Evaluation of Structural and Optical Properties of Graphene Oxide-Polyvinyl Alcohol Thin Film and Its Potential for Pesticide Detection Using an Optical Method
Abstract
:1. Introduction
2. Experimental
2.1. Chemicals
2.2. Preparation of GO–PVA and Pesticide Solution
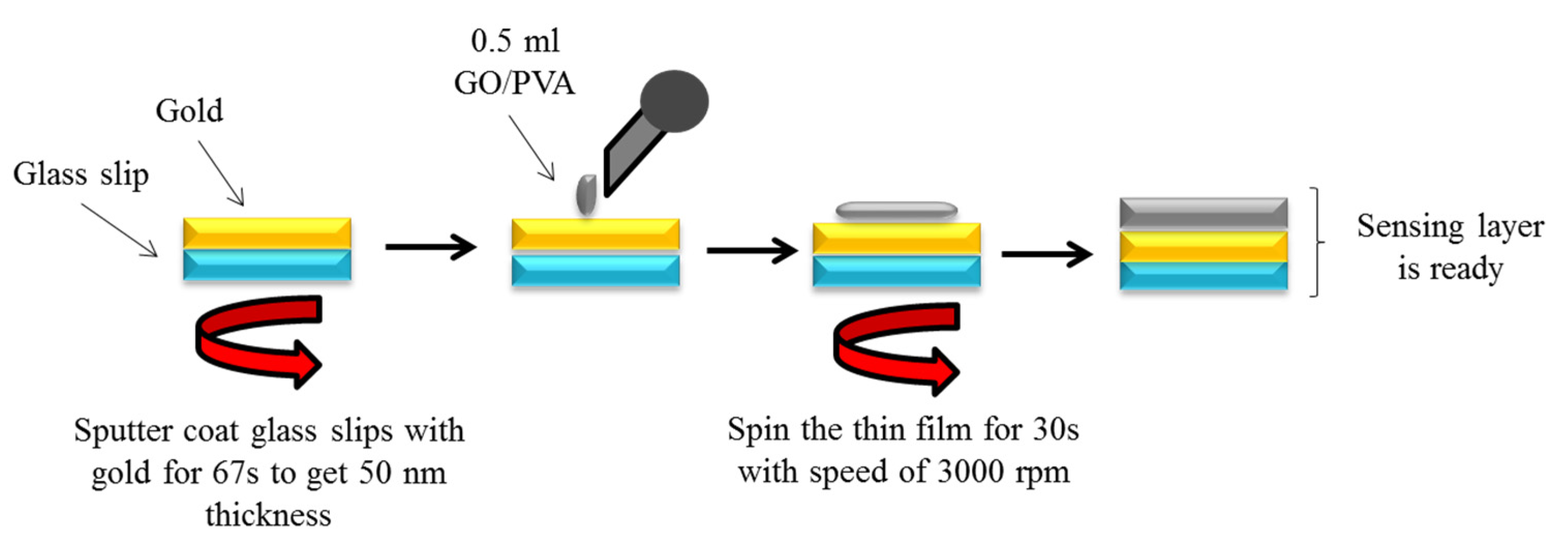
2.3. Deposition of Thin Films
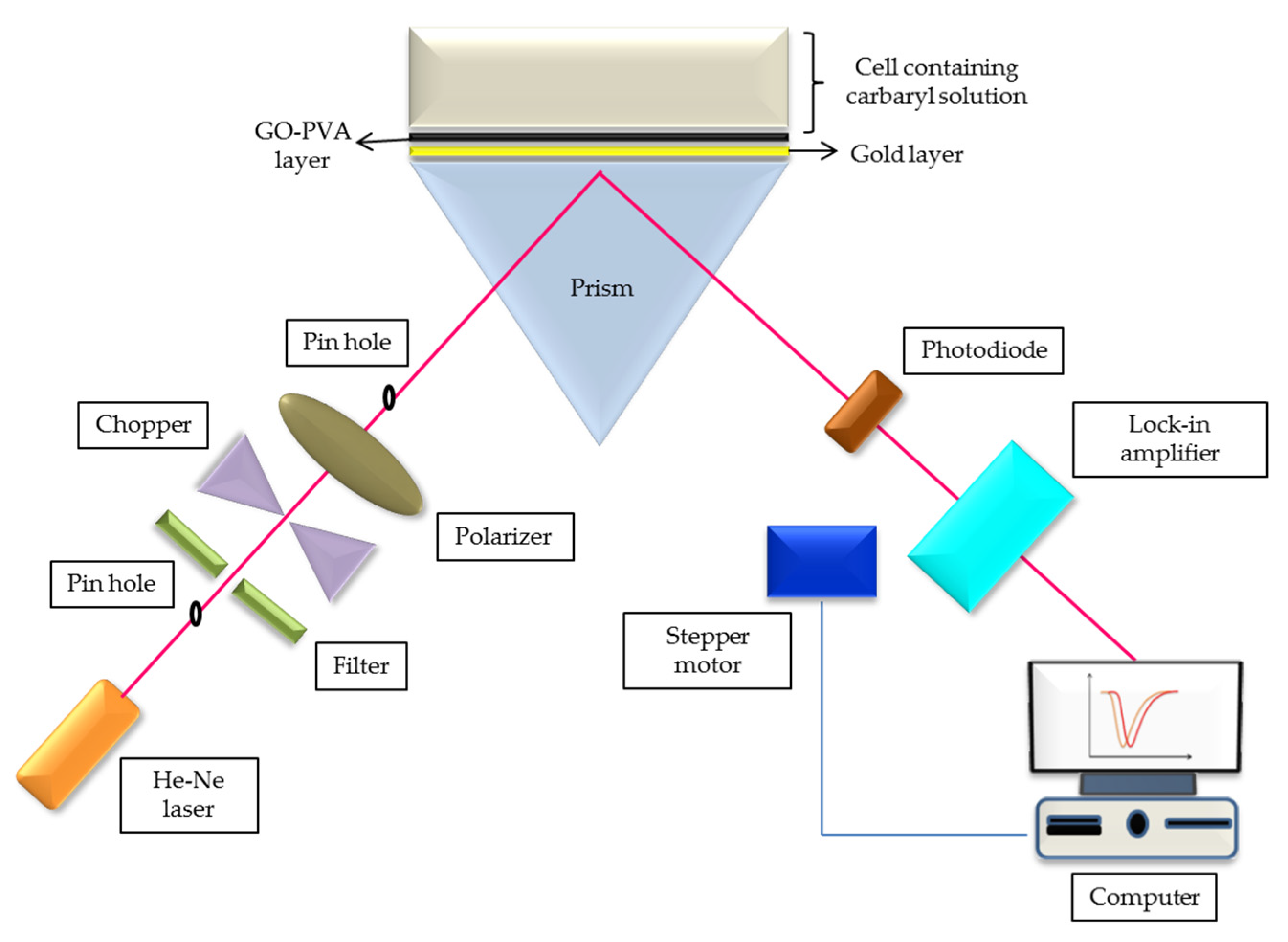
2.4. Characterization and Potential Sensing
3. Results
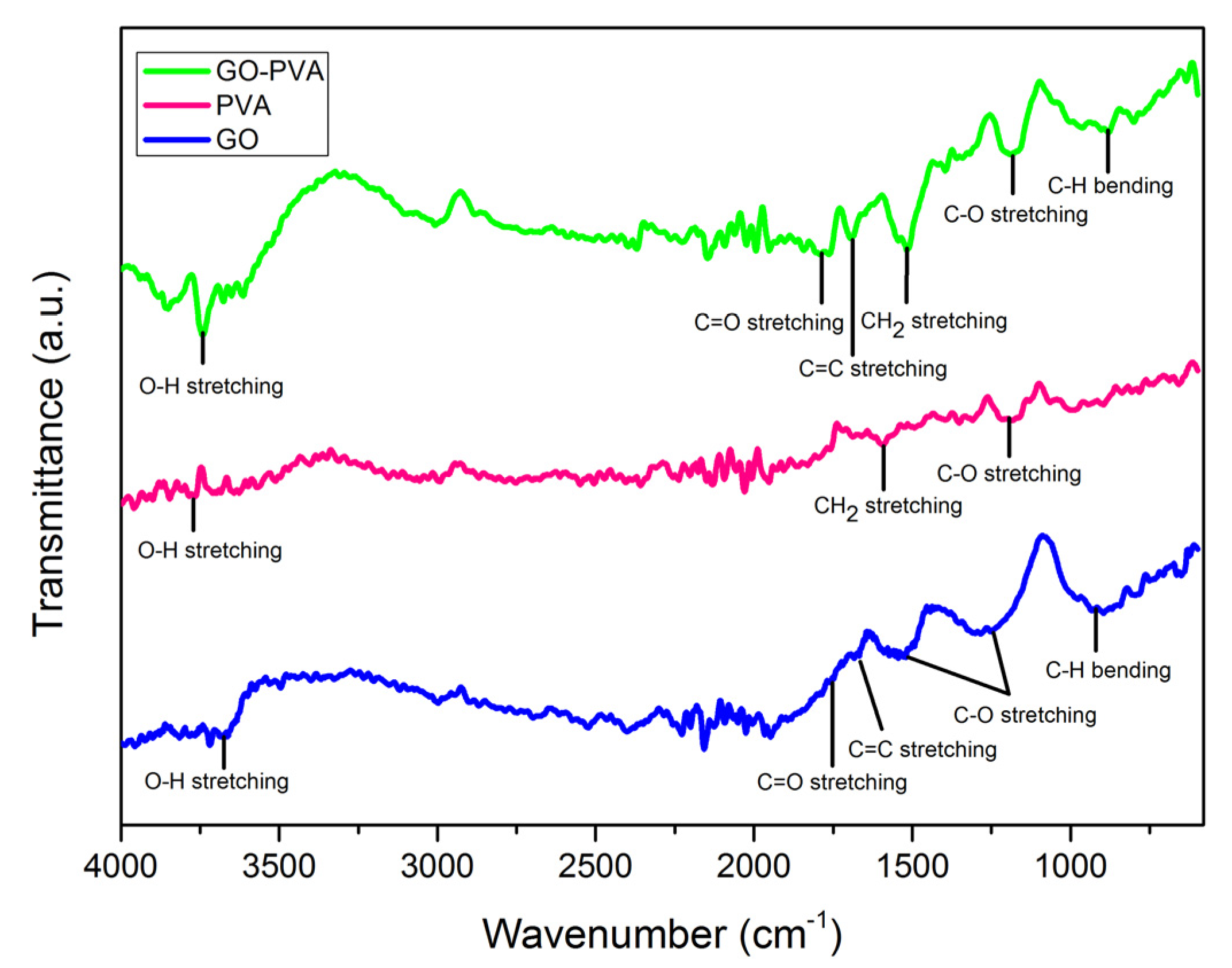
3.1. Structural Properties
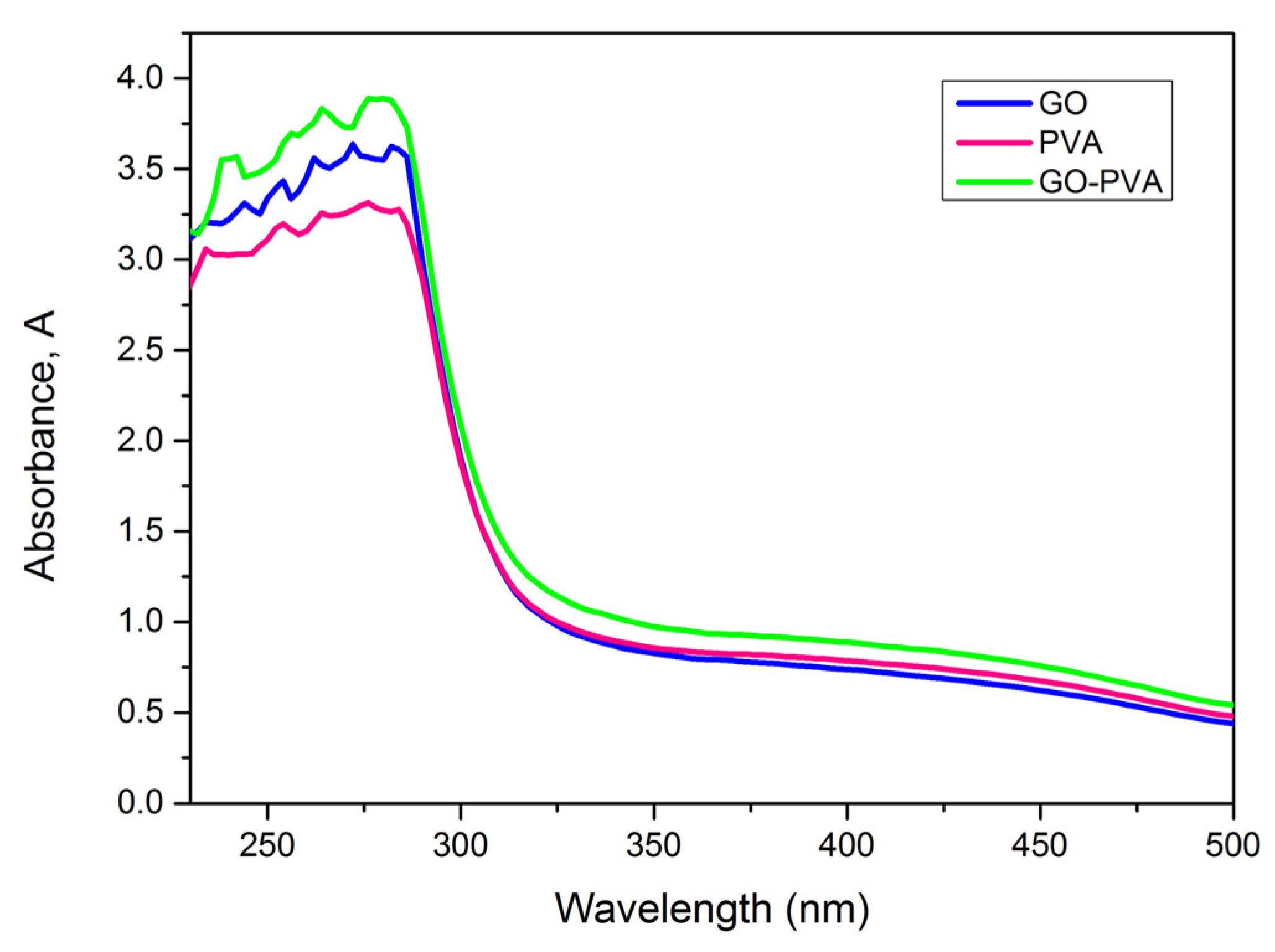
3.2. Optical Properties
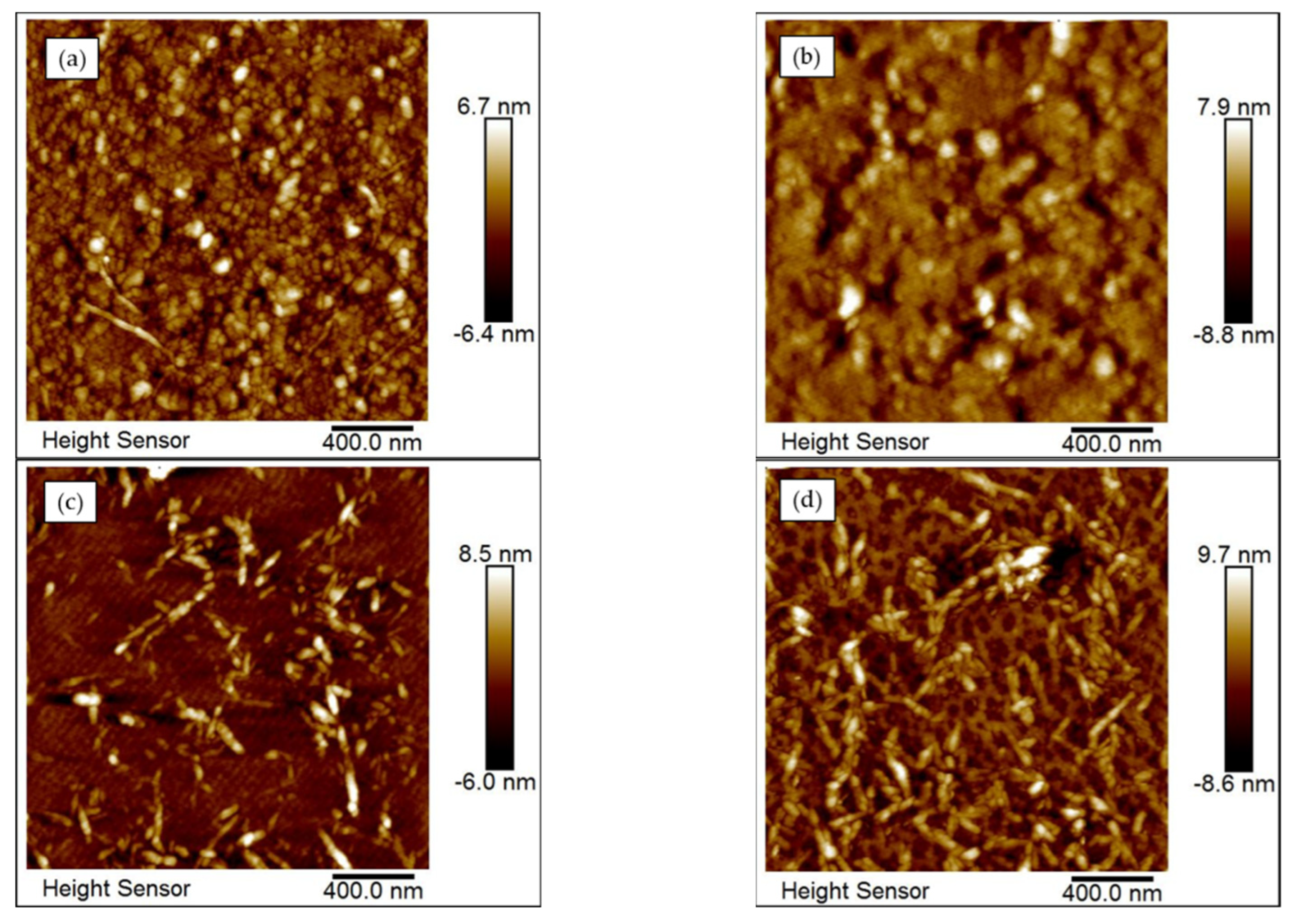
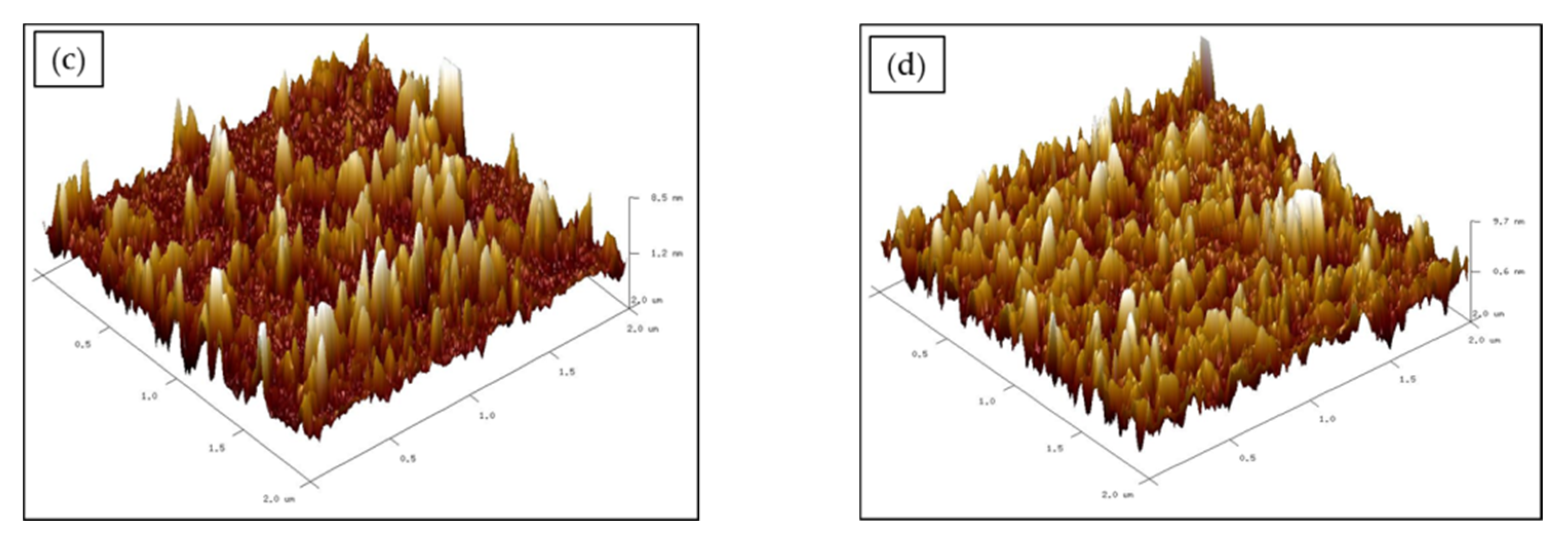
3.3. Surface Morphology
3.4. Potential Sensing of Pesticide
4. Discussion
5. Comparison GO–PVA with Other Materials-Based Optical Methods to Detect Carbaryl
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lieberzeit, P.A.; Dickert, F.L. Sensor technology and its application in environmental analysis. Anal. Bioanal. Chem. 2007, 387, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Kerschen, G.; De Boe, P.; Golinval, J.; Worden, K. Sensor validation using principal component analysis. Smart Mater. Struct. 2005, 14, 36–42. [Google Scholar] [CrossRef]
- Abdollahzadeh, S.; Navimipour, N.J. Deployment strategies in the wireless sensor network: A comprehensive review. Comput. Commun. 2016, 91–92, 1–16. [Google Scholar]
- Zakaria, R.; Zainuddin, N.M.; Fahri, M.A.S.A.; Thirunavakkarasu, P.M.; Patel, S.K.; Harun, S.W. High sensitivity refractive index sensor in long-range surface plasmon resonance based on side polished optical fiber. Opt. Fiber Technol. 2021, 61, 102449. [Google Scholar]
- Souto, D.E.; Volpe, J.; Goncalves, C.d.C.; Ramos, C.H.I.; Kubota, L.T. A brief review on the strategy of developing SPR-based biosensors for application to the diagnosis of neglected tropical diseases. Talanta 2019, 205, 120122. [Google Scholar] [CrossRef] [PubMed]
- Verma, R.K.; Suwalka, P.; Yadav, J. Detection of adulteration in diesel and petrol by kerosene using SPR based fiber optic technique. Opt. Fiber Technol. 2018, 43, 95–100. [Google Scholar] [CrossRef]
- Takemura, K. Surface Plasmon Resonance (SPR)- and localized spr (lspr)-based virus sensing systems: Optical vibration of nano- and micro-metallic materials for the development of next-generation virus detection technology. Biosensors 2021, 11, 250. [Google Scholar] [CrossRef]
- Semwal, V.; Gupta, B.D. Highly selective SPR based fiber optic sensor for the detection of hydrogen peroxide. Sens. Actuators B Chem. 2020, 329, 129062. [Google Scholar] [CrossRef]
- Xie, S.; Wang, X.; Shang, H. Security analysis on wireless sensor network in the data center for energy internet of things. Int. J. Saf. Secur. Eng. 2020, 10, 397–402. [Google Scholar] [CrossRef]
- Wang, S.; Ding, L.; Wang, Y.; Gong, X. Multifunctional triboelectric nanogenerator towards impact energy harvesting and safeguards. Nano Energy 2019, 59, 434–442. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, S.; Aziz, F. Synthesis and toxicity of graphene oxide nanoparticles: A literature review of in vitro and in vivo studies. BioMed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef] [PubMed]
- Aliyev, E.; Filiz, V.; Khan, M.M.; Lee, Y.J.; Abetz, C.; Abetz, V. Structural characterization of graphene oxide: Surface functional groups and fractionated oxidative debris. Nanomaterials 2019, 9, 1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahriary, L.; Athawale, A.A. Graphene oxide synthesized by using modified hummers approach. Int. J. Renew. Energy Environ. Eng. 2014, 2, 58–63. [Google Scholar]
- Claramunt, S.; Varea, A.; Lopez-Diaz, D.; Velazquez, M.M.; Cornet, A.; Cirera, A. The importance of interbands on the interpretation of the raman spectrum of graphene oxide. J. Phys. Chem. 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Mattevi, C.; Eda, G.; Agnoli, S.; Miller, S.; Mkhoyan, K.A.; Celik, O.; Mastrogiovanni, D.; Granozzi, G.; Garfunkel, E.; Chhowalla, M. Evolution of electrical, chemical, and structural properties of transparent and conducting chemically derived graphene thin films. Adv. Funct. Mater. 2009, 19, 2577–2583. [Google Scholar] [CrossRef]
- Perrozzi, F.; Prezioso, S.; Ottaviano, L. Graphene oxide: From fundamentals to applications. J. Phys. Condens. Matter 2015, 27, 13002. [Google Scholar] [CrossRef] [Green Version]
- Ling, S. Structure and synthesis of graphene oxide. Chin. J. Chem. Eng. 2019, 27, 2251–2260. [Google Scholar]
- Huang, X.; Liu, L.; Zhou, S.; Zhao, J. Physical properties and device applications of graphene oxide. Front. Phys. 2019, 15, 33301. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Li, J. Graphene oxide membranes: Functional structures, preparation and environmental applications. Nano Today 2018, 20, 121–137. [Google Scholar] [CrossRef]
- Zhao, J.; Dehbari, N.; Han, W.; Huang, L.; Tang, Y. Electrospun multi-scale hybrid nano fiber/net with enhanced water swelling ability in rubber composites. Mater. Des. 2015, 86, 14–21. [Google Scholar] [CrossRef]
- Chen, X.; Qiu, M.; Ding, H.; Fu, K.; Fan, Y. Reduced graphene oxide nanofiltration membrane intercalated by well-dispersed carbon nanotubes for drinking water purification. Nanoscale 2016, 8, 5696–5705. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tu, Y.; Li, L.; Shang, S.; Tao, X. Well-dispersed chitosan/graphene oxide nanocomposites. Appl. Mater. Interfaces 2010, 2, 1707–1713. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Hu, Y.; Li, C.; Qin, C.; Ye, M. Synthesis of amphiphilic graphene nanoplatelets. Communications 2009, 4, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Cote, L.J.; Huang, J. Graphene Oxide: Surface Activity and Two-Dimensional assembly. Adv. Mater. 2010, 22, 1954–1958. [Google Scholar] [CrossRef]
- Huang, T.; Zeng, X.; Yao, Y.; Sun, R.; Meng, F.; Xu, J.; Wong, C. Boron nitride@graphene oxide hybrids for epoxy composites with enhanced thermal conductivity. RSC Adv. 2016, 6, 35847–35854. [Google Scholar] [CrossRef]
- Yeh, C.; Raidongia, K.; Shao, J.; Yang, Q.; Huang, J. On the origin of the stability of graphene oxide membranes in water. Nat. Chem. 2015, 7, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Mohan, V.B.; Lau, K.; Hui, D.; Bhattacharyya, D. Graphene-based materials and their composites: A review on production, applications and product limitations. Compos. Part B 2018, 142, 200–220. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene oxide sheets at interfaces. JACS Artic. 2010, 132, 8180–8186. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Huang, J. Two dimensional soft material: New faces of graphene oxide. Acc. Chem. Res. 2012, 45, 1356–1364. [Google Scholar] [CrossRef]
- Achari, A.; Datta, K.K.R.; De, M.; Dravid, V.P.; Eswaramoorthy, M. Amphiphilic Aminoclay-rGO hybrids: A simple strategy to disperse a high concentration of rGO in water. Nanoscale 2010, 5, 5316–5320. [Google Scholar] [CrossRef]
- Dong, X.; Huang, W.; Chen, P. In situ synthesis of reduced graphene oxide and gold nanocomposites for nanoelectronics and biosensing. Nanoscale Res. Lett. 2011, 6, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, K.; Huang, C.; Liao, J.; Zhang, W.; Li, L.; Lai, C.; Su, C. Fluorinated graphene as high performance dielectric materials and the applications for graphene nanoelectronics. Sci. Rep. 2014, 4, 5893. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Huang, J.; Li, G.; Cao, J.; Zhang, B.; Chen, X.; Zhang, H.; Jia, L. Preparation and catalytic behavior of reduced graphene oxide supported cobalt oxide hybrid nanocatalysts for CO oxidation. Trans. Nonferrous Met. Soc. China 2018, 28, 2265–2273. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, M.; Zhang, A.; Zhang, C. Graphene oxide: An ideal support for gold nanocatalysts. J. Phys. Chem. 2012, 116, 22336–22340. [Google Scholar] [CrossRef]
- Gupta, V.K.; Atar, N.; Yola, M.L.; Üstündağ, Z.; Uzun, L. A novel magnetic Fe@Au core-shell nanoparticles anchored graphene oxide recyclable nanocatalyst for the reduction of nitrophenol compounds. Water Res. 2013, 48, 210–217. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, S.; Si, Y.; Zhang, N.; Sun, Z.; Wu, H.; Lin, Y. Platinum nanocatalysts loaded on graphene oxide- dispersed carbon nanotubes with greatly enhanced peroxidase-like catalysis and electrocatalysis. Nanoscale 2014, 6, 17–19. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.; Chen, W.; Tzou, D.; Roy, A.K.; Hsiao, H. Atomic layer deposition of Pt nanocatalysts on graphene oxide nanosheets for electro-oxidation of formic acid. Int. J. Hydrogen Energy 2012, 37, 17837–17843. [Google Scholar] [CrossRef]
- Maleki, A.; Paydar, R. Graphene oxide–chitosan bionanocomposite: A highly efficient nanocatalyst for the one-pot three- component synthesis of trisubstituted imidazoles under solvent-free conditions. RSC Adv. 2015, 5, 33177–33184. [Google Scholar] [CrossRef]
- Lin, B.; Chen, H.; Liang, D.; Lin, W.; Qi, X.; Liu, H.; Deng, X. Acidic pH and High-H2O2 dual tumor microenvironment-responsive nanocatalytic graphene oxide for cancer selective therapy and recognition. ACS Appl. Mater. Interfaces 2019, 11, 11157–11166. [Google Scholar] [CrossRef]
- Smith, A.T.; Marie, A.; Zeng, S.; Liu, B.; Sun, L. Nano materials science synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and graphene oxide: Synthesis, properties, and applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2004, 112, 6027–6053. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Xing, G.; Chan-park, M.B.; Shi, W.; Xiao, N.; Wang, J.; Yan, Q.; Sum, T.C.; Huang, W.; Chen, P. The formation of a carbon nanotube–graphene oxide core–shell structure and its possible applications. Carbon 2011, 49, 5071–5078. [Google Scholar] [CrossRef]
- Zuo, X.; He, S.; Li, D.; Peng, C.; Huang, Q.; Song, S.; Fan, C. Graphene oxide-facilitated electron transfer of metalloproteins at electrode surfaces. Langmuir 2010, 26, 1936–1939. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bai, H.; Li, L. Graphene oxide–chitosan composite hydrogels as broad-spectrum adsorbents for water purification. J. Mater. Chem. A 2013, 1, 1992–2001. [Google Scholar] [CrossRef]
- Dinda, D.; Gupta, A.; Saha, S.K. Removal of toxic Cr(VI) by UV-active functionalized graphene oxide for water purification. J. Mater. C 2013, 1, 11221–11228. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.; Deng, B. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, H.; Chen, J.; Lv, Y.; Dong, C.; Sreeprasad, T.S. Graphene and graphene oxide: Advanced membranes for gas separation and water purification. Inorg. Chem. Front. 2015, 2, 417–424. [Google Scholar] [CrossRef]
- Ain, Q.; Farooq, M.U.; Jalees, M.I. Application of magnetic graphene oxide for water purification: Heavy metals removal and disinfection. J. Water Process Eng. 2020, 33, 101044. [Google Scholar] [CrossRef]
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide—TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Fathizadeh, M.; Xu, W.L.; Zhou, F.; Yoon, Y.; Yu, M. Graphene Oxide: A novel 2-dimensional material in membrane separation for water purification. Adv. Mater. Interfaces 2017, 4, 1600918. [Google Scholar] [CrossRef]
- Sun, X.; Qin, J.; Xia, P.; Guo, B.; Yang, C.; Song, C.; Wang, S.-G. Graphene oxide-silver nanoparticle membrane for biofouling control and water purification. Chem. Eng. J. 2015, 281, 53–59. [Google Scholar] [CrossRef]
- Al-gamal, A.Q.; Falath, W.S.; Saleh, T.A. Enhanced efficiency of polyamidemembranes by incorporating TiO2-Graphene oxide for water purificatio. J. Mol. Liq. 2021, 323, 114922. [Google Scholar] [CrossRef]
- Chung, C.; Kim, Y.; Shin, D.; Ryoo, S.; Hong, B.H.; Min, D. Biomedical applications of graphene and graphene oxide. Acc. Chem. Res. 2013, 46, 2211–2224. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cui, L.; Losic, D. Graphene and graphene oxide as new nano-carriers for drug delivery applications. Acta Biomater. 2013, 9, 9243–9257. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Mao, X.; Xu, J.; Kong, J.; Hu, X. Functional graphene oxide nanocarriers for drug delivery. Int. J. Polym. Sci. 2019, 2019, 8453493. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.L.; Larosa, J.M.; Luo, X.; Cui, X.T. Electrically controlled drug delivery from graphene oxide nanocomposite films. ACS Nano 2014, 8, 1834–1843. [Google Scholar] [CrossRef]
- Cheng, S.-J.; Chiu, H.-Y.; Kumar, P.V.; Hsieh, K.Y.; Yang, J.-W.; Lin, Y.-R.; Shen, Y.-C.; Chen, G.-Y. Simultaneous drug delivery and cellular imaging using graphene oxide. Biomater. Sci. 2018, 6, 813–819. [Google Scholar] [CrossRef]
- Makharza, S.; Cirillo, G.; Bachmatiuk, A.; Ibrahim, I.; Ioannides, N.; Trzebicka, B.; Hampel, S.; Rummeli, M.H. Graphene oxide-based drug delivery vehicles: Functionalization, characterization, and cytotoxicity evaluation. J. Nanopart. Res. 2013, 15, 2099. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; Chen, Y.; Zhao, D.; Chen, J.; Liu, Y. Construction of a graphene oxide based noncovalent multiple nanosupramolecular assembly as a scaffold for drug delivery. Chem. A Eur. J. 2012, 18, 4208–4215. [Google Scholar] [CrossRef] [PubMed]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Label-free optical spectroscopy for characterizing binding properties of highly sensitive nanocrystalline cellulose-graphene oxide based nanocomposite towards nickel ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 212, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S. Structural, optical and sensing properties of ionophore doped graphene based bionanocomposite thin film. Optik 2017, 144, 308–315. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Abdullah, J.; Daniyal, W.M.E.M.M.; Saleviter, S. Detection of phenol by incorporation of gold modified-enzyme based graphene oxide thin film with surface plasmon resonance technique. Opt. Express 2020, 28, 9738–9752. [Google Scholar] [CrossRef] [PubMed]
- Roshidi, M.D.A.; Fen, Y.W.; Omar, N.A.S.O.; Saleviter, S.; Daniyal, W.M.E.M.M. Optical studies of graphene oxide/poly(amidoamine) dendrimer composite thin film and its potential for sensing Hg2+ using surface plasmon resonance spectroscopy. Sens. Mater. 2019, 31, 1147–1156. [Google Scholar]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Saleviter, S.; Omar, N.A.S. Preparation and characterization of hexadecyltrimethylammonium bromide modified nanocrystalline cellulose/graphene oxide composite thin film and its potential in sensing copper ion using surface plasmon resonance technique. Opt.-Int. J. Light Electron. Opt. 2018, 173, 71–77. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Saleviter, S.; Daniyal, W.M.E.M.M.; Anas, N.A.A.; Ramdzan, N.S.M.; Roshidi, M.D.A. Development of a graphene-based surface plasmon resonance optical sensor chip for potential biomedical application. Materials 2019, 12, 1928. [Google Scholar] [CrossRef] [Green Version]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S. Development of graphene quantum dots-based optical sensor for toxic metal ion detection. Sensors. 2019, 19, 3850. [Google Scholar] [CrossRef] [Green Version]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Zainudin, A.A.; Daniyal, W.M.E.M.M. Optical and structural characterization of immobilized 4-(2-pyridylazo)resorcinol in chitosan-graphene oxide composite thin film and its potential for Co2+ sensing using surface plasmon resonance technique. Results Phys. 2018, 11, 118–122. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainuddin, A.A. Optical properties of chitosan/hydroxyl-functionalized graphene quantum dots thin film for potential optical detection of ferric (III) ion. Opt. Laser Technol. 2019, 120, 105724. [Google Scholar] [CrossRef]
- Saleviter, S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Abdullah, J.; Zaid, M.H.M. Structural and optical studies of cadmium sulfide quantum dot- graphene oxide-chitosan nanocomposite thin film as a novel spr spectroscopy active layer. J. Nanomater. 2018, 2018, 4324072. [Google Scholar] [CrossRef]
- Anas, N.A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M. Investigating the properties of cetyltrimethylammonium bromide/hydroxylated graphene quantum dots thin film for potential optical detection of heavy metal ions. Materials 2020, 13, 2591. [Google Scholar]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Fauzi, N.I.M.; Daniyal, W.M.E.M.M. Recent advances of priority phenolic compounds detection using phenol oxidases-based electrochemical and optical sensors. Measurement 2021, 184, 109855. [Google Scholar] [CrossRef]
- Zainudin, A.A.; Fen, Y.W.; Yusof, N.A.; Al-Rekabi, S.H.; Mahdi, M.A.; Omar, N.A.S. Incorporation of surface plasmon resonance with novel valinomycin doped chitosan-graphene oxide thin film for sensing potassium ion. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2018, 191, 111–115. [Google Scholar] [CrossRef]
- Li, D.; Wang, T.; Li, Z.; Xu, X.; Wang, C.; Duan, Y. Application of graphene-based materials for detection of nitrate and nitrite in water—A Review. Sensors 2019, 20, 54. [Google Scholar] [CrossRef] [Green Version]
- Joshi, D.J.; Janardhan, K.R.; Malek, N.I.; Hussain, C.M.; Kumar, K.S. Surface modifications and analytical applications of graphene oxide: A review. Trends Anal. Chem. 2021, 144, 116448. [Google Scholar] [CrossRef]
- Hegab, H.M.; Zou, L. Graphene oxide-assisted membranes: Fabrication and potential applications in desalination and water purification. J. Memb. Sci. 2015, 484, 95–106. [Google Scholar] [CrossRef]
- Harsanyi, G. Polymer films in sensor applications: A review of present uses and future possibilities. Sens. Rev. 2000, 20, 98–105. [Google Scholar] [CrossRef]
- Arul, K.T.; Manikandan, E.; Ladchumananandasivam, R.; Maaza, M. Novel PVA polymer based nanostructure with ferrities co-doped with nickel and cobalt ions for Magneto-Sensor application. Polym. Int. 2016, 65, 1482–1485. [Google Scholar] [CrossRef]
- Ansari, S. Combination of molecularly imprinted polymers and carbon nanomaterials as a versatile biosensing tool in sample analysis: Recent applications and challenges. Trends Anal. Chem. 2017, 93, 134–151. [Google Scholar] [CrossRef]
- Feig, V.R.; Tran, H.; Bao, Z. Biodegradable polymeric materials in degradable electronic devices. ACS Cent. Sci. 2018, 4, 337–348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Cao, S.; Whitcombe, M.J.; Piletsky, S.A. Size matters: Challenges in imprinting macromolecules. Prog. Polym. Sci. 2014, 39, 145–163. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. Progress in synthetic polymers based on natural amino progress in synthetic polymers based on natural amino acids. J. Macromol. Sci. Part A Pure Appl. Chem. 2011, 48, 37–41. [Google Scholar] [CrossRef]
- Hu, K.; Kulkarni, D.D.; Choi, I.; Tsukruk, V.V. Graphene–polymer nanocomposites for structural and functional applications. Prog. Polym. Sci. 2014, 39, 1934–1972. [Google Scholar] [CrossRef]
- Cai, D.; Song, M. Recent advance in functionalized graphene/polymer nanocomposites. J. Mater. Chem. 2010, 20, 7906. [Google Scholar] [CrossRef]
- Potts, J.R.; Dreyer, D.R.; Bielawski, C.W.; Ruoff, R.S. Graphene-based polymer nanocomposites. Polymer 2011, 52, 5–25. [Google Scholar] [CrossRef] [Green Version]
- Verdejo, R.; Bernal, M.M.; Romasanta, L.J.; Lopez-manchado, M.A. Graphene filled polymer nanocomposites. J. Mater. Chem. 2011, 21, 3301–3310. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Li, Y.; Su, Z.; Wei, G. Recent advances in the synthesis and applications of graphene-polymer nanocomposites. Polym. Chem. 2015, 6, 6107–6124. [Google Scholar] [CrossRef]
- Cui, Y.; Kundalwal, S.I.; Kumar, S. Gas barrier performance of graphene/polymer nanocomposites. Carbon 2016, 98, 313–333. [Google Scholar] [CrossRef] [Green Version]
- Vickery, J.L.; Patil, A.J.; Mann, S. Fabrication of graphene—Polymer nanocomposites with higher-order three-dimensional architectures. Adv. Mater. 2009, 21, 2180–2184. [Google Scholar] [CrossRef]
- Ramanathan, T.; Abdala, A.A.; Stankovich, S.; Dikin, D.A.; Herrera-Alonso, M.; Piner, R.D.; Adamson, D.H.; Schniepp, H.C.; Chen, X.; Ruoff, R.S.; et al. Functionalized graphene sheets for polymer nanocomposites. Letters 2008, 3, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ye, Y.; Xue, Y.; Xie, X.; Mai, Y. Recent Advances in covalent functionalization of carbon nanomaterials with polymers: Strategies and perspectives. J. Polym. Sci. Part A Polym. Chem. 2016, 55, 622–631. [Google Scholar] [CrossRef] [Green Version]
- Yoo, B.M.; Shin, H.J.; Yoon, H.W.; Park, H.B. Graphene and graphene oxide and their uses in barrier polymers. J. Polym. Sci. Part A Polym. Chem. 2013, 131, 1–23. [Google Scholar] [CrossRef]
- Li, D.; Huang, J.; Kaner, R.B. Polyaniline nanofibers: A unique polymer nanostructure for versatile applications. Acc. Chem. Res. 2009, 42, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Gaaz, T.S.; Sulong, A.B.; Akhtar, M.N.; Kadhum, A.A.H.; Mohamad, A.B.; Al-Amiery, A.A. Properties and applications of polyvinyl alcohol, halloysite nanotubes and their nanocomposites. Molecules 2015, 20, 22833–22847. [Google Scholar] [CrossRef] [Green Version]
- Chang, I.; Kim, C.; Nam, B. The influence of poly-vinyl-alcohol (PVA) characteristics on the physical stability of encapsulated immobilization media for advanced wastewater treatment. Process Biochem. 2005, 40, 3050–3054. [Google Scholar] [CrossRef]
- Aziz, S.B.; Hassan, A.Q.; Mohammed, S.J.; Karim, W.O.; Kadir, M.F.Z.; Tajuddin, H.A.; Chan, N.N.M.Y. Structural and optical characteristics of PVA: C-Dot Composites: Tuning the absorption of ultra violet (UV) region. Nanomaterials 2019, 9, 216. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Xu, K.; Ding, W.; Qiao, Y.; Wang, L. Microstructural characteristics and their impact on mechanical properties of steel-PVA fiber reinforced concrete. Cem. Concr. Compos. 2021, 123, 104196. [Google Scholar] [CrossRef]
- Rudra, R.; Kumar, V.; Kundu, P.P. Acid catalysed cross-linking of poly vinyl alcohol (PVA) by glutaraldehyde: Effect of crosslink density on the characteristics of PVA membrane used in single chambered microbial fuel cell. RSC Adv. 2015, 5, 83436–83447. [Google Scholar] [CrossRef]
- Hakalahti, M.; Salminen, A.; Seppälä, J.; Tammelin, T.; Hanninen, T. Effect of interfibrillar PVA bridging on water stability and mechanical properties of TEMPO/NaClO2 oxidized cellulosic nanofibril films. Carbohydr. Polym. 2015, 126, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Kochkina, N.E.; Lukin, N.D. Structure and properties of biodegradable maize starch/chitosan composite films as affected by PVA additions. Int. J. Biol. Macromol. 2020, 157, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.; Araby, S.; Gibson, C.T.; Meng, Q.; Zhu, S.; Ma, J. Graphene platelets and their polymer composites: Fabrication, structure, properties, and applications. Adv. Funct. Mater. 2018, 28, 1706705. [Google Scholar] [CrossRef]
- Gudarzi, M.M.; Sharif, F. Enhancement of dispersion and bonding of graphene-polymer through wet transfer of functionalized graphene oxide. Express Polym. Lett. 2012, 6, 1017–1031. [Google Scholar] [CrossRef]
- Gudarzi, M.M.; Moghadam, M.H.M.; Sharif, F. Spontaneous exfoliation of graphite oxide in polar aprotic solvents as the route to produce graphene oxide—Organic solvents liquid crystals. Carbon 2013, 64, 403–415. [Google Scholar] [CrossRef]
- Khan, Z.U.; Kausar, A.; Ullah, H.; Badshah, A. A review of graphene oxide, graphene buckypaper, and polymer/graphene composites: Properties and fabrication techniques. J. Plast. Film Sheeting 2016, 32, 336–379. [Google Scholar] [CrossRef]
- Bhawal, P.; Ganguly, S.; Chaki, T.K.; Das, N.C. Synthesis and characterization of graphene oxide filled ethylene methyl acrylate hybrid nanocomposites. RSC Adv. 2016, 6, 20781–20790. [Google Scholar] [CrossRef]
- Badri, A.; Whittaker, M.R.; Zetterlund, P.B. Modification of graphene/graphene oxide with polymer brushes using controlled/living radical polymerization. Polym. Chem. 2012, 50, 2981–2992. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, P.; Zhao, X.; Xu, J. Preparation of poly (vinyl alcohol)-grafted graphene oxide/poly (vinyl alcohol) Nanocomposites via in-situ low-temperature emulsion polymerization and their thermal and mechanical characterization. Appl. Surf. Sci. 2016, 396, 1098–1107. [Google Scholar] [CrossRef]
- Govindaraj, P.; Sokolova, A.; Salim, N.; Juodkazis, S.; Konstantin, F.; Fox, B.; Hameed, N. Distribution states of graphene in polymer nanocomposites: A review. Compos. Part B 2021, 226, 109353. [Google Scholar] [CrossRef]
- Hurayra-Lizu, A.K.M.; Bari, W.M.D.; Gulshan, F.; Islam, R.M. GO based PVA nanocomposites: Tailoring of optical and structural properties of PVA with low percentage of GO nano fillers. Heliyon 2021, 7, e06983. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, Y.; Zhang, L.; Wang, Y.; Ma, Y.; Guo, T.; Chen, Y. Molecular-level dispersion of graphene into poly(vinyl alcohol) and effective reinforcement of their nanocomposites. Adv. Funct. Mater. 2009, 19, 2297–2302. [Google Scholar] [CrossRef]
- Al Sheheri, S.Z.; Al-amshany, Z.M.; Al Sulami, Q.A.; Tashkandi, N.Y.; Hussein, M.A.; El-shishtawy, R.M. The preparation of carbon nanofillers and their role on the performance of variable polymer nanocomposites. Des. Monomers Polym. 2019, 22, 8–53. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Xiong, D.; Li, J.; Wang, N. In situ reduction of graphene oxide nanosheets in poly(vinyl alcohol) hydrogel by γ-ray irradiation and its influence on mechanical and tribological properties. J. Phys. Chem. 2016, 120, 19442–194543. [Google Scholar] [CrossRef]
- Qi, Y.Y.; Tai, Z.X.; Sun, D.F.; Chen, J.T.; Ma, H.B.; Yan, X.B.; Liu, B.; Xue, Q.J. Fabrication and characterization of poly(vinyl alcohol)/graphene oxide nanofibrous biocomposite scaffolds. J. Appl. Polym. 2012, 127, 1885–1894. [Google Scholar] [CrossRef]
- Sun, Y.; Lu, J.; Ai, C.; Wen, D. Nonvolatile memory devices based on poly(vinyl alcohol) + graphene oxide hybrid composites. Phys. Chem. Chem. Phys. 2016, 18, 11341–11347. [Google Scholar] [CrossRef]
- Debnath, D.; Zhao, X.; Anas, M.; Kulhanek, D.L.; Oh, J.H.; Green, M.J. Radio frequency heating and reduction of graphene oxide and graphene oxide-polyvinyl alcohol composites. Carbon 2020, 169, 475–481. [Google Scholar] [CrossRef]
- Shuai, C.; Feng, P.; Gao, C.; Shuai, X.; Xiao, T.; Peng, S. Graphene oxide reinforced poly(vinyl alcohol): Nanocomposite scaffolds for tissue engineering applications. RSC Adv. 2015, 5, 25416–25423. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, C.; Lou, W.; Shentu, F. Fiber optic humidity sensor based on the graphene oxide/PVA composite film. Opt. Commun. 2016, 372, 229–234. [Google Scholar] [CrossRef]
- Syuhada, A.; Shamsudin, M.S.; Daud, S.; Krishnan, G.; Harun, S.W.; Aziz, M.S.A. Single-mode modified tapered fiber structure functionalized with GO–PVA composite layer for relative humidity sensing. Photonic Sens. 2021, 11, 314–324. [Google Scholar] [CrossRef]
- Kaur, R.; Arora, A.; Tripathi, S.K. Fabrication and characterization of metal insulator semiconductor Ag/PVA/GO/PVA/n-Si/Ag device. Microelectron. Eng. 2020, 233, 111419. [Google Scholar] [CrossRef]
- Hmar, J.J.L. Non-volatile resistive switching memory device based on ZnO-graphene oxide embedded in a polymer matrix fabricated on a flexible PET substrate. Microelectron. Eng. 2020, 233, 111436. [Google Scholar] [CrossRef]
- Ngo, H.T.; Thi, M.T.N.; Do, D.P.; Tran, K.M.; Thi, K.H.T.; Phan, B.T.; Pham, K.N. Low operating voltage resistive random access memory based on graphene oxide—Polyvinyl alcohol nanocomposite thin films. J. Sci. Adv. Mater. Devices 2020, 5, 199–206. [Google Scholar] [CrossRef]
- Hwang, S.; Kang, D.; Ruoff, R.S.; Shin, H.S.; Park, Y. Poly (vinyl alcohol) Reinforced and toughened with poly(dopamine) -treated graphene oxide, and its use for humidity. ACS Nano 2014, 8, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Trigona, C.; Al-hamry, A.; Kanoun, O.; Baglio, S. Hybrid micro electro mechanical sensor based on graphene oxide/polyvinyl alcohol for humidity. Proceedings 2018, 2, 1011. [Google Scholar]
- Lim, M.; Shin, H.; Shin, D.M.; Lee, S.; Lee, J. Poly(vinyl alcohol) nanocomposites containing reduced graphene oxide coated with tannic acid for humidity sensor. Polymer 2016, 84, 89–98. [Google Scholar] [CrossRef]
- Lee, M.E.; Jin, H. Nanocomposite films of poly(vinyl alcohol)-grafted graphene oxide/poly(vinyl alcohol) for gas barrier film applications. J. Nanosci. Nanotechnol. 2015, 15, 8348–8352. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Kamil, Y.M.; Daniyal, W.M.E.M.M.; Sadrolhosseini, A.R.; Mahdi, M.A. Sensitive detection of dengue virus type 2 E-Proteins signals using self-assembled monolayers/reduced graphene oxide-PAMAM dendrimer thin film-SPR optical sensor. Sci. Rep. 2020, 10, 2374. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A.; Yusof, N.A. Development of surface plasmon resonance sensor for determining zinc ion using novel active nanolayers as probe. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 134, 48–52. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Utilization of chitosan-based sensor thin films for the detection of lead ion by surface plasmon resonance optical sensor. IEEE Sens. J. 2013, 13, 1413–1418. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Saleviter, S.; Zainudin, A.A. Optik Optical and surface plasmon resonance sensing properties for chitosan/carboxyl-functionalized graphene quantum dots thin film. Optik 2019, 178, 802–812. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A.; Ishak, N.S.; Omar, N.A.S.; Zainudin, A.A. Preparation, characterization and optical properties of ionophore doped chitosan biopolymer thin film and its potential application for sensing metal ion. Optik 2015, 126, 4688–4692. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Moksin, M.M.; Talib, Z.A.; Yusof, N.A. Surface plasmon resonance optical sensor for mercury ion detection by crosslinked chitosan thin film. J. Optoelectron. Adv. Mater. 2011, 13, 279–285. [Google Scholar]
- Zainuddin, N.H.; Fen, Y.W.; Alwahib, A.A.; Yaacob, M.H.; Bidin, N.; Omar, N.A.S.; Mahdi, M.A. Detection of adulterated honey by surface plasmon resonance optical sensor. Optik 2018, 168, 134–139. [Google Scholar] [CrossRef]
- Eddin, F.B.K.; Fen, Y.W.; Omar, N.A.S.; Liew, J.Y.C.; Daniyal, W.M.E.M.M. Femtomolar detection of dopamine using surface plasmon resonance sensor based on chitosan/graphene quantum dots thin film. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 263, 120202. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Ramli, I.; Sadrolhosseini, A.R.; Abdullah, J.; Yusof, N.A.; Kamil, Y.M.; Mahdi, M.A. An optical sensor for dengue envelope proteins using polyamidoamine dendrimer biopolymer-based nanocomposite thin film: Enhanced sensitivity, selectivity, and recovery studies. Polymers 2021, 13, 762. [Google Scholar] [CrossRef]
- Mitchell, J. Small molecule immunosensing using surface plasmon resonance. Sensors 2010, 10, 7323–7346. [Google Scholar] [CrossRef] [Green Version]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Surface plasmon resonance optical sensor for detection of Pb2+ based on immobilized p-tert-butylcalix [4] arene-tetrakis in chitosan thin film as an active layer. Sens. Actuators B Chem. 2012, 171–172, 287–293. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M.; Talib, Z.A. Analysis of Pb (II) ion sensing by crosslinked chitosan thin film using surface plasmon resonance spectroscopy. Opt.-Int. J. Light Electron. Opt. 2013, 124, 126–133. [Google Scholar] [CrossRef]
- Fen, Y.W.; Yunus, W.M.M. Surface plasmon resonance spectroscopy as an alternative for sensing heavy metal ions: A review. Sens. Rev. 2009, 33, 305–314. [Google Scholar]
- Fen, Y.W.; Mahmood, W.; Yunus, M.; Yusof, N.A. Detection of mercury and copper ions using surface plasmon resonance optical sensor. Sens. Mater. 2011, 23, 325–334. [Google Scholar]
- Zaid, M.H.M.; Matori, K.A.; Aziz, S.H.A.; Kamari, H.M.; Wahab, Z.A.; Fen, Y.W.; Alibe, I.M. Synthesis and characterization of low cost willemite based glass—Ceramic for opto-electronic applications. J. Mater. Sci. Mater. Electron. 2016, 27, 11158–11167. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Saleviter, S.; Fen, Y.W. Development of surface plasmon resonance spectroscopy for metal ion development of surface plasmon resonance spectroscopy for metal ion detection. Sens. Mater. 2018, 30, 2023–2028. [Google Scholar]
- Fen, Y.W.; Yunus, W.M.M.; Yusof, N.A. Optical properties of cross-linked chitosan thin film for copper ion detection using surface plasmon resonance technique. Opt. Appl. 2011, 41, 999–1013. [Google Scholar]
- Eddin, F.B.K.; Fen, Y.W. Recent advances in electrochemical and optical sensing of dopamine. Sensors 2020, 20, 1039. [Google Scholar] [CrossRef] [Green Version]
- Ramdzan, N.S.M.; Fen, Y.W.; Anas, N.A.A.; Omar, N.A.S.; Saleviter, S. Development of biopolymer and conducting polymer-based optical sensors for heavy metal ion detection. Molecules 2020, 25, 2548. [Google Scholar] [CrossRef]
- Roshidi, M.D.A.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Omar, N.A.S.; Zulholinda, M. Structural and optical properties of chitosan—Poly(amidoamine) dendrimer composite thin film for potential sensing Pb2+ using an optical spectroscopy. Opt.-Int. J. Light Electron. Opt. 2019, 185, 351–358. [Google Scholar] [CrossRef]
- Eddin, F.B.K.; Fen, Y.W. The principle of nanomaterials based surface plasmon resonance biosensors and its potential for dopamine detection. Molecules 2020, 25, 2769. [Google Scholar] [CrossRef]
- Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Saleviter, S.; Daniyal, W.M.E.M.M.; Hashim, H.S.; Nasrullah, M. Nanostructured chitosan/maghemite composites thin film for potential optical detection of mercury ion by surface plasmon resonance investigation. Polymers 2020, 12, 1497. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Fauzi, N.I.M.; Hashim, H.S.; Ramdzan, N.S.; Omar, N.A.S. Recent advances in surface plasmon resonance optical sensors for potential application in environmental monitoring. Sens. Mater. 2020, 32, 4191–4200. [Google Scholar] [CrossRef]
- Daniyal, W.M.E.M.M.; Fen, Y.W.; Abdullah, J.; Sadrolhosseini, A.R.; Saleviter, S.; Omar, N.A.S. Exploration of surface plasmon resonance for sensing copper ion based on nanocrystalline cellulose-modified thin film. Opt. Express 2018, 26, 34880–34893. [Google Scholar] [CrossRef] [PubMed]
- Saleviter, S.; Fen, Y.W.; Daniyal, W.M.E.M.M.; Abdullah, J.; Sadrolhosseini, A.R.; Omar, N.A.S. Design and analysis of surface plasmon resonance optical sensor for determining cobalt ion based on chitosan-graphene oxide decorated quantum dots-modified gold active layer. Opt. Express 2019, 27, 32294–32307. [Google Scholar] [CrossRef] [PubMed]
- Anas, N.A.A.; Fen, Y.W.; Yusof, N.A.; Omar, N.A.S.O.; Daniyal, W.M.E.M.M.; Ramdzan, N.S.M. Highly sensitive surface plasmon resonance optical detection of ferric ion using CTAB/hydroxylated graphene quantum dots thin film. J. Appl. Phys. 2020, 128, 83105. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Fen, Y.W.; Abdullah, J.; Zaid, M.H.M.; Mahdi, M.A. Structural, optical and sensing properties of CdS-NH2GO thin film as a dengue virus E-protein sensing material. Opt.-Int. J. Light Electron. Opt. 2018, 171, 934–940. [Google Scholar] [CrossRef]
- Hashim, H.S.; Fen, Y.W.; Omar, N.A.S.; Daniyal, W.M.E.M.M.; Saleviter, S.; Abdullah, J. Structural, optical and potential sensing properties of tyrosinase immobilized graphene oxide thin film on gold surface. Opt.-Int. J. Light Electron. Opt. 2020, 212, 164786. [Google Scholar] [CrossRef]
- Ramdzan, N.S.M.; Fen, Y.W.; Omar, N.A.S.; Anas, N.A.A.; Liew, J.Y.C.; Daniyal, W.M.E.M.M.; Hashim, H.S. Detection of mercury ion using surface plasmon resonance spectroscopy based on nanocrystalline cellulose/poly(3,4-ethylenedioxythiophene) thin film. Measurement 2021, 182, 109728. [Google Scholar] [CrossRef]
- Bao, C.; Guo, Y.; Song, L.; Hu, Y. Poly(vinyl alcohol) nanocomposites based on graphene and graphite oxide: A comparative investigation of property and mechanism. Chem. J. Mater. 2011, 21, 13942–13950. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Shang, S.; Tao, X. Synthesis and characterization of layer-aligned poly(vinyl alcohol)/graphene nanocomposites. Polymer 2010, 51, 3431–3435. [Google Scholar] [CrossRef]
- Islam, M.R. Enhanced electrochemical performance of solution-processed single-wall carbon nanotube reinforced polyvinyl alcohol nanocomposite synthesized via solution-cast method Enhanced electrochemical performance of solution-processed single-wall carbon nanotube. Nano Express 2020, 1, 30013. [Google Scholar] [CrossRef]
- Yahia, I.S.; Mohammed, M.I. Facile synthesis of graphene oxide/PVA nanocomposites for laser optical limiting: Band gap analysis and dielectric constants. J. Mater. Sci. Mater. Electron. 2018, 29, 8555–8563. [Google Scholar] [CrossRef]
- Wu, T.; Chen, B. Facile fabrication of porous conductive thermoplastic polyurethane nanocomposite films via solution casting. Sci. Rep. 2017, 7, 17470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.R.; Mollik, I. Enhanced electrochemical performance of flexible and eco-friendly starch/graphene oxide nanocomposite. Heliyon 2020, 6, e05292. [Google Scholar] [CrossRef] [PubMed]
- Mo, S.; Peng, L.; Yuan, C.; Zhao, C.; Tang, W.; Ma, C.; Shen, J.; Yang, W.; Yu, Y.; Min, Y.; et al. Enhanced properties of poly(vinyl alcohol) composite films with functionalized graphene. RSC Adv. 2015, 5, 97738–97745. [Google Scholar] [CrossRef]
- Omar, N.A.S.; Irmawati, R.; Fen, Y.W.; Muhamad, E.N.; Eddin, F.B.K.; Anas, N.A.A.; Ramdzan, N.S.M.; Fauzi, N.I.M.; Mahdi, M.A. Surface refractive index sensor based on titanium dioxide composite thin film for detection of cadmium ions. Measurement 2021, 187, 110287. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, F.; Zhang, Y.; Xiong, Y.; Han, S. Recent advances in aptamer-based optical and electrochemical biosensors for detection of pesticides and veterinary drugs. Food Control 2022, 131, 108399. [Google Scholar] [CrossRef]
- Gong, C.; Fan, Y.; Zhao, H. Recent advances and perspectives of enzyme-based optical biosensing for organophosphorus pesticides detection. Talanta 2022, 240, 123145. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Zhang, X.; Zhou, T.; Cui, Z.; Zhang, K. A novel terahertz metasurface based on a single-walled carbon nanotube film for sensing application. J. Mater. Chem. A 2022, 10, 1780–1787. [Google Scholar] [CrossRef]
- Byrne, B.; Stack, E.; Gilmartin, N.; O’Kennedy, R. Antibody-based sensors: Principles, problems and potential for detection of pathogens and associated toxins. Sensors 2009, 9, 4407–4445. [Google Scholar] [CrossRef] [Green Version]
- Ramdzan, N.S.M.; Fen, Y.W.; Liew, J.Y.C.; Omar, N.A.S.; Anas, N.A.A.; Daniyal, W.M.E.M.M.; Fauzi, I.M.F. Exploration on structural and optical properties of nanocrystalline cellulose/poly(3,4-ethylenedioxythiophene) thin film for potential plasmonic sensing application. Photonics 2021, 8, 419. [Google Scholar] [CrossRef]
- Sun, Z.; Cui, Z.; Li, H. p-Amino benzenesulfonic acid functionalized gold nanoparticles: Synthesis, colorimetric detection of carbaryl and mechanism study by zeta potential assays. Sens. Actuators B Chem. 2013, 183, 297–302. [Google Scholar] [CrossRef]
- Tsagkaris, A.S.; Uttl, L.; Pulkrabova, J.; Hajslova, J. Screening of carbamate and organophosphate pesticides in food matrices using an affordable and simple spectrophotometric acetylcholinesterase assay. Appl. Sci. 2020, 10, 565. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gao, L.; Ma, S.; Hu, T. Porous MB@Cd-MOF Obtained by Post-Modification: Self-Calibrated Fluorescent Turn-on Sensor for Highly Sensitive Detection of carbaryl. Cryst. Growth Des. 2022, 22, 2662–2669. [Google Scholar] [CrossRef]
- Hossain, S.Z.; Luckham, R.E.; McFadden, M.J.; Brennan, J.D. Reagentless bidirectional lateral flow bioactive paper sensors for detection of pesticides in beverage and food samples. Anal. Chem. 2009, 81, 9055–9064. [Google Scholar] [CrossRef] [PubMed]
- Minh, P.N.; Hoang, V.; Dinh, N.X.; Hoang, O.V.; Cuong, N.V.; Hop, D.T.B.; Tuan, T.Q.; Khi, N.T.; Huy, T.Q.; Le, A. Reduced graphene oxide-wrapped silver nanoparticles for applications to ultrasensitive colorimetric detection of Cr (VI) ions and cabaryl pesticide. New J. Chem. 2020, 44, 7611–7620. [Google Scholar] [CrossRef]
- Mauriz, E.; Calle, A.; Manclus, J.J.; Montoya, A.; Escuela, A.M.; Sendra, J.R.; Lechuga, L.M. Single and multi-analyte surface plasmon resonance assays for simultaneous detection of cholinesterase inhibiting pesticides. Sens. Actuators B 2006, 118, 399–407. [Google Scholar] [CrossRef]
- Mauriz, E.; Calle, A.; Lechuga, L.M.; Quintana, J.; Montoya, A.; Mancl, J.J. Real-time detection of chlorpyrifos at part per trillion levels in ground, surface and drinking water samples by a portable surface plasmon resonance immunosensor. Anal. Chim. Acta 2006, 561, 40–47. [Google Scholar] [CrossRef]
- Chen, J.; Liu, Z.; Fang, J.; Wang, Y.; Cao, Y.; Xu, W.; Ma, Y.; Meng, X.; Wang, B. A turn-on fluorescence biosensor for sensitive detection of carbaryl using flavourzyme-stabilized gold nanoclusters. LWT 2022, 157, 113099. [Google Scholar] [CrossRef]
- Soriano, M.L.; Jimenez-Sanchez, A.; Cardenas, S. Passivated graphene quantum dots for carbaryl determination in juices. J. Sep. Sci. 2021, 44, 1652–1661. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, X.; Yuan, C.; Shi, R.; Wang, Y. Double responsive analysis of carbaryl pesticide based on carbon quantum dots and Au nanoparticles. Dyes Pigm. 2020, 181, 108529. [Google Scholar] [CrossRef]
- Shahdost-fard, F.; Fahimi-Kashani, N.; Hormozi-nezhad, M.R. A ratiometric fluorescence nanoprobe using CdTe QDs for fast detection of carbaryl insecticide in apple. Talanta 2020, 221, 121467. [Google Scholar] [CrossRef]
- Cervera-chiner, L.; March, C.; Arnau, A. Detection of DDT and carbaryl pesticides in honey by means of immunosensors based on High Fundamental Frequency Quartz Crystal Microbalance (HFF-QCM). J. Sci. Food Agric. 2020, 100, 2468–2472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Cui, H.; Cai, J.; Duan, Y.; Liu, Y. Development of fluorescence sensing material based on cdse/zns quantum dots and molecularly imprinted polymer for the detection of carbaryl in rice and chinese cabbage. J. Agric. Food Chem. 2015, 63, 5–11. [Google Scholar] [CrossRef] [PubMed]
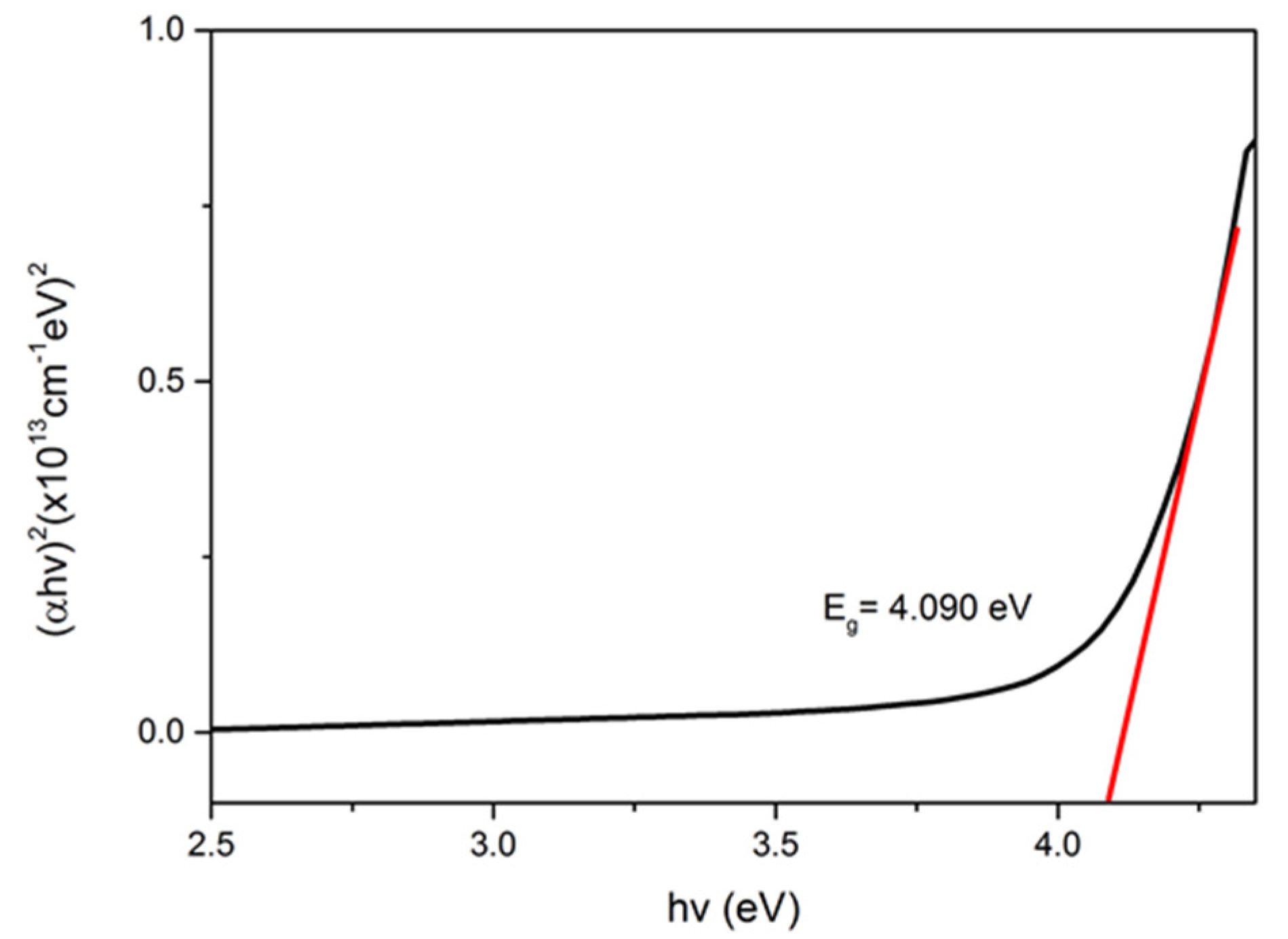
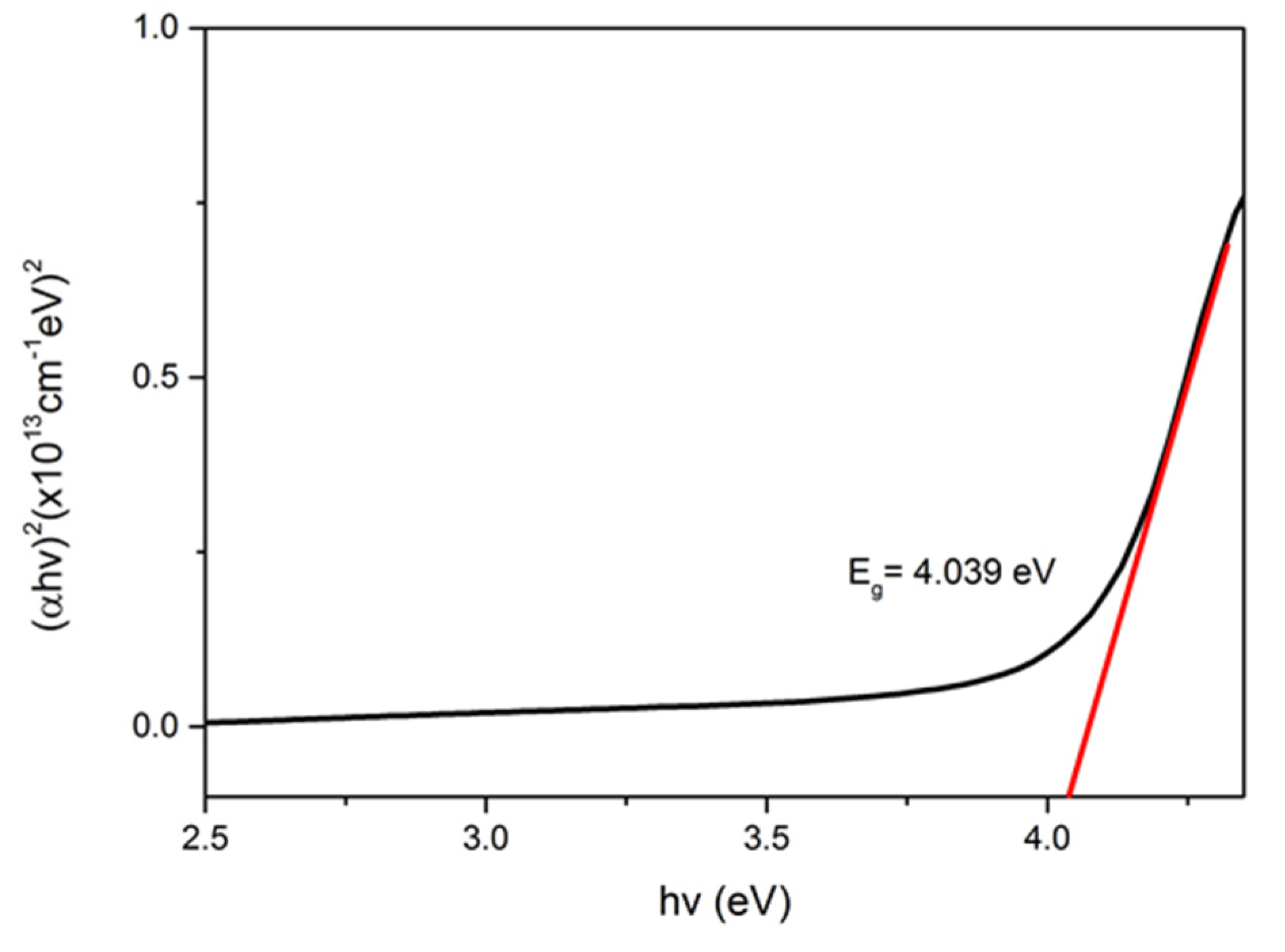
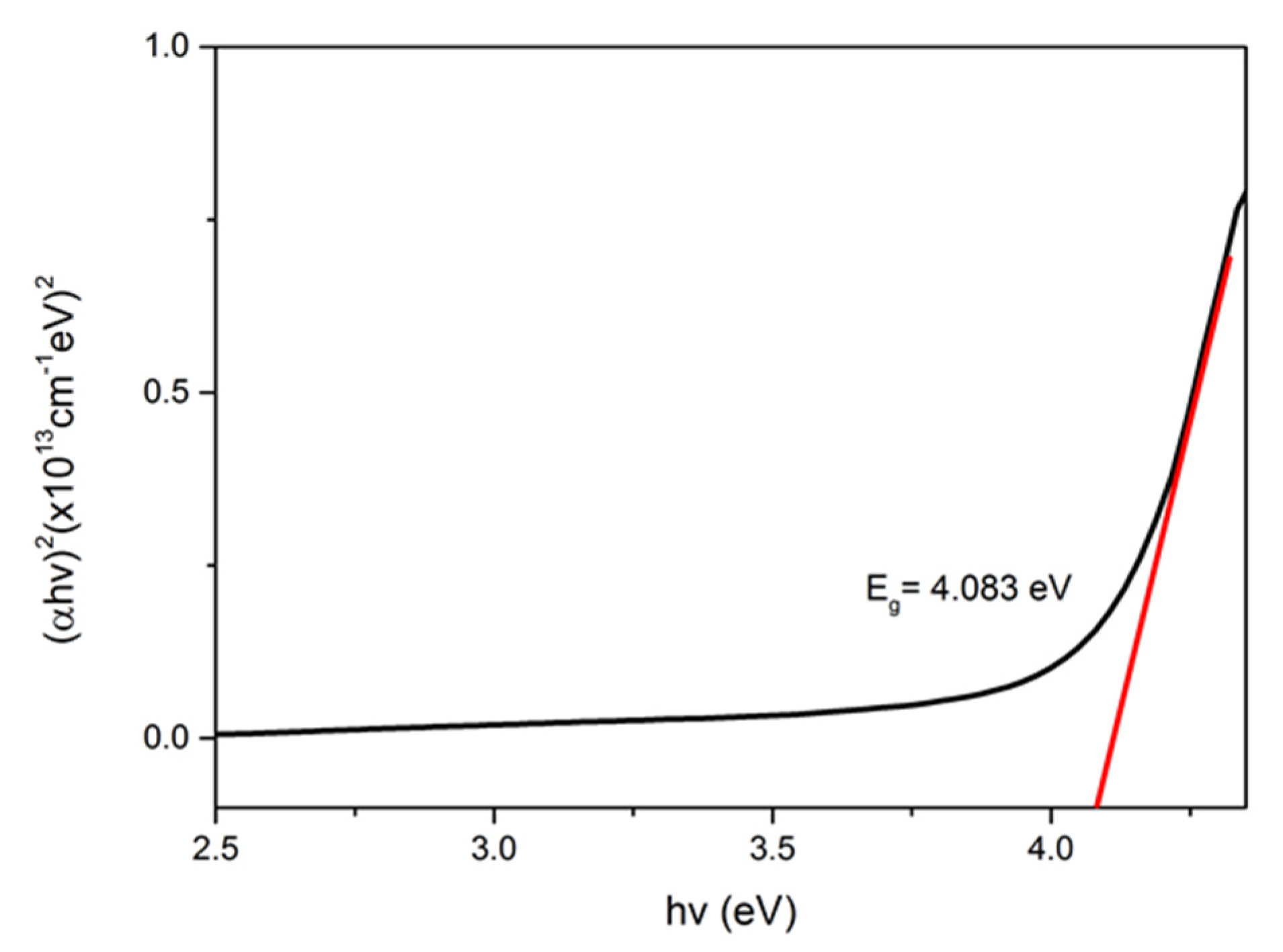


| Materials | Limit of Detection | References |
|---|---|---|
| 4-acetamidobenzenesulfonyl azide-AuNPs | 50.00 ppb | [170] |
| acetylcholinesterase | 20.00 ppb | [171] |
| 3,5-di(2′,5′-dicarboxylphenyl)pyridine | 6.70 ppb | [172] |
| idophenyl acetate-acetylcholinesterase | 2.01 ppb | [173] |
| silver reduced-graphene oxide | 1.50 ppb | [174] |
| monoclonal antibody | 1.41 ppb | [175] |
| monoclonal antibody | 1.38 ppb | [176] |
| flavourzyme-stabilized gold nanoclusters | 0.47 ppb | [177] |
| graphene quantum dots | 0.36 ppb | [178] |
| carbon quantum dots-AuNPs-acetylcholinesterase | 0.20 ppb | [179] |
| cadmium tellurite quantum dots | 0.12 ppb | [180] |
| monoclonal antibody | 0.05 ppb | [181] |
| cadmium selenide/zinc sulfide quantum dots | 0.03 ppb | [182] |
| graphene oxide-polyvinyl alcohol | 0.02 ppb | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauzi, N.I.M.; Fen, Y.W.; Abdullah, J.; Kamarudin, M.A.; Omar, N.A.S.; Eddin, F.B.K.; Ramdzan, N.S.M.; Daniyal, W.M.E.M.M. Evaluation of Structural and Optical Properties of Graphene Oxide-Polyvinyl Alcohol Thin Film and Its Potential for Pesticide Detection Using an Optical Method. Photonics 2022, 9, 300. https://doi.org/10.3390/photonics9050300
Fauzi NIM, Fen YW, Abdullah J, Kamarudin MA, Omar NAS, Eddin FBK, Ramdzan NSM, Daniyal WMEMM. Evaluation of Structural and Optical Properties of Graphene Oxide-Polyvinyl Alcohol Thin Film and Its Potential for Pesticide Detection Using an Optical Method. Photonics. 2022; 9(5):300. https://doi.org/10.3390/photonics9050300
Chicago/Turabian StyleFauzi, Nurul Illya Muhamad, Yap Wing Fen, Jaafar Abdullah, Mazliana Ahmad Kamarudin, Nur Alia Sheh Omar, Faten Bashar Kamal Eddin, Nur Syahira Md Ramdzan, and Wan Mohd Ebtisyam Mustaqim Mohd Daniyal. 2022. "Evaluation of Structural and Optical Properties of Graphene Oxide-Polyvinyl Alcohol Thin Film and Its Potential for Pesticide Detection Using an Optical Method" Photonics 9, no. 5: 300. https://doi.org/10.3390/photonics9050300
APA StyleFauzi, N. I. M., Fen, Y. W., Abdullah, J., Kamarudin, M. A., Omar, N. A. S., Eddin, F. B. K., Ramdzan, N. S. M., & Daniyal, W. M. E. M. M. (2022). Evaluation of Structural and Optical Properties of Graphene Oxide-Polyvinyl Alcohol Thin Film and Its Potential for Pesticide Detection Using an Optical Method. Photonics, 9(5), 300. https://doi.org/10.3390/photonics9050300







