1. Introduction
As China’s economy continues to grow, the demand for energy is becoming increasingly urgent. Coal is and will remain one of China’s main energy sources [
1]. From 2014 to 2021, coal consumption in China’s energy consumption increased from 4.02649 × 10
9 tons of standard coal to 4.79161 × 10
9 tons of standard coal [
2]. However, safety issues still exist in coal storage and transportation. Although coal mine accidents are decreasing year by year, it is predicted that 4.5 coal mine accidents will result in 14 deaths in 2025 [
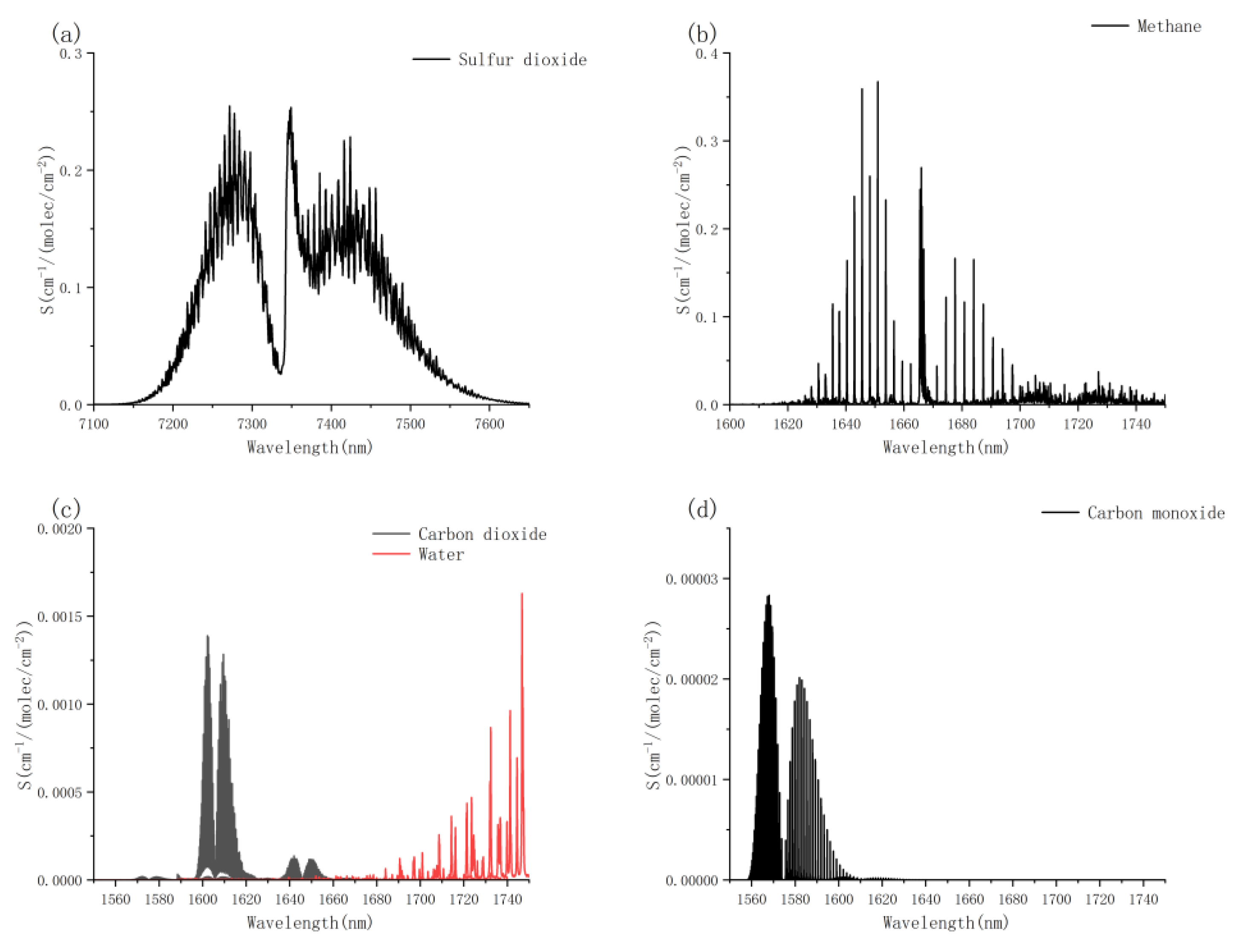
3]. Coal, as an organic material with a complex composition, releases various gases when burned [
4]. As methane is one of the characteristic gases for the early warning of coal smoldering, the accurate and flexible telemetry of methane concentration is an important means to prevent safety hazards such as spontaneous combustion. Currently, few studies have investigated gas emissions during the coal smoldering stage. However, it can be anticipated that the release rates of various gases during smoldering are generally low, which significantly increases the difficulty of early warning detection for coal smoldering.
Currently, traditional methane sensors include combustion catalytic sensors, semiconductor sensors, thermoelectric sensors, etc. [
5]. Spectroscopic sensors, on the other hand, include DOAS (Differential Optical Absorption Spectroscopy), FTIR (Fourier Transform Infrared), and TDLAS, among others. Catalytic combustion sensors usually consist of a temperature sensor, a catalytic burner, and a heater unit, which works on the principle of the presence of a catalyst in the surface layer that interacts with the gas and releases heat through combustion at low temperatures [
6]. The methane concentration is measured by detecting the temperature difference. Although they are cost-effective, these sensors are unable to achieve the required level of sensitivity to prevent coal smoldering due to the limitations of their detection principle. Yang Ziyang et al. pointed out that the zero-point drift of catalytic combustion methane detectors should not exceed 0.1%, yet this range of drift remains excessive for detecting coal smoldering [
7]. In addition, due to its chemical reaction, it is not as safe as a physical approach. The resistance of semiconductor sensors changes when exposed to methane [
8]. Tao et al. developed a novel optical fiber sensing element with a detection limit as low as 0.1%. However, this sensitivity still proves to be inadequate for coal smoldering early-warning purposes [
9]. Overall, non-spectral sensors have a lower cost, but poor environmental adaptability and limited detection accuracy [
10]. For comparison, Yanan Cao et al. employed deep learning residual networks to achieve high-performance filtering and the high-sensitivity concentration retrieval of methane in photoacoustic spectroscopy, attaining a measurement precision of 0.0626 ppm and a minimum detection limit (MDL) of 1.47 ppb. [
11] DOAS and FTIR technologies, on the other hand, are based on a spectral approach to detection. DOAS technology has a wide range of applications [
12]. However, its detection distance range is too far and often passive, making it unsuitable for the telemetry of smoldering coal. FTIR detection accuracy is lower compared to the TDLAS and is not suitable for coal smoldering telemetry as well.
In comparison, laser absorption spectroscopy based on TDLAS technology has advantages such as being non-contact, having no chemical reaction, high sensitivity, strong anti-interference, and a fast response speed. Therefore, it has been widely used in gas detection [
13]. Dong Lei et al. conducted the harmonic detection of methane gas using a digital lock-in amplifier, which achieved a methane concentration measurement with an ultimate sensitivity of 2.9 ppm·m at a distance of 1.63 m [
14]. However, 1.63 m is too close for coal mine telemetry to meet the requirements of coal smoldering detection. Jun Xu designed a new methane telemetry device to realize the detection of a methane concentration with a minimum detection limit of 70.5 ppm [
15] which is not enough to realize the purpose of coal smoldering warning. By introducing a focusable lens into the telemetry collimation system, Li Guolin dynamically adjusted the perfusion dispersion performance so that the methane telemetry system receives light maximization while avoiding stray light interference to a certain extent [
16]. Although it achieves the purpose of methane telemetry concentration, due to its simulation of natural gas leakage, the methane concentration, which is more than 1000 ppm, is too much for coal smoldering. So, it cannot be applied well in the lower-concentration methane detection of coal smoldering detection. Yang Haoqing et al. investigated methane remote sensing using TDLAS, achieving a detection range of 30 m. However, there are significant signal fluctuations due to environmental interference, as evident in their experimental data [
17]. Wu Qi et al. focused on miniaturizing methane sensors and improving response speed, but their study did not sufficiently account for environmental factors, particularly temperature variations, which can critically affect sensor accuracy [
18]. To enable the early remote detection of coal smoldering, distance measurement is equally crucial. Hansemann et al. pioneered an integrated approach combining Tunable Diode Laser Absorption Spectroscopy (TDLAS) with optical ranging techniques to achieve the high-spatial-resolution measurement of gas-phase properties in particle-laden flows. This technical approach offers great advantages for coal storage safety monitoring [
19].
In summary, remote measurement of low-concentration methane has rarely been achieved to date. Additionally, there is still a lack of effective methods to mitigate interference from external factors, particularly variations in ambient temperature. This article presents the methane concentration required for the smoldering detection of coal, as determined through experimental means. Concurrently, a novel methane concentration detection device has been conceptualized. Temperature compensation algorithms have been invented to improve the system’s immunity to interference. In this way, the long-distance warning of coal cloudy combustion is realized, contributing to coal safety.
4. Methane Telemetry System and Performance
4.1. System Structure
The structure and device of the methane telemetry system are shown in
Figure 5, including the optical part, the electrical part, and the software algorithms.
In the optical part, the DFB laser (NEL NLK1U5FAAA, 1653 nm laser) emits a laser signal which is passed through a variable fiber optic attenuator to control the signal strength. The variable fiber optic attenuator is connected to a laser collimator, which collimates the laser light into parallel light that passes through a cavity with a length of 30 cm. The gas cavity is flanked on both sides by infrared wavelength windows with a transmittance of more than 99%. After passing through the cavity, a diffuse reflecting plate is set up at a distance of 12 m so that the laser light is diffusely reflected. A portion of the light is reflected and converged to the detector (PDAPC4, Thorlabs) by a transmission telescope head.
A DAQ (USB-6211, National Instruments) is under the control of a computer, and it outputs a sawtooth wave signal at 10 Hz, superimposed on a sinusoidal modulation signal at 2 kHz. The laser driver board scans a current range of 50 mA to 70 mA to control the laser wavelength. The laser’s internal thermistor and thermoelectric cooler (MTD415T, Thorlabs) are managed by a temperature controller, which maintains the internal temperature of the laser measurement point at a constant 23.5 °C with an accuracy of 0.002 °C. This is performed to avoid changes in the laser wavelength due to temperature variations. The analog signal received by the detector is converted to a digital signal by the DAQ and subsequently input to the computer. The computer program then decodes the digital signal and calculates the concentration value along the measured path. In this system, the generation of modulation signals, the acquisition of detection signals, and the lock-in amplifiers are realized through MATLAB R2022b for continuous, automatic, and full-process operation.
4.2. Situation Simulation and Calculation of Readings
To simulate the situation, the cavity is positioned in front of the collimator, with dimensions of 30 cm in length, 50 mm in diameter, and a volume of approximately 0.6 L. As the coal undergoes combustion, the light traverses the measured gas in a back-and-forth motion. The cavity utilized in the experiment is exclusively traversed by the signal emitted, thereby ensuring that the thickness of the air mass simulated by the 30 cm cavity in this experiment is equivalent to 15 cm around the coal. The methane concentration in the laboratory is 2 ppm and the optical range length is 12 m.
Let
represent the total gas absorption capacity of the optical path, and
is the methane concentration in the gas cavity (unit: ppm). Thus, the methane concentration
(ppm) in the cavity can be converted to the telemetry-common unit
(ppm·m). Here,
denotes the methane concentration integrated along the optical path, a key parameter for telemetry.
The gas can be introduced and extracted using valves located at opposing ends of the cavity. The gas distribution within the cavity is facilitated by a gas distributor, which enables the modulation of the gas ratio. The flow rate is 400 mL/min, and the gas in the cavity can be completely replaced in two minutes. The second valve is connected to the external environment, thereby ensuring that waste gases do not impact the laboratory’s atmosphere.
It is imperative to reduce the potential impact of extrinsic vibrations, laser energy attenuation, stray light, and detector response fluctuations on the outcomes obtained by the direct absorption method. In order to improve the signal-to-noise ratio, and improve the detection accuracy and detection limit of the gas, the second harmonic and the first harmonic of the wavelength modulation technology are detected and normalized.
5. Results
5.1. Performance of the Instrument
A methane-filled gas cavity with different standard concentrations is used to simulate the occurrence of smoldering around the coal. The raw signal is shown in
Figure 6a and the second harmonic plots for different methane concentrations are presented in
Figure 6b. To enhance the effect, in this step of the experiment, the detector is placed directly behind the cavity to avoid interference from atmospheric methane concentrations. It can be seen that the peak of the second harmonic signal still shows a clear correlation with the methane concentration.
The experimental set is used to simulate the concentration of methane released when smoldering. The simulated gases range from 0 ppm to 300 ppm and the air pressure is 1 atm to test whether it can realize the warning of coal smoldering. The lock-in amplifier output signal is averaged, normalized, filtered and amplified to obtain the
signal, and the results are calibrated. During the experiment, the system detects the gas concentration at a frequency of 10 Hz and then averages it to reduce the noise’s interference. Each gas proportioning is performed for a total of 15 min. Due to the large volume of the gas cavity, it takes approximately 2 min to fill, so the first 3 min of data is removed and the concentration calculated for the next 12 min is averaged. A total of 5 sets of data including 0 ppm, 50 ppm, 100 ppm, 150 ppm, 200 ppm, and 300 ppm are included in the experiment and the results are shown in
Figure 6c,d. The fitting function is y=0.99347x + 1.03384 and R
2 = 0.998, which shows that there is a good linear relationship between the methane concentration values and the
signal.
Since the second harmonic signal has already been linearly related to the methane concentration, the purpose of methane content measurement can also be achieved by directly adopting the peak of the second harmonic for methane concentration calculation, if we do not take into account that the strength of the signal leads to changes in the readings. In practical engineering applications, the use of the second harmonic (
) peak amplitude for methane quantification does not necessarily degrade measurement accuracy compared to alternative methods. However, the additional computational overhead required for simultaneously extracting the
signal and calculating the
ratio can introduce resource constraints in real-time monitoring scenarios. A comparative analysis of these two approaches is thus justified to optimize system performance versus measurement precision trade-offs. We can compare the stability of the two ways. The comparison results are shown in
Figure 7.
The variances of methane concentration calibration by second harmonic peak and signals are 3.686 and 1.905, respectively. This finding indicates that the normalization processing of the signal can significantly reduce data variability, enhance overall detection accuracy, and decrease the lower detection limit.
5.2. Suppress Temperature-Induced Signal Drift
The tunable laser is very sensitive to temperature changes. Every 2 °C change in laser shell temperature causes a 2.67 pm shift in the emitted laser light from the 1653 nm laser [
27]. Changes in ambient temperature can also result in a change in the signal. When the temperature changes, the gas pattern changes, which leads to a change in its reading.
The variation in gas absorption spectral lines can be determined through calculations using a relatively complex formula [
28]. However, we may alternatively obtain methane absorption intensities at different temperatures from the HITRAN database, as illustrated in
Figure 8. The data reveal an approximately linear relationship between methane absorption intensity and temperature within common operational ranges. Specifically, a 10 °C temperature increase corresponds to approximately a 5% decrease in methane spectral absorption intensity. This relationship significantly facilitates the compensation of temperature-induced reading drift in practical applications.
This problem was also found in our preliminary experiments. Since the ambient temperature can be obtained from the temperature sensor, it is possible to suppress the drift caused by temperature by conducting experiments at different temperatures to obtain the relationship between the readings and the temperatures. In the laboratory, while the ambient temperature can be adjusted by air conditioning, the relevant data were recorded and analyzed.
We conducted a three-day temperature monitoring experiment in the laboratory. The measurement results are shown in
Figure 9. During the daytime, the indoor temperature gradually increased due to personnel activity and rising outdoor temperatures. At night, the temperature progressively decreased as staff departed and solar radiation diminished. The monitoring data demonstrate that even in an enclosed room, temperature is not constant, exhibiting a diurnal fluctuation range of approximately 2 °C.
As can be seen from
Figure 10, there is a strong linear relationship between the room temperature and the instrumental indications in this temperature interval, enabling a linear fit to be performed to minimize temperature-induced errors. In the end, the standard deviation decreased from 2.3 in
Figure 11a to 1.6 in
Figure 11b with the help of the temperature sensor through the algorithmic correction, proving that the method is successful in suppressing the signal variation caused by the temperature change in both the atmosphere and the laser.
5.3. Allan Analysis of Variance
The Allan variance of the entire system is also calculated in
Figure 12. Allan deviation analysis is a method of assessing the error level across various time scales, frequently employed in the analysis of instabilities in the domain of gas detection.
Allan deviation plotted in a log–log scale form indicated that an LoD of 22.59 ppm is achieved with a 2 s integration time and a measurement precision of 1.47 ppm is estimated with integration times at 147 s in
Figure 12. Allan variance analysis shows that the methane telemetry system has good stability. Notably, the Allan deviation fails to exhibit the characteristic
scaling law prior to reaching its minimum value. This anomalous behavior is likely due to excessive flicker noise (
noise) and random walk noise within the system, which could be caused by laser-related instabilities, electronic noise, or other factors. Consequently, the telemetry system designed in this paper is capable of both rapid responsiveness and ensuring high detection performance, thereby meeting the requirements for warning of coal spontaneous combustion.
5.4. Stability Difference Under Different Signal Strengths
In the context of methane telemetry, signal interference arising from factors such as light intensity fluctuation, ambient gas disturbance, and the coal surface reflection coefficient frequently compromise the accuracy of measurement outcomes. To investigate the impact of signal strength on the measurement value, the intensity of the light is regulated by employing an optical fiber attenuator, thereby enabling the simulation under varying signal strengths.
In order to verify the influence of signal strength on the solution value, the experiments of no signal attenuation, 30% attenuation, and 60% attenuation are carried out by using an optical fiber attenuator. The gas cavity was filled with 500 ppm of methane, and the three groups of experiments were tested for 5 min. As is shown in
Figure 13, The standard deviations were 2.14, 6.97 and 13.02, respectively.
Figure 14 illustrates the light reflection characteristics at varying distances. Given the minimal angle
in practical scenarios, the reflection intensity can be approximated as uniform across different angles, with
exhibiting a proportional relationship to the received signal strength.
Given the lens’s diameter of 50 mm, the blue curve represents the reflection profile at a lens-to-surface distance of 12 m. The angle
can be derived from Equation (17):
Using the signal at 12 m as the reference baseline, when the signal attenuation reaches 30% and 60%, respectively, the corresponding distances can be determined as follows:
Substituting θ₂ and θ₃ into the calculation yields corresponding distances of 17 m and 30 m for 70% and 40% of the reference signal intensity, respectively. If the units are converted to ppm·m, they are 8.25 ppm·m, 6.41 ppm·m, and 4.5 ppm·m, respectively. As mentioned above, the lower limit of coal smolder detection should be 50 ppm, so the difference between the highest detection value and the lowest detection value should be controlled within 50 ppm as far as possible, which can improve the reliability of an early warning and shorten the response time from smolder to alarm. When the distance becomes longer, the detection accuracy of methane concentration is rather higher, taking into account the effect of atmospheric background methane concentration. Measurements at 17 m and 30 m require 1.42 and 2.5 times more detection deviation than at 12 m. At the same time, considering the differences between the actual situation and the laboratory, the working distance of the coal smolder warning system designed in this paper should be within 17 m, and the effect is better when it is within 12 m. When the working distance exceeds 17 m, the detection accuracy will be greatly reduced, and the response time will be greatly increased. Beyond 30 m, the system’s efficacy is significantly diminished, its response time is substantially prolonged, and the credibility is significantly diminished. The underlying cause of this phenomenon is that even in the absence of visible smoke, the detection system may erroneously interpret a methane reading exceeding 50 ppm as a false positive.