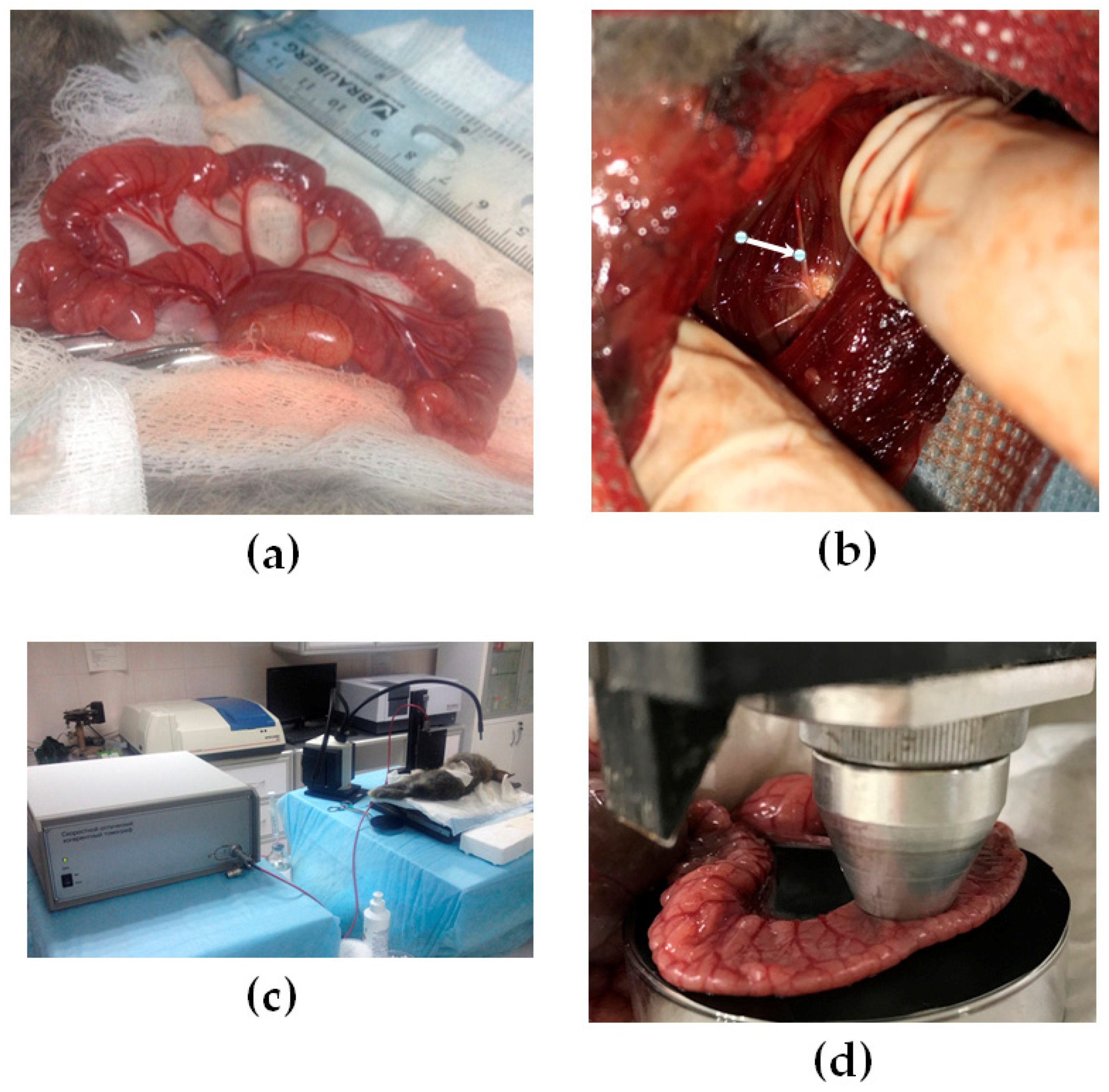
2.1. Animals and Sympathectomy Model of Small Intestine
2.2. Histology
2.3. OCT Experimental Setup and Data Acquisition
2.4. Data Processing for OCT Angiography (OCTA)
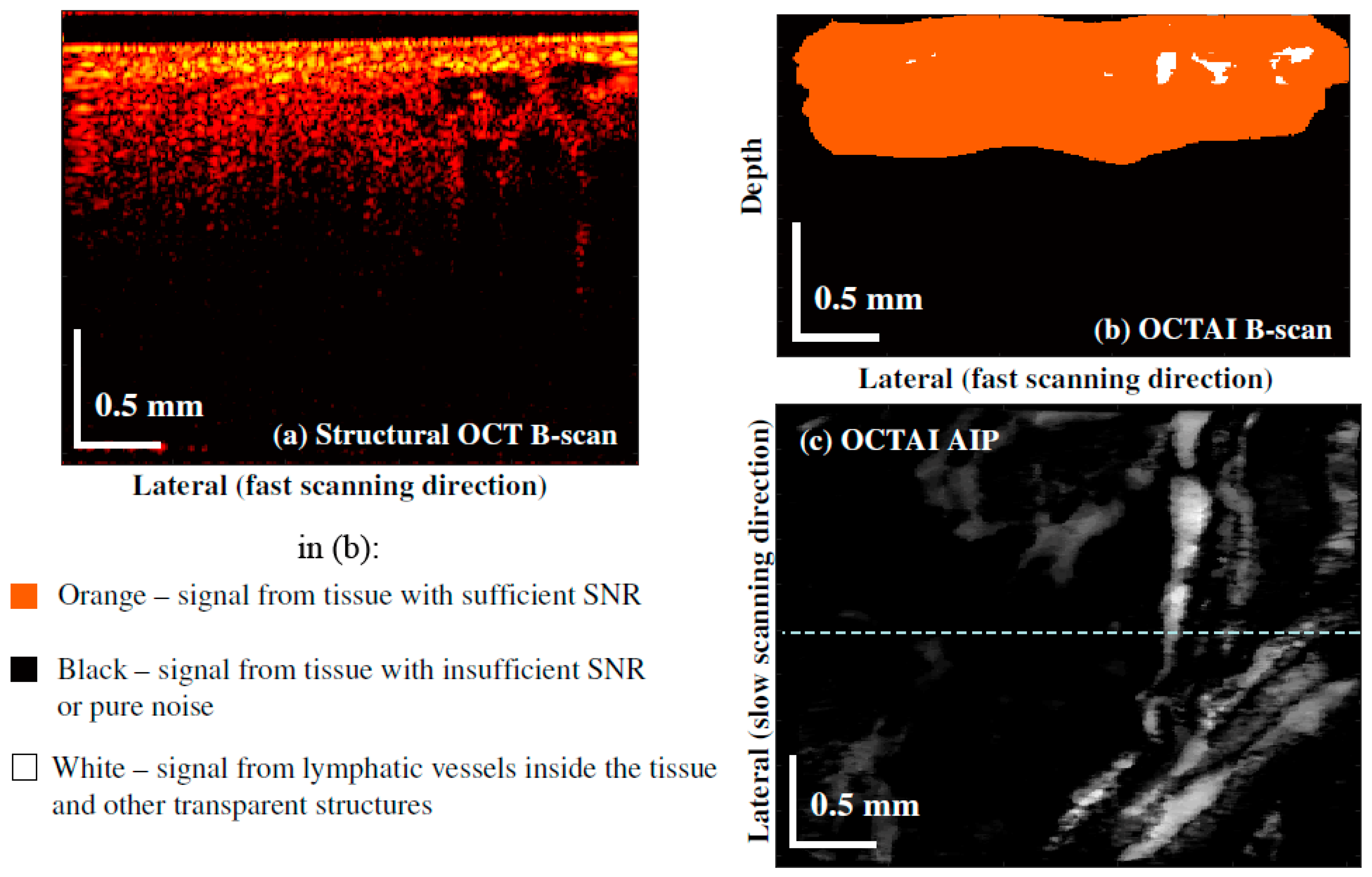
2.5. Data Processing of OCTAI
2.6. Statistical Analysis
3. Results
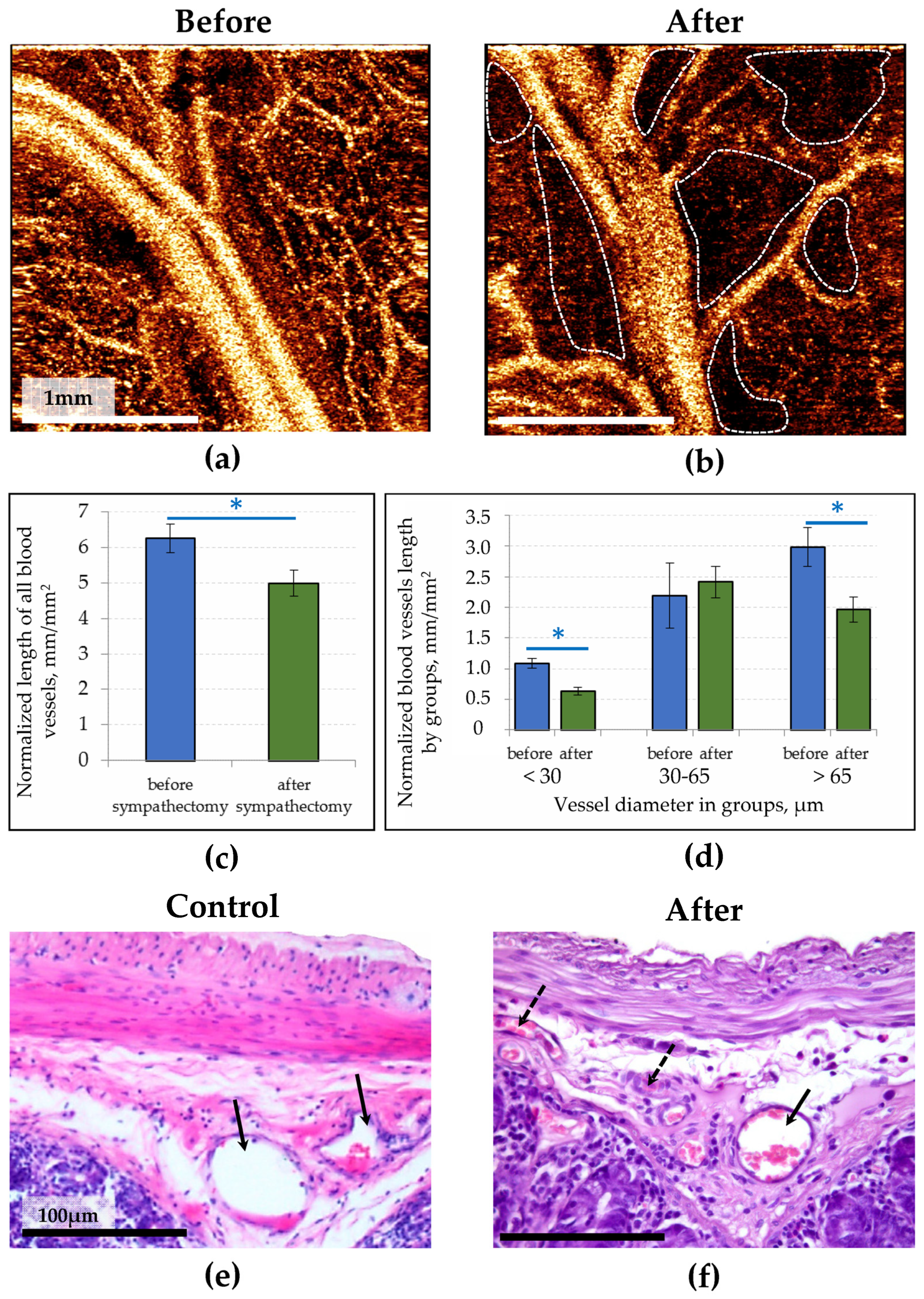
3.1. OCTA Study of the Healthy and Denervated Small Intestine
3.2. OCT Visualization of Lymphatic Vesssels in the Healthy Small Intestine and Its Response to Sympathectomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drexler, W.; Liu, M.; Kumar, A.; Kamali, T.; Unterhuber, A.; Leitgeb, R.A. Optical Coherence Tomography Today: Speed, Contrast, and Multimodality. J. Biomed. Opt. 2014, 19, 071412. [Google Scholar] [CrossRef] [PubMed]
- Leitgeb, R.A.; Placzek, F.; Rank, E.A.; Krainz, L.; Haindl, R.; Li, Q.; Liu, M.; Liu, M.; Unterhuber, A.; Schmoll, T.; et al. Enhanced Medical Diagnosis for DOCTors: A Perspective of Optical Coherence Tomography. J. Biomed. Opt. 2021, 26, 100601. [Google Scholar] [CrossRef] [PubMed]
- Hagag, A.; Gao, S.; Jia, Y.; Huang, D. Optical Coherence Tomography Angiography: Technical Principles and Clinical Applications in Ophthalmology. Taiwan J. Ophthalmol. 2017, 7, 115. [Google Scholar] [PubMed]
- Liu, M.; Drexler, W. Optical Coherence Tomography Angiography and Photoacoustic Imaging in Dermatology. Photochem. Photobiol. Sci. 2019, 18, 945–962. [Google Scholar] [CrossRef]
- Gong, P.; Es’haghian, S.; Harms, K.-A.; Murray, A.; Rea, S.; Wood, F.M.; Sampson, D.D.; McLaughlin, R.A. In Vivo Label-Free Lymphangiography of Cutaneous Lymphatic Vessels in Human Burn Scars Using Optical Coherence Tomography. Biomed. Opt. Express 2016, 7, 4886–4898. [Google Scholar] [CrossRef] [PubMed]
- Lai, P.-Y.; Chang, C.-H.; Chang, C.-H.; Su, H.-R.; Kuo, W.-C. Lymphatic Vessel Segmentation in Optical Coherence Tomography by Adding U-Net-Based CNN for Artifact Minimization. Biomed. Opt. Express 2020, 11, 2679–2693. [Google Scholar] [CrossRef]
- Demidov, V.; Matveev, L.A.; Demidova, O.; Matveyev, A.L.; Zaitsev, V.Y.; Flueraru, C.; Vitkin, I.A. Analysis of Low-Scattering Regions in Optical Coherence Tomography: Applications to Neurography and Lymphangiography. Biomed. Opt. Express 2019, 10, 4207–4219. [Google Scholar] [CrossRef]
- Carolus, A.E.; Möller, J.; Hofmann, M.R.; van de Nes, J.A.P.; Welp, H.; Schmieder, K.; Brenke, C. Comparison between Optical Coherence Tomography Imaging and Histological Sections of Peripheral Nerves. J. Neurosurg. 2019, 134, 270–277. [Google Scholar] [CrossRef]
- Kennedy, B.F. Front matter. In Optical Coherence Elastography; AIP Publishing Books; AIP Publishing LLC: Melville, NY, USA, 2021; pp. i–xviii. [Google Scholar]
- Zaitsev, V.Y.; Matveyev, A.L.; Matveev, L.A.; Sovetsky, A.A.; Hepburn, M.S.; Mowla, A.; Kennedy, B.F. Strain and Elasticity Imaging in Compression Optical Coherence Elastography: The Two-decade Perspective and Recent Advances. J. Biophotonics 2021, 14, e202000257. [Google Scholar] [CrossRef]
- Zaitsev, V.Y.; Ksenofontov, S.Y.; Sovetsky, A.A.; Matveyev, A.L.; Matveev, L.A.; Zykov, A.A.; Gelikonov, G.V. Real-Time Strain and Elasticity Imaging in Phase-Sensitive Optical Coherence Elastography Using a Computationally Efficient Realization of the Vector Method. Photonics 2021, 8, 527. [Google Scholar] [CrossRef]
- Granger, D.N.; Holm, L.; Kvietys, P. The gastrointestinal circulation: Physiology and pathophysiology. In Comprehensive Physiology; Terjung, R., Ed.; Wiley: Hoboken, NJ, USA, 2015; pp. 1541–1583. [Google Scholar]
- Norden, P.R.; Kume, T. The Role of Lymphatic Vascular Function in Metabolic Disorders. Front. Physiol. 2020, 11, 404. [Google Scholar] [CrossRef]
- Sato, H.; Higashiyama, M.; Hozumi, H.; Sato, S.; Furuhashi, H.; Takajo, T.; Maruta, K.; Yasutake, Y.; Narimatsu, K.; Yoshikawa, K.; et al. Platelet Interaction with Lymphatics Aggravates Intestinal Inflammation by Suppressing Lymphangiogenesis. Am. J. Physiol. Gastrointest. Liver Physiol. 2016, 311, G276–G285. [Google Scholar] [CrossRef]
- Xu, P.; Guo, S.; Xie, Y.; Liu, Z.; Liu, C.; Zhang, X.; Yang, D.; Gong, H.; Chen, Y.; Du, L.; et al. Effects of Highly Selective Sympathectomy on Neurogenic Bowel Dysfunction in Spinal Cord Injury Rats. Sci. Rep. 2021, 11, 15892. [Google Scholar] [CrossRef]
- Khochanskiy, D.N.; Mkhitarov, V.A.; Sladkopevtsev, A.S.; Chernikov, V.P.; Mikhailova, L.P.; Dobrynina, M.T.; Makarova, O.V. Structural Changes in the Nervous Fibers of the Colon Mucosa in Experimental Acute Colitis. Bull. Exp. Biol. Med. 2020, 169, 104–109. [Google Scholar] [CrossRef]
- Ranzenberger, L.R.; Pai, R.B. Lymphoscintigraphy; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Pervukhin, S.A.; Lebedeva, M.A.; Elistratov, A.A.; Ivanova, E.Y.; Statsenko, I.A.; Palmash, A.V.; Fomichev, N.G. Results of Intensive Therapy of Complicated Trauma of the Thoracic Spine. Polytrauma 2017, 3, 30–37. [Google Scholar]
- Sentell, K.T.; Ferroni, M.C.; Abaza, R. Near-infrared Fluorescence Imaging for Intraoperative Margin Assessment during Robot-assisted Partial Nephrectomy. BJU Int. 2020, 126, 259–264. [Google Scholar] [CrossRef]
- Mahieu, R.; Krijger, G.C.; Ververs, F.F.T.; de Roos, R.; de Bree, R.; de Keizer, B. [68Ga]Ga-Tilmanocept PET/CT Lymphoscintigraphy: A Novel Technique for Sentinel Lymph Node Imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 963–965. [Google Scholar] [CrossRef]
- Cifarelli, V.; Eichmann, A. The Intestinal Lymphatic System: Functions and Metabolic Implications. Cell. Mol. Gastroenterol. Hepatol. 2019, 7, 503–513. [Google Scholar] [CrossRef]
- Hsu, M.C.; Itkin, M. Lymphatic Anatomy. Tech. Vasc. Interv. Radiol. 2016, 19, 247–254. [Google Scholar] [CrossRef]
- Ross, M.H.; Pawlina, W. Histology: A Text and Atlas: With Correlated Cell and Molecular Biology, 7th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2016. [Google Scholar]
- Dori, Y. Novel Lymphatic Imaging Techniques. Tech. Vasc. Interv. Radiol. 2016, 19, 255–261. [Google Scholar] [CrossRef]
- Itkin, M.; Nadolski, G.J. Modern Techniques of Lymphangiography and Interventions: Current Status and Future Development. Cardiovasc. Interv. Radiol. 2018, 41, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Matter, D.; Grosshans, E.; Muller, J.; Furderer, C.; Mathelin, C.; Warter, S.; Bellocq, J.P.; Maillot, C. Apport de l’échographie à l’imagerie des vaisseaux lymphatiques par rapport aux autres méthodes [Sonographic imaging of lymphatic vessels compared to other methods]. J. Radiol. 2002, 83, 599–609. [Google Scholar] [PubMed]
- Alitalo, K. The Lymphatic Vasculature in Disease. Nat. Med. 2011, 17, 1371–1380. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, H.; Saharinen, P.; Zarkada, G.; Zheng, W.; Robciuc, M.R.; Alitalo, K. VEGF-C Is Required for Intestinal Lymphatic Vessel Maintenance and Lipid Absorption. EMBO Mol. Med. 2015, 7, 1418–1425. [Google Scholar] [CrossRef]
- Kigerl, K.A.; Mostacada, K.; Popovich, P.G. Gut Microbiota Are Disease-Modifying Factors After Traumatic Spinal Cord Injury. Neurotherapeutics 2018, 15, 60–67. [Google Scholar] [CrossRef]
- Matveev, L.A.; Zaitsev, V.Y.; Gelikonov, G.V.; Matveyev, A.L.; Moiseev, A.A.; Ksenofontov, S.Y.; Gelikonov, V.M.; Sirotkina, M.A.; Gladkova, N.D.; Demidov, V.; et al. Hybrid M-Mode-like OCT Imaging of Three-Dimensional Microvasculature in Vivo Using Reference-Free Processing of Complex Valued B-Scans. Opt. Lett. 2015, 40, 1472. [Google Scholar] [CrossRef]
- Moiseev, A.; Ksenofontov, S.; Sirotkina, M.; Kiseleva, E.; Gorozhantseva, M.; Shakhova, N.; Matveev, L.; Zaitsev, V.; Matveyev, A.; Zagaynova, E.; et al. Optical Coherence Tomography-Based Angiography Device with Real-Time Angiography B-Scans Visualization and Hand-Held Probe for Everyday Clinical Use. J. Biophotonics 2018, 11, e201700292. [Google Scholar] [CrossRef]
- Kiseleva, E.B.; Ryabkov, M.; Bederina, E.; Shirmanova, M.V.; Baleev, M.; Lukina, M.; Sirotkina, M.A.; Chilipenok, A.S.; Beschastnov, V.; Moiseev, A.A.; et al. Observations of the Bowel Wall in the Case of Acute Ischemia: Optical Coherence Tomography, FLIM Macro-Imaging and Histological Analysis Data. In Multimodal Biomedical Imaging XV; Azar, F.S., Intes, X., Fang, Q., Eds.; SPIE: San Francisco, CA, USA, 2020; p. 22. [Google Scholar]
- Kiseleva, E.B.; Ryabkov, M.G.; Sizov, M.A.; Bederina, E.L.; Komarova, A.D.; Moiseev, A.A.; Bagryantsev, M.V.; Vorobiev, A.N.; Gladkova, N.D. Effect of Surgical Technique on the Microstructure and Microcirculation of the Small Intestine Stump during Delayed Anastomosis: Multimodal OCT Data. Sovrem. Tehnol. Med. 2021, 13, 36. [Google Scholar] [CrossRef]
- Moiseev, A.A.; Sirotkina, M.A.; Potapov, A.L.; Matveev, L.A.; Vagapova, N.N.; Kuznetsova, I.A.; Gladkova, N.D. Lymph Vessels Visualization from Optical Coherence Tomography Data Using Depth-resolved Attenuation Coefficient Calculation. J. Biophotonics 2021, 14, e202100055. [Google Scholar] [CrossRef]
- Lu, Q.; Hua, J.; Kassir, M.M.; Delproposto, Z.; Dai, Y.; Sun, J.; Haacke, M.; Hu, J. Imaging Lymphatic System in Breast Cancer Patients with Magnetic Resonance Lymphangiography. PLoS ONE 2013, 8, e69701. [Google Scholar] [CrossRef]
- Sevick-Muraca, E.M.; Sharma, R.; Rasmussen, J.C.; Marshall, M.V.; Wendt, J.A.; Pham, H.Q.; Bonefas, E.; Houston, J.P.; Sampath, L.; Adams, K.E.; et al. Imaging of Lymph Flow in Breast Cancer Patients after Microdose Administration of a Near-Infrared Fluorophore: Feasibility Study. Radiology 2008, 246, 734–741. [Google Scholar] [CrossRef]
- Hayashi, K.; Jiang, P.; Yamauchi, K.; Yamamoto, N.; Tsuchiya, H.; Tomita, K.; Moossa, A.R.; Bouvet, M.; Hoffman, R.M. Real-Time Imaging of Tumor-Cell Shedding and Trafficking in Lymphatic Channels. Cancer Res. 2007, 67, 8223–8228. [Google Scholar] [CrossRef]
- Semyachkina-Glushkovskaya, O.; Abdurashitov, A.; Dubrovsky, A.; Klimova, M.; Agranovich, I.; Terskov, A.; Shirokov, A.; Vinnik, V.; Kuzmina, A.; Lezhnev, N.; et al. Photobiomodulation of Lymphatic Drainage and Clearance: Perspective Strategy for Augmentation of Meningeal Lymphatic Functions. Biomed. Opt. Express 2020, 11, 725. [Google Scholar] [CrossRef]
- Vakoc, B.J.; Lanning, R.M.; Tyrrell, J.A.; Padera, T.P.; Bartlett, L.A.; Stylianopoulos, T.; Munn, L.L.; Tearney, G.J.; Fukumura, D.; Jain, R.K.; et al. Three-Dimensional Microscopy of the Tumor Microenvironment in Vivo Using Optical Frequency Domain Imaging. Nat. Med. 2009, 15, 1219–1223. [Google Scholar] [CrossRef]
- Xie, W.; Chen, S.; Strong, J.A.; Li, A.-L.; Lewkowich, I.P.; Zhang, J.-M. Localized Sympathectomy Reduces Mechanical Hypersensitivity by Restoring Normal Immune Homeostasis in Rat Models of Inflammatory Pain. J. Neurosci. 2016, 36, 8712–8725. [Google Scholar] [CrossRef]
- Gelikonov, V.M.; Romashov, V.N.; Shabanov, D.V.; Ksenofontov, S.Y.; Terpelov, D.A.; Shilyagin, P.A.; Gelikonov, G.V.; Vitkin, I.A. Cross-Polarization Optical Coherence Tomography with Active Maintenance of the Circular Polarization of a Sounding Wave in a Common Path System. Radiophys. Quantum El. 2018, 60, 897–911. [Google Scholar] [CrossRef]
- Maslennikova, A.V.; Sirotkina, M.A.; Moiseev, A.A.; Finagina, E.S.; Ksenofontov, S.Y.; Gelikonov, G.V.; Matveev, L.A.; Kiseleva, E.B.; Zaitsev, V.Y.; Zagaynova, E.V.; et al. In-Vivo Longitudinal Imaging of Microvascular Changes in Irradiated Oral Mucosa of Radiotherapy Cancer Patients Using Optical Coherence Tomography. Sci. Rep. 2017, 7, 16505. [Google Scholar] [CrossRef]
- Sirotkina, M.A.; Matveev, L.A.; Shirmanova, M.V.; Zaitsev, V.Y.; Buyanova, N.L.; Elagin, V.V.; Gelikonov, G.V.; Kuznetsov, S.S.; Kiseleva, E.B.; Moiseev, A.A.; et al. Photodynamic Therapy Monitoring with Optical Coherence Angiography. Sci. Rep. 2017, 7, 41506. [Google Scholar] [CrossRef]
- Yousefi, S.; Zhongwei, Z.; Wang, R.K. Label-Free Optical Imaging of Lymphatic Vessels Within Tissue Beds IN VIVO. IEEE J. Select. Topics Quantum Electron. 2014, 20, 15–24. [Google Scholar] [CrossRef]
- Smithson, M. Confidence Intervals; Sage Publications, Inc.: Thousand Oaks, CA, USA, 2003. [Google Scholar]
- Vermeer, K.A.; Mo, J.; Weda, J.J.A.; Lemij, H.G.; de Boer, J.F. Depth-Resolved Model-Based Reconstruction of Attenuation Coefficients in Optical Coherence Tomography. Biomed. Opt. Express 2014, 5, 322. [Google Scholar] [CrossRef]
- Ma, Z.; Meng, Z.; Tian, Y.; Liu, J.; Li, A.; Lin, Y.; Yu, Y.; Luan, J.; Wang, H.; Zhao, Y.; et al. Evaluation of Mannitol Intervention Effects on Ischemic Cerebral Edema in Mice Using Swept Source Optical Coherence Tomography. Photonics 2022, 9, 81. [Google Scholar] [CrossRef]
- Moiseev, A.A.; Achkasova, K.A.; Kiseleva, E.B.; Yashin, K.S.; Potapov, A.L.; Bederina, E.L.; Kuznetsov, S.S.; Sherstnev, E.P.; Shabanov, D.V.; Gelikonov, G.V.; et al. Brain White Matter Morphological Structure Correlation with Its Optical Properties Estimated from Optical Coherence Tomography (OCT) Data. Biomed. Opt. Express 2022, 13, 2393. [Google Scholar] [CrossRef]
- McMillan, D.W.; Henderson, G.C.; Nash, M.S.; Jacobs, K.A. Effect of Paraplegia on the Time Course of Exogenous Fatty Acid Incorporation into the Plasma Triacylglycerol Pool in the Postprandial State. Front. Physiol. 2021, 12, 626003. [Google Scholar] [CrossRef]
- Zuvarox, T.; Belletieri, C. Malabsorption syndromes. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK553106/ (accessed on 22 November 2021).
- Hokari, R.; Tomioka, A. The Role of Lymphatics in Intestinal Inflammation. Inflamm. Regen. 2021, 41, 25. [Google Scholar] [CrossRef] [PubMed]
- La Fountaine, M.F.; Cirnigliaro, C.M.; Hobson, J.C.; Lombard, A.T.; Specht, A.F.; Dyson-Hudson, T.A.; Kirshblum, S.C.; Bauman, W.A. A Four Month Randomized Controlled Trial on the Efficacy of Once-Daily Fenofibrate Monotherapy in Persons with Spinal Cord Injury. Sci. Rep. 2019, 9, 17166. [Google Scholar] [CrossRef] [PubMed]
- Hatipoglu, S. Effect of Laparoscopic Abdominal Surgery on Splanchnic Circulation: Historical Developments. World J. Gastroenterol. 2014, 20, 18165. [Google Scholar] [CrossRef] [PubMed]
- Baran, U.; Qin, W.; Qi, X.; Kalkan, G.; Wang, R.K. OCT-Based Label-Free in Vivo Lymphangiography within Human Skin and Areola. Sci. Rep. 2016, 6, 21122. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Q.; Wang, J.; Kim, A.; Liu, Q.; Watts, R.; Hoopfer, E.; Mitchison, T.; Luo, L.; He, Z. Involvement of the Ubiquitin-Proteasome System in the Early Stages of Wallerian Degeneration. Neuron 2003, 39, 217–225. [Google Scholar] [CrossRef]
- Oliveira, H.A.; Ximenes, M.; Filho, F.B.; Carvalho, P.H.C.; GamaFilho, J.B.; Parra, E.R.; Capelozzi, V.L.; DeCampos, J.R.M. Experimental Selective Sympathicotomy (Ramicotomy) and Sympathetic Regeneration. Interact. CardioVascular Thorac. Surg. 2009, 9, 411–415. [Google Scholar] [CrossRef][Green Version]
- Thomsen, L.L.; Mikkelsen, R.T.; Derejko, M.; Schrøder, H.D.; Licht, P.B. Sympathetic Block by Metal Clips May Be a Reversible Operation. Interact. CardioVascular Thorac. Surg. 2014, 19, 908–913. [Google Scholar] [CrossRef][Green Version]


| Cases of a Significant Decrease in the VD Values | Cases of a Slight Decrease/Increase in the VD Values | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROI | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 |
| VD/10−2 before sympathectomy | 4.14 | 4.31 | 2.75 | 3.46 | 5.00 | 1.93 | 5.44 | 4.37 | 5.61 | 2.80 | 0.73 | 0.57 | 1.78 | 0.68 | 1.65 |
| VD/10−2 after sympathectomy | 0.49 | 1.28 | 0.88 | 1.14 | 2.30 | 0.97 | 2.79 | 3.33 | 5.02 | 2.58 | 0.71 | 0.58 | 1.83 | 0.86 | 2.17 |
| Change, % | −88.2 | −70.3 | −68.0 | −67.1 | −54.0 | −49.7 | −48.7 | −23.8 | −10.5 | −7.9 | −2.74 | 1.75 | 2.81 | 26.5 | 31.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matveev, L.; Kiseleva, E.; Baleev, M.; Moiseev, A.; Ryabkov, M.; Potapov, A.; Bederina, E.; Sirotkina, M.; Shalin, V.; Smirnov, I.; et al. Optical Coherence Tomography Angiography and Attenuation Imaging for Label-Free Observation of Functional Changes in the Intestine after Sympathectomy: A Pilot Study. Photonics 2022, 9, 304. https://doi.org/10.3390/photonics9050304
Matveev L, Kiseleva E, Baleev M, Moiseev A, Ryabkov M, Potapov A, Bederina E, Sirotkina M, Shalin V, Smirnov I, et al. Optical Coherence Tomography Angiography and Attenuation Imaging for Label-Free Observation of Functional Changes in the Intestine after Sympathectomy: A Pilot Study. Photonics. 2022; 9(5):304. https://doi.org/10.3390/photonics9050304
Chicago/Turabian StyleMatveev, Lev, Elena Kiseleva, Mikhail Baleev, Alexander Moiseev, Maxim Ryabkov, Arseniy Potapov, Evgeniya Bederina, Marina Sirotkina, Vladislav Shalin, Igor Smirnov, and et al. 2022. "Optical Coherence Tomography Angiography and Attenuation Imaging for Label-Free Observation of Functional Changes in the Intestine after Sympathectomy: A Pilot Study" Photonics 9, no. 5: 304. https://doi.org/10.3390/photonics9050304
APA StyleMatveev, L., Kiseleva, E., Baleev, M., Moiseev, A., Ryabkov, M., Potapov, A., Bederina, E., Sirotkina, M., Shalin, V., Smirnov, I., Gladkova, N., & Zaitsev, V. (2022). Optical Coherence Tomography Angiography and Attenuation Imaging for Label-Free Observation of Functional Changes in the Intestine after Sympathectomy: A Pilot Study. Photonics, 9(5), 304. https://doi.org/10.3390/photonics9050304










