Effects of Er:YAG and Nd:YAG Lasers with Photobiomodulation on Alveolar Bone Preservation Post-Extraction: A Randomized Clinical Control Trial
Abstract
1. Introduction
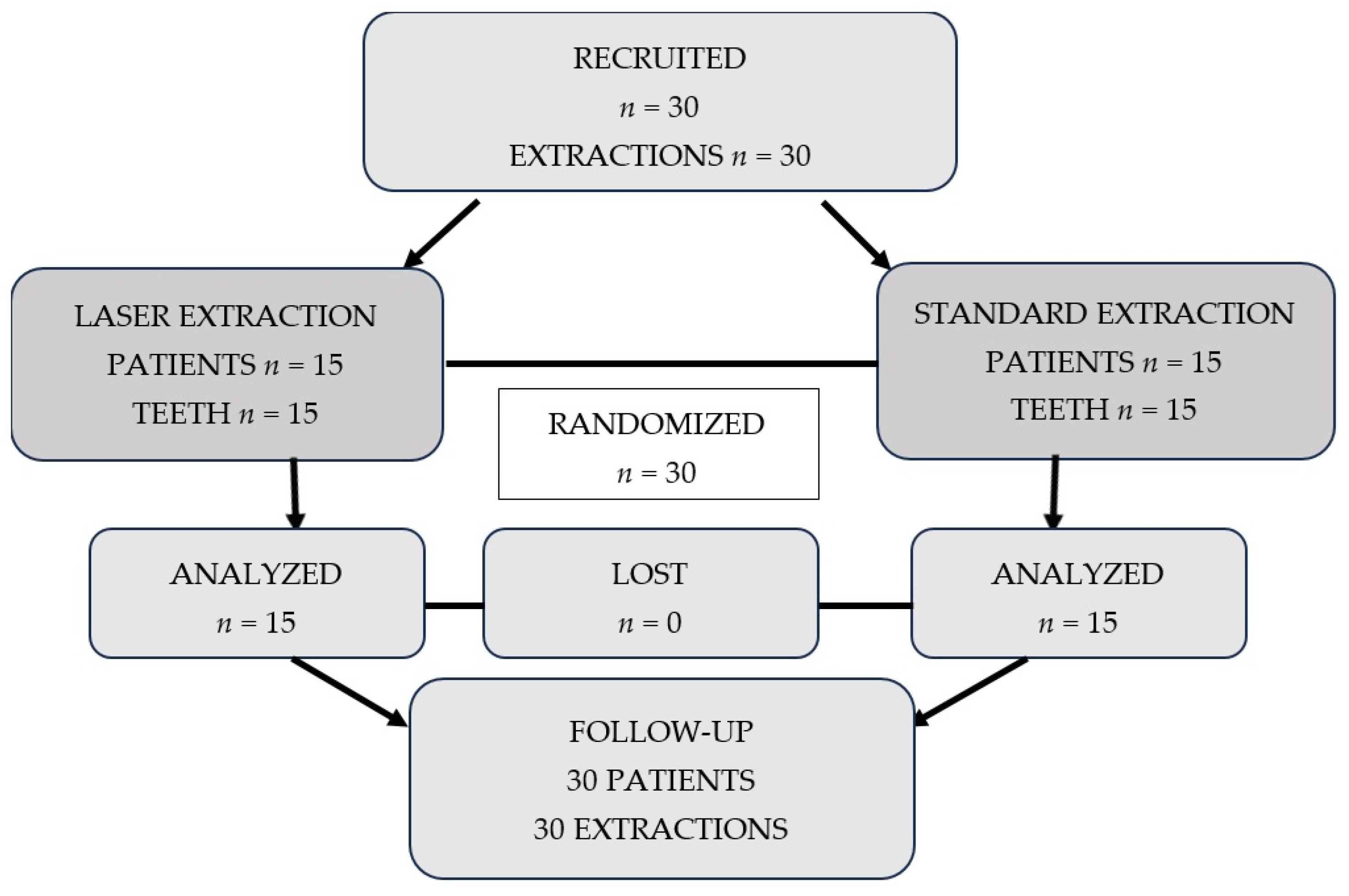
2. Materials and Methods
2.1. Ethical Approval
2.2. Patients
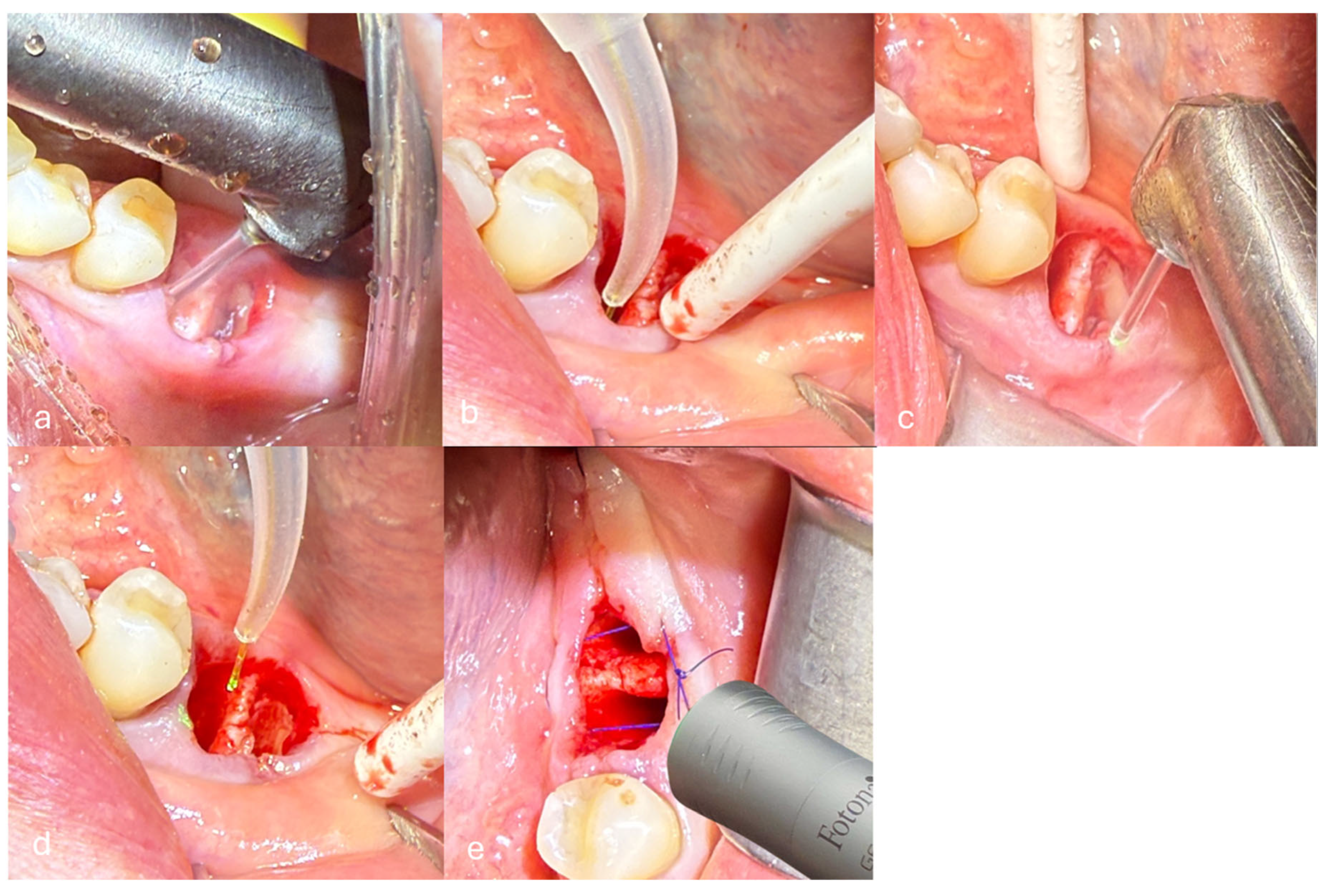
2.3. Laser Procedure
- Removal of inflamed granulation tissue and debridement of the alveolus were performed using the Er:YAG laser with the H14 handpiece and a cylindrical tip of 1.3 mm diameter in non-contact mode with a non-activated tip. The laser operated with a pulse duration of 300 µs (Short Pulse mode, SP), energy of 160 mJ, frequency of 15 Hz, and water/air settings of 4/2. The calculated fluence was approximately 12.05 J/cm2, and the power density was 184.61 W/cm2 (Figure 2a).
- Deep disinfection of the alveolus was performed using the Nd:YAG laser with a 300 μm fiber in non-contact and non-activated mode. The laser operated with a pulse duration of 300 µs (SP), power of 2 W, and frequency of 20 Hz. The calculated fluence was approximately 143 J/cm2, and the power density was 2829.42 W/cm2 (Figure 2b).
- De-epithelialization of the keratinized gingiva to the mucogingival junction was performed using the Er:YAG laser with the H14 handpiece and a cylindrical tip of 1.3 mm diameter in non-contact mode and a non-activated tip. The laser operated with a pulse duration of 300 µs (SP), energy of 120 mJ, frequency of 20 Hz, and water/air settings of 4/2. The calculated fluence was approximately 9.04 J/cm2, and the power density was 184.61 W/cm2 (Figure 2c).
- Stabilization of the blood clot was performed using the Nd:YAG laser with a 300 μm fiber in non-contact and non-activated mode. The laser operated with a pulse duration of 500 µs (Long Pulse mode, LP), power of 4 W, and frequency of 15 Hz. The calculated fluence was approximately 377 J/cm2, and the power density was 5658.84 W/cm2 (Figure 2d).
- Photobiomodulation (PBM) was performed using the Nd:YAG laser with the Genova flat-top handpiece (spot size 0.95 cm2) in Micro Short Pulse (MSP) mode. The laser operated with a power of 0.5 W and a frequency of 10 Hz. PBM was applied to the post-extraction socket in three points/locations: buccally (vestibular side), lingually, and occlusally (from the top of the socket). Each point was irradiated for 60 s per session. The delivered fluence per session was approximately 31.58 J/cm2, and the power density was 0.53 W/cm2. PBM was performed on days 0, 3, 5, and 7 post-extraction (Figure 2e).
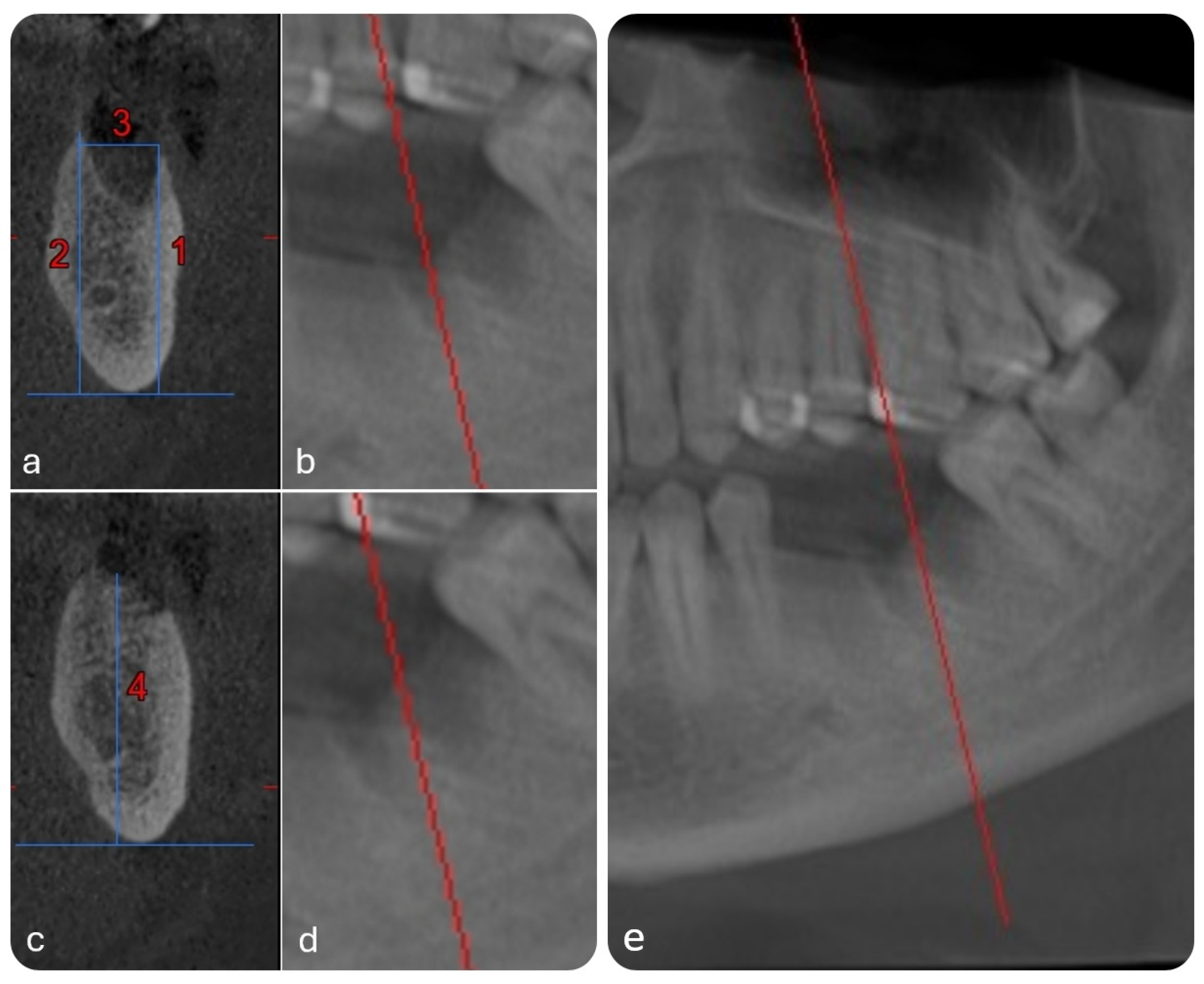
2.4. Radiographic Measurement and Pain Assessment
- -
- Buccal bone height (BBH)—distance from the mandibular base to the crest of the buccal alveolar wall (Line 1 in Figure 3a);
- -
- Lingual bone height (LBH)—distance from the mandibular base to the crest of the lingual alveolar wall (Line 2 in Figure 3a);
- -
- Alveolar width (AW)—distance between the buccal and lingual crests (Line 3 in Figure 3a,b);
- -
- Interradicular septum height (ISH)—vertical distance from the mandibular base to the peak of the septum between roots (Line 4 in Figure 3c).
2.5. Statistical Analysis
3. Results
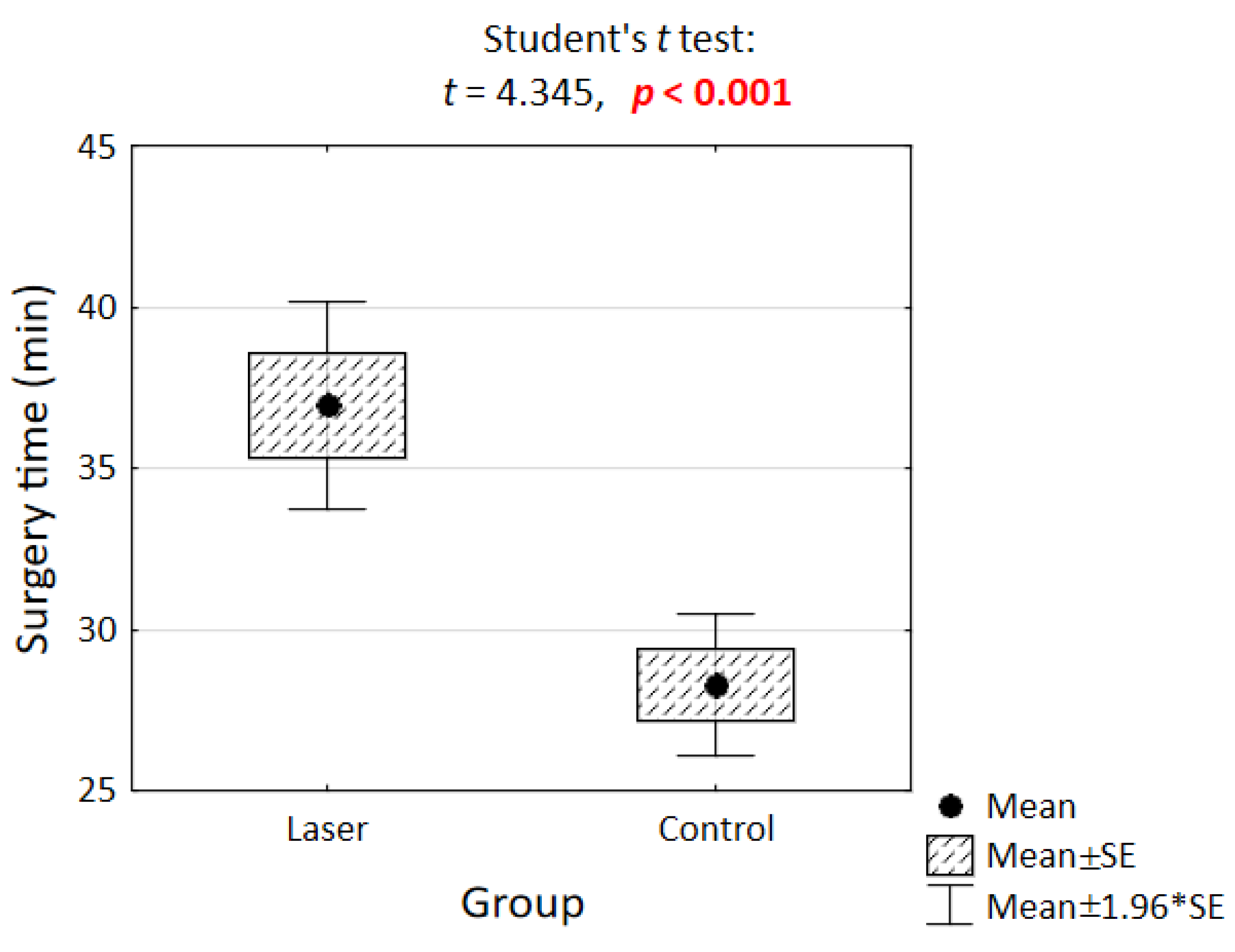
3.1. Surgery Time
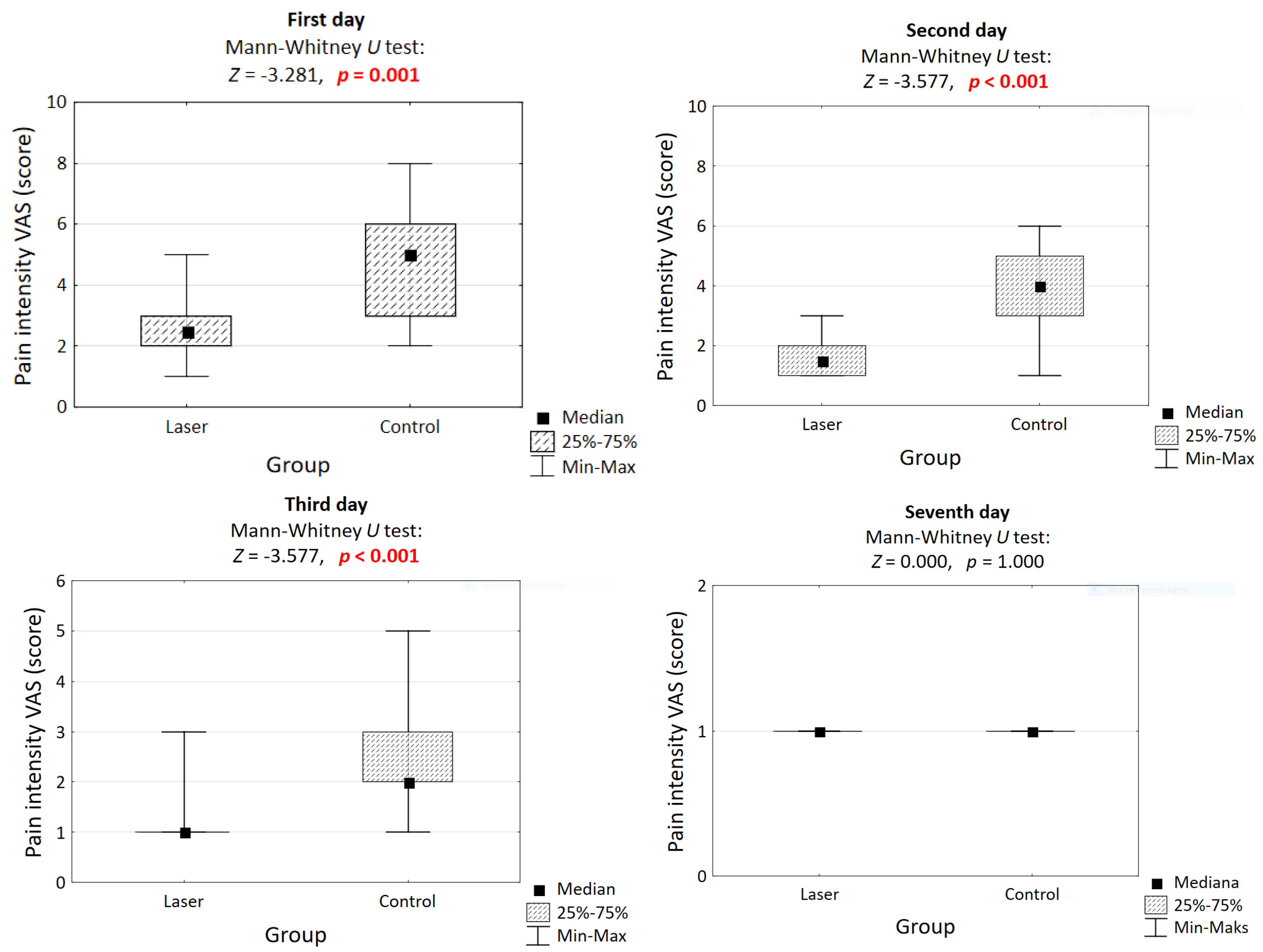
3.2. Pain After Procedure
3.3. The Relationship Between the Duration of the Procedure and the Intake of Analgesics
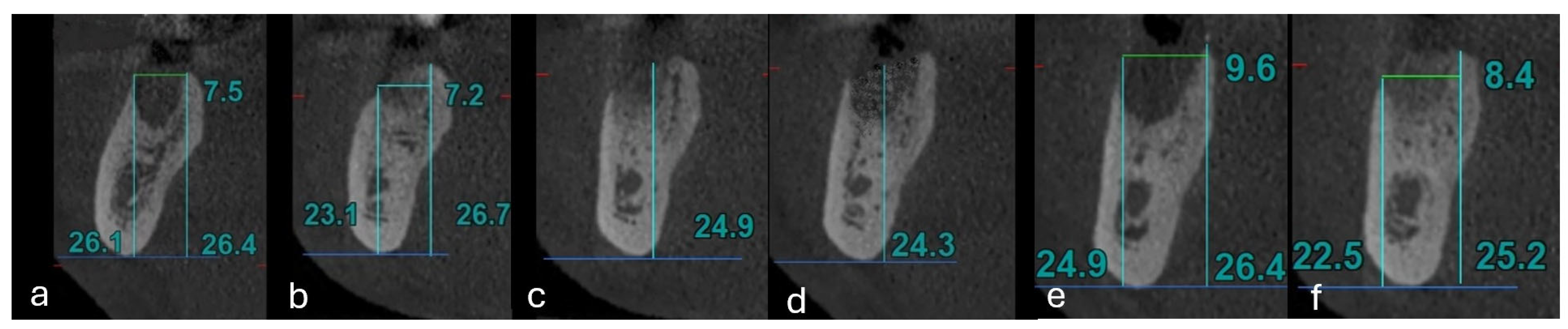
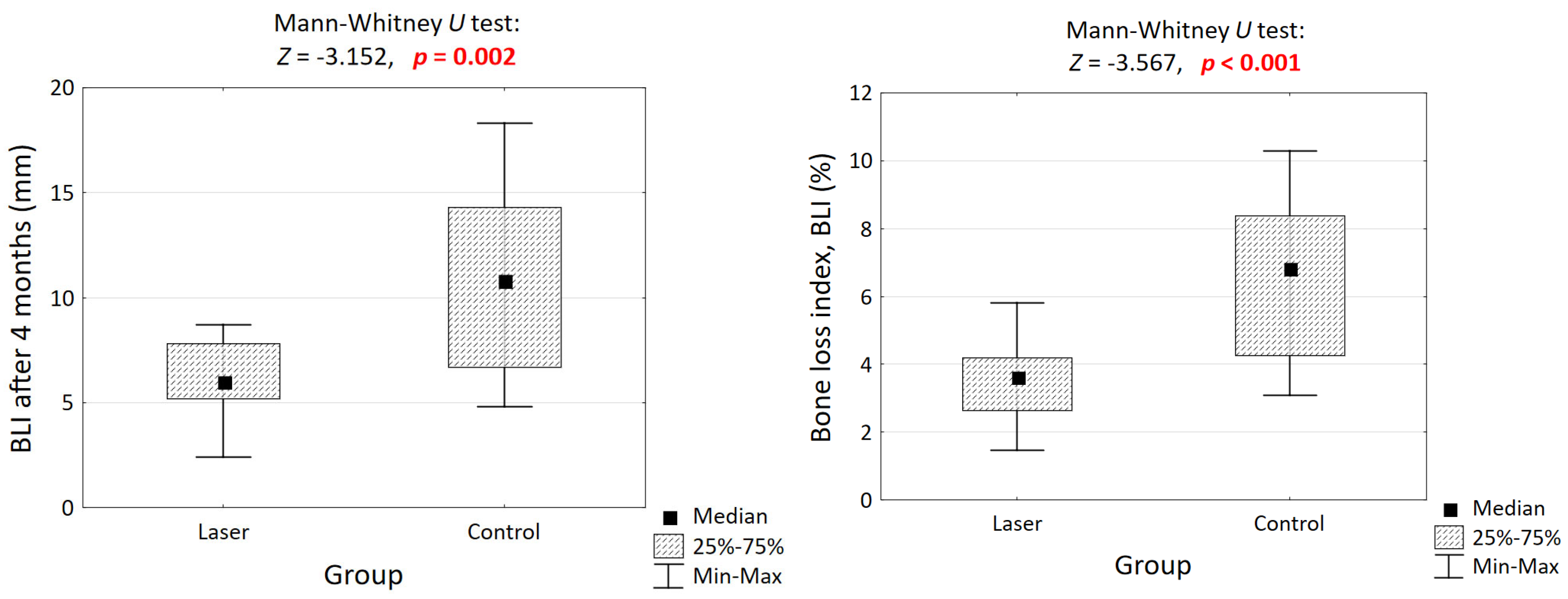
3.4. Measurement of the Alveolar Wall
3.5. Cumulative Bone Loss Assessment Using Bone Loss Index (BLI4)
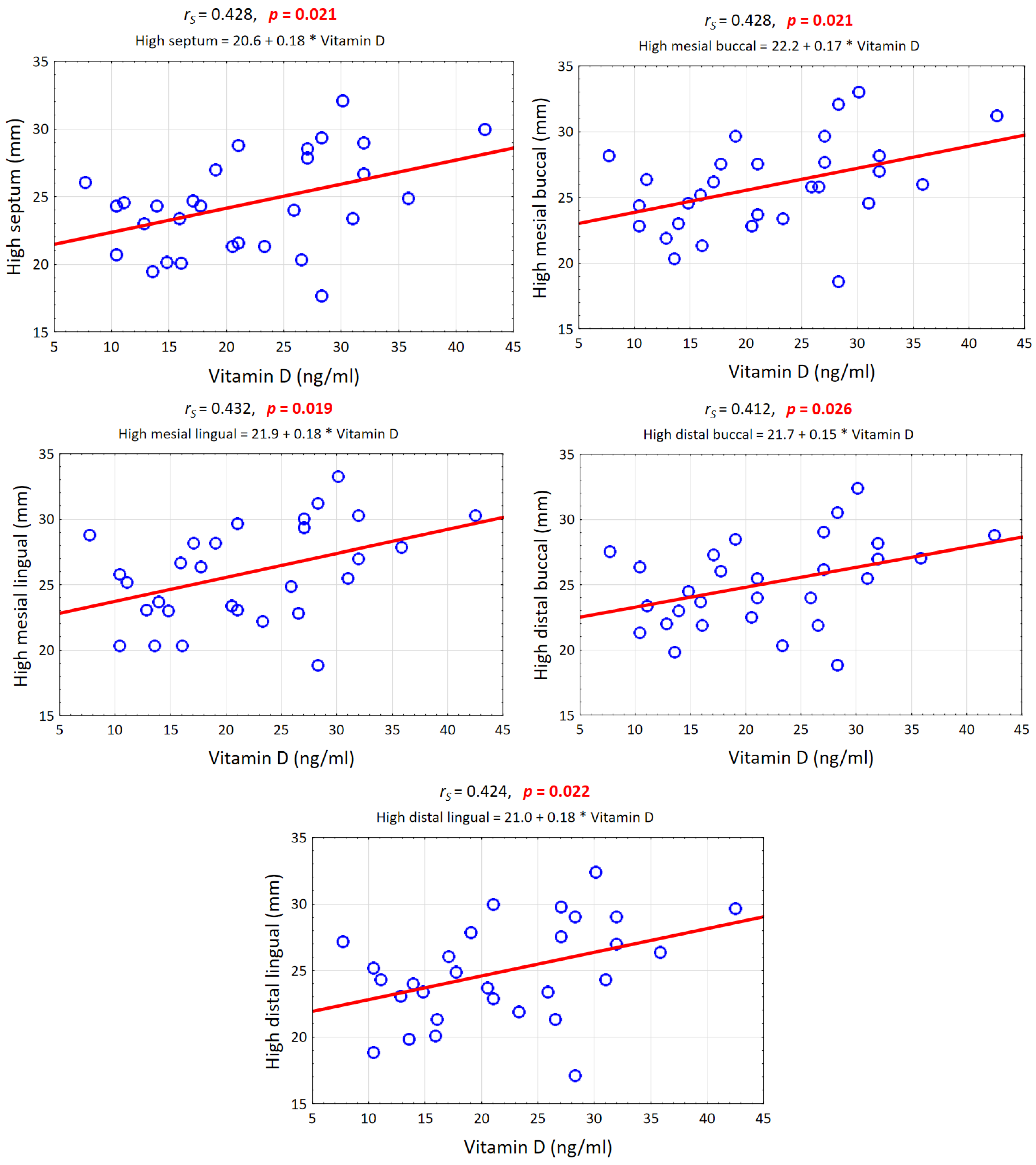
3.6. Vitamin D Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Creanor, S. Essential Clinical Oral Biology (Essentials (Dentistry)); Wiley: Hoboken, NJ, USA, 2016; p. 51. [Google Scholar]
- Kulkarni, S.; Meer, M.; George, R. Efficacy of photobiomodulation on accelerating bone healing after tooth extraction: A systematic review. Lasers Med. Sci. 2019, 34, 685–692. [Google Scholar] [CrossRef]
- Huebsch, R.; Coleman, R.; Frandsen, A.; Becks, H. The healing process following molar extraction. 1. Normal male rats (Long-Evans strain). Oral Surg. Oral. Mcd. Oral. Pathol. 1952, 5, 864–876. [Google Scholar] [CrossRef]
- Amler, M. The time sequence of tissue regeneration in human extraction wounds. Oral Surg. Oral. Mcd. Oral Pathol. 1969, 27, 309–318. [Google Scholar] [CrossRef]
- Kajiyama, K.; Murakami, T.; Yokota, S. Gingival reaction after experimentally induced extrusion of the upper inciosors in monkeys. Am J. Orthod. 1993, 104, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Salama, H.; Salama, M. The role of orthodontic extrusive remodeling in the enhancement of soft and hard tissue profiles prior to implant placement. A systematic approach to the management of extraction site defects. Int. J. Periodontics Restor. Dent. 1993, 13, 313–333. [Google Scholar]
- Hidding, J.; Lazar, F.; Zoller, J. The vertical distraction of the alveolar bone. J. Caniomaxillofac. Surg. 1998, 26, 72–76. [Google Scholar] [CrossRef]
- Quayle, A. Atraumatic removal of teeth and root fragments in dental implantology. Int. J. Oral Macillofac. Implant. 1990, 5, 293–296. [Google Scholar]
- Cardaropoli, G.; Araujo, M.; Hayacibara, R.; Sukekava, F.; Lindhe, J. Healing of extraction sockets ridge defects prior to implant placement Clinical results histologic evidence of osteoblastic osteoclastic acitivties in, D.F.D.B.A. Int. J. Periodontics Restor. Dent. 1999, 19, 259–267. [Google Scholar]
- Carmagnola, D.; Adriaens, P.; Berglundh, T. Healing of human extraction sockets filled with Bio-Oss. Clin. Oral Implant. Res. 2003, 14, 137–143. [Google Scholar] [CrossRef]
- Becker, W.; Becker, B.E.; Caffesse, R. A comparison of demineralized freeze-dried bone and autologous bone to induce bone formation in human extraction sockets. J. Periodontol. 1994, 65, 1128–1133, Erratum in J. Periodontol. 1995, 66, 309. [Google Scholar] [CrossRef]
- Artzi, Z.; Tal, H.; Dayan, D. Porous bovine bone mineral in healing of human extraction sockets. Part 1: Histomorphometric evaluations at 9 months. J. Periodontol. 2000, 71, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Adams, J.S.; Hewison, M. Update in vitamin D. J. Clin. Endocrinol. Metab. 2010, 95, 471–478. [Google Scholar] [CrossRef] [PubMed]
- Krawiec, M.; Dominiak, M. The role of vitamin D in the human body with a special emphasis on dental issues: Literature review. Dent. Med. Probl. 2018, 55, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Hujoel, P. Vitamin D and dental caries in controlled clinical trials: Systematic review and meta-analysis. Nutr. Rev. 2013, 71, 88–97. [Google Scholar] [CrossRef]
- Glerup, H.; Mikkelsen, K.; Poulsen, L.; Hass, E.; Overbeck, S.; Andersen, H.; Charles, P.; Eriksen, E.F. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif. Tissue Int. 2000, 66, 419–424. [Google Scholar] [CrossRef]
- McMahon, L.; Schwartz, K.; Yilmaz, O.; Brown, E.; Ryan, L.K.; Diamond, G. Vitamin D—Mediated induction of innate immunity in gingival epithelial cells. Infect Immun. 2011, 79, 2250–2256. [Google Scholar] [CrossRef]
- Perayil, J.; Menon, K.S.; Kurup, S.; Thomas, A.E.; Fenol, A.; Vyloppillil, R.; Bhaskar, A.; Megha, S. Influence of Vitamin D & Calcium Supplementation in the Management of Periodontitis. J. Clin. Diagn. Res. 2015, 9, ZC35–ZC38. [Google Scholar] [CrossRef]
- Kassi, E.N.; Stavropoulos, S.; Kokkoris, P.; Galanos, A.; Moutsatsou, P.; Dimas, C.; Papatheodorou, A.; Zafeiris, C.; Lyritis, G. Smoking is a significant determinant of low serum vitamin D in young and middle-aged healthy males. Hormones 2015, 14, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.; Santos, A.; Bernardes, M.; Ramalho, A.; Martins, M.J. Vitamin D metabolism in human adipose tissue: Could it explain low vitamin D status in obesity? Horm. Mol. Biol. Clin. Investig. 2017, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Lou, Y.R.; Toh, T.C.; Tee, Y.H.; Yu, H. 25-Hydroxyvitamin D3induces osteogenic differentiation of human mesenchymal stem cells. Sci. Rep. 2017, 17, 42816. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar] [CrossRef] [PubMed]
- Deeb, J.G.; Grzech-Leśniak, K.; Weaver, C.; Matys, J.; Bencharit, S. Retrieval of Glass Fiber Post Using Er: YAG Laser and Conventional Endodontic Ultrasonic Method: An In Vitro Study. J. Prosthodont. 2019, 28, 1024–1028. [Google Scholar] [CrossRef] [PubMed]
- Deeb, J.G.; Grzech-Leśniak, K.; Brody, E.R.; Matys, J.; Bencharit, S. Erbium laser-assisted ceramic debonding: A scoping review. J. Prosthodont. 2022, 31, e100–e124. [Google Scholar] [CrossRef] [PubMed]
- Grzech-Leśniak, Z.; Pyrkosz, J.; Szwach, J.; Lelonkiewicz, M.; Pajączkowska, M.; Nowicka, J.; Matys, J.; Grzech-Leśniak, K. In vitro evaluation of the effect of Er:YAG laser with a fractional PS04 handpiece on microbial biofilm survival. Dent Med Probl 2025. [Google Scholar] [CrossRef] [PubMed]
- Golob Deeb, J.; Smith, J.; Belvin, B.R.; Lewis, J.; Grzech-Leśniak, K. Er: YAG Laser Irradiation Reduces Microbial Viability When Used in Combination with Irrigation with Sodium Hypochlorite, Chlorhexidine, and Hydrogen Peroxide. Microorganisms 2019, 7, 612. [Google Scholar] [CrossRef]
- Križaj Dumić, A.; Pajk, F.; Olivi, G. The effect of post-extraction socket preservation laser treatment on bone density 4 months after extraction: Randomized controlled trial. Clin. Implant. Dent. Relat. Res. 2021, 23, 309–316. [Google Scholar] [CrossRef]
- Yukna, R.A.; Carr, R.L.; Evans, G.H. Histologic evaluation of an Nd: YAG laser-assisted new attachment procedure in humans. Int. J. Periodontics Restor. Dent. 2007, 27, 577–587. [Google Scholar]
- Yukna, R.A. Clinical evaluation of Laser-Assisted New Attachment Procedure® (LANAP®) surgical treatment of chronic periodontitis: A retrospective case series of 1-year results in 22 consecutive patients. J. Periodontal Implant. 2023, 53, 173–183. [Google Scholar] [CrossRef]
- Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Yukna, R.A.; Takasaki, A.A.; Romanos, G.E.; Taniguchi, Y.; Sasaki, K.M.; Zeredo, J.L.; et al. Periodontal and peri-implant wound healing following laser therapy. Periodontol 2000 2015, 68, 217–269. [Google Scholar] [CrossRef]
- Pinheiro, A.L.; Gerbi, M.E. Photoengineering of bone repair processes. Photomed. Laser Surg. 2006, 24, 169–178. [Google Scholar] [CrossRef]
- Ozawa, Y.; Shimizu, N.; Kariya, G.; Abiko, Y. Low-energy laser irradiation stimulates bone nodule formation at early stages of cell culture in rat calvarial cells. Bone 1998, 22, 347–354. [Google Scholar] [CrossRef]
- Stein, A.; Benayahu, D.; Maltz, L.; Oron, U. Low-level laser irradiation promotes proliferation and differentiation of human osteoblasts in vitro. Photomed. Laser Surg. 2005, 23, 161–166. [Google Scholar] [CrossRef]
- Ninomiya, T.; Miyamoto, Y.; Ito, T.; Yamashita, A.; Wakita, M.; Nishisaka, T. High-intensity pulsed laser irradiation accel-erates bone formation in metaphyseal trabecular bone in rat femur. J. Bone Miner. Metab. 2003, 21, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Mergoni, G.; Vescovi, P.; Sala, R.; Merigo, E.; Passerini, P.; Maestri, R.; Corradi, D.; Govoni, P.; Nammour, S.; Bianchi, M.G. The effect of laser therapy on the expression of osteocalcin and osteopontin after tooth extraction in rats treated with zoledronate and dexamethasone. Support. Care Cancer 2016, 24, 807–813. [Google Scholar] [CrossRef]
- Rosero, K.A.V.; Sampaio, R.M.F.; Deboni, M.C.Z.; Corrêa, L.; Marques, M.M.; Ferraz, E.P.; da Graça Naclério-Homem, M. Photobiomodulation as an adjunctive therapy for alveolar socket preservation: A preliminary study in humans. Lasers Med. Sci. 2020, 35, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhaleem Othman, M.A.; Zaky, A.A.; Eltayeb, E.A.; Khalil, N.M. A radiographic and histological study to compare red (650 nm) versus near infrared (810 nm) diode lasers photobiomodulation for alveolar socket preservation. Sci. Rep. 2024, 22, 6871. [Google Scholar] [CrossRef]
- Daigo, Y.; Daigo, E.; Hasegawa, A.; Fukuoka, H.; Ishikawa, M.; Takahashi, K. Utility of High-Intensity Laser Therapy Combined with Photobiomodulation Therapy for Socket Preservation After Tooth Extraction. Photobiomodul. Photomed. Laser Surg. 2020, 38, 75–83. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef]
- Kim, K.; Kim, I.S.; Cho, T.H.; Seo, Y.K.; Hwang, S.J. High-intensity Nd:YAG laser accelerates bone regeneration in calvarial defect models. J. Tissue Eng. Regen. Med. 2013. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Matys, J. The Effect of Er:YAG Lasers on the Reduction of Aerosol Formation for Dent.al Workers. Materials 2021, 14, 2857. [Google Scholar] [CrossRef]
- Grzech-Leśniak, Z.; Szwach, J.; Lelonkiewicz, M.; Migas, K.; Pyrkosz, J.; Szwajkowski, M.; Kosidło, P.; Pajączkowska, M.; Wiench, R.; Matys, J.; et al. Effect of Nd: YAG Laser Irradiation on the Growth of Oral Biofilm. Microorganisms 2024, 12, 2231. [Google Scholar] [CrossRef] [PubMed]
- Matys, J.; Grzech-Leśniak, K.; Flieger, R.; Dominiak, M. Assessment of an Impact of a Diode Laser Mode with Wavelength of 980 nm on a Temperature Rise Measured by Means of k-02 Thermocouple: Preliminary Results. Dent. Med. Probl. 2016, 53, 345–351. [Google Scholar] [CrossRef]
- Kensy, J.; Dobrzyński, M.; Wiench, R.; Grzech-Leśniak, K.; Matys, J. Fibroblasts Adhesion to Laser-Modified Titanium Surfaces—A Systematic Review. Materials 2021, 14, 7305. [Google Scholar] [CrossRef]
- Sterczała, B.; Grzech-Leśniak, K.; Michel, O.; Trzeciakowski, W.; Dominiak, M.; Jurczyszyn, K. Assessment of Human Gingival Fibroblast Proliferation after Laser Stimulation In Vitro Using Different Laser Types and Wavelengths (1064, 980, 635, 450, and 405 nm)-Preliminary Report. J. Pers. Med. 2021, 11, 98. [Google Scholar] [CrossRef]
- Kocherova, I.; Bryja, A.; Błochowiak, K.; Kaczmarek, M.; Stefańska, K.; Matys, J.; Grzech-Leśniak, K.; Dominiak, M.; Mozdziak, P.; Kempisty, B.; et al. Photobiomodulation with Red and Near-Infrared Light Improves Viability and Modulates Expression of Mesenchymal and Apoptotic-Related Markers in Human Gingival Fibroblasts. Materials 2021, 14, 3427. [Google Scholar] [CrossRef]
- El Mobadder, M.; Grzech-Lesniak, Z.; El Mobadder, W.; Rifai, M.; Ghandour, M.; Nammour, S. Management of Medication-Related Osteonecrosis of the Jaw with Photobiomodulation and Minimal Surgical Intervention. Dent. J. 2023, 11, 127. [Google Scholar] [CrossRef]
- Sezer, U.; Eltas, A.; Ustun, K.; Senyurt, S.Z.; Erciyas, K.; Aras, M.H. Effects of low-level laser therapy as an adjunct to standard therapy in acute pericoronitis, and its impact on oral health-related quality of life. Photomed. Laser Surg. 2012, 30, 592–597. [Google Scholar] [CrossRef]
- Gil, A.C.; Díaz, L.; Von Martens, A.; Sotomayor, C.; Basualdo, J.; Beltrán, V.; Jorquera, G.; Caviedes, R.; Fernández, E. The efficacy of low-level laser therapy in oral surgery: A systematic review of randomized controlled trials. Photodiagnosis Photodyn. Ther. 2025, 53, 104594. [Google Scholar] [CrossRef]
- Le, J.M.; Wu, J.H.; Jaw, F.S.; Su, C.T. The effect of bone remodeling with photobiomodulation in dentistry: A review study. Lasers Med. Sci. 2023, 38, 265. [Google Scholar] [CrossRef]
- Özer, H.; İnci, M.A. Effect of low-level laser therapy in wound healing of primary molar teeth extraction. BMC Oral Health 2024, 24, 348. [Google Scholar] [CrossRef]
- Reza, B.; Soheil, N.; Ehsan, B.; Kourosh, S.; Reza, F. Efficacy of photo bio-modulation therapy for pain relief and soft tissue wound healing after dental implant surgery: A double-blind randomized clinical trial. J. Photochem. Photobiol. 2021, 8, 100062. [Google Scholar] [CrossRef]
- Liu, G.Q.; Chen, X.X.; Gong, K. Impact of 810nm diode laser, intraoral and extraoral applications, on postoperative pain, swelling, wound healing, and patient satisfaction following mandibular third molar extraction: A comparative study. Laser Dent. Sci. 2024, 8, 1. [Google Scholar] [CrossRef]
- Kanazirski, N.; Vladova, D.; Neychev, D.; Raycheva, R.; Kanazirska, P. Effect of Er:YAG Laser Exposure on the Amorphous Smear Layer in the Marginal Zone of the Osteotomy Site for Placement of Dental Screw Implants: A Histomorphological Study. J. Funct. Biomater. 2023, 14, 376. [Google Scholar] [CrossRef] [PubMed]
- Rossmann, J.A.; Israel, M. Laser de-epithelialization for enhanced guided tissue regeneration. A paradigm shift? Dent. Clin. N. Am. 2000, 44, 793–809. [Google Scholar] [CrossRef]
- Grzech-Leśniak, K.; Matys, J.; Jurczyszyn, K.; Ziółkowski, P.; Dominiak, M.; Junior, A.B.; Romeo, U. Histological and Thermometric Examination of Soft Tissue De-Epithelialization Using Digitally Controlled Er:YAG Laser Handpiece: An Ex Vivo Study. Photomed. Laser Surg. 2018, 36, 313–319. [Google Scholar] [CrossRef]
- El Mobadder, M.; Nammour, S.; Namour, M.; Namour, A.; Grzech-Leśniak, K. Disinfection Potential of 980 nm Diode Laser and Hydrogen Peroxide (3%) in "Critical Probing Depths" Periodontal Pockets: Retrospective Study. Life 2022, 12, 370. [Google Scholar] [CrossRef]
- Michalak, F.; Hnitecka, S.; Dominiak, M.; Grzech-Leśniak, K. Schemes for Drug-Induced Treatment of Osteonecrosis of Jaws with Particular Emphasis on the Influence of Vitamin D on Therapeutic Effects. Pharmaceutics 2021, 13, 354. [Google Scholar] [CrossRef]
- Michalak, F.; Dominiak, M.; Kiryk, J.; Popecki, P.; Kubicki, D.; Matys, J.; Grzech-Leśniak, K. The Influence of Vitamin D Levels and Supplementation on the Treatment of Patients Affected by MRONJ. Appl. Sci. 2025, 15, 670. [Google Scholar] [CrossRef]
- Krawiec, M.; Dominiak, M. Prospective evaluation of vitamin D levels in dental treated patients: A screening study. Dent. Med. Probl. 2021, 58, 321–326. [Google Scholar] [CrossRef]
- Płudowski, P.; Karczmarewicz, E.; Bayer, M.; Carter, G.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dębski, R.; Decsi, T.; Dobrzańska, A.; Franek, E.; et al. Vitamin D supplementation in healthy population and risk groups of vitamin D deficiency—Practice guidelines for Central Europe 2013. Endokrynol. Pol. 2013, 10, 573–578. (In Polish) [Google Scholar] [CrossRef]
- Krall, E.A.; Wehler, C.; Garcia, R.I.; Harris, S.S.; Dawson-Hughes, B. Calcium and vitamin D supplements reduce tooth loss in the elderly. Am. J. Med. 2001, 111, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Tehranchi, A.; Sadighnia, A.; Younessian, F.; Abdi, A.H.; Shirvani, A. Correlation of Vitamin D status and orthodontic-induced external apical root resorption. Dent. Res. J. 2017, 14, 403–411. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, L.; Clarke, L.; Khalilidehkordi, E.; Butzkueven, H.; Taylor, B.; Broadley, S.A. Vitamin D for the treatment of multiple sclerosis: A meta-analysis. J. Neurol. 2018, 265, 2893–2905. [Google Scholar] [CrossRef]

| Group | Patients | Mean Age | Min–Max Age | Male | Female | Tooth 36 | Tooth 46 | Teeth 47 and 37 |
|---|---|---|---|---|---|---|---|---|
| Control | 15 | 45 years 1 months | 30–59 years | 7 | 8 | 4 | 9 | 2 |
| Laser | 15 | 49 years 6 months | 32–70 years | 8 | 7 | 8 | 7 | 0 |
Inclusion dental criteria were as follows:
| Inclusion general criteria were as follows:
|
Exclusion dental criteria were as follows:
| Exclusion general criteria were as follows:
|
| Day | Laser | Control | p-Value |
|---|---|---|---|
| 1st | 2.5 [2, 3] | 5 [3, 6] | 0.001 |
| 2nd | 1.5 [1, 2] | 4 [3, 5] | <0.001 |
| 3rd | 1 [1, 1] | 2 [2, 3] | 0.001 |
| 7th | 1 [1, 1] | 1 [1, 1] | 1.000 |
| The Day After the Procedure | Group | Taking Painkillers | Result of Test | |
|---|---|---|---|---|
| Yes | No | |||
| First day | All | n = 9 36.2 ± 5.5 | n = 21 31.1 ± 7.0 | p = 0.067 |
| Laser | n = 4 40.6 ± 5.2 | n = 11 35.6 ± 6.4 | p = 0.196 | |
| Control | n = 5 32.6 ± 2.2 | n = 10 26.1 ± 3.4 | p = 0.002 | |
| Result of test | p = 0.017 | p = 0.001 | × | |
| Second day | All | n = 5 35.7 ± 5.0 | n = 25 32.0 ± 7.2 | p = 0.285 |
| Laser | n = 1 44.5 ± 0.0 | n = 14 36.4 ± 6.3 | p = 0.233 | |
| Control | n = 4 33.5 ± 1.3 | n = 11 26.4 ± 3.3 | p = 0.001 | |
| Result of test | p = 0.004 | p < 0.001 | × | |
| Third day | All | n = 1 34.0 ± 0.0 | n = 29 32.6 ± 7.0 | p = 0.841 |
| Laser | - | n = 15 37.0 ± 6.4 | - | |
| Control | n = 1 34.0 ± 0.0 | n = 14 27.9 ± 4.2 | p = 0.179 | |
| Result of test | - | p < 0.001 | × | |
| Dimension (mm) | On the Day of the Procedure | 4 Months After the Procedure | Result of the Test |
|---|---|---|---|
| High septum—with laser | 24.7 (23.4–27.9) | 24.2 (22.0–26.8) | p = 0.001 |
| High septum—no laser | 23.0 (20.7–26.1) | 20.2 (20.0–24.8) | p <0.001 |
| Result of the test | p = 0.206 | p = 0.125 | × |
| High mesial buccal—with laser | 26.2 (24.4–29.7) | 25.8 (23.7–28.6) | p = 0.001 |
| High mesial buccal—no laser | 24.6 (22.8–27.6) | 22.5 (20.8–26.3) | p = 0.001 |
| Result of the test | p = 0.245 | p = 0.056 | × |
| High mesial lingual—with laser | 27.9 (24.9–30.3) | 25.8 (23.8–28.3) | p = 0.001 |
| High mesial lingual—no laser | 23.7 (23.0–28.8) | 21.9 (21.0–26.4) | p = 0.001 |
| Result of the test | p = 0.184 | p = 0.125 | × |
| Width mesial root—with laser | 8.8 (8.1–9.4) | 8.1 (7.8–8.7) | p = 0.001 |
| Width mesial root—no laser | 9.6 (7.5–10.5) | 8.7 (7.3–10.2) | p = 0.001 |
| Result of the test | p = 0.395 | p = 0.648 | × |
| High distal buccal—with laser | 27.0 (24.0–28.5) | 25.4 (23.2–27.3) | p = 0.001 |
| High distal buccal—no laser | 23.7 (22.0–25.5) | 21.3 (20.1–25.5) | p = 0.001 |
| Result of the test | p = 0.081 | p = 0.051 | × |
| High distal lingual—with laser | 26.1 (23.4–27.9) | 25.4 (22.8–27.6) | p = 0.001 |
| High distal lingual—no laser | 23.7 (21.3–27.2) | 21.7 (20.2–24.9) | p = 0.001 |
| Result of the test | p = 0.237 | p = 0.046 | × |
| Width distal lingual—with laser | 8.4 (8.1–9.6) | 8.1 (7.5–8.7) | p = 0.001 |
| Width distal lingual—no laser | 9.0 (7.8–10.7) | 8.7 (7.5–9.9) | p = 0.001 |
| Result of the test | p = 0.468 | p = 0.384 | × |
| Parameter | Δ Laser (mm) | Δ Control (mm) | Between-Group Δ (mm) | p-Value |
|---|---|---|---|---|
| Septum height | −0.5 | −2.8 | 2.3 | 0.125 |
| Mesial buccal wall height | −0.4 | −2.1 | 1.7 | 0.056 |
| Mesial lingual wall height | −2.1 | −1.8 | 0.3 | 0.125 |
| Mesial socket width | −0.7 | −0.9 | 0.2 | 0.648 |
| Distal buccal wall height | −1.6 | −2.4 | 0.8 | 0.051 |
| Distal lingual wall height | −0.7 | −2.0 | 1.3 | 0.046 |
| Distal socket width | −0.3 | −0.3 | 0.0 | 0.384 |
| Vitamin D (ng/mL) Versus: | rS | t (N − 2) | p-Value |
|---|---|---|---|
| High septum (mm) | 0.428 | 2.458 | 0.021 |
| High mesial buccal (mm) | 0.428 | 2.460 | 0.021 |
| High mesial lingual (mm) | 0.432 | 2.488 | 0.019 |
| Width mesial root (mm) | 0.019 | 0.061 | 0.316 |
| High distal buccal (mm) | 0.412 | 2.349 | 0.026 |
| High distal lingual (mm) | 0.424 | 2.433 | 0.022 |
| Width distal root (mm) | −0.047 | −0.244 | 0.809 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryka-Deszczyńska, M.; Grzech-Leśniak, Z.; Dembicka-Mączka, D.; Wiench, R.; Dominiak, M.; Matys, J.; Grzech-Leśniak, K. Effects of Er:YAG and Nd:YAG Lasers with Photobiomodulation on Alveolar Bone Preservation Post-Extraction: A Randomized Clinical Control Trial. Photonics 2025, 12, 817. https://doi.org/10.3390/photonics12080817
Gryka-Deszczyńska M, Grzech-Leśniak Z, Dembicka-Mączka D, Wiench R, Dominiak M, Matys J, Grzech-Leśniak K. Effects of Er:YAG and Nd:YAG Lasers with Photobiomodulation on Alveolar Bone Preservation Post-Extraction: A Randomized Clinical Control Trial. Photonics. 2025; 12(8):817. https://doi.org/10.3390/photonics12080817
Chicago/Turabian StyleGryka-Deszczyńska, Magdalena, Zuzanna Grzech-Leśniak, Diana Dembicka-Mączka, Rafał Wiench, Marzena Dominiak, Jacek Matys, and Kinga Grzech-Leśniak. 2025. "Effects of Er:YAG and Nd:YAG Lasers with Photobiomodulation on Alveolar Bone Preservation Post-Extraction: A Randomized Clinical Control Trial" Photonics 12, no. 8: 817. https://doi.org/10.3390/photonics12080817
APA StyleGryka-Deszczyńska, M., Grzech-Leśniak, Z., Dembicka-Mączka, D., Wiench, R., Dominiak, M., Matys, J., & Grzech-Leśniak, K. (2025). Effects of Er:YAG and Nd:YAG Lasers with Photobiomodulation on Alveolar Bone Preservation Post-Extraction: A Randomized Clinical Control Trial. Photonics, 12(8), 817. https://doi.org/10.3390/photonics12080817








