1. Introduction
Interferometric scattering microscopy (iSCAT) has emerged as a powerful tool for visualizing nanoscale objects, offering distinct advantages over fluorescence-based techniques. By leveraging the scattering of light from nanoparticles or single molecules, iSCAT achieves enhanced contrast without the limitations of fluorescence saturation [
1], photo-bleaching [
2] or photo-blinking [
3], which hinder long-term, reliable tracking of the samples [
4,
5,
6]. Previous publication has demonstrated the feasibility of tracking 5 nm gold nanoparticles (GNPs) with iSCAT [
7], enabling the identification of molecular attachments such as lipids [
8,
9,
10] and proteins [
11,
12,
13]. Subsequent advancements extended label-free tracking to single proteins through innovations in contrast enhancement. Central to these improvements is extending the shot-noise limit [
14], a critical bottleneck in iSCAT systems. In the early stage of iSCAT, non-polarizing beam splitters were widely used [
15]. In the later iterations of iSCAT setups, polarizing beam splitters and wave plates were employed to suppress reference light intensity [
16,
17,
18], effectively minimizing background noise while preserving scattered light signals. For instance, spatial filtering of transmitted or reflected components reduced reference light dominance, enhancing extinction contrast for scatterers near interfaces [
19,
20,
21]. Similarly, dark-field configurations, which block reflected light using a sub-aperture mask in the objective’s back focal plane, localized GNPs at an exposure time of 6 μs [
22].
Despite these advancements, the intrinsic trade-off between reference light suppression and scattering signal reduction remains a challenge. While reducing reference light intensity mitigates background noise, it simultaneously weakens the scattered light from the target, compromising the dynamic range of detectable signals [
19]. To address this, computational approaches have been developed to extract weak scattering signals from noisy backgrounds. For example, frame averaging [
23], differential rolling averages [
24] and ratio metric [
25] algorithms are designed to stabilize noise by averaging multiple scans of the same imaging region at the expense of temporal resolution. The iterative noise estimation method further refined this process but often requires extensive computational resources [
26]. More recently, self-supervised machine learning has been applied to particle identification, offering high sensitivity, though at the cost of interpretability [
27]. These solutions either rely on frame-consuming averaging or prior labeled training.
A transformative approach to overcoming these barriers lies in deconvolution algorithms, which reverse the blurring effects of the imaging system. Deconvolution is particularly effective in iSCAT, which enhances SNR and contrast by reconstructing sharp images from noisy data. The Richardson–Lucy (RL) deconvolution framework, for instance, has been validated in diverse imaging modalities, from underwater acoustic imaging [
28] to high-resolution X-ray tomography [
29], demonstrating its versatility in extracting fine structural details. Deconvolutions have not yet been introduced in iSCAT; we demonstrated that a spatial–frequency domain deconvolution (SF) not only improves contrast but also sharpens particle localization, making it ideal for quantitative mass photometric analysis. By reducing nanoparticle localization errors through SNR enhancement, deconvolution enables more precise tracking and dynamic analysis. Comparative studies of deconvolution and denoising algorithms reveal that SF outperforms conventional methods in preserving spatial resolution, underscoring its potential to advance iSCAT’s capabilities in single-molecule and nanoscale imaging. Collectively, with these optical and computational innovations, the inherent challenges of low-light imaging with iSCAT can be efficiently mitigated, paving the way for molecular dynamics and biophysical processes tracking with deep insights.
3. Results and Discussion
To evaluate the deconvolution performance in signal recovery and background suppression for particle imaging in iSCAT, we imaged a gold nanoparticle (GNP) sample and compared the raw data with deconvolved results. A spatial and frequency domain that combined deconvolution and filtering algorithm (SF) was employed to mitigate high-frequency noise (artifact speckles in the spatial domain), using a Gaussian function as the convolution kernel, derived via OpenSIM [
30]. The SF deconvolution method combined Wiener deconvolution, RL deconvolution and a low-pass filter in the frequency domain, thereby processing signal and background noise in both spatial and frequency domains.
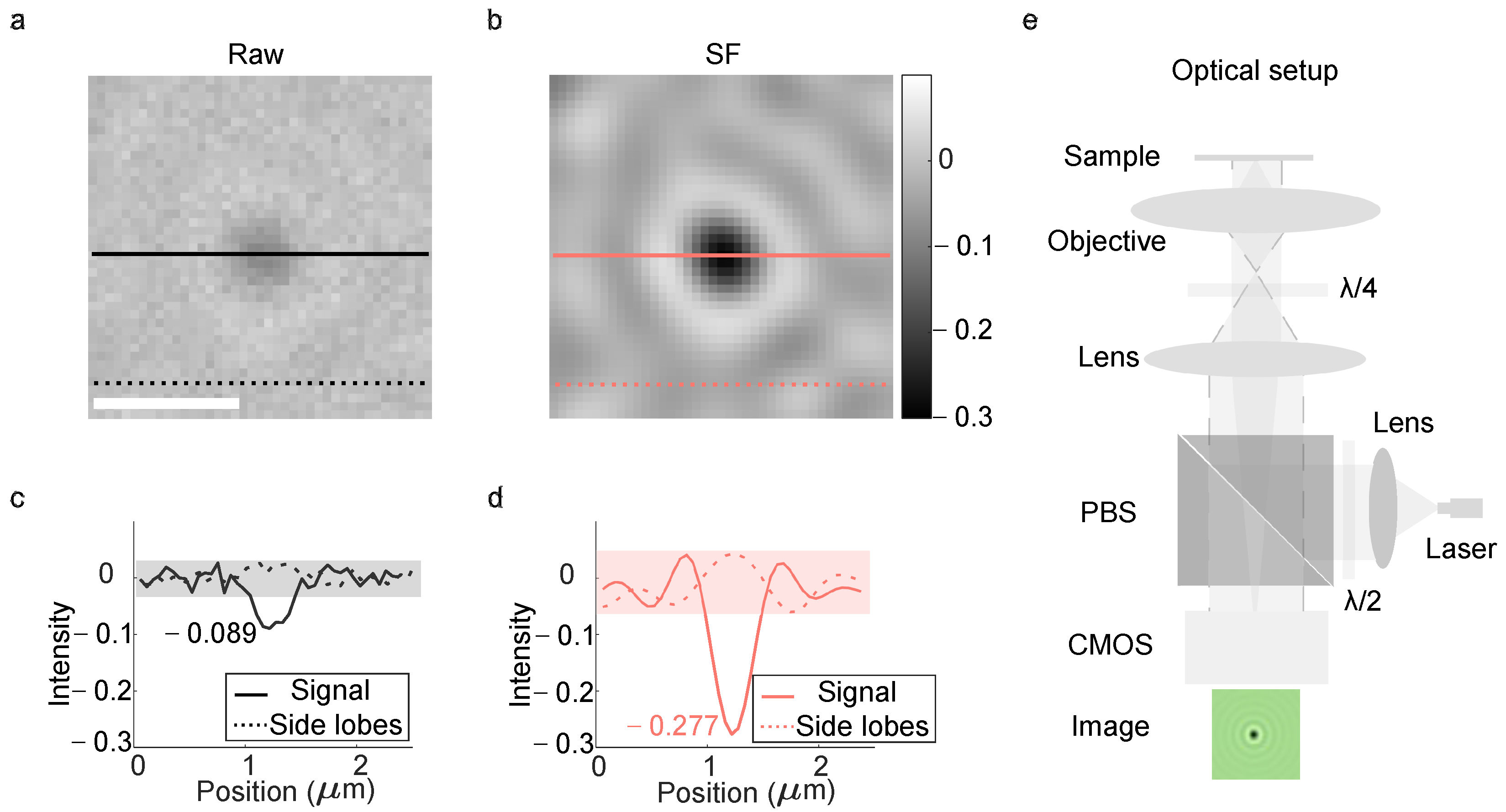
Figure 1a shows the raw GNP image. The corresponding deconvolved image using the SF method is presented in
Figure 1b, where the interference signal appears darker compared to
Figure 1a (as indicated by the color bar).
Figure 1c provides quantitative intensity profiles: the raw image’s signal contrast (solid dark line) was approximately −0.089, whereas deconvolution improved this to −0.277 (solid red line). Intensity profiles along the solid dark and red lines in
Figure 1a and
Figure 1b were displayed in
Figure 1c and
Figure 1d, respectively (same color coding). Notably, the raw image’s dashed-dark line exhibits intensity fluctuations with pronounced spikes (dashed line in
Figure 1c), while the deconvolved image’s dashed-red line shows a smoother profile with distinct peaks and valleys (dashed line in
Figure 1c), indicating reduced noise artifacts. The optical setup is depicted in
Figure 1e.
A collimated laser (wavelength of 520 nm) was reflected by the polarized beam splitter and focused on the back focal plane of the objective, resulting in a plane-wave reference illuminated on the cover glass and GNPs sample. The half-wave plate was inserted between the collimation lens and the polarized beam splitter to change the laser’s polarization into a s polarization for high reflection. The quarter-wave plate between the imaging lens and the objective was to maintain the symmetry of the point spread function in the image plane, since the shape of the point spread function changes with the polarization.
Deconvolution improved the signal contrast by threefold (
Figure 1c), demonstrating its effectiveness in iSCAT imaging. In optical imaging, the image formation process is modeled as the convolution of an object with a point spread function (PSF) kernel, superimposed with noise. Signal attenuation occurs due to the PSF’s non-uniformity, often modeled as a Gaussian function. SF deconvolution compensates for this attenuation in both spatial and frequency domains. In iSCAT, the interference of spherical and plane waves generate concentric dark–bright ring “side lobes” around the central dark spot (termed the interference PSF). In
Figure 1a, these side lobes are obscured by random noise, appearing as spikes in the dashed-dark line profile (
Figure 1c). Deconvolution suppresses this noise, revealing the underlying side lobes as distinct peaks and valleys in the dashed-red line profile (
Figure 1d), thereby reconstructing the true interference pattern.
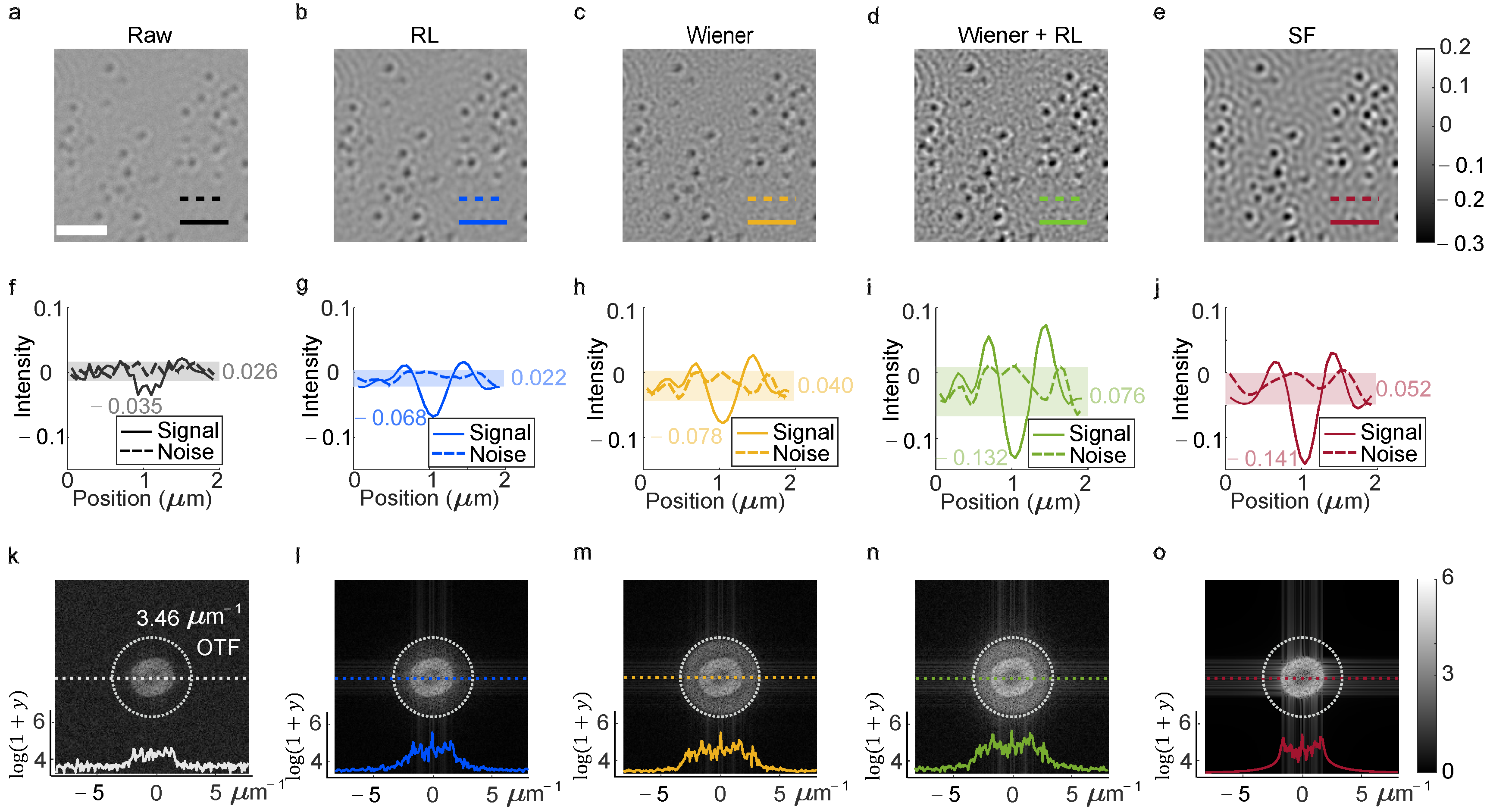
To evaluate deconvolution techniques across spatial and frequency domains, Wiener, RL, Wiener-assisted RL and SF deconvolutions were applied to raw image data (
Figure 2a–e). Wiener deconvolution operates in the frequency domain (
Figure 2b), while RL deconvolution is a spatial-domain method (
Figure 2c). The Wiener-assisted RL approach integrates both domains (
Figure 2d), and SF deconvolution incorporates a low-pass filter to suppress noise (
Figure 2e). Spatial-domain metrics, such as signal contrast and background noise fluctuations, were quantified in
Figure 2f (intensity along cut-lines in
Figure 2a) as a baseline. Deconvoluted images (
Figure 2g–j) revealed enhanced features and modulated backgrounds: central dark spots and noise became more distinct compared to unprocessed images. Frequency-domain analysis (
Figure 2k–o) demonstrated how each method addressed attenuated spectral components.
In
Figure 2f,g, background noise along the dashed line in
Figure 2a was reduced from 0.026 to 0.022, and signal contrast improved from −0.035 to −0.068. RL deconvolution doubled the contrast without increasing noise (
Figure 2g). Wiener deconvolution (
Figure 2h) achieved a contrast of −0.078 but introduced speckles in the background, raising noise to 0.040. The combined Wiener-assisted RL method (
Figure 2d,i) sharpened the image, enhanced contrast to −0.132 and increased intensity along the cut-line to 0.076, though background speckles persisted. SF deconvolution (
Figure 2e,j) further reduced noise while preserving signal contrast, outperforming the combined method.
The raw image’s refraction-limited frequency spectrum (
Figure 2k) showed peripheral components above zero, indicating residual high-frequency noise from the aperture-limited system. Deconvolution (
Figure 2l–o) suppressed these artifacts, with brighter central regions (
Figure 2l–o) revealing recovered signal, consistent with enhanced spatial and spectral contrast (
Figure 2b–e,l–o). The dark ring in
Figure 2o did not degrade signal contrast in
Figure 2e,j, suggesting the effective OTF region in iSCAT is narrower than the system’s theoretical limit. The white dashed circle represented the cutoff frequency of the OTF in
Figure 2k–o. The cutoff frequency was 3.462 μm
−1. The horizontal coordinates represent the frequency and the vertical coordinates represent the intensity of frequency; these coordinates were labeled in
Figure 2.
The RL deconvolution reduced the noise based on a Poisson noise model and the cut-line intensity variance was reduced from 0.026 in
Figure 2f to 0.022 in
Figure 2g. The rest of the variance of the background with low frequency is the fringe generated from interference which can be treated as the interference feather for detection. Additionally, noise in the frequency domain was introduced by Wiener deconvolution, which was suppressed by the low-pass filter in the frequency domain.
While deconvolution methods compensated for signal loss due to aperture limitations, their noise performance varied. Wiener deconvolution (
Figure 2b,l) amplified background noise via an imprecise two-parameter power spectrum model, introducing speckles. RL deconvolution (
Figure 2g) preserved spatial detail without adding high-frequency noise. The SF approach (
Figure 2e–j) balanced noise reduction and contrast enhancement through low-pass filtering. In contrast, frame-averaging techniques (e.g., differential rolling average or ratio metric) reduced noise but required processing hundreds of frames, whereas deconvolution improved contrast directly without averaging for each frame.
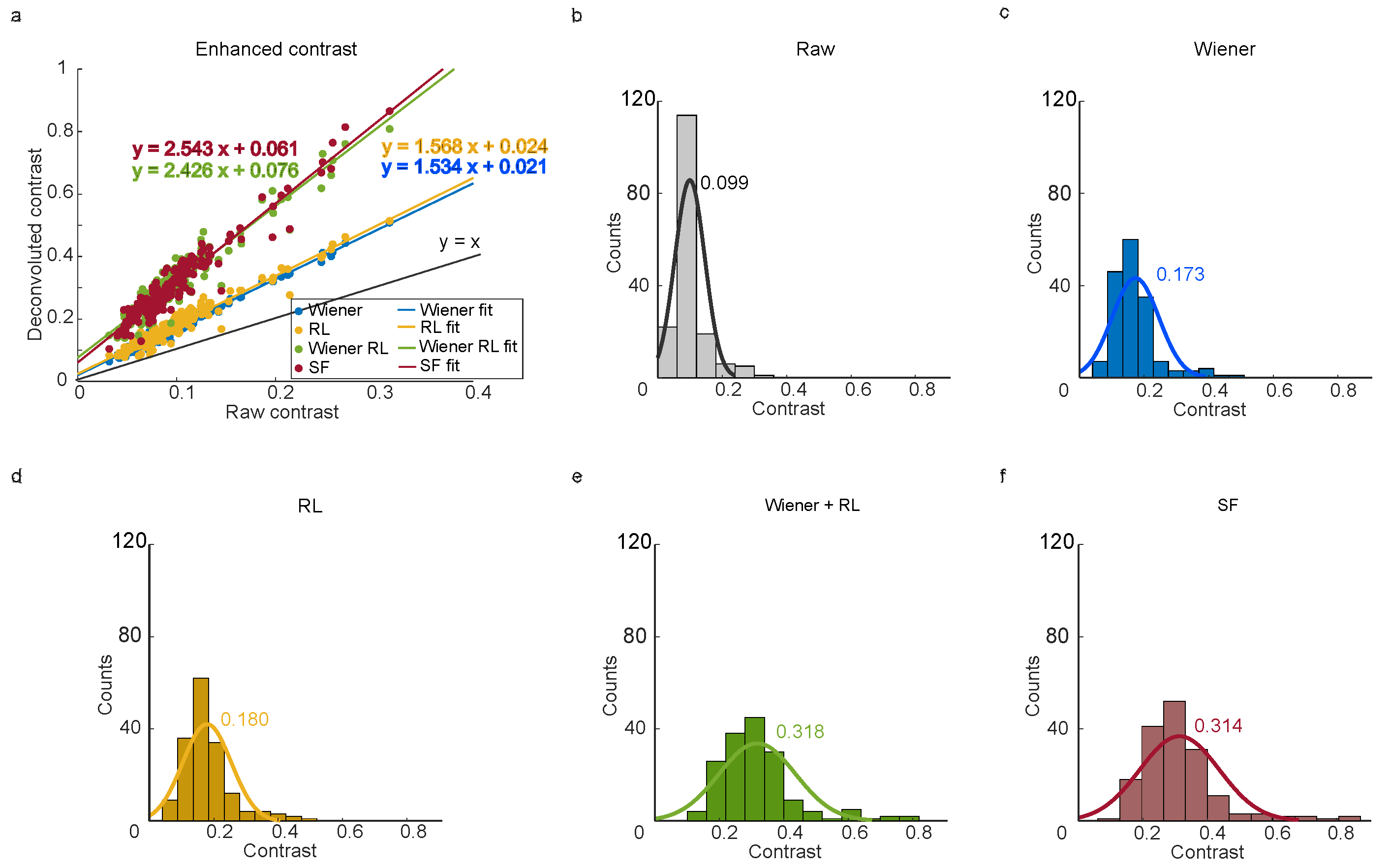
Quantitative analysis is central to iSCAT-based functional measurements, such as determining nanoparticle diameter, mass and surface charge. To assess the impact of image processing techniques, 10 nm gold nanoparticles (GNPs) were analyzed, with statistical comparisons between the raw and the deconvolved images (
Figure 3). Deconvolution was applied to enhance image quality and resolve low-contrast features critical for accurate quantification.
The deconvolved images showed marked improvements in contrast compared to raw images. Linear regression analysis of raw and deconvolved data (
Figure 3a, colored lines) revealed consistent slopes of 1.5, 1.5, 2.5 and 2.5, with coefficients of determination (R
2) of 0.976, 0.935, 0.906 and 0.924, respectively. Statistical histograms of contrast distributions (
Figure 3b–f) further demonstrated deconvolution’s effectiveness. For example, the Gaussian-fitted peak contrast in raw images (0.099,
Figure 3b) increased to 0.173 (Wiener deconvolution,
Figure 3c) and 0.180 (RL deconvolution,
Figure 3d), representing a 1.7-fold improvement in contrast. Wiener-assisted RL deconvolution achieved a contrast of 0.318, with a comparable 0.314 contrast in the spatial–frequency (SF) deconvolved image, representing a ∼ 3-fold improvement in contrast. These enhancements enabled clearer resolution of nanoparticle features, as evidenced by the higher peak contrasts in processed data.
The strong linear correlation between raw and deconvolved contrast, supported by high R2 values, highlights the deconvolution algorithms’ ability to preserve quantitative accuracy while amplifying signal strength. These findings are particularly valuable for high-sensitivity applications, such as nanoparticle characterization. In raw images, low-contrast particles are often masked by background noise, limiting detection sensitivity. Frame-averaging methods yield similar contrast but require hundreds of frames, whereas deconvolution suppresses noise and sharpens particle signals, improving both contrast and signal-to-noise ratios. This dual benefit—enhanced contrast and reduced noise—enables the detection of smaller or sparsely distributed nanoparticles within single frames. By addressing these challenges, deconvolution emerges as a vital tool for advancing iSCAT’s precision in quantitative nanoscale imaging. Future work could explore adaptive deconvolution strategies to account for spatially variant point spread functions (PSFs), further refining the accuracy of functional measurements.
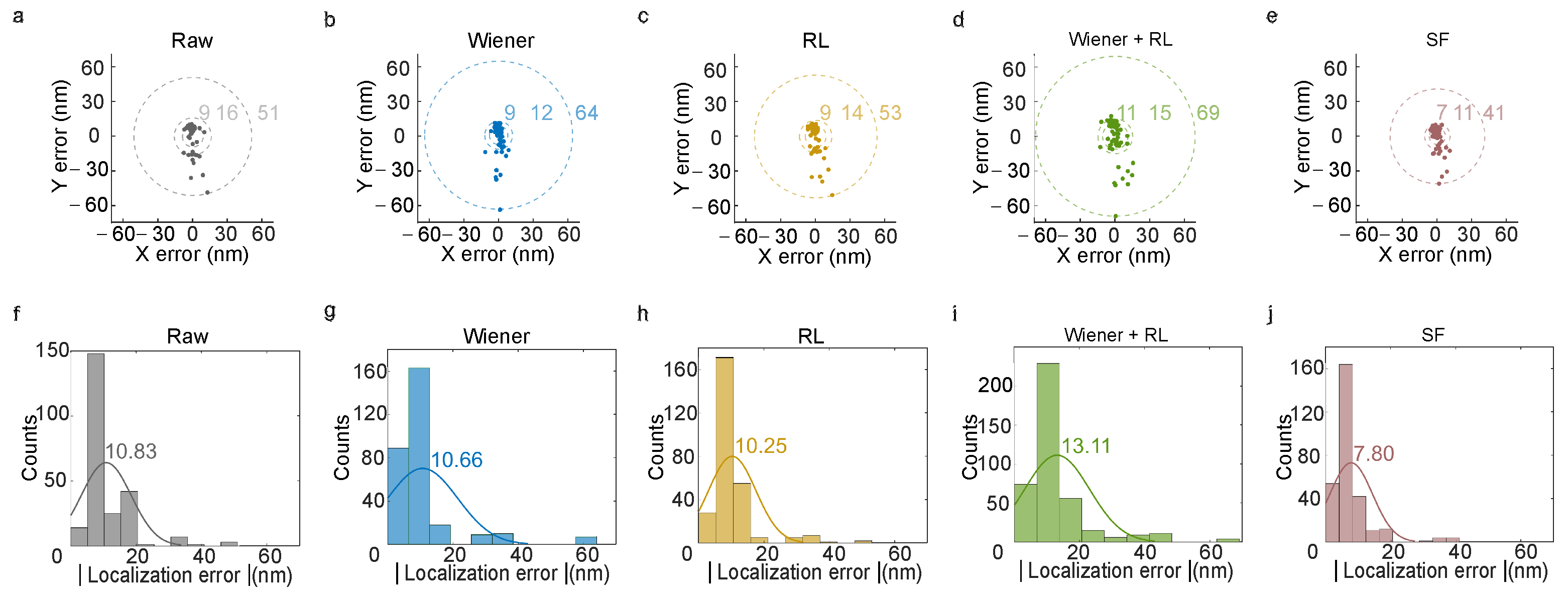
Localization precision is fundamentally tied to the signal-to-noise ratio (SNR). Deconvolution techniques enhance image contrast and reduce noise artifacts, directly benefiting localization accuracy. In this study, localization errors were quantified by measuring the mean shift in individual particles in raw and deconvoluted images. Error values were calculated as the difference between each particle’s shift distance and the overall mean shift. Sample particles were displaced by a piezo stage.
For raw images (
Figure 4a), 50% of particles exhibited localization errors below 9 nm, 80% below 16 nm and the maximum error remained under 51 nm. After Wiener deconvolution (
Figure 4b), these thresholds improved to 11 nm (50%), 15 nm (80%) and 64 nm (100%). RL deconvolution (
Figure 4c) further refined these to 9 nm (50%), 13 nm (80%) and 53 nm (100%), respectively. However, combining Wiener and RL deconvolution (
Figure 4d) paradoxically increased localization errors to 11 nm (50%), 15 nm (80%) and 69 nm (100%). Strikingly, applying the SF deconvolution method (
Figure 4e) restored and improved these values to 7 nm (50%), 11 nm (80%) and 41 nm (100%).
Histograms and Gaussian-fitted distributions (
Figure 4f–j) further illustrate these trends. The fitted peak localization error in raw images was 10.83 nm. While Wiener and RL deconvolution achieved 10.66 nm (
Figure 4g) and 10.25 nm (
Figure 4h), respectively, the hybrid Wiener-RL method increased the peak error to 13.11 nm (
Figure 4i). SF deconvolution, however, reduced this to 7.80 nm (
Figure 4j), demonstrating its superior performance.
The systematic reduction in localization errors highlights the efficacy of SF deconvolution in refining spatial resolution. By concentrating errors into narrower ranges, SF deconvolution aligns with theoretical expectations: enhanced SNR and contrast directly correlate with improved localization accuracy (
Figure 1,
Figure 2 and
Figure 3). While the raw image median error was 10.83 nm, SF deconvolution reduced this to 7.80 nm. These findings reveal a trade-off between computational complexity (e.g., RL’s iterative approach) and error suppression, with SF deconvolution striking an optimal balance by progressively mitigating noise while preserving critical signal features.
This trend validates the hypothesis that SF deconvolution optimizes SNR through the suppression of out-of-focus light while retaining in-plane detail. The improved performance is particularly evident in SF-deconvoluted images, where sub-diffraction imaging achieves greater statistical confidence. Collectively, these results demonstrate that deconvolution not only sharpens image contrast but also quantifiably enhances the reliability of single-particle localization. Future work could explore adaptive deconvolution strategies to further tailor error profiles for specific imaging conditions.
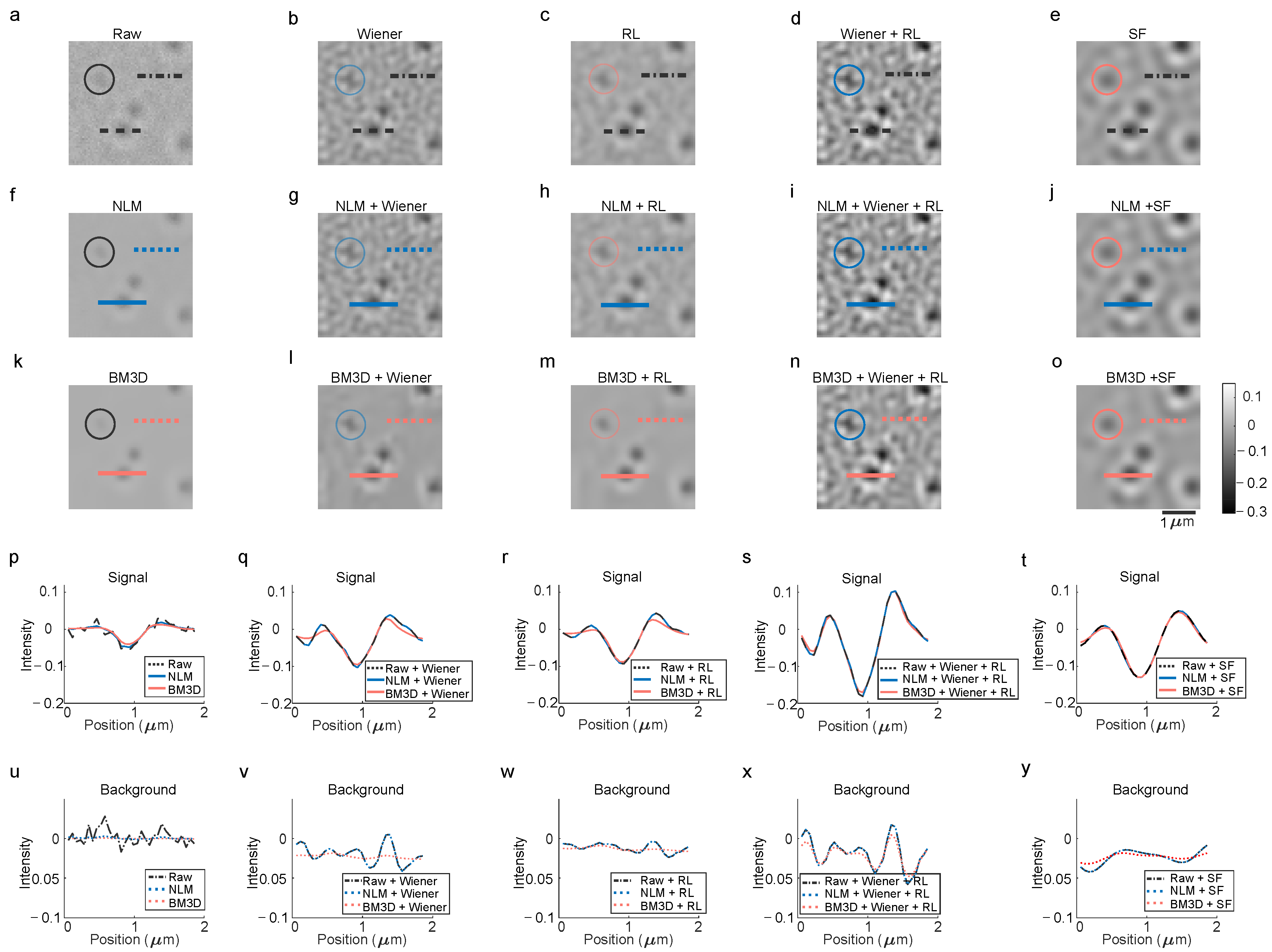
To systematically evaluate deconvolution algorithms, raw images and their processed counterparts (via deconvolution and denoising) were compared in a structured matrix format, as illustrated in
Figure 5a–o. Signal intensity and background noise along designated lines in these images were quantified to analyze contrast and noise behavior (
Figure 5p–y). The evaluation included Non-Local Means (NLMs) [
31] and Block-Matching 3D (BM3D) [
32] denoising algorithms, alongside spatial and frequency domain deconvolution methods: Wiener, RL, Wiener-assisted RL and SF.
In the raw image (
Figure 5a), a weak particle spot transformed into a sharp, asymmetric pattern under Wiener deconvolution (
Figure 5b), while RL deconvolution (
Figure 5c) produced a softer, more diffuse shape. The Wiener-assisted RL approach (
Figure 5d) further enhanced asymmetry, which was symmetrized by the SF method (
Figure 5e). NLM denoising (
Figure 5f–j) preserved particle patterns from the raw image (
Figure 5a–e) but showed minimal improvement in background noise suppression (
Figure 5b–e vs.
Figure 5g–j). Similarly, BM3D denoising (
Figure 5k–o) smoothed the background while maintaining consistent particle patterns, though residual side lobes—interference feathers from plane-wave and spherical-wave interference—remained in the SF-deconvoluted image (
Figure 5e).
Signal intensity profiles along particle-crossing lines (
Figure 5a–o) were plotted in
Figure 5p–t, with the corresponding background noise analyzed in
Figure 5u–y. For example, raw (
Figure 5a), NLM-denoised (
Figure 5f) and BM3D-denoised (
Figure 5k) images were compared in
Figure 5p. Subsequent plots (
Figure 5q–t) detailed the effects of combining deconvolution with NLM or BM3D denoising. While NLM and BM3D reduced background noise and smoothed signals, they also blurred fine contrasts and suppressed side lobes. Notably, speckle artifacts introduced by Wiener-based deconvolutions were partially filtered but introduced a bias depending on speckle density.
Under iSCAT imaging, where signals manifest as discrete intensity peaks (typically 0–1 contrast), noise fluctuations often obscure weak features. This highlights a critical trade-off: deconvolution enhances resolution but may amplify noise, while aggressive denoising risks signal loss. The results underscore the need for balanced preprocessing strategies that optimize contrast preservation and noise reduction to maintain fidelity in high-resolution imaging.