Recycling Technologies for Extracting Gallium from Light-Emitting Diodes
Abstract
1. Introduction
2. Role of Gallium in LEDs
3. Recycling Methods
3.1. Hydrometallurgy Methods
3.1.1. Recycling of Gallium by Oxalic Acid Leaching
3.1.2. Recycling of Gallium by Oxidation and Subsequent Leaching
3.1.3. Recycling of Gallium by HCL Acid Leaching of Coal Fly Ash
3.2. Other Methods
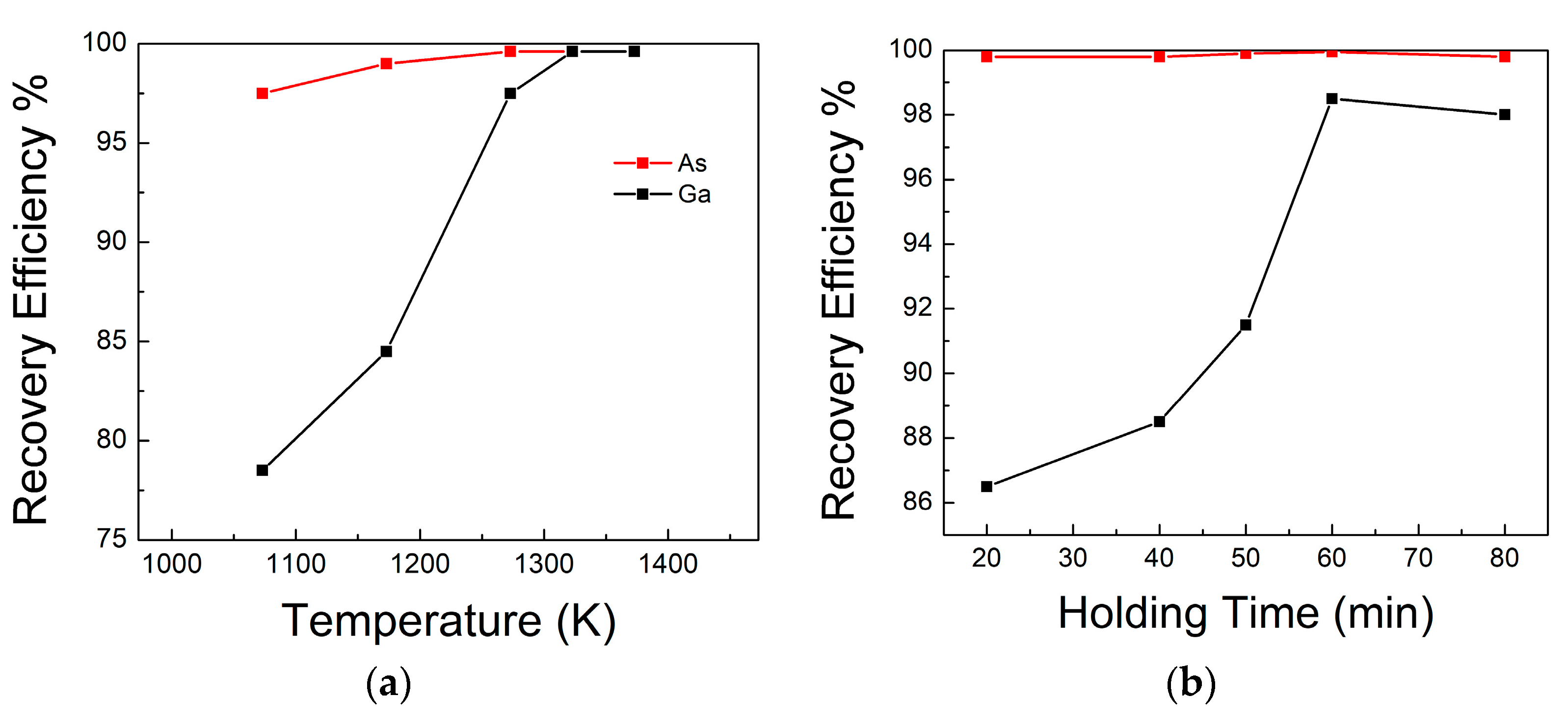
3.2.1. Recycling of Gallium by Pyrolysis
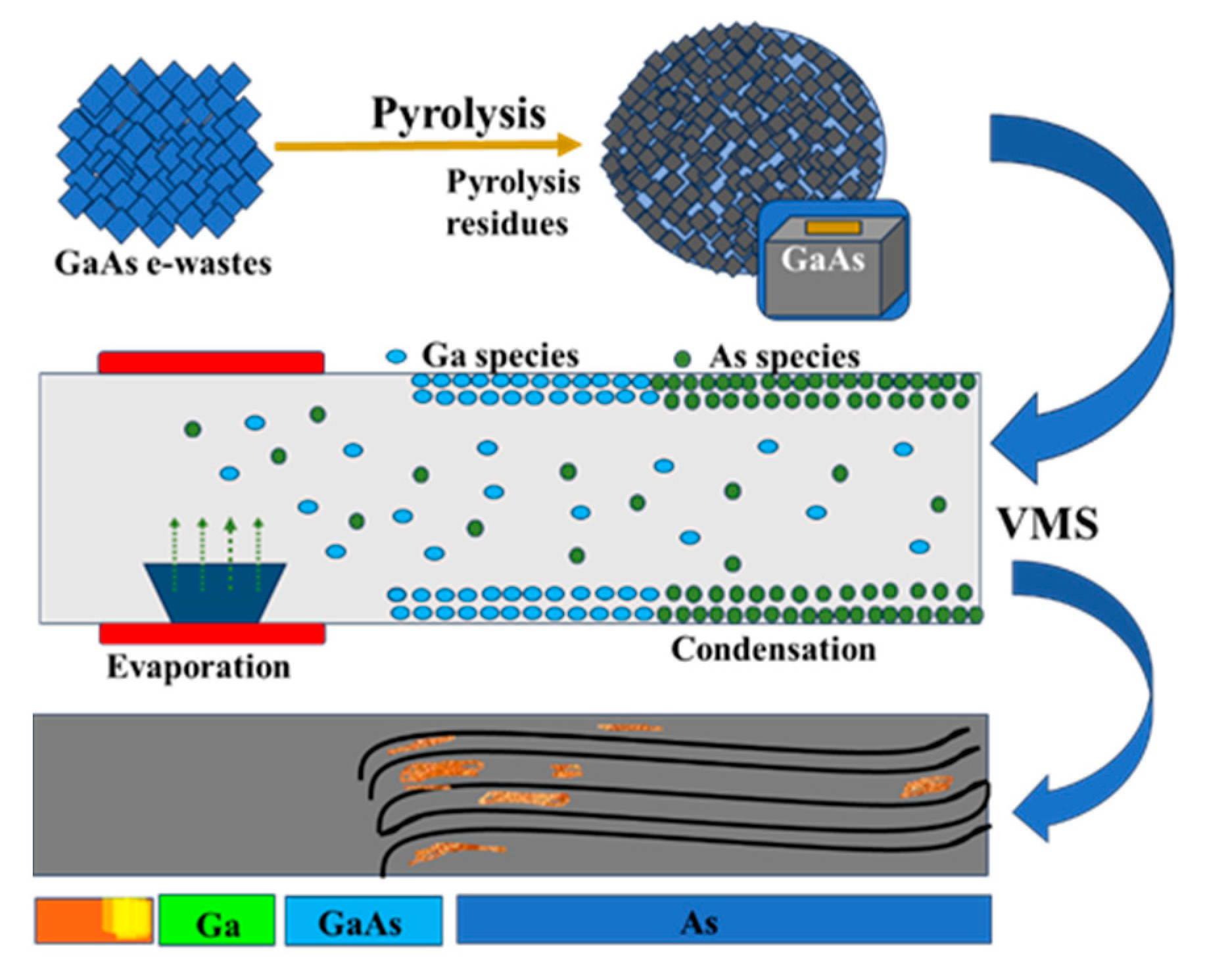
3.2.2. Recycling of Gallium by Subcritical Water Treatment
3.2.3. Recycling of Gallium by Supercritical Ethanol
3.2.4. Recycling of Gallium by Bayer Process
3.2.5. Emerging Green Methods for Recycling Gallium
4. Conclusions
5. Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grandell, L.; Lehtilä, A.; Kivinen, M.; Koljonen, T.; Kihlman, S.; Lauri, L.S. Role of critical metals in the future markets of clean energy technologies. Renew. Energy 2016, 95, 53–62. [Google Scholar] [CrossRef]
- Mishra, U.K.; Parikh, P.; Wu, Y.-F. AlGaN/GaN HEMTs-an overview of device operation and applications. Proc. IEEE 2002, 90, 1022–1031. [Google Scholar] [CrossRef]
- Moskalyk, R.R. Gallium: The backbone of the electronics industry. Miner. Eng. 2003, 16, 921–929. [Google Scholar] [CrossRef]
- Pavel, C.C.; Marmier, A.; Tzimas, E.; Schleicher, T.; Schüler, D.; Buchert, M.; Blagoeva, D. Critical raw materials in lighting applications: Substitution opportunities and implication on their demand. Phys. Status Solidi A 2016, 213, 2937–2946. [Google Scholar] [CrossRef]
- Stevenson, R. Endangered elements. Phys. World 2019, 32, 12. [Google Scholar] [CrossRef]
- Sverdrup, H.U.; Haraldsson, H.V. Gallium: Assessing the long term future extraction, supply, recycling and price of using WORLD7, in relation to future technology visions in the European Union. Preprints 2024. [Google Scholar] [CrossRef]
- European Union Aviation Safety Agency. Priorities for Critical Materials for a Circular Economy; ESAC Secretariat: Madrid, Spain, 2016. [Google Scholar]
- Lu, F.; Xiao, T.; Lin, J.; Ning, Z.; Long, Q.; Xiao, L.; Huang, F.; Wang, W.; Xiao, Q.; Lan, X. Resources and extraction of gallium: A review. Hydrometallurgy 2017, 174, 105–115. [Google Scholar] [CrossRef]
- Kumar, A.; Kuppusamy, V.K.; Holuszko, M.; Song, S.; Loschiavo, A. LED lamps waste in Canada: Generation and characterization. Resour. Conserv. Recycl. 2019, 146, 329–336. [Google Scholar] [CrossRef]
- Scholand, M.; Dillon, H.E. Life-Cycle Assessment of Energy and Environmental Impacts of LED Lighting Products Part 2: LED Manufacturing and Performance; Pacific Northwest National Lab. (PNNL): Richland, WA, USA, 2012.
- Zhao, L.; Zhu, S.; Wu, C.; Yang, C.; Yu, Z.; Yang, H.; Liu, L. GaN-based LEDs for light communication. Sci. China Phys. Mech. Astron. 2016, 59, 1–10. [Google Scholar] [CrossRef]
- Martin-Ramos, P.; Ramos-Silva, M. Lanthanide-Based Multifunctional Materials: From OLEDs to SIMs; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
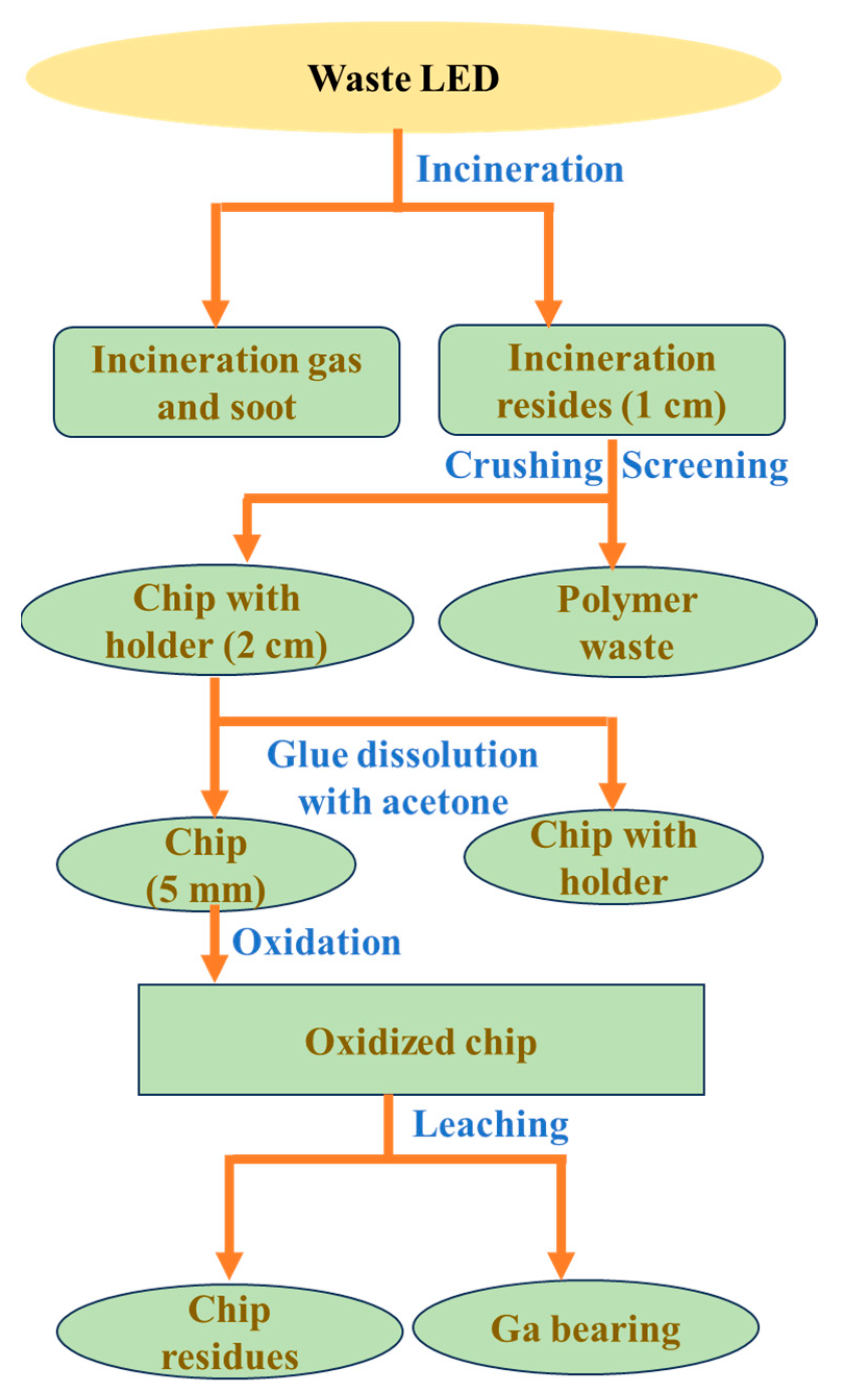
- Fang, S.; Yan, W.; Cao, H.; Song, Q.; Zhang, Y.; Sun, Z. Evaluation on end-of-life LEDs by understanding the criticality and recyclability for metals recycling. J. Clean. Prod. 2018, 182, 624–633. [Google Scholar] [CrossRef]
- Chou, W.-L.; Wang, C.-T.; Yang, K.-C.; Huang, Y.-H. Removal of gallium (III) ions from acidic aqueous solution by supercritical carbon dioxide extraction in the green separation process. J. Hazard. Mater. 2008, 160, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Gupta, B.; Mudhar, N.; Singh, I. Separations and recovery of indium and gallium using bis (2, 4, 4-trimethylpentyl) phosphinic acid (Cyanex 272). Sep. Purif. Technol. 2007, 57, 294–303. [Google Scholar] [CrossRef]
- Ali, S.; Prasad, D.; Munirathnam, N.; Prakash, T. Purification of tellurium by single-run multiple vacuum distillation technique. Sep. Purif. Technol. 2005, 43, 263–267. [Google Scholar] [CrossRef]
- Ueberschaar, M.; Geiping, J.; Zamzow, M.; Flamme, S.; Rotter, V.S. Assessment of element-specific recycling efficiency in WEEE pre-processing. Resour. Conserv. Recycl. 2017, 124, 25–41. [Google Scholar] [CrossRef]
- Steranka, F.; Bhat, J.; Collins, D.; Cook, L.; Craford, M.; Fletcher, R.; Gardner, N.; Grillot, P.; Goetz, W.; Keuper, M. High power LEDs–Technology status and market applications. Phys. Status Solidi A 2002, 194, 380–388. [Google Scholar] [CrossRef]
- Zhan, L.; Xia, F.; Ye, Q.; Xiang, X.; Xie, B. Novel recycle technology for recovering rare metals (Ga, In) from waste light-emitting diodes. J. Hazard. Mater. 2015, 299, 388–394. [Google Scholar] [CrossRef]
- DenBaars, S.P.; Feezell, D.; Kelchner, K.; Pimputkar, S.; Pan, C.-C.; Yen, C.-C.; Tanaka, S.; Zhao, Y.; Pfaff, N.; Farrell, R. Development of gallium-nitride-based light-emitting diodes (LEDs) and laser diodes for energy-efficient lighting and displays. Acta Mater. 2013, 61, 945–951. [Google Scholar] [CrossRef]
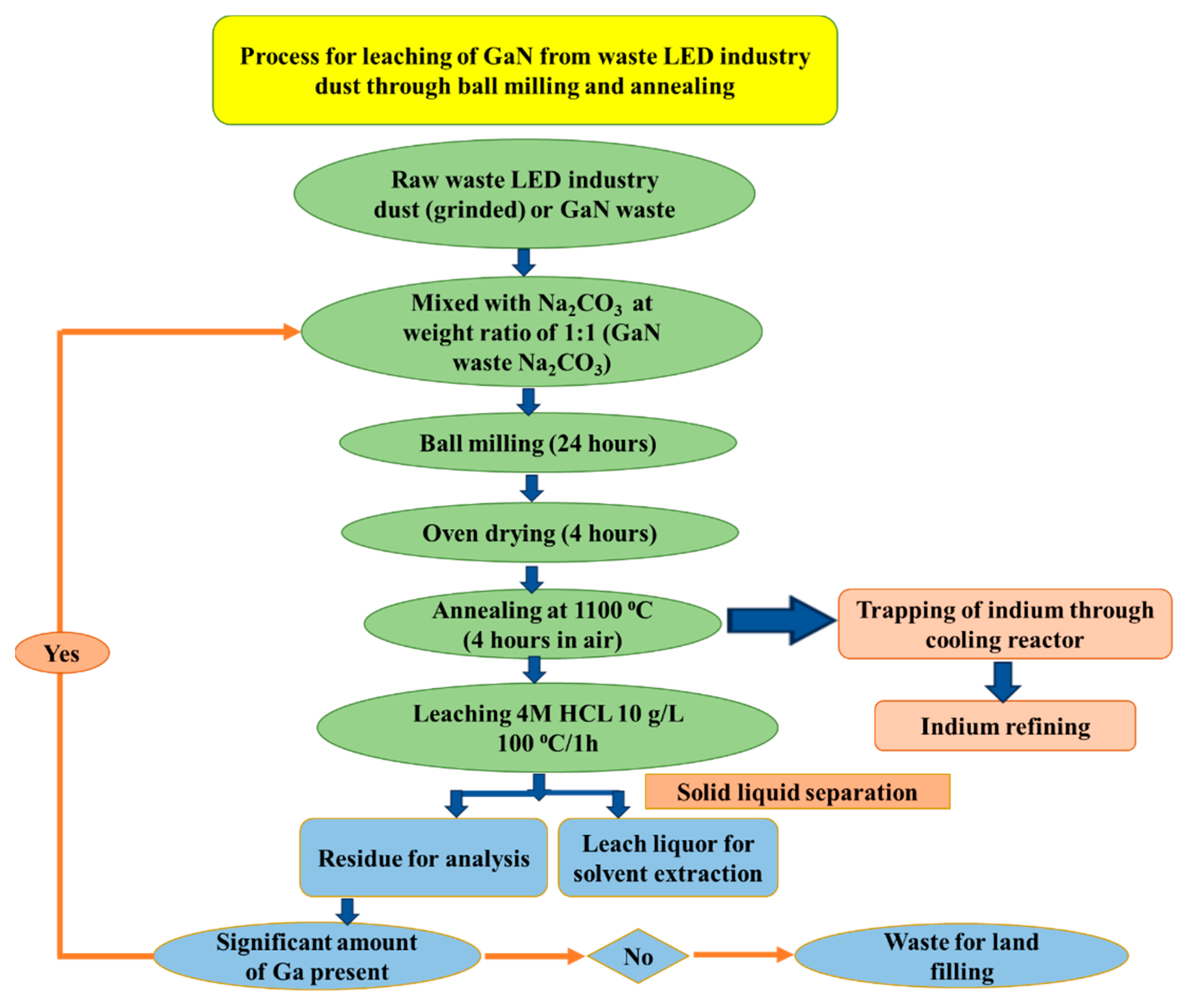
- Swain, B.; Mishra, C.; Kang, L.; Park, K.-S.; Lee, C.G.; Hong, H.S. Recycling process for recovery of gallium from GaN an e-waste of LED industry through ball milling, annealing and leaching. Environ. Res. 2015, 138, 401–408. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, L.; Liu, H.; Qian, P.; Ye, S.; Chen, Y. Acid leaching pretreatment on two-stage roasting pyrite cinder for gold extraction and co-precipitation of arsenic with iron. Hydrometallurgy 2018, 179, 192–197. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhan, L.; Xu, Z. Recycling Ag, As, Ga of waste light-emitting diodes via subcritical water treatment. J. Hazard. Mater. 2021, 408, 124409. [Google Scholar] [CrossRef] [PubMed]
- Xue, B.; Wei, B.; Ruan, L.; Li, F.; Jiang, Y.; Tian, W.; Su, B.; Zhou, L. The influencing factor study on the extraction of gallium from red mud. Hydrometallurgy 2019, 186, 91–97. [Google Scholar] [CrossRef]
- Lu, F.; Xiao, T.; Lin, J.; Li, A.; Long, Q.; Huang, F.; Xiao, L.; Li, X.; Wang, J.; Xiao, Q. Recovery of gallium from Bayer red mud through acidic-leaching-ion-exchange process under normal atmospheric pressure. Hydrometallurgy 2018, 175, 124–132. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Z.; Li, Y.; Wilson, B.P.; Lundström, M. Recovery and separation of gallium (III) and germanium (IV) from zinc refinery residues: Part I: Leaching and iron (III) removal. Hydrometallurgy 2017, 169, 564–570. [Google Scholar] [CrossRef]
- De Oliveira, R.; Benvenuti, J.; Espinosa, D. A review of the current progress in recycling technologies for gallium and rare earth elements from light-emitting diodes. Renew. Sustain. Energy Rev. 2021, 145, 111090. [Google Scholar] [CrossRef]
- Zhou, J.; Zhu, N.; Liu, H.; Wu, P.; Zhang, X.; Zhong, Z. Recovery of gallium from waste light emitting diodes by oxalic acidic leaching. Resour. Conserv. Recycl. 2019, 146, 366–372. [Google Scholar] [CrossRef]
- Cui, J.; Zhu, N.; Luo, D.; Li, Y.; Wu, P.; Dang, Z.; Hu, X. The role of oxalic acid in the leaching system for recovering indium from waste liquid crystal display panels. ACS Sustain. Chem. Eng. 2019, 7, 3849–3857. [Google Scholar] [CrossRef]
- Verma, A.; Kore, R.; Corbin, D.R.; Shiflett, M.B. Metal recovery using oxalate chemistry: A technical review. Ind. Eng. Chem. Res. 2019, 58, 15381–15393. [Google Scholar] [CrossRef]
- Schmitz, D.; Prasetyo, H.; Birich, A.; Yeetsorn, R.; Friedrich, B. Co-Precipitation of Metal Oxalates from Organic Leach Solution Derived from Spent Lithium-Ion Batteries (LIBs). Metals 2024, 14, 80. [Google Scholar] [CrossRef]
- Zheng, D.; Zhang, Y.; Liu, T.; Huang, J.; Cai, Z.; Zhang, R. Selective leaching of valuable metals from spent fluid catalytic cracking catalyst with oxalic acid. Minerals 2022, 12, 748. [Google Scholar] [CrossRef]
- Tan, K.S.; Chong, S.C.; Lin, B.; Eze, U.C. Internet-based ICT adoption: Evidence from Malaysian SMEs. Ind. Manag. Data Syst. 2009, 109, 224–244. [Google Scholar] [CrossRef]
- Cheng, Z.; Fang, M.; Chen, X.; Zhang, Y.; Wang, Y.; Li, H.; Qian, J. Thermal stability and flame retardancy of a cured trifunctional epoxy resin with the synergistic effects of silicon/titanium. ACS Omega 2020, 5, 4200–4212. [Google Scholar] [CrossRef]
- Zhuang, D.; Edgar, J. Wet etching of GaN, AlN, and SiC: A review. Mater. Sci. Eng. R Rep. 2005, 48, 1–46. [Google Scholar] [CrossRef]
- Jorger, R.; Kolarik, Z. Extraction of gallium (III) and accompanying elements with tributyl phosphate from chloride media. Solvent Extr. Ion Exch. 1993, 11, 33–49. [Google Scholar] [CrossRef]
- Maarefvand, M.; Sheibani, S.; Rashchi, F. Recovery of gallium from waste LEDs by oxidation and subsequent leaching. Hydrometallurgy 2020, 191, 105230. [Google Scholar] [CrossRef]
- Gesser, H.D.; Bock, E.; Baldwin, W.; Chow, A.; McBride, D.; Lipinsky, W. Open-cell polyurethane foam sponge as a “solvent extractor” for gallium and iron. Sep. Sci. Technol. 1976, 11, 317–327. [Google Scholar] [CrossRef]
- Chernoburova, O.; Chagnes, A. 3—Processing and extraction of critical raw materials from residues. In Mining and Processing Residues; Chernoburova, O., Chagnes, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 71–183. [Google Scholar]
- Fang, Z.; Gesser, H. Recovery of gallium from coal fly ash. Hydrometallurgy 1996, 41, 187–200. [Google Scholar] [CrossRef]
- Zhao, Z.; Cui, L.; Guo, Y.; Gao, J.; Li, H.; Cheng, F. A stepwise separation process for selective recovery of gallium from hydrochloric acid leach liquor of coal fly ash. Sep. Purif. Technol. 2021, 265, 118455. [Google Scholar] [CrossRef]
- Gutiérrez, B.; Pazos, C.; Coca, J. Recovery of Gallium from Coal Fly Ash By a Dual Reactive Extraction Process. Waste Manag. Res. 1997, 15, 371–382. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Ismail, Z.H.; Hamed, M.M. Extraction and separation of Ga (III) from hydrochloric acid solution by Cyanex-921 in sulfonated kerosene. J. Radioanal. Nucl. Chem. 2018, 317, 969–976. [Google Scholar] [CrossRef]
- Baldé, C.; Wang, F.; Kuehr, R.; Huisman, J. The Global E-Waste Monitor; United Nations University, IAS–SCYCLE: Bonn, Germany, 2014. [Google Scholar]
- Amankwah-Amoah, J. Global business and emerging economies: Towards a new perspective on the effects of e-waste. Technol. Forecast. Soc. Change 2016, 105, 20–26. [Google Scholar] [CrossRef]
- Zhan, L.; Xia, F.; Xia, Y.; Xie, B. Recycle gallium and arsenic from GaAs-based e-wastes via pyrolysis–vacuum metallurgy separation: Theory and feasibility. ACS Sustain. Chem. Eng. 2018, 6, 1336–1342. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, Z.; Zhang, Y.; Xu, Z. Recycling of metals (Ga, In, As and Ag) from waste light-emitting diodes in sub/supercritical ethanol. Resour. Conserv. Recycl. 2020, 155, 104695. [Google Scholar] [CrossRef]
- Figueiredo, A.; Avristcher, W.; Masini, E.; Diniz, S.; Abrão, A. Determination of lanthanides (La, Ce, Nd, Sm) and other elements in metallic gallium by instrumental neutron activation analysis. J. Alloys Compd. 2002, 344, 36–39. [Google Scholar] [CrossRef]
- Gray, F.; Kramer, D.A.; Bliss, J.D. Gallium and gallium compounds. In Kirk-Othmer Encyclopedia of Chemical Technology; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar]
- Coakley, G.J. The mineral industry of Namibia. In Minerals Yearbook; Bureau of Mines: Nagpur, India, 2003; Volume 3, p. 17. [Google Scholar]
- Guo, Z.; Qin, Z.; Liu, S.; Zhang, W.; Zheng, C.; Wang, H. Solvent Extraction of Gallium and Germanium Using a Novel Hydroxamic Acid Extractant. Minerals 2024, 14, 1147. [Google Scholar] [CrossRef]
- Maneesuwannarat, S.; Teamkao, P.; Vangnai, A.S.; Yamashita, M.; Thiravetyan, P. Possible mechanism of gallium bioleaching from gallium nitride (GAN) by Arthrobacter creatinolyticus: Role of amino acids/peptides/proteins bindings with gallium. Process Saf. Environ. Prot. 2016, 103, 36–45. [Google Scholar] [CrossRef]
- Sun, Z.; Tao, Z.; Li, H.; Pittman, A.S.; Zhou, F.; Zhang, G.; Wu, J.; Cheng, H.; Feng, E.; Chen, Z. Recovery of rare earth elements, gallium and germanium from fly ash and red mud via ultra-fast flash Joule heating. Chem. Eng. Sci. 2025, 315, 121879. [Google Scholar] [CrossRef]
- Rachel Winn, Q.W.; Robert, V.F. Gallium Separation and Processing Method; US Critical Materials: Salt Lake City, UT, USA, 2025. [Google Scholar]
- ISO 14001:2015; Environmental Management Systems—Requirements with Guidance for Use. International Organization for Standardization: Geneva, Switzerland, 2015.


| Leaching Condition | ||||||
|---|---|---|---|---|---|---|
| Gallium Resource | Agent | S:L | Temperature (°C) | Time (h) | Ga Extraction (%) | Ref. |
| Coal fly ash red mud | 200 g L−1 NaOH | 1:5 | 120 | 12 | 91 | [24] |
| Bayer red mud | 159 g L−1 HCL | 1:8 | 100 | 4 | 98 | [25] |
| 219 g L−1 H2SO4 | 91 | |||||
| 261 g L−1 NaOH | 78 | |||||
| Zinc residue | 70 g L−1 H2C2O | 1:10 | 90 | 2 | 96 | [26] |
| Parameters | ||||
|---|---|---|---|---|
| Concentration (M) | Temperature (°C) | Time (Minutes) | Gallium Recovery (%) | |
| 1 | 2.2 | 31 | 57 | 5.9 |
| 2 | 4.0 | 70 | 57 | 14.5 |
| 3 | 4.0 | 93 | 120 | 91.4 |
| 4 | 2.2 | 73 | 56 | 9.7 |
| 5 | 4.0 | 33 | 15 | 4.3 |
| 6 | 2.2 | 68 | 120 | 16.1 |
| 7 | 1.0 | 33 | 120 | 6.5 |
| 8 | 1.0 | 53 | 15 | 4.8 |
| 9 | 1.0 | 94 | 82 | 26.9 |
| 10 | 2.9 | 53 | 15 | 7 |
| 11 | 3.7 | 64 | 120 | 27.4 |
| 12 | 4.0 | 33 | 120 | 7.5 |
| 13 | 4.0 | 33 | 15 | 4.8 |
| 14 | 4.0 | 69 | 57 | 21 |
| 15 | 2.8 | 95 | 86 | 82.3 |
| 16 | 4.0 | 31 | 120 | 7.5 |
| 17 | 1.0 | 33 | 120 | 6.5 |
| 18 | 1.0 | 89 | 15 | 6.5 |
| 19 | 3.0 | 93 | 15 | 14 |
| 20 | 4.0 | 93 | 120 | 90.3 |
| SN | Solid Waste | Technique | Temperature | Time | Ga Recovery | Ref. |
|---|---|---|---|---|---|---|
| 1 | Waste from electrical and electronic components | Vacuum metallurgy separation | 100 °C | 1 h | 90% | [19] |
| 2 | SMD LEDs | Oxalic acid leaching | 733 K | 1 h | 83.2% | [28] |
| 3 | Waste LEDs | Oxidation and subsequent leaching | 93 °C | 120 min | 91.4% | [37] |
| 4 | Adsorbent PUF | HCL acid leaching of coal fly ash | Room temperature | 15 min | 90–95% | [40] |
| 5 | GaAs-based e-wastes | Pyrolysis | 1273 K | 1 h | 95% | [46] |
| 6 | 3528 SMD LEDs | Subcritical water treatment | 300 °C | 400 min | 80.5% | [23] |
| 7 | 3528 SMD LEDs | Supercritical ethanol | 300 °C | 240 min | 93.10% | [47] |
| SN | Technique | Toxicity | Energy (kWh/kg Ga) | Ga Purity (%) | Capital Cost (USD/kg Ga) | Environmental Compliance | Emerging Alternatives (TRL) |
|---|---|---|---|---|---|---|---|
| 1 | Vacuum metallurgy separation | Moderate | 210 ± 30 | 99.9 ± 0.1 | 380–450 | RoHS/REACH-compliant | Plasma-assisted vacuum (TRL5) |
| 2 | Oxalic acid leaching | Low | 55 ± 15 | 95.2 ± 1.3 | 180–220 | Zero liquid discharge | Electro-assisted leaching (TRL6) |
| 3 | Oxidation and subsequent leaching | Moderate | 115 ± 25 | 97.5 ± 0.8 | 250–320 | ISO 14001-certified [55] | Photocatalytic oxidation (TRL4) |
| 4 | HCL acid leaching of coal fly ash | High | 85 ± 20 | 92.1 ± 2.1 | 150–200 | EPA Part 266-compliant | Deep eutectic solvents (TRL7) |
| 5 | Pyrolysis | High | 280 ± 50 | 88.7 ± 3.5 | 300–400 | Meets WEEE Directive | Microwave pyrolysis (TRL6) |
| 6 | Subcritical water treatment | Low | 150 ± 20 | 98.4 ± 0.5 | 220–280 | GreenCircle-certified | Nanocatalyzed hydrolysis (TRL5) |
| 7 | Supercritical ethanol | Very Low | 190 ± 25 | 99.2 ± 0.3 | 350–500 | Cradle-to-Cradle Gold | CO2-expanded ethanol (TRL4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mustafa, L.; Usman, M.; Ali, S.; Ali, A.; Naveed, A. Recycling Technologies for Extracting Gallium from Light-Emitting Diodes. Photonics 2025, 12, 808. https://doi.org/10.3390/photonics12080808
Mustafa L, Usman M, Ali S, Ali A, Naveed A. Recycling Technologies for Extracting Gallium from Light-Emitting Diodes. Photonics. 2025; 12(8):808. https://doi.org/10.3390/photonics12080808
Chicago/Turabian StyleMustafa, Laraib, Muhammad Usman, Shazma Ali, Ahmed Ali, and Anis Naveed. 2025. "Recycling Technologies for Extracting Gallium from Light-Emitting Diodes" Photonics 12, no. 8: 808. https://doi.org/10.3390/photonics12080808
APA StyleMustafa, L., Usman, M., Ali, S., Ali, A., & Naveed, A. (2025). Recycling Technologies for Extracting Gallium from Light-Emitting Diodes. Photonics, 12(8), 808. https://doi.org/10.3390/photonics12080808





