Characterization of Collagen I Fiber Thickness, Density, and Orientation in the Human Skin In Vivo Using Second-Harmonic Generation Imaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Two-Photon Tomography
2.2. SHG-to-AF Aging Index of Dermis (SAAID)
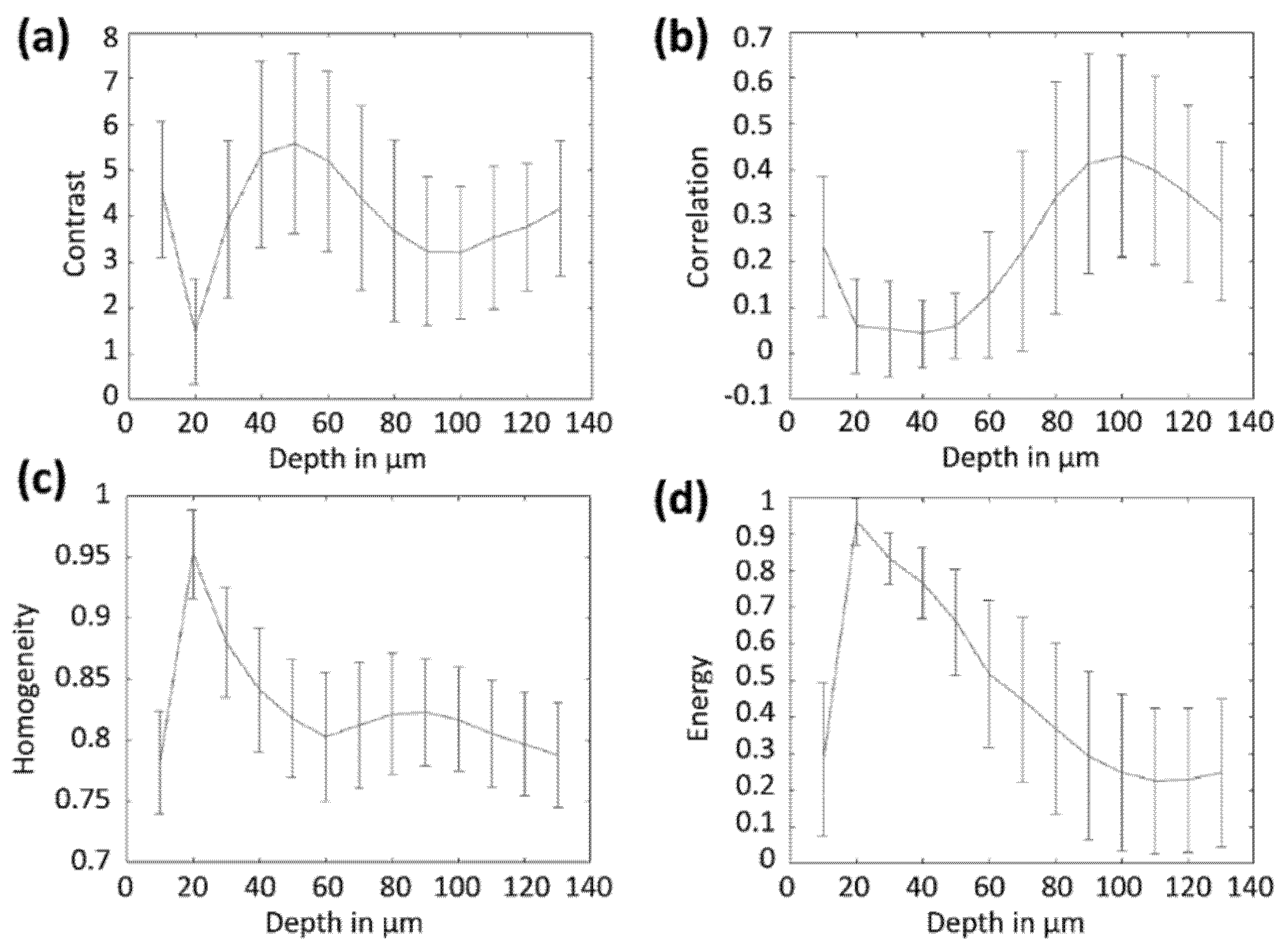
2.3. Grey Level Co-Occurrence Matrix (GLCM)
2.4. Hilbert Transformation
2.5. Directionality of Altered Extracellular Matrix
2.6. Statistical Analysis
2.7. Ethics
2.8. Volunteers
3. Results
3.1. Depth-Analysis of Human Skin with AF and SHG Detection Methods
3.2. Construction of Training Datasets of Thin, Convoluted Collagen Structures and Thick, Oriented Collagen Structures
3.3. Collagen I Containing ECM Structures, and Analysis of SAAID, FFT Directionality, and Edge Detection in Native Grey Value Images
3.4. Grey Level Co-Occurrence Matrix Analysis in Native Grey Values and in Hilbert Transform
3.5. Distinction of Collagen I Structures in Skin with Similar SAAID Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pfisterer, K.; Shaw, L.E.; Symmank, D.; Weninger, W. The Extracellular Matrix in Skin Inflammation and Infection. Front. Cell Dev. Biol. 2021, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Jackson, C.J. Extracellular Matrix Reorganization During Wound Healing and Its Impact on Abnormal Scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Shirshin, E.A.; Gurfinkel, Y.I.; Priezzhev, A.V.; Fadeev, V.V.; Lademann, J.; Darvin, M.E. Two-photon autofluorescence lifetime imaging of human skin papillary dermis in vivo: Assessment of blood capillaries and structural proteins localization. Sci. Rep. 2017, 7, 1171. [Google Scholar] [CrossRef]
- Carlson, M.A.; Longaker, M.T. The fibroblast-populated collagen matrix as a model of wound healing: A review of the evidence. Wound Repair Regen. 2004, 12, 134–147. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.; Dallon, J.; Sherratt, J.; Maini, P. Fibroblast migration and collagen deposition during dermal wound healing: Mathematical modelling and clinical implications. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 1385–1405. [Google Scholar] [CrossRef] [PubMed]
- Marcos-Garcés, V.; Molina Aguilar, P.; Bea Serrano, C.; García Bustos, V.; Benavent Seguí, J.; Ferrández Izquierdo, A.; Ruiz-Saurí, A. Age-related dermal collagen changes during development, maturation and ageing—A morphometric and comparative study. J. Anat. 2014, 225, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Fisher, G.J.; Kang, S.; Varani, J.; Bata-Csorgo, Z.; Wan, Y.; Datta, S.; Voorhees, J.J. Mechanisms of Photoaging and Chronological Skin Aging. Arch. Dermatol. 2002, 138, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Tanaka, H.; Okada, T.; Konishi, H.; Takahashi, M.; Ito, M.; Asai, J. The effects of ultraviolet A and reactive oxygen species on the mRNA expression of 72-kDa type IV collagenase and its tissue inhibitor in cultured human dermal fibroblasts. Arch. Dermatol. Res. 1996, 288, 39–44. [Google Scholar] [CrossRef]
- Darvin, M.E.; Richter, H.; Ahlberg, S.; Haag, S.F.; Meinke, M.C.; Le Quintrec, D.; Doucet, O.; Lademann, J. Influence of sun exposure on the cutaneous collagen/elastin fibers and carotenoids: Negative effects can be reduced by application of sunscreen. J. Biophotonics 2014, 7, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Meigel, W.N.; Gay, S.; Weber, L. Dermal architecture and collagen type distribution. Arch. Dermatol. Res. 1977, 259, 1–10. [Google Scholar] [CrossRef]
- Koehler, M.J.; König, K.; Elsner, P.; Bückle, R.; Kaatz, M. In vivo assessment of human skin aging by multiphoton laser scanning tomography. Opt. Lett. 2006, 31, 2879. [Google Scholar] [CrossRef] [PubMed]
- Ueck, C. Charakterisierung Humaner Diabetischer Haut und Diabetischer Modellsysteme Bezüglich Barrierefunktion, Dermisstruktur und der Wirkung von Triterpenen auf die Wundheilung. Ph.D. Thesis, University Hamburg, Hamburg, Germany, 13 May 2016. [Google Scholar]
- König, K. Clinical multiphoton tomography. J. Biophotonics 2008, 1, 13–23. [Google Scholar] [CrossRef]
- Darvin, M.E.; Richter, H.; Zhu, Y.J.; Meinke, M.C.; Knorr, F.; Gonchukov, S.A.; Koenig, K.; Lademann, J. Comparison of in vivo and ex vivo laser scanning microscopy and multiphoton tomography application for human and porcine skin imaging. Quantum Electron. 2014, 44, 646–651. [Google Scholar] [CrossRef]
- Tilbury, K.; Campagnola, P.J. Applications of second-harmonic generation imaging microscopy in ovarian and breast cancer. Perspect. Med. Chem. 2015, 7, 21–32. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Sasaki, K.; Tohno, Y.; Araki, T. Tomographic imaging of collagen fiber orientation in human tissue using depth-resolved polarimetry of second-harmonic-generation light. Opt. Quantum Electron. 2005, 37, 1397–1408. [Google Scholar] [CrossRef]
- Vanakker, O.M.; Hemelsoet, D.; De Paepe, A. Hereditary connective tissue diseases in young adult stroke: A comprehensive synthesis. Stroke Res. Treat. 2011, 2011, 712903. [Google Scholar] [CrossRef]
- Malfait, F.; Castori, M.; Francomano, C.A.; Giunta, C.; Kosho, T.; Byers, P.H. The Ehlers–Danlos syndromes. Nat. Rev. Dis. Prim. 2020, 6, 64. [Google Scholar] [CrossRef]
- Shiferaw, B.; Miro, V.; Smith, C.; Akella, J.; Chua, W.; Kim, Z. Goodpasture’s Disease: An Uncommon Disease With an Atypical Clinical Course. J. Clin. Med. Res. 2016, 8, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Pittet, J.-C.; Freis, O.; Vazquez-Duchêne, M.-D.; Périé, G.; Pauly, G. Evaluation of Elastin/Collagen Content in Human Dermis in-Vivo by Multiphoton Tomography—Variation with Depth and Correlation with Aging. Cosmetics 2014, 1, 211–221. [Google Scholar] [CrossRef]
- Schenke-Layland, K. Non-invasive multiphoton imaging of extracellular matrix structures. J. Biophotonics 2008, 1, 451–462. [Google Scholar] [CrossRef]
- Diaspro, A.; Chirico, G. Two-photon excitation microscopy. Adv. Imaging Electron Phys. 2003, 126, 399–429. [Google Scholar] [CrossRef]
- Lin, S.-J.; Wu, R.-J.; Tan, H.-Y.; Lo, W.; Lin, W.-C.; Young, T.-H.; Hsu, C.-J.; Chen, J.-S.; Jee, S.-H.; Dong, C.-Y. Evaluating cutaneous photoaging by use of multiphoton fluorescence and second-harmonic generation microscopy. Opt. Lett. 2005, 30, 2275. [Google Scholar] [CrossRef]
- Meinke, M.C.; Nowbary, C.K.; Schanzer, S.; Vollert, H.; Lademann, J.; Darvin, M.E. Influences of orally taken carotenoid-rich curly kale extract on collagen I/elastin index of the skin. Nutrients 2017, 9, 775. [Google Scholar] [CrossRef]
- Lehmann, P.; Zheng, P.; Lavker, R.M.; Kligman, A.M. Corticosteroid atrophy in human skin. A study by light, scanning, and transmission electron microscopy. J. Investig. Dermatol. 1983, 81, 169–176. [Google Scholar] [CrossRef]
- Jung, S.; Lademann, J.; Darvin, M.E.; Richter, C.; Pedersen, C.B.; Richter, H.; Schanzer, S.; Kottner, J.; Blume-Peytavi, U.; Røpke, M.A. In vivo characterization of structural changes after topical application of glucocorticoids in healthy human skin. J. Biomed. Opt. 2017, 22, 076018. [Google Scholar] [CrossRef] [PubMed]
- Noorlander, M.L.; Melis, P.; Jonker, A.; Van Noorden, C.J.F. A quantitative method to determine the orientation of collagen fibers in the dermis. J. Histochem. Cytochem. 2002, 50, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Li, H.; Yang, H.; Zhang, X.; Li, Z.; Xu, S. Quantitative analysis on collagen morphology in aging skin based on multiphoton microscopy. J. Biomed. Opt. 2011, 16, 040502. [Google Scholar] [CrossRef]
- Mostaço-Guidolin, L.; Rosin, N.L.; Hackett, T.L. Imaging collagen in scar tissue: Developments in second harmonic generation microscopy for biomedical applications. Int. J. Mol. Sci. 2017, 18, 1772. [Google Scholar] [CrossRef]
- Ducourthial, G.; Affagard, J.S.; Schmeltz, M.; Solinas, X.; Lopez-Poncelas, M.; Bonod-Bidaud, C.; Rubio-Amador, R.; Ruggiero, F.; Allain, J.M.; Beaurepaire, E.; et al. Monitoring dynamic collagen reorganization during skin stretching with fast polarization-resolved second harmonic generation imaging. J. Biophotonics 2019, 12, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pijanka, J.K.; Markov, P.P.; Midgett, D.; Paterson, N.G.; White, N.; Blain, E.J.; Nguyen, T.D.; Quigley, H.A.; Boote, C. Quantification of collagen fiber structure using second harmonic generation imaging and two-dimensional discrete Fourier transform analysis: Application to the human optic nerve head. J. Biophotonics 2019, 12, 1–19. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man. Cybern 1973, SMC-3, 610–621. [Google Scholar] [CrossRef]
- De Vries, H.J.C.; Enomoto, D.N.H.; Van Marle, J.; Van Zuijlen, P.P.M.; Mekkes, J.R.; Bos, J.D. Dermal organization in scleroderma: The fast fourier transform and the laser scatter method objectify fibrosis in nonlesional as well as lesional skin. Lab. Investig. 2000, 80, 1281–1289. [Google Scholar] [CrossRef][Green Version]
- Osman, O.S.; Selway, J.L.; Harikumar, P.E.; Stocker, C.J.; Wargent, E.T.; Cawthorne, M.A.; Jassim, S.; Langlands, K. A novel method to assess collagen architecture in skin. BMC Bioinform. 2013, 14, 260. [Google Scholar] [CrossRef]
- Reznikov, N.; Almany-Magal, R.; Shahar, R.; Weiner, S. Three-dimensional imaging of collagen fibril organization in rat circumferential lamellar bone using a dual beam electron microscope reveals ordered and disordered sub-lamellar structures. Bone 2013, 52, 676–683. [Google Scholar] [CrossRef]
- Yang, K.; Yu, K.; Li, Q. Modal parameter extraction based on Hilbert transform and complex independent component analysis with reference. Mech. Syst. Signal Process. 2013, 40, 257–268. [Google Scholar] [CrossRef]
- Weinigel, M.; Breunig, H.G.; Kellner-Höfer, M.; Bückle, R.; Darvin, M.E.; Klemp, M.; Lademann, J.; König, K. In vivo histology: Optical biopsies with chemical contrast using clinical multiphoton/coherent anti-Stokes Raman scattering tomography. Laser Phys. Lett. 2014, 11, 055601. [Google Scholar] [CrossRef]
- Zhu, Y.; Choe, C.-S.; Ahlberg, S.; Meinke, M.C.; Alexiev, U.; Lademann, J.; Darvin, M.E. Penetration of silver nanoparticles into porcine skin ex vivo using fluorescence lifetime imaging microscopy, Raman microscopy, and surface-enhanced Raman scattering microscopy. J. Biomed. Opt. 2015, 20, 051006. [Google Scholar] [CrossRef] [PubMed]
- Kröger, M.; Scheffel, J.; Nikolaev, V.V.; Shirshin, E.A.; Siebenhaar, F.; Schleusener, J.; Lademann, J.; Maurer, M.; Darvin, M.E. In vivo non-invasive staining-free visualization of dermal mast cells in healthy, allergy and mastocytosis humans using two-photon fluorescence lifetime imaging. Sci. Rep. 2020, 10, 14930. [Google Scholar] [CrossRef]
- Czekalla, C.; Schönborn, K.H.; Döge, N.; Jung, S.; Darvin, M.E.; Lademann, J.; Meinke, M.C. Impact of Body Site, Age, and Gender on the Collagen/Elastin Index by Noninvasive in vivo Vertical Two-Photon Microscopy. Ski. Pharmacol. Physiol. 2017, 30, 260–267. [Google Scholar] [CrossRef]
- Quinn, K.P.; Golberg, A.; Broelsch, G.F.; Khan, S.; Villiger, M.; Bouma, B.; Austen, W.G.; Sheridan, R.L.; Mihm, M.C.; Yarmush, M.L.; et al. An automated image processing method to quantify collagen fibre organization within cutaneous scar tissue. Exp. Dermatol. 2015, 24, 78–80. [Google Scholar] [CrossRef]
- Chaudhuri, D. A simple least squares method for fitting of ellipses and circles depends on border points of a two-tone image and their 3-D extensions. Pattern Recognit. Lett. 2010, 31, 818–829. [Google Scholar] [CrossRef]
- Davis, J.A.; McNamara, D.E.; Cottrell, D.M.; Campos, J. Image processing with the radial Hilbert transform: Theory and experiments. Opt. Lett. 2000, 25, 99. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.I. Hubert transform associated with the fractional fourier transform. IEEE Signal Process. Lett. 1998, 5, 206–208. [Google Scholar] [CrossRef]
- ČÍŽEK, Vá. Discrete Hilbert Transform. IEEE Trans. Audio Electroacoust. 1970, 18, 340–343. [Google Scholar] [CrossRef]
- Molina, N.; Aguirre, J.; Walczak, M. Application of FFT analysis for the study of directionality of wear scars in exposure to slurry flow of varying velocity. Wear 2019, 426–427, 589–595. [Google Scholar] [CrossRef]
- Fitzpatrick, T.B. The Validity and Practicality of Sun-Reactive Skin Types I Through VI. Arch. Dermatol. 1988, 124, 869–871. [Google Scholar] [CrossRef]
- Cicchi, R.; Kapsokalyvas, D.; De Giorgi, V.; Maio, V.; Van Wiechen, A.; Massi, D.; Lotti, T.; Pavone, F.S. Scoring of collagen organization in healthy and diseased human dermis by multiphoton microscopy. J. Biophotonics 2010, 3, 34–43. [Google Scholar] [CrossRef]


| Papillary Dermis | Reticular Dermis | |||
|---|---|---|---|---|
| Group 1: Thin, Convoluted | Group 2: Thick, Oriented | Group 3: Thin, Convoluted | Group 4: Thick, Oriented | |
| SAAID | 0.7 ± 0.1 | 0.45 ± 0.08 | 0.42 ± 0.08 | 0.38 ± 0.06 |
| Grey value | 21 ± 7 | 13 ± 4 | 16 ± 5 | 10 ± 3 |
| Edge Canny | 7500 ± 900 | 6500 ± 2000 | 7900 ± 1100 | 8800 ± 800 |
| Edge Sobel | 6600 ± 500 | 6100 ± 1600 | 6400 ± 400 | 6500 ± 500 |
| FFT directionality | 0.95 ± 0.04 | 0.82 ± 0.08 | 0.96 ± 0.03 | 0.89 ± 0.03 |
| Third angular momentum | 0.016 ± 0.003 | 0.054 ± 0.006 | 0.014 ± 0.003 | 0.054 ± 0.006 |
| Papillary Dermis | Reticular Dermis | |||
|---|---|---|---|---|
|
Group 1: Thin, Convoluted |
Group 2: Thick, Oriented |
Group 3: Thin, Convoluted |
Group 4: Thick, Oriented | |
| Correlation | 0.26 ± 0.03 | 0.14 ± 0.01 | 0.75 ± 0.07 | 0.67 ± 0.09 |
| Contrast | 1.5 ± 0.4 | 1.24 ± 0.50 | 1.6 ± 0.5 | 2.0 ± 0.7 |
| Energy | 0.5 ± 0.2 | 0.8 ± 0.1 | 0.12 ± 0.05 | 0.11 ± 0.07 |
| Homogeneity | 0.71 ± 0.03 | 0.77 ± 0.02 | 0.70 ± 0.05 | 0.63 ± 0.02 |
| Papillary Dermis | Reticular Dermis | |||
|---|---|---|---|---|
| Group 1: Thin, Convoluted | Group 2: Thick, Oriented | Group 3: Thin, Convoluted | Group 4: Thick, Oriented | |
| Correlation | 0.5 ± 0.1 | 0.6 ± 0.2 | 0.5 ± 0.1 | 0.45 ± 0.10 |
| Contrast | 12 ± 3 | 10 ± 4 | 12 ± 3 | 13 ± 3 |
| Energy | 0.30 ± 0.03 | 0.33 ± 0.04 | 0.31 ± 0.03 | 0.29 ± 0.02 |
| Homogeneity | 0.77 ± 0.05 | 0.81 ± 0.06 | 0.79 ± 0.04 | 0.74 ± 0.04 |
| Third angular momentum | −5461 ± 5284 | 2995 ± 478 | −6287 ± 5272 | 2891 ± 330 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kröger, M.; Schleusener, J.; Jung, S.; Darvin, M.E. Characterization of Collagen I Fiber Thickness, Density, and Orientation in the Human Skin In Vivo Using Second-Harmonic Generation Imaging. Photonics 2021, 8, 404. https://doi.org/10.3390/photonics8090404
Kröger M, Schleusener J, Jung S, Darvin ME. Characterization of Collagen I Fiber Thickness, Density, and Orientation in the Human Skin In Vivo Using Second-Harmonic Generation Imaging. Photonics. 2021; 8(9):404. https://doi.org/10.3390/photonics8090404
Chicago/Turabian StyleKröger, Marius, Johannes Schleusener, Sora Jung, and Maxim E. Darvin. 2021. "Characterization of Collagen I Fiber Thickness, Density, and Orientation in the Human Skin In Vivo Using Second-Harmonic Generation Imaging" Photonics 8, no. 9: 404. https://doi.org/10.3390/photonics8090404
APA StyleKröger, M., Schleusener, J., Jung, S., & Darvin, M. E. (2021). Characterization of Collagen I Fiber Thickness, Density, and Orientation in the Human Skin In Vivo Using Second-Harmonic Generation Imaging. Photonics, 8(9), 404. https://doi.org/10.3390/photonics8090404






