Photodynamic Therapy in the Management of MDR Candida spp. Infection Associated with Palatal Expander: In Vitro Evaluation
Abstract
1. Introduction
- Orthodontic appliances and impact on oral microbiota
- Oral microbiota differences in fixed orthodontic appliances and aligners
- Clinical approach against oral infections due to palatal expanders
- Photodynamic Therapy (PDT) in orthodontics
2. Materials and Methods
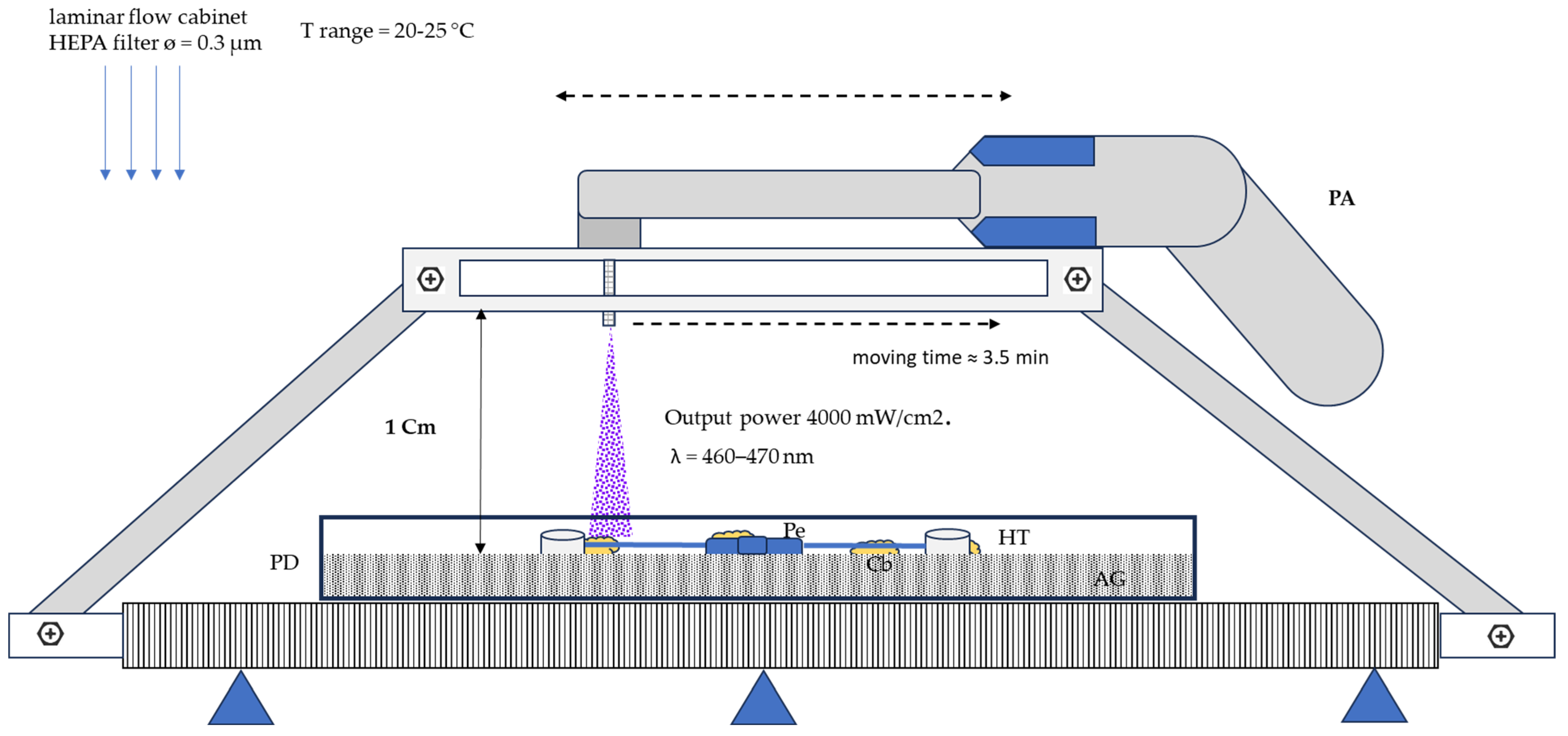
2.1. Photodynamic Therapy Treatment Procedure
2.2. In Vitro Experiment
- ✔
- ωt = medium oscillations min per cm2, regulated by tilting apparatus.
- ✔
- A = occupied cross-sectional area of the ø 90 mm Petri dish in cm2.
- ✔
- V = the available volume of the liquid medium (6.5 mL).
2.3. Irradiation Experimental Setup
- Output power: 4000 mW/cm2.
- Wavelength range from 460 to 470 nm.
- Irradiation cone (about 1 cm) for each treatment point.
- The distance from the PFT apparatus to the palatal expander: 2 cm.
- Time of exposition for each treatment point = 10 s.
- Total irradiation time/prosthesis = 3.5 min.
2.4. Statistical Analysis
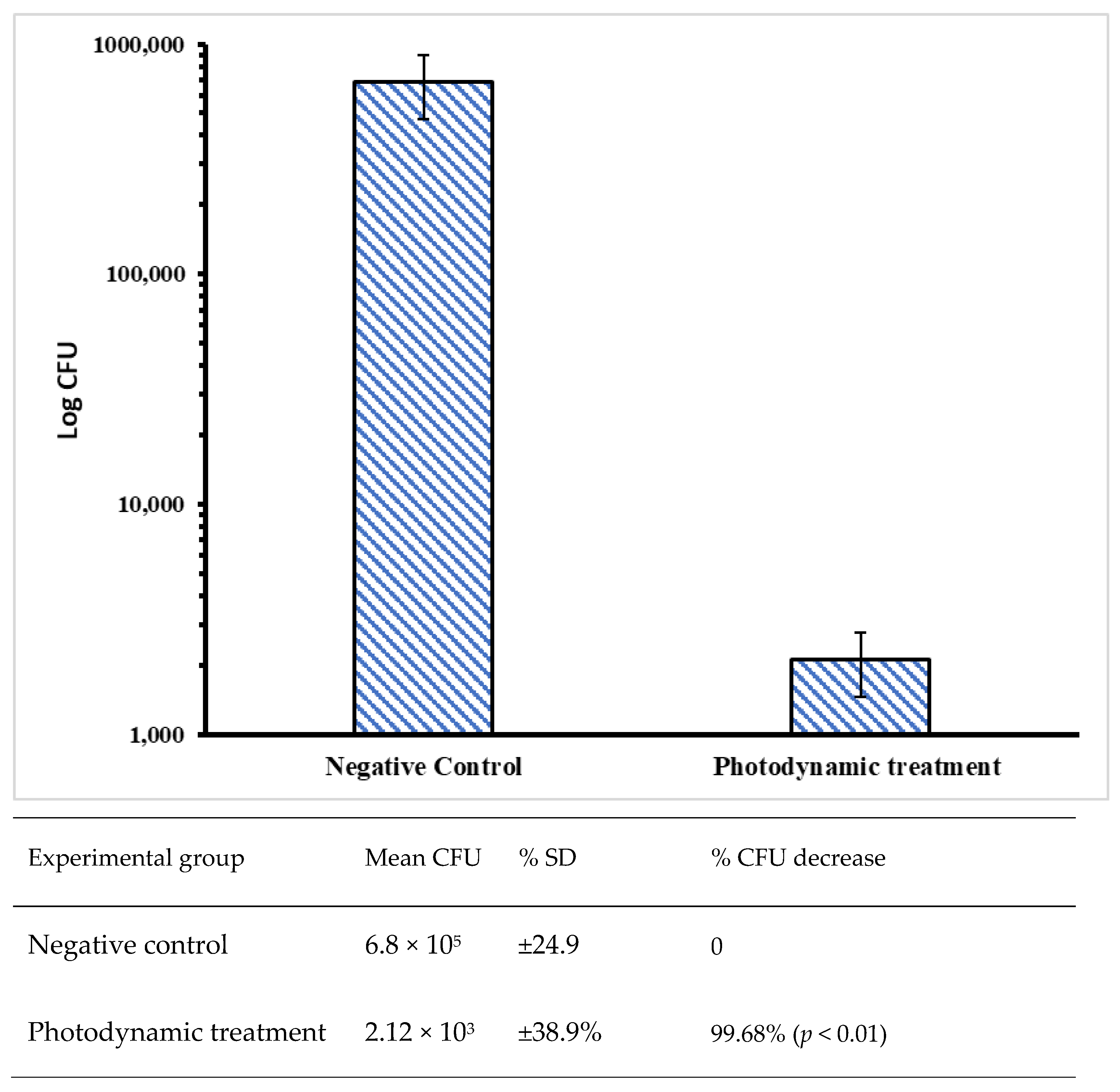
3. Results
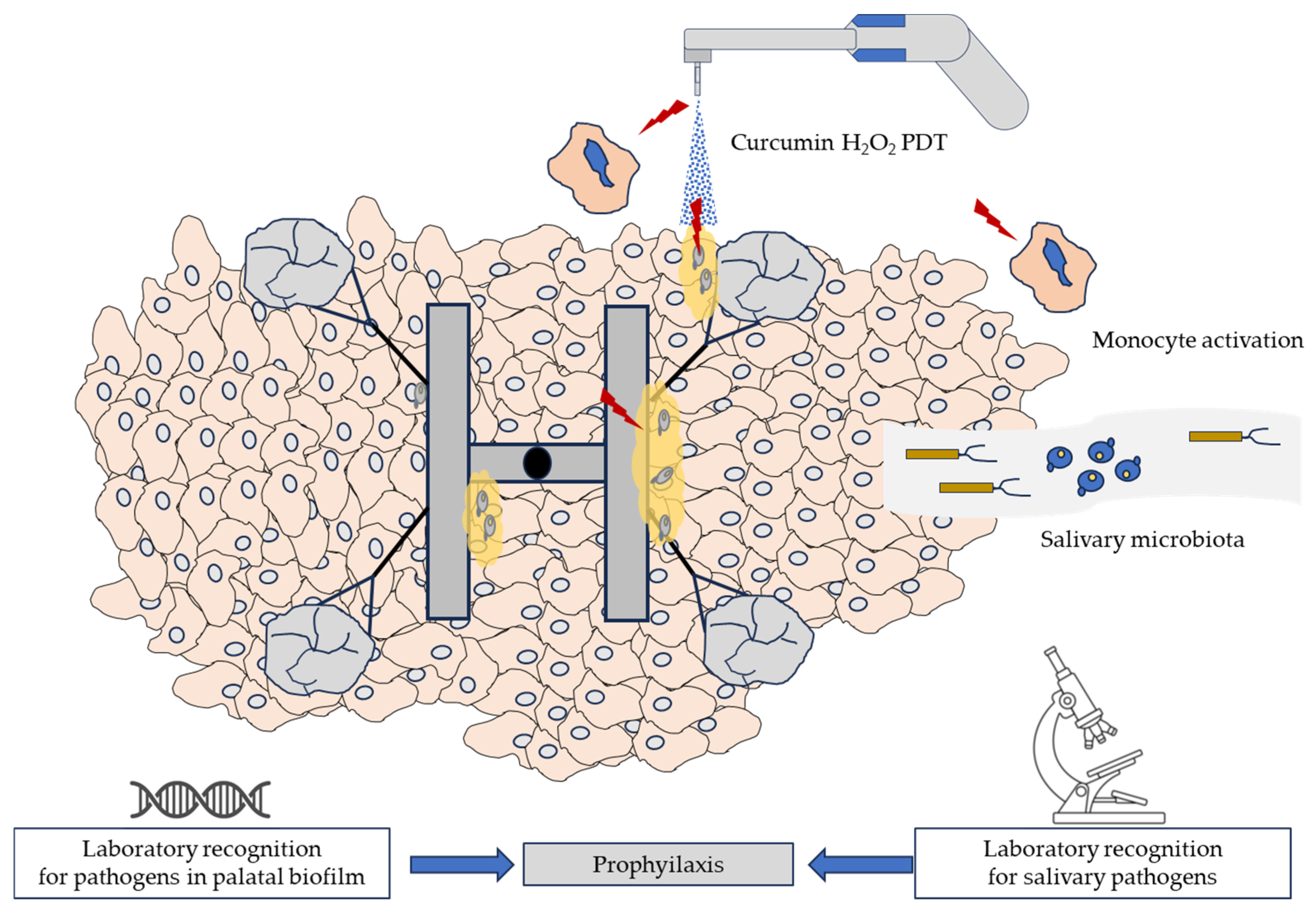
4. Discussion
- Study limitations and future perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Klug, B.; Rodler, C.; Koller, M.; Wimmer, G.; Kessler, H.H.; Grube, M.; Santigli, E. Oral biofilm analysis of palatal expanders by fluorescence in-situ hybridization and confocal laser scanning microscopy. J. Vis. Exp. 2011, 56, 2967. [Google Scholar] [CrossRef]
- Ferro, R.; Besostri, A.; Olivieri, A.; Quinzi, V.; Scibetta, D. Prevalence of cross-bite in a sample of Italian preschoolers. Eur. J. Paediatr. Dent. 2016, 17, 307–309. [Google Scholar] [PubMed]
- Carli, E.; Fambrini, E.; Lardani, L.; Derchi, G.; Defabianis, P. Early orthodontic treatment need in paediatric age: A prospective observational study in Italian school-children. Eur. J. Paediatr. Dent. 2023, 24, 94–98. [Google Scholar] [CrossRef]
- Wysocki, M.; Kobus, K.; Szotek, S.; Kobielarz, M.; Kuropka, P.; Będziński, R. Biomechanical effect of rapid mucoperiosteal palatal tissue expansion with the use of osmotic expanders. J. Biomech. 2011, 44, 1313–1320. [Google Scholar] [CrossRef]
- Lemos, M.M.; Cattaneo, P.M.; Melsen, B.; Faveri, M.; Feres, M.; Figueiredo, L.C. Impact of Treatment with Full-fixed Orthodontic Appliances on the Periodontium and the Composition of the Subgingival Microbiota. J. Int. Acad. Periodontol. 2020, 22, 174–181. [Google Scholar]
- Lovrov, S.; Hertrich, K.; Hirschfelder, U. Enamel Demineralization during Fixed Orthodontic Treatment - Incidence and Correlation to Various Oral-hygiene Parameters. J. Orofac. Orthop. 2007, 68, 353–363. [Google Scholar] [CrossRef]
- Sharab, L.; Loss, C.; Kluemper, A.; Nagaoka, H.; Hawk, G.; Beeman, C. Effect of patients’ attitude and perception of oral hygiene on white spot lesion development and plaque accumulation during orthodontic treatment: A survey of patients with fixed appliances. J. Orofac. Orthop. 2024, 85 (Suppl. S1), 34–40. [Google Scholar] [CrossRef]
- Bud, E.; Vlasa, A.; Pacurar, M.; Matei, A.; Bud, A.; Szoke, A.-R.; Minervini, G. A Retrospective Histological Study on Palatal and Gingival Mucosa Changes during a Rapid Palatal Expansion Procedure. Biomedicines 2023, 11, 3246. [Google Scholar] [CrossRef]
- Brzezińska-Zając, A.; Sycińska-Dziarnowska, M.; Spagnuolo, G.; Szyszka-Sommerfeld, L.; Woźniak, K. Candida Species in Children Undergoing Orthodontic Treatment with Removable Appliances: A Pilot Study. Int. J. Environ. Res. Public Health 2023, 20, 4824. [Google Scholar] [CrossRef]
- Grzegocka, K.; Krzyściak, P.; Hille-Padalis, A.; Loster, J.E.; Talaga-Ćwiertnia, K.; Loster, B.W. Candida prevalence and oral hygiene due to orthodontic therapy with conventional brackets. BMC Oral Health 2020, 20, 277. [Google Scholar] [CrossRef] [PubMed]
- Nowak, R.; Przywitowski, S.; Golusiński, P.; Olejnik, A.; Zawiślak, E. Complications of Surgically Assisted Rapid Maxillary/Palatal Expansion (SARME/SARPE)—A Retrospective Analysis of 185 Cases Treated at a Single Center. J. Clin. Med. 2024, 13, 2053. [Google Scholar] [CrossRef]
- Kado, I.; Hisatsune, J.; Tsuruda, K.; Tanimoto, K.; Sugai, M. The impact of fixed orthodontic appliances on oral microbiome dynamics in Japanese patients. Sci. Rep. 2020, 10, 21989. [Google Scholar] [CrossRef] [PubMed]
- Lucchese, A.; Bondemark, L.; Marcolina, M.; Manuelli, M. Changes in oral microbiota due to orthodontic appliances: A systematic review. J. Oral Microbiol. 2018, 10, 1476645. [Google Scholar] [CrossRef]
- Topaloglu-Ak, A.; Ertugrul, F.; Eden, E.; Ates, M.; Bulut, H. Effect of orthodontic appliances on oral microbiota—6 month follow-up. J. Clin. Pediatr. Dent. 2011, 35, 433–436. [Google Scholar] [CrossRef] [PubMed]
- Türkkahraman, H.; Sayin, M.O.; Bozkurt, F.Y.; Yetkin, Z.; Kaya, S.; Onal, S. Archwire ligation techniques, microbial colonization, and periodontal status in orthodontically treated patients. Angle Orthod. 2005, 75, 231–236. [Google Scholar] [PubMed]
- Santonocito, S.; Polizzi, A. Oral Microbiota Changes during Orthodontic Treatment. Front. Biosci.-Elite 2022, 14, 19. [Google Scholar] [CrossRef]
- Mummolo, S.; Nota, A.; Albani, F.; Marchetti, E.; Gatto, R.; Marzo, G.; Quinzi, V.; Tecco, S. Salivary levels of Streptococcus mutans and Lactobacilli and other salivary indices in patients wearing clear aligners versus fixed orthodontic appliances: An observational study. PLoS ONE 2020, 15, e0228798. [Google Scholar] [CrossRef]
- Ahn, S.-J.; Lim, B.-S.; Lee, S.-J. Prevalence of cariogenic streptococci on incisor brackets detected by polymerase chain reaction. Am. J. Orthod. Dentofac. Orthop. 2007, 131, 736–741. [Google Scholar] [CrossRef]
- Bergamo, A.Z.N.; Nelson-Filho, P.; Andrucioli, M.C.D.; do Nascimento, C.; Pedrazzi, V.; Matsumoto, M.A.N. Microbial complexes levels in conventional and self-ligating brackets. Clin. Oral Investig. 2017, 21, 1037–1046. [Google Scholar] [CrossRef]
- Maruo, I.T.; Rosa, E.A.R.; Maruo, H.; Tanaka, O.; Guariza Filho, O.; Ignácio, S.A.; Camargo, E.S. Effect of chlorhexidine mouth rinse on Streptococci counts of tooth-tissue-borne palatal expander biofilm. Orthod. Craniofacial Res. 2008, 11, 136–142. [Google Scholar] [CrossRef]
- Pellissari, B.A.; Sabino, G.S.P.; de Souza Lima, R.N.; Motta, R.H.L.; Suzuki, S.S.; Garcez, A.S.; Basting, R.T.; Barbosa, J.A.; Martins Montalli, V.A. Antimicrobial resistance of bacterial strains in patients undergoing orthodontic treatment with and without fixed appliances. Angle Orthod. 2021, 91, 672–679. [Google Scholar] [CrossRef] [PubMed]
- Abid, S.; Uzair, B.; Niazi, M.B.K.; Fasim, F.; Bano, S.A.; Jamil, N.; Batool, R.; Sajjad, S. Bursting the Virulence Traits of MDR Strain of Candida albicans Using Sodium Alginate-based Microspheres Containing Nystatin-loaded MgO/CuO Nanocomposites. Int. J. Nanomed. 2021, 16, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.M.; Silva, T.P.; Lemos, A.S.d.O.; Diniz, I.O.M.; Palazzi, C.; da Rocha, V.N.; Araújo, M.G.d.F.; Melo, R.C.; Fabri, R.L. Antibiofilm potential of Annona muricata L. ethanolic extract against multi-drug resistant Candida albicans. J. Ethnopharmacol. 2023, 315, 116682. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Gomes, A.S.; Curvelo, J.A.R.; Soares, R.M.A.; Ferreira-Pereira, A. Curcumin acts synergistically with fluconazole to sensitize a clinical isolate of Candida albicans showing a MDR phenotype. Med. Mycol. 2012, 50, 26–32. [Google Scholar] [CrossRef]
- Sharifzadeh, S.S.; Lotfali, E.; Lesan, S.; Farrokhnia, T. Antifungal Effect of Probiotic Lactobacillus casei on Drug-Resistant Oral Candida albicans Isolated from Patients with Hematological Malignancy: An in vitro Study. J. Dent. 2023, 24, 389–394. [Google Scholar] [CrossRef]
- Sareen, S.; Gupta, S.; Goyal, L. Ozone therapy as a complementary approach in periodontal therapeutics. Evid.-Based Dent. 2025, 26, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Butera, A.; Giordano, A.; Gallo, S.; Pascadopoli, M.; Scribante, A.; Albanese, M. Photodynamic Therapy in Non-Surgical Treatment of Periodontitis: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 1086. [Google Scholar] [CrossRef]
- Haque, T.; Albagieh, H.; Akhter, F.; Alkahwaji, A. Effectiveness of Photodynamic Therapy in the Treatment of Oral Diseases: A Reality or Myth? Photobiomodul. Photomed. Laser Surg. 2025, 43, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Casu, C.; Orrù, G.; Scano, A. Curcumin/H2O2 photodynamically activated: An antimicrobial time-response assessment against an MDR strain of Candida albicans. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 8841–8851. [Google Scholar] [CrossRef]
- Jing, Y.; Shu, R.; Wu, T.; Liu, D.; Luo, X.; Sun, J.; Chen, F. Clinical efficacy of photodynamic therapy of oral potentially malignant disorder. Photodiagn. Photodyn. Ther. 2024, 46, 104026. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Q.; Liu, D. Development of photodynamic therapy in treating oral diseases. Front. Oral Health. 2025, 5, 1506407. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tkaczyk, M.; Mertas, A.; Kuśka-Kiełbratowska, A.; Fiegler-Rudol, J.; Bobela, E.; Cisowska, M.; Morawiec, T.; Skaba, D.; Wiench, R. In Vitro Evaluation of Candida spp. And Staphylococcus aureus Sensitivity to 450 nm Diode Laser-Mediated Antimicrobial Photodynamic Therapy with Curcumin and Riboflavin. Int. J. Mol. Sci. 2025, 26, 5645. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Olek, M.; Machorowska-Pieniążek, A.; Stós, W.; Kalukin, J.; Bartusik-Aebisher, D.; Aebisher, D.; Cieślar, G.; Kawczyk-Krupka, A. Photodynamic Therapy in Orthodontics: A Literature Review. Pharmaceutics 2021, 13, 720. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Haddadi-Asl, V.; Ghafari, H.-A.; Ghorbanzadeh, R.; Mazlum, Y.; Bahador, A. Shear bond strength, adhesive remnant index, and anti-biofilm effects of a photoexcited modified orthodontic adhesive containing curcumin doped poly lactic-co-glycolic acid nanoparticles: An ex-vivo biofilm model of S. mutans on the enamel slab bonded brackets. Photodiagn. Photodyn. Ther. 2020, 30, 101674. [Google Scholar] [CrossRef]
- Alqerban, A. Effectiveness of Riboflavin and Rose Bengal Photosensitizer Modified Adhesive Resin for Orthodontic Bonding. Pharmaceuticals 2021, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- García-Salazar, E.; Acosta-Altamirano, G.; Betancourt-Cisneros, P.; Reyes-Montes, M.D.R.; Rosas-De-Paz, E.; Duarte-Escalante, E.; Sánchez-Conejo, A.R.; Hernández, E.O.; Frías-De-León, M.G. Detection and Molecular Identification of Eight Candida Species in Clinical Samples by Simplex PCR. Microorganisms 2022, 10, 374. [Google Scholar] [CrossRef]
- Kanbe, T.; Horii, T.; Arishima, T.; Ozeki, M.; Kikuchi, A. PCR-based identification of pathogenic Candida species using primer mixes specific to Candida DNA topoisomerase II genes. Yeast 2002, 19, 973–989. [Google Scholar] [CrossRef]
- Healey, K.R.; Perlin, D.S. Fungal Resistance to Echinocandins and the MDR Phenomenon in Candida glabrata. J. Fungi 2018, 4, 105. [Google Scholar] [CrossRef]
- Hameed, S.; Fatima, Z. Novel Regulatory Mechanisms of Pathogenicity and Virulence to Combat MDR in Candida albicans. Int. J. Microbiol. 2013, 2013, 240209. [Google Scholar] [CrossRef]
- Medaglia, S.; Otri, I.; Bernardos, A.; Marcos, M.D.; Aznar, E.; Sancenón, F.; Martínez-Máñez, R. Synergistic antimicrobial photodynamic therapy using gated mesoporous silica nanoparticles containing curcumin and polymyxin B. Int. J. Pharm. 2024, 654, 123947. [Google Scholar] [CrossRef]
- Ravazzi, T.P.Q.; de Jesus, I.M.; de Oliveira Santos, G.P.; Reis, T.A.; Rosa, L.P.; Rosa, F.C.S. The effects of antimicrobial photodynamic therapy (aPDT) with nanotechnology-applied curcumin and 450nm blue led irradiation on multi-species biofilms in root canals. Lasers Med. Sci. 2023, 38, 254. [Google Scholar] [CrossRef]
- Etemadi, A.; Hamidain, M.; Parker, S.; Chiniforush, N. Blue Light Photodynamic Therapy with Curcumin and Riboflavin in the Management of Periodontitis: A Systematic Review. J. Lasers Med. Sci. 2021, 12, e15. [Google Scholar] [CrossRef]
- Casu, C.; Butera, A.; Piga, A.; Scribante, A.; Fais, S.; Orrù, G. Lactoferrin Solution as a New Natural Photosensitizer in Photodynamic Therapy Against Oral Candida spp. Multidrug-Resistant Isolates: A Preliminary In Vitro Study. Microorganisms 2025, 13, 1255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leite, D.P.V.; Paolillo, F.R.; Parmesano, T.N.; Fontana, C.R.; Bagnato, V.S. Effects of photodynamic therapy with blue light and curcumin as mouth rinse for oral disinfection: A randomized controlled trial. Photomed. Laser Surg. 2014, 32, 627–632. [Google Scholar] [CrossRef] [PubMed]
- Oda, D.F.; Duarte, M.A.H.; Andrade, F.B.; Moriyama, L.T.; Bagnato, V.S.; de Moraes, I.G. Antimicrobial action of photodynamic therapy in root canals using LED curing light, curcumin and carbopol gel. Int. Endod. J. 2019, 52, 1010–1019. [Google Scholar] [CrossRef] [PubMed]
- El-Gendy, A.O.; Ezzat, S.; Samad, F.A.; Dabbous, O.A.; Dahm, J.; Hamblin, M.R.; Mohamed, T. Studying the viability and growth kinetics of vancomycin-resistant Enterococcus faecalis V583 following femtosecond laser irradiation (420–465 nm). Lasers Med. Sci. 2024, 39, 144. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Gendy, A.O.; Obaid, Y.; Ahmed, E.; Enwemeka, C.S.; Hassan, M.; Mohamed, T. The Antimicrobial Effect of Gold Quantum Dots and Femtosecond Laser Irradiation on the Growth Kinetics of Common Infectious Eye Pathogens: An In Vitro Study. Nanomaterials 2022, 12, 3757. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wiench, R.; Skaba, D.; Matys, J.; Grzech-Leśniak, K. Efficacy of Toluidine Blue—Mediated Antimicrobial Photodynamic Therapy on Candida spp. A Systematic Review. Antibiotics 2021, 10, 349. [Google Scholar] [CrossRef]
- Bilal, H.; Shafiq, M.; Hou, B.; Islam, R.; Khan, M.N.; Khan, R.U.; Zeng, Y. Distribution and antifungal susceptibility pattern of Candida species from mainland China: A systematic analysis. Virulence 2022, 13, 1573–1589. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, A.; Roudbary, M.; Mohammadi, R.; Černáková, L.; Rodrigues, C.F. Overview on the Infections Related to Rare Candida Species. Pathogens 2022, 11, 963. [Google Scholar] [CrossRef]
- Mosca, C.O.; Moragues, M.D.; Brena, S.; Rosa, A.C.; Pontón, J. Isolation of Candida dubliniensis in a teenager with denture stomatitis. Med. Oral Patol. Oral Cir. Bucal 2005, 10, 28–31, 25-8. [Google Scholar] [PubMed]
- Casu, C.; Orrù, G.; Fais, S.; Mazur, M.; Grassi, R.; Grassi, R.F.; Nardi, G.M. Efficacy of ozonated water as a PS in photodynamic therapy: A tool for dental caries management? An in vitro study. J. Public Health Res. 2023, 12, 22799036231182267. [Google Scholar] [CrossRef]
- Rad, A.H.; Pourjafar, H.; Mirzakhani, E. A comprehensive review of the application of probiotics and postbiotics in oral health. Front. Cell. Infect. Microbiol. 2023, 13, 1120995. [Google Scholar] [CrossRef]
- Butera, A.; Pascadopoli, M.; Nardi, M.G.; Ogliari, C.; Chiesa, A.; Preda, C.; Perego, G.; Scribante, A. Clinical Use of Paraprobiotics for Pregnant Women with Periodontitis: Randomized Clinical Trial. Dent. J. 2024, 12, 116. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casu, C.; Butera, A.; Scano, A.; Scribante, A.; Fais, S.; Ladu, L.; Siotto-Pintor, A.; Orrù, G. Photodynamic Therapy in the Management of MDR Candida spp. Infection Associated with Palatal Expander: In Vitro Evaluation. Photonics 2025, 12, 786. https://doi.org/10.3390/photonics12080786
Casu C, Butera A, Scano A, Scribante A, Fais S, Ladu L, Siotto-Pintor A, Orrù G. Photodynamic Therapy in the Management of MDR Candida spp. Infection Associated with Palatal Expander: In Vitro Evaluation. Photonics. 2025; 12(8):786. https://doi.org/10.3390/photonics12080786
Chicago/Turabian StyleCasu, Cinzia, Andrea Butera, Alessandra Scano, Andrea Scribante, Sara Fais, Luisa Ladu, Alessandra Siotto-Pintor, and Germano Orrù. 2025. "Photodynamic Therapy in the Management of MDR Candida spp. Infection Associated with Palatal Expander: In Vitro Evaluation" Photonics 12, no. 8: 786. https://doi.org/10.3390/photonics12080786
APA StyleCasu, C., Butera, A., Scano, A., Scribante, A., Fais, S., Ladu, L., Siotto-Pintor, A., & Orrù, G. (2025). Photodynamic Therapy in the Management of MDR Candida spp. Infection Associated with Palatal Expander: In Vitro Evaluation. Photonics, 12(8), 786. https://doi.org/10.3390/photonics12080786







