The Effect of Deposition Parameters on Morphological and Optical Properties of Cu2S Thin Films Grown by Chemical Bath Deposition Technique
Abstract
:1. Introduction
2. Experiment
2.1. Cleaning Process
2.2. Deposition of Cu2S Thin Films
2.3. Characterization of Cu2S Thin Films
3. Results and Discussion
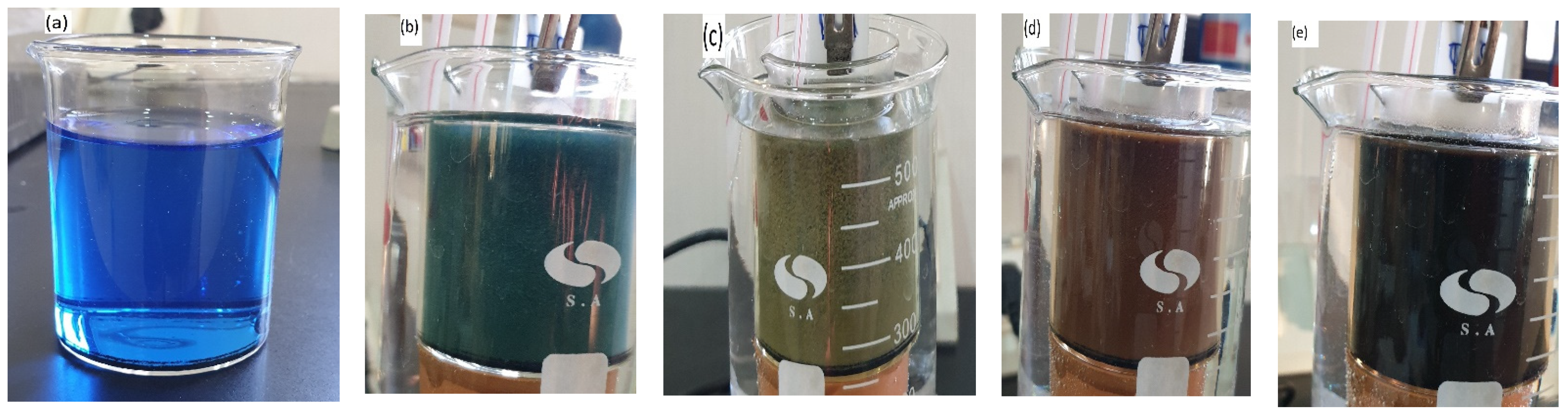
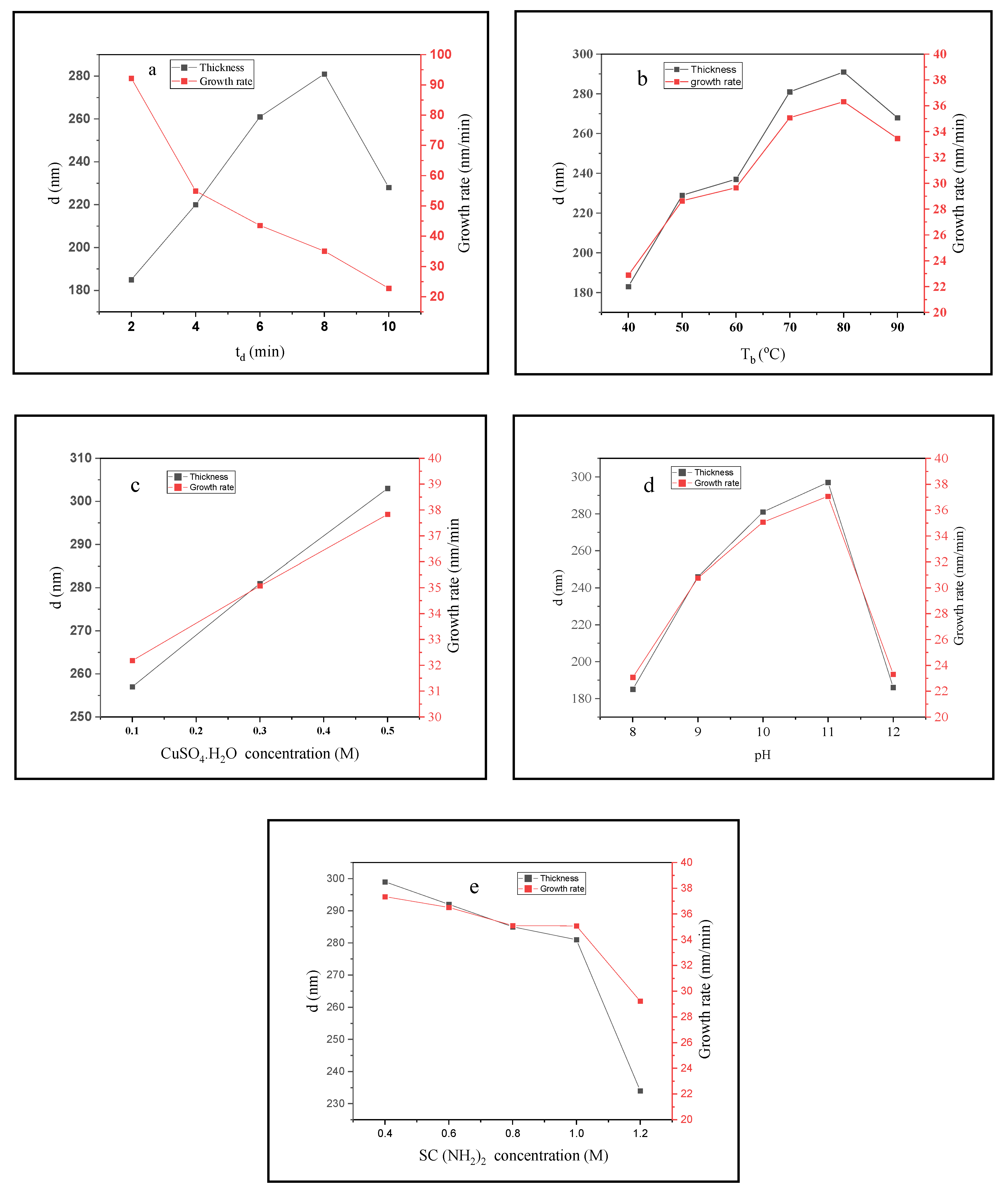
3.1. Deposition Parameters
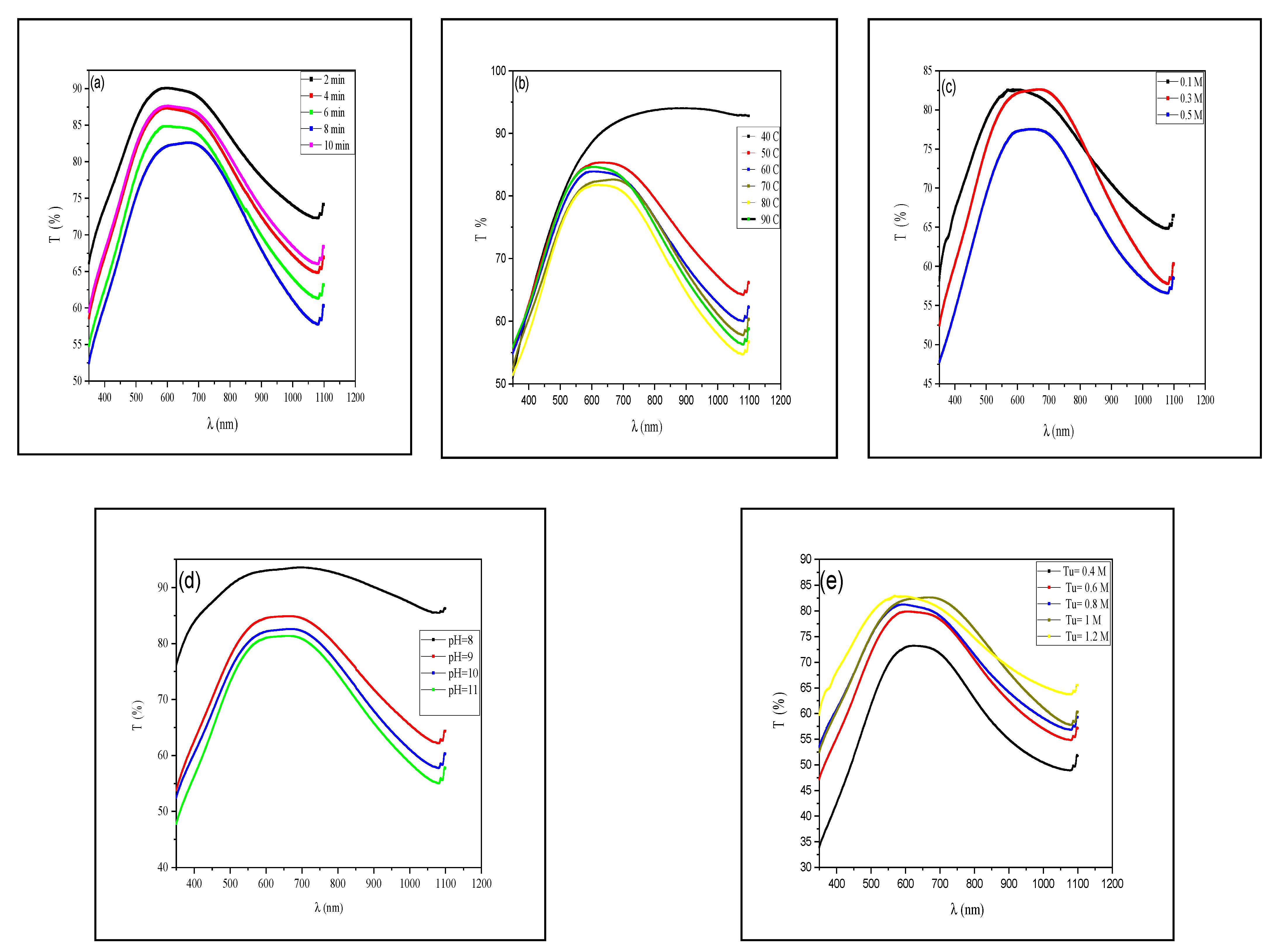
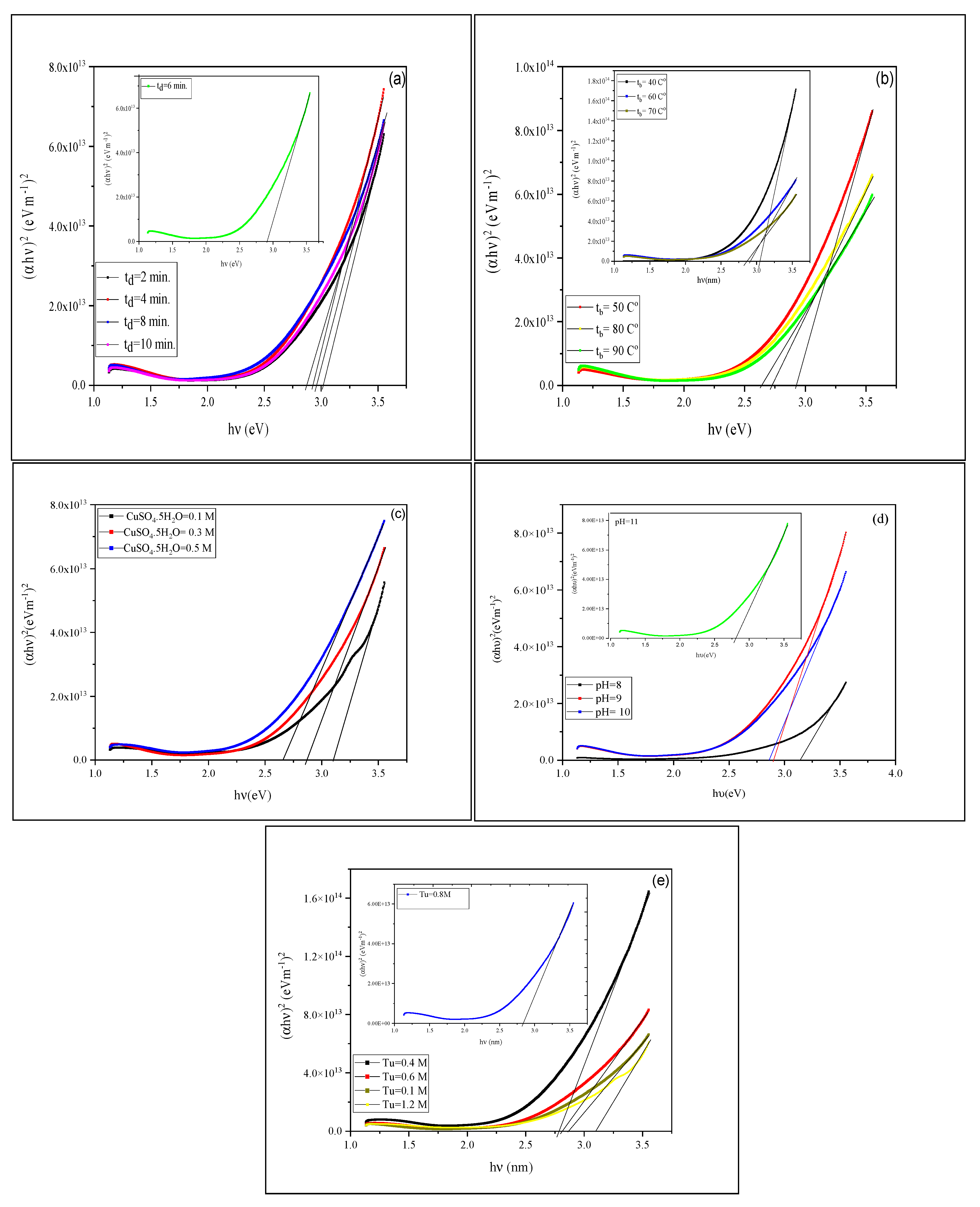
3.2. Physical Properties
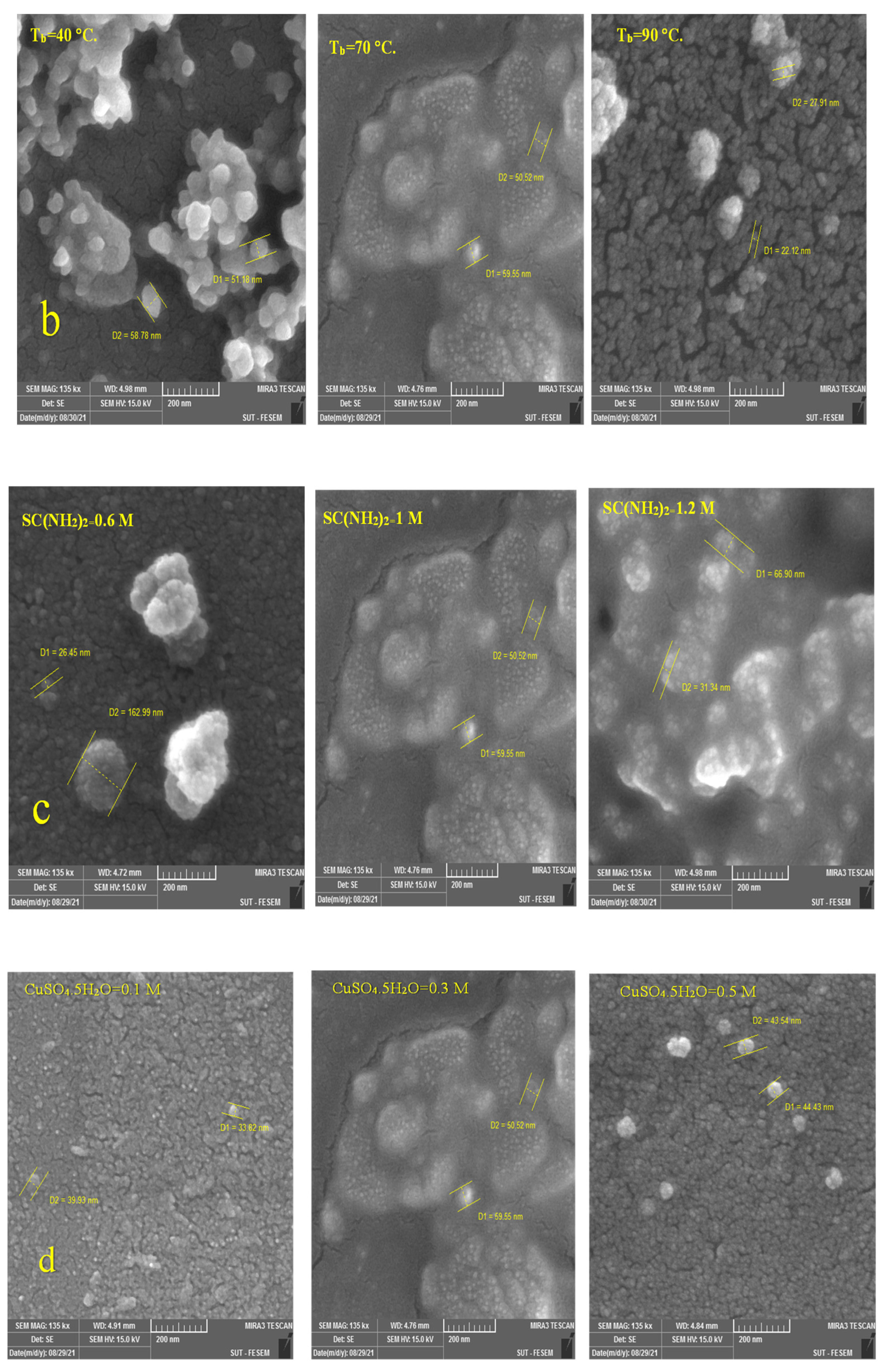
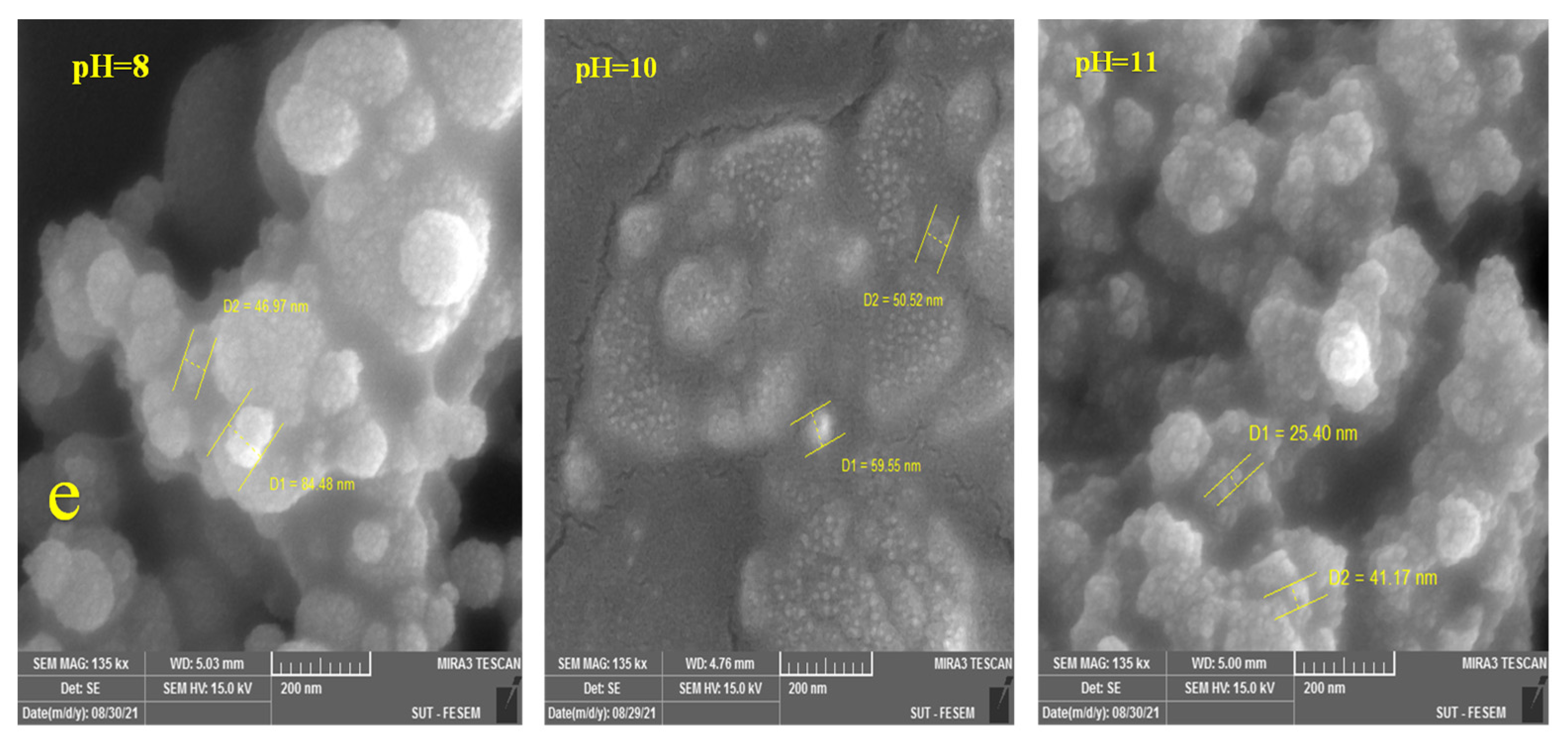
3.2.1. Morphological Properties
3.2.2. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz, J.S.; Hernández, S.M.; Coronel, J. Characterization of CuxS thin films obtained by CBD technique at differents annealing temperatures. Chalcogenide Lett. 2012, 9, 85–91. [Google Scholar]
- Munce, C.G. Chemical Bath Deposition of Copper Sulfide Thin Films; Griffith University: Queensland, Australia, 2008. [Google Scholar]
- Sagade, A.; Sharma, R. Copper sulphide (CuxS) as an ammonia gas sensor working at room temperature. Sensors Actuators B Chem. 2008, 133, 135–143. [Google Scholar] [CrossRef]
- Adelifard, M.; Eshghi, H.; Mohagheghi, M.M.B. Comparative studies of spray pyrolysis deposited copper sulfide nanostructural thin films on glass and FTO coated glass. Bull. Mater. Sci. 2012, 35, 739–744. [Google Scholar] [CrossRef]
- More, P.; Dhanayat, S.; Gattu, K.; Mahajan, S.; Upadhye, D.; Sharma, R. Annealing effect on Cu2S thin films prepared by chemical bath deposition. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2016. [Google Scholar]
- Dhondge, A.D.; Gosavi, S.R.; Gosavi, N.M.; Sawant, C.P.; Patil, A.M.; Shelke, A.R.; Deshpande, N.G. Influence of Thickness on the Photosensing Properties of Chemically Synthesized Copper Sulfide Thin Films. World J. Condens. Matter Phys. 2015, 05, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sabah, F.A.; Ahmed, N.M.; Hassan, Z.; Rasheed, H.S. Effect of Annealing on the Electrical Properties of CuxS Thin Films. Procedia Chem. 2016, 19, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Bharathi, B.; Thanikaikarasan, S.; Kollu, P.; Chandrasekar, P.V.; Sankaranarayanan, K.; Shajan, S. Effect of substrate on electroplated copper sulphide thin films. J. Mater. Sci. Mater. Electron. 2014, 25, 5338–5344. [Google Scholar] [CrossRef]
- Naji, I.; Sleman, U. Influence of precursor molar ratios on the physical properties of nanocrystalline Cu2−xS thin films prepared by chemical bath deposition method. Chalcogenide Lett. 2018, 15, 317–328. [Google Scholar]
- Wang, L.-W. High ChalcociteCu2S: A Solid-Liquid Hybrid Phase. Phys. Rev. Lett. 2012, 108, 085703. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Wu, Q.; Li, Y.; Lu, N.; Mao, K.; Bai, Y.; Zhao, J.; Wang, J.; Zeng, X.C. Copper (i) sulfide: A two-dimensional semiconductor with superior oxidation resistance and high carrier mobility. Nanoscale Horiz. 2019, 4, 223–230. [Google Scholar] [CrossRef]
- Hedlund, J.K.; Estrada, T.G.; Walker, A.V. Chemical Bath Deposition of Copper Sulfide on Functionalized SAMs: An Unusual Selectivity Mechanism. Langmuir 2020, 36, 3119–3126. [Google Scholar] [CrossRef]
- Patil, M.; Sharma, D.; Dive, A.; Mahajan, S.; Sharma, R. Synthesis and Characterization of Cu2S Thin Film Deposited by Chemical Bath Deposition Method. Procedia Manuf. 2018, 20, 505–508. [Google Scholar] [CrossRef]
- Yamamoto, T.; Kubota, E.; Taniguchi, A.; Dev, S.; Tanaka, K.; Osakada, K.; Sumita, M. Electrically conductive metal sulfide-polymer composites prepared by using organosols of metal sulfides. Chem. Mater. 1992, 4, 570–576. [Google Scholar] [CrossRef]
- Kassim, A.N.; Min, H.S.; Siang, L.K.; Nagalingam, S.A. SEM, EDAX and UV-Visible studies on the properties of Cu2S thin films. Chalcogenide Lett. 2011, 8, 405–410. [Google Scholar]
- Li, H.; Wang, Y.; Jiang, J.; Zhang, Y.; Peng, Y.; Zhao, J. CuS Microspheres as High-Performance Anode Material for Na-ion Batteries. Electrochimica Acta 2017, 247, 851–859. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Z.; Zhang, S.; Luo, W.; Zhang, G. Controllable synthesis of CuS hollow microflowers hierarchical structures for asymmetric supercapacitors. Appl. Surf. Sci. 2018, 442, 711–719. [Google Scholar] [CrossRef]
- Guo, K.; Chen, X.; Han, J.; Liu, Z. Synthesis of ZnO/Cu2S core/shell nanorods and their enhanced photoelectric performance. J. Sol-Gel Sci. Technol. 2014, 72, 92–99. [Google Scholar] [CrossRef]
- Ismail, R.A.; Al-Samarai, A.-M.E.; Muhammed, A.M. High-performance nanostructured p-Cu2S/n-Si photodetector prepared by chemical bath deposition technique. J. Mater. Sci. Mater. Electron. 2019, 30, 11807–11818. [Google Scholar] [CrossRef]
- Zhan, Y.; Shao, Z.; Jiang, T.; Ye, J.; Wu, X.; Zhang, B.; Ding, K.; Wu, D.; Jie, J. Cation exchange synthesis of two-dimensional vertical Cu2S/CdS heterojunctions for photovoltaic device applications. J. Mater. Chem. A 2019, 8, 789–796. [Google Scholar] [CrossRef]
- Ramya, M.; Ganesan, S. Study of thickness dependent characterictics of Cu2S thin film for various applications. Iran. J. Mater. Sci. Eng. 2011, 8, 34–40. [Google Scholar]
- Podder, J.; Kobayashi, R.; Ichimura, M. Photochemical deposition of CuxS thin films from aqueous solutions. Thin Solid Films 2004, 472, 71–75. [Google Scholar] [CrossRef]
- Aniefuna, N.K.; Ezenwaka, L.; Umeokwonna, N. Effect of deposition time on the optical properties of Copper Sulphide thin films fabricated by chemical bath deposition method. In Proceedings of the 1st African International Conference/Workshop on Applications of Nanotechnology to Energy, Health and Environment, Enugu, Nigeria, 23–29 March 2014; pp. 275–279. [Google Scholar]
- Ismail, R.A.; Al-Samarai, A.E.; Ali, A.M.M. Effect of molar concentration of CuCl2 on the characteristics of Cu2S film. Opt. Quantum Electron. 2020, 52, 1–14. [Google Scholar] [CrossRef]
- He, Y.; Polity, A.; Österreicher, I.; Pfisterer, D.; Gregor, R.; Meyer, B.; Hardt, M. Hall effect and surface characterization of Cu2S and CuS films deposited by RF reactive sputtering. Phys. B Condens. Matter 2001, 308, 1069–1073. [Google Scholar] [CrossRef]
- Kosoljitkul, P.; Rakkroekkong, P.; Vas-Umnuay, P. Parameter-controllable microchannel reactor for enhanced deposition of copper sulfide thin films. Thin Solid Films 2018, 646, 150–157. [Google Scholar] [CrossRef]
- Su, Y.-W.; Paul, B.K.; Chang, C.-H. Investigation of CdS nanoparticles formation and deposition by the continuous flow microreactor. Appl. Surf. Sci. 2019, 472, 158–164. [Google Scholar] [CrossRef]
- Isac, L.A.; Duta, A.; Kriza, A.; Nanu, M.; Schoonman, J. Crystal order in Cu2S thin films obtained by spray pyrolysis. J. Optoelectron. Adv. Mater. 2007, 9, 1265–1268. [Google Scholar]
- Nho, P.V.; Ngan, P.H.; Tien, N.Q.; Viet, H.D. Preparation and characterization of low resistivity CuS films using spray pyrolysis. Chalcogenide Lett. 2012, 9, 397–402. [Google Scholar]
- Zhuge, F.; Li, X.; Gao, X.; Gan, X.; Zhou, F. Synthesis of stable amorphous Cu2S thin film by successive ion layer adsorption and reaction method. Mater. Lett. 2009, 63, 652–654. [Google Scholar] [CrossRef]
- Pathan, H.; Desai, J.; Lokhande, C. Modified chemical deposition and physico-chemical properties of copper sulphide (Cu2S) thin films. Appl. Surf. Sci. 2002, 202, 47–56. [Google Scholar] [CrossRef]
- Omwoyo, J.G. Characterization of Cu2S/SnO2: F pn Junction for Solar CEll Applications; Kenyatta University: Nairobi, Kenya, 2019. [Google Scholar]
- Gashaw Hone, F.; Abza, T. Short Review of Factors Affecting Chemical Bath Deposition Method for Metal Chalcogenide Thin Films. Int. J. Thin Film. Sci. Technol. 2019, 8, 3. [Google Scholar]
- Allouche, N.K.; Ben Nasr, T.; Guasch, C.; Turki, N.K. Optimization of the synthesis and characterizations of chemical bath deposited Cu2S thin films. Comptes Rendus. Chim. 2010, 13, 1364–1369. [Google Scholar] [CrossRef]
- Hone, F.G.; Dejene, F.B. Cationic concentration and pH effect on the structural, morphological and optical band gap of chemically synthesized lead sulfide thin films. J. Mater. Res. Technol. 2019, 8, 467–474. [Google Scholar] [CrossRef]
- Kırmızıgül, F.; Güneri, E.; Gümüş, C. Effects of different deposition conditions on the properties of Cu2S thin films. Philos. Mag. 2013, 93, 511–523. [Google Scholar] [CrossRef]
- Patil, M.; Sharma, D.; Dive, A.; Mahajan, S.; Sharma, R. Study of various synthesis techniques of nanomaterials. In AIP Conference Proceedings; AIP Publishing LLC: Melville, NY, USA, 2018. [Google Scholar]
- Chopra, K.L. Thin Film Phenomena; McGraw-Hill: New York, NY, USA, 1969. [Google Scholar]
- Khalil, M.H.; Mohammed, R.Y.; Ibrahem, M.A. The Influence of CBD Parameters on the Energy Gap of ZnS Narcissus-Like Nanostructured Thin Films. Coatings 2021, 11, 1131. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. Investigation of optical properties of the PbS/CdS thin films by thermal Evaporation. J. Electron Devices 2012, 12, 761–766. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How to Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra; ACS Publications: Washington, DC, USA, 2018. [Google Scholar]
- Mohammed, K.A.; Ahmed, S.M.; Mohammed, R.Y.; Mohammed, K. Investigation of Structure, Optical, and Electrical Properties of CuS Thin Films by CBD Technique. Crystals 2020, 10, 684. [Google Scholar] [CrossRef]
- Shinde, M.S.; Ahirrao, P.B.; Patil, I.J.; Patil, R.S. Thickness Dependent Electrical and Optical Properties of Nanocrystalline Copper Sulphide Thin Films Grown by Simple Chemical Route; NISCAIR-CSIR: New Delhi, India, 2012. [Google Scholar]
- Ouachtari, F.; Rmili, A.; Elidrissi, B.; Bouaoud, A.; Erguig, H.; Elies, P. Influence of Bath Temperature, Deposition Time and S/Cd Ratio on the Structure, Surface Morphology, Chemical Composition and Optical Properties of CdS Thin Films Elaborated by Chemical Bath Deposition. J. Mod. Phys. 2011, 02, 1073–1082. [Google Scholar] [CrossRef] [Green Version]
- Sangamesha, M.; Pushapalatha, K.; Shekar, G. Effect of concentration on structural and optical properties of CuS thin films. Int. J. Pure Appl. Res. Eng. Technol. 2013, 2, 227. [Google Scholar]
- Vas-Umnuay, P.; Chang, C.-H. Growth Kinetics of Copper Sulfide Thin Films by Chemical Bath Deposition. ECS J. Solid State Sci. Technol. 2013, 2, P120–P129. [Google Scholar] [CrossRef]
- Manjulavalli, T.; Kannan, A. Effects of deposition time on structural, optical and electrical properties of chemically deposited Cu2S thin films. J. Chem. Tech. Res. 2015, 8, 607–616. [Google Scholar]
- Xu, H.; Dong, J.; Chen, C. One-step chemical bath deposition and photocatalytic activity of Cu2O thin films with orientation and size controlled by a chelating agent. Mater. Chem. Phys. 2014, 143, 713–719. [Google Scholar] [CrossRef]
- Yu, M.; Draskovic, T.I.; Wu, Y. Understanding the crystallization mechanism of delafossite CuGaO2 for controlled hydrothermal synthesis of nanoparticles and nanoplates. Inorg. Chem. 2014, 53, 5845–5851. [Google Scholar] [CrossRef] [PubMed]
- Rondiya, S.; Rokade, A.; Gabhale, B.; Pandharkar, S.; Chaudhari, M.; Date, A.; Chaudhary, M.; Pathan, H.; Jadkar, S. Effect of Bath Temperature on Optical and Morphology Properties of CdS Thin Films Grown by Chemical Bath Deposition. Energy Procedia 2017, 110, 202–209. [Google Scholar] [CrossRef]
- Ahmed, H.S.; Mohammed, R.Y.; Khalil, M.H. Effects of Deposition Time And PH on The Characterization of Chemically Synthesized Composite Nano-Wires of Cu2S Thin Films. Sci. J. Univ. Zakho 2021, 9, 184–192. [Google Scholar] [CrossRef]
- Muhammed, A.M.; Ibrahim, A.E.; Ismail, R.A. Study an Effect of Thiourea Concentration on the Structural, Optical and Electrical Properties of (Cu2S) film Prepared by Chemical Bath Deposition (CBD). Kirkuk Univ. J.-Sci. Stud. 2019, 14, 97–115. [Google Scholar] [CrossRef]


| Bath No. | t (min) | CuSO4.5H2O (M) | SC (NH2)2 (M) | T (°C) | pH Value | TEA (mL) |
|---|---|---|---|---|---|---|
| 1 | 2–10 step 2 | 0.3 | 1 | 70 | 10 | 2 |
| 2 | 8 | 0.1, 0.5 | 1 | 70 | 10 | 2 |
| 3 | 8 | 0.3 | 0.4–1.2 step 0.2 | 70 | 10 | 2 |
| 4 | 8 | 0.3 | 1 | 70 | 8, 9, and 11 | 2 |
| 5 | 8 | 0.3 | 1 | 40, 50, 60, 80, and 90 | 10 | 2 |
| Bath No. | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Deposition time (min) | 2 | 4 | 6 | 8 | 10 | - |
| Eg (eV) | 3.01 | 2.92 | 2.9 | 2.86 | 2.95 | - |
| Bath temprature (°C) | 40 | 50 | 60 | 70 | 80 | 90 |
| Eg (eV) | 3.04 | 2.92 | 2.9 | 2.86 | 2.72 | 2.63 |
| CuSO4.5H2O concentration (M) | 0.1 | 0.3 | 0.5 | - | - | - |
| Eg (eV) | 3.1 | 2.86 | 2.65 | |||
| pH Value | 8 | 9 | 10 | 11 | - | - |
| Eg (eV) | 3.14 | 2.9 | 2.86 | 2.75 | ||
| SC (NH2)2 concentration (M) | 0.4 | 0.6 | 0.8 | 1 | 1.2 | - |
| Eg (eV) | 2.79 | 2.81 | 2.83 | 2.86 | 3.09 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, H.S.; Mohammed, R.Y. The Effect of Deposition Parameters on Morphological and Optical Properties of Cu2S Thin Films Grown by Chemical Bath Deposition Technique. Photonics 2022, 9, 161. https://doi.org/10.3390/photonics9030161
Ahmed HS, Mohammed RY. The Effect of Deposition Parameters on Morphological and Optical Properties of Cu2S Thin Films Grown by Chemical Bath Deposition Technique. Photonics. 2022; 9(3):161. https://doi.org/10.3390/photonics9030161
Chicago/Turabian StyleAhmed, Honar S., and Raghad Y. Mohammed. 2022. "The Effect of Deposition Parameters on Morphological and Optical Properties of Cu2S Thin Films Grown by Chemical Bath Deposition Technique" Photonics 9, no. 3: 161. https://doi.org/10.3390/photonics9030161
APA StyleAhmed, H. S., & Mohammed, R. Y. (2022). The Effect of Deposition Parameters on Morphological and Optical Properties of Cu2S Thin Films Grown by Chemical Bath Deposition Technique. Photonics, 9(3), 161. https://doi.org/10.3390/photonics9030161






