1. Introduction
According to the World Health Organization, approximately one billion people worldwide suffer from vision disorders that cause irreversible loss of vision, often leading to low vision [
1]. Low vision is defined as a condition in which visual function remains impaired despite treatment and/or refractive correction, interfering with daily activities. Typically, a visual acuity of less than 6/18 to light perception or a visual field narrower than 10° from the point of fixation characterizes low vision. Common ocular conditions causing low vision include age-related macular degeneration, glaucoma, diabetic eye disease, and retinitis pigmentosa, all of which are associated with progressive visual field loss (VFL) [
2]. The prevalence of VFL increases with age, rising from 3% in individuals aged 55–64 years to 19% in those aged 85 years and older [
3]. VFL significantly reduces quality of life and restricts participation in daily activities.
Retinitis pigmentosa (RP) is a leading cause of visual disability, with a global prevalence of 1 in 4000 individuals [
4]. RP encompasses a spectrum of hereditary retinal dystrophies characterized by a gradual loss of vision caused by photoreceptor degeneration. In most cases, degeneration primarily affects the photoreceptor rods, with secondary involvement of the cones. For this reason, typical RP is often referred to as rod-cone dystrophy, as photoreceptor rods are more severely affected than cones [
5]. Early symptoms include reduced night vision or nyctalopia, followed by progressive peripheral field loss (PFL), which impairs orientation and mobility. In one study, 80% of individuals with RP reported mobility difficulties [
6], which severely limited their ability to navigate unfamiliar environments and avoid obstacles outdoors. Restricted visual fields increase the risk of accidents, thereby compromising patients’ independence, health, and quality of life.
Visual rehabilitation plays a critical role in the enhancement of daily functioning among individuals with irreversible vision loss that cannot be addressed by conventional medical or surgical treatment. Orientation and mobility (O&M) training helps visually impaired individuals to use their residual vision and navigate safely and independently. However, traditional optical field extension aids, such as prisms, amorphic lenses, and inverted Galilean telescopes, have significant limitations [
7,
8,
9]. These devices often fail to provide meaningful field expansion, reduce image quality (e.g., causing blur, haziness, or dimness), and produce disorienting binocular visual confusion [
10,
11]. In addition, the social stigma associated with low-vision devices contributes to low adoption rates [
12]. Consequently, there is an urgent need for innovative, user-friendly solutions that can enhance the mobility and quality of life of individuals with RP.
Head-mounted displays (HMDs) are wearable electronic visual aids that position a visual display directly in front of the user’s eyes, providing immersive or enhanced viewing experiences. HMDs can be categorized as virtual reality (VR) devices, which fully immerse the user in a computer-generated environment, or augmented reality (AR) devices, which overlay virtual information onto the physical world in real time [
2]. (AR HMDs employ either optical-see-through (OST) displays or video-see-through technology. While video-see-through displays introduce delays and reduce direct interaction with the environment, OST displays use transparent or semi-transparent lenses, allowing users to maintain natural stereo vision while viewing digital information projected onto the lenses [
13].
AR technology has rapidly advanced and has been applied in ophthalmology, particularly for conditions such as visual field defects, low vision, nyctalopia, metamorphopsia, and amblyopia [
14]. By combining real-world contexts with augmented information, AR can improve functionality and quality of life. AR systems have also emerged as alternatives to traditional optical aids for visually impaired individuals, offering features such as variable magnification [
14], enhanced contrast and edge detection [
15], obstacle avoidance [
16], and text-to-speech functionality [
17]. These features help users better perceive and interact with their surroundings, provide navigational cues, and extend the visual field, thereby facilitating autonomy and daily tasks for individuals with peripheral vision loss [
2].
Considering these advancements, AR technologies can be a promising approach for low-vision rehabilitation in patients with RP. This study aimed to evaluate the effectiveness of Retiplus, a new commercial optical-see-through AR head-mounted visual aid, in improving the visual function of patients with RP. Additionally, the participants’ satisfaction and their ability to perform daily activities using the AR aid were assessed.
We hypothesized that using Retiplus would significantly enhance the functional vision and spatial awareness of patients with RP compared with their performance in the absence of the aid. Furthermore, we expected that participants would report higher satisfaction levels when performing daily activities with the AR device.
2. Materials and Methods
2.1. Subjects
Thirteen patients (10 females and 3 males) diagnosed with typical RP participated in this study. Their ages ranged from 21 to 76 years (mean: 55.2 ± 14.3 years), with a mean spherical equivalent of −0.72 ± 3.70 D. All participants were evaluated at the Optometric Clinic of the Faculty of Optics and Optometry, Complutense University of Madrid, Spain. A summary of the participants’ demographic and clinical data is presented in
Table 1. The exclusion criteria were central visual field loss, motor impairment, and any ocular pathology other than retinitis pigmentosa. This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the institutional review board (21/115-E). Written informed consent was obtained from all participants prior to their inclusion in the study.
2.2. Device
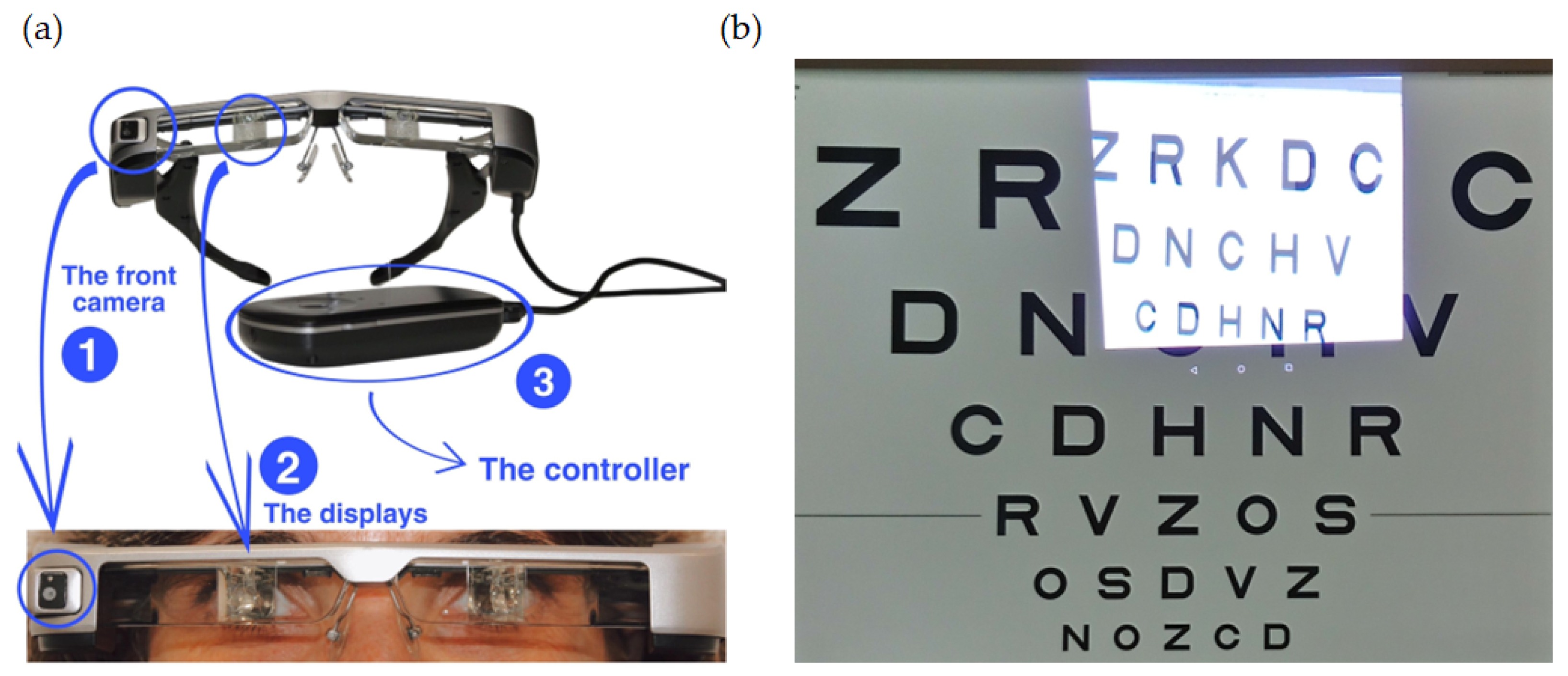
Retiplus (Plusindes SL, Madrid, Spain) is a new low-vision aid based on AR, specifically designed for patients with peripheral visual field defects caused by conditions such as retinitis pigmentosa, glaucoma, or hemianopia. The device comprised an optical-see-through display (Epson Moverio BT-350, Seiko Epson Corporation, Suwa, Nagano, Japan) equipped with a front-facing camera and a controller (
Figure 1a). The Epson Moverio smart glasses feature a wide-field-of-view camera with a 23° capture range and a high-definition display resolution of 1280 × 720 pixels operating at 30 Hz. The smart glasses weigh 119 g, making them lightweight and portable.
The 5-megapixel camera on the right side of the case captures images displayed on the optical-see-through lenses. Since the device utilizes a semi-transparent display, the patient’s natural field of vision beyond the screen remains unaffected. This allows them to perceive their surroundings and objects below by shifting their gaze. Additionally, because the display overlays information rather than obstructing the view, there is no complete occlusion of the environment, ensuring that the patient can still use their residual peripheral vision effectively. This device is a commercial, pre-configured system that does not require manual calibration by the user or the researchers to ensure correct alignment between the world camera and the projection on the OST-HMD. For users with refractive errors, corrective lenses can be inserted into the device. Additionally, the detachable visor reduces ambient light and enhances viewing comfort in brightly lit environments.
The Retiplus system also includes a tablet that allows specialists to assess the patient’s visual function, perform digital calibration and customization of the optical-see-through displays, and analyze usage data.
This AR aid incorporates several vision enhancement techniques, including variable magnification via digital zoom, contrast and brightness adjustments, and minification (
Figure 1b). A “contour mode” is also available, which highlights object edges and is particularly useful for detecting obstacles, stairs, or objects in the environment. The controller allows patients to select their preferred viewing mode and adjust settings according to their needs. During the training sessions, participants were able to familiarize themselves with the use of the remote controller to adjust the zoom, contrast, and brightness. During the execution of ADL tasks, they could also modify these parameters as needed. No participants reported difficulties in handling it, suggesting that this type of interaction did not interfere with task execution. This ease of use suggests that the system could be easily adopted in daily life without adding an extra cognitive burden.
In this study, the configuration included a filter, full-screen mode, and a 5x minification display mode. The filter used is a category 3 neutral density filter designed to ensure that the patient perceives only the augmented reality image. This setup encourages a bioptic viewing strategy, where the patient consciously shifts their gaze to access their residual vision. In the final training session, the patient lifts the filter to perceive both the augmented reality and their natural vision simultaneously. Regarding the full-screen mode, it allows the augmented reality information to occupy the entire display. In the 5x minification display mode, the captured wide-angle video feed is digitally reduced (minified) by a factor of five and overlaid onto the transparent display of the HMD. This minified image is shown within the user’s intact central field of vision, effectively compressing peripheral scene information into a smaller area that can be perceived centrally. For patients with peripheral visual field loss, such as those with retinitis pigmentosa, this mode compensates for their restricted FOV by bringing peripheral elements of the scene into the central visual area without requiring head or eye movements to scan the environment. Thus, the user gains spatial awareness of obstacles, people, or hazards located outside their residual field. The enhancements remained consistent after the initial calibration by the low-vision specialist, and the participants did not manually adjust the enhancements for different tasks.
2.3. Protocol
The AR training comprised five 1 h sessions with a 1-week interval between each session to ensure consistency in adaptation and skill acquisition. In the first session, the visual aid is introduced, the training objectives are explained, and the subject’s views are calibrated on the tablet. Different viewing modes are configured: bioptic mode, which minimizes the image (5x) and expands the visual field; tracking mode, which displays a magnified image on half of the screen to facilitate localization; and contour mode, which highlights the contours of objects and structures. Static exercises are performed to locate objects and spatial references with and without the visual aid in different configurations. In the second session, previous exercises are reviewed, and the contour mode is introduced for object and spatial localization. Additionally, a puzzle completion task is added, where pieces on a table must be placed on a wall-mounted puzzle, performed with and without the visual aid in bioptic mode. The third session combines static and dynamic exercises, including object localization with the tracking mode without an external filter, puzzle completion in the same configuration, and a task where the subject must approach and touch the examiner using the bioptic mode. A color cone circuit is introduced, requiring the subject to navigate obstacles, find an object, and return, comparing their performance with and without the visual aid. In the fourth session, mobility exercises in more complex environments are reinforced. The cone circuit is repeated, first without the visual aid and then using the tracking mode. A guided walk is conducted through the university facilities, including the clinic, stairs, lobby, and cafeteria, using the bioptic mode. Additionally, stair identification is practiced using the contour mode. The fifth session evaluates the patient’s performance in outdoor environments and the effectiveness of a 450 nm filter adapted to the AR aid. The university circuit is repeated, and an urban route is introduced, including pedestrian crossings, traffic lights, and street/store localization in the bioptic mode. Finally, an orientation task in a supermarket is performed, locating different sections using the tracking mode without an external filter.
No subjects were excluded due to their inability to complete the training. An experienced optometrist performed both low-vision training and an AR assessment.
Binocular photopic and mesopic (33 lux) distant visual acuity (VA) were measured using the ETDRS (Early Treatment of Diabetic Retinopathy Study) chart under high low-contrast conditions at 2 m. VA was expressed as the logarithm of the minimum angle of resolution (logMAR). Prior to the VA measurement, each participant underwent both objective refraction (Visionix VX120 system, Luneau Technology, Chartres, France) and subjective refraction to determine their best-corrected VA. The binocular visual fields were measured using a tangent screen at 1 m under photopic illumination. Visual field testing involved a clinician moving a 15.7-mm white target on the end of a black wand to identify areas where the patient could not detect the target (the dynamic visual field detection method). This method was used due to the impossibility of performing a full-field perimetry while the patients were wearing the AR aid. The horizontal and vertical field diameters of each participant were recorded both without visual aid and while using Retiplus 1.0.
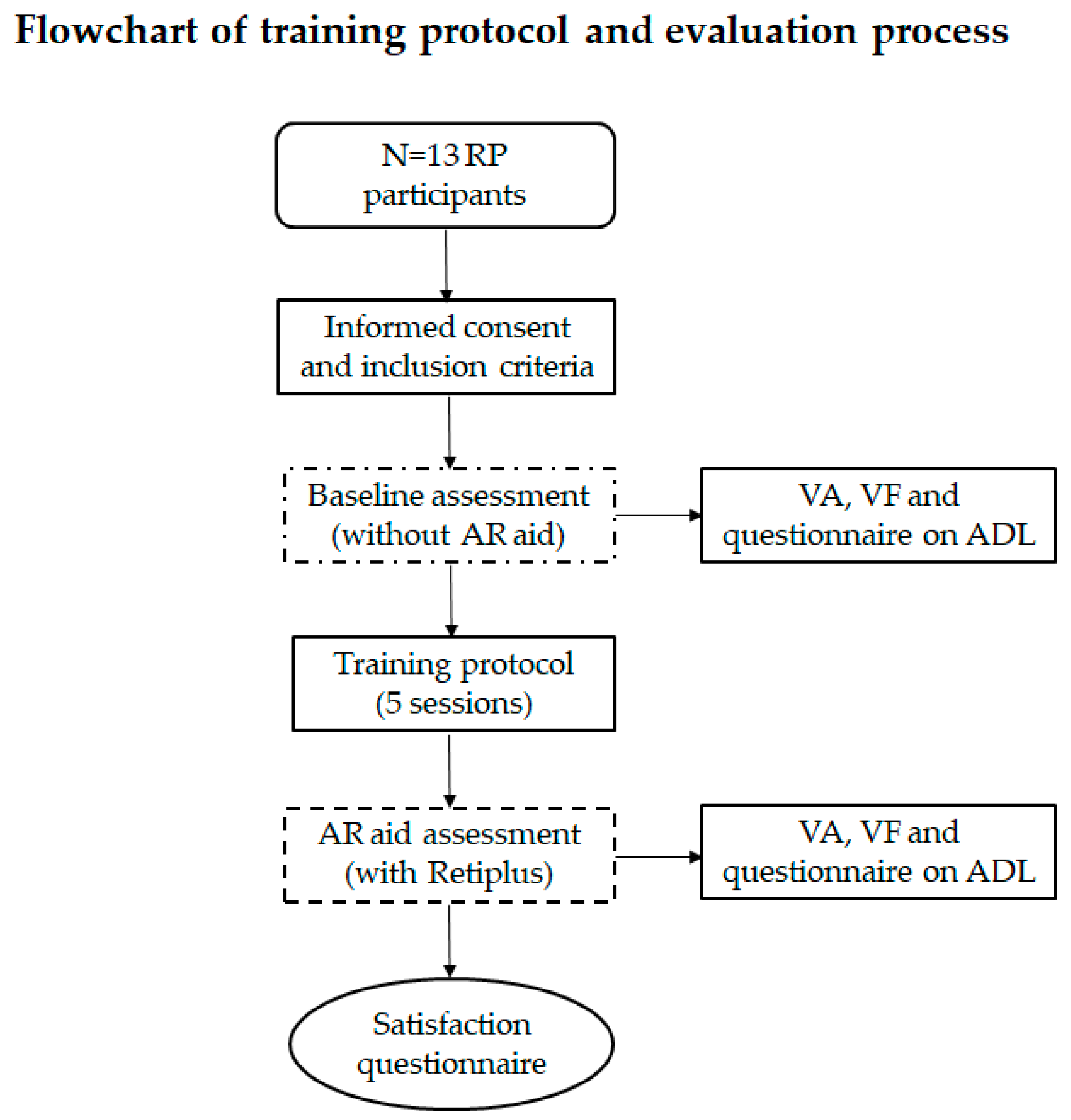
At the initial visit, the participants were informed about the study, and after signing an informed consent form, their baseline visual function was assessed without the AR device. After completing the training sessions, the participants reassessed their visual function using the AR device. Additionally, all participants completed a questionnaire on their activities of daily living (ADL), in which they rated the difficulty of performing activities related to peripheral vision and mobility. The questionnaire used a 0–3 scale: 0 = never, 1 = occasionally, 2 = quite often, and 3 = very often, with higher scores indicating greater difficulty in performing daily activities. This questionnaire was completed twice: once without using any low-vision aids and once while using the AR device.
At the conclusion of the study, the participants completed a short satisfaction survey consisting of five items rated on a 1–4 scale (1 = none, 2 = a little, 3 = somewhat, and 4 = a lot). The survey assessed the participants’ experiences with AR low-vision aids in various situations (
Table 2). Responses were recorded and summarized for analysis. A schematic of the experimental procedure is shown in
Figure 2.
2.4. Statistical Analysis
All statistical analyses were performed using SPSS software version 26.0 (IBM Corp., Chicago, IL, USA). The normality of the data distribution was assessed using the Shapiro–Wilk test. Scores from the peripheral vision and mobility questionnaire were compared between conditions (baseline vs. AR aid) using the Wilcoxon test. The visual parameters between conditions were compared using either the paired t-test or the Wilcoxon test, depending on whether the assumption of normality was met. Pearson correlation coefficients were calculated to evaluate the associations between parametric variables. The significance level was set at 0.05 for all tests.
3. Results
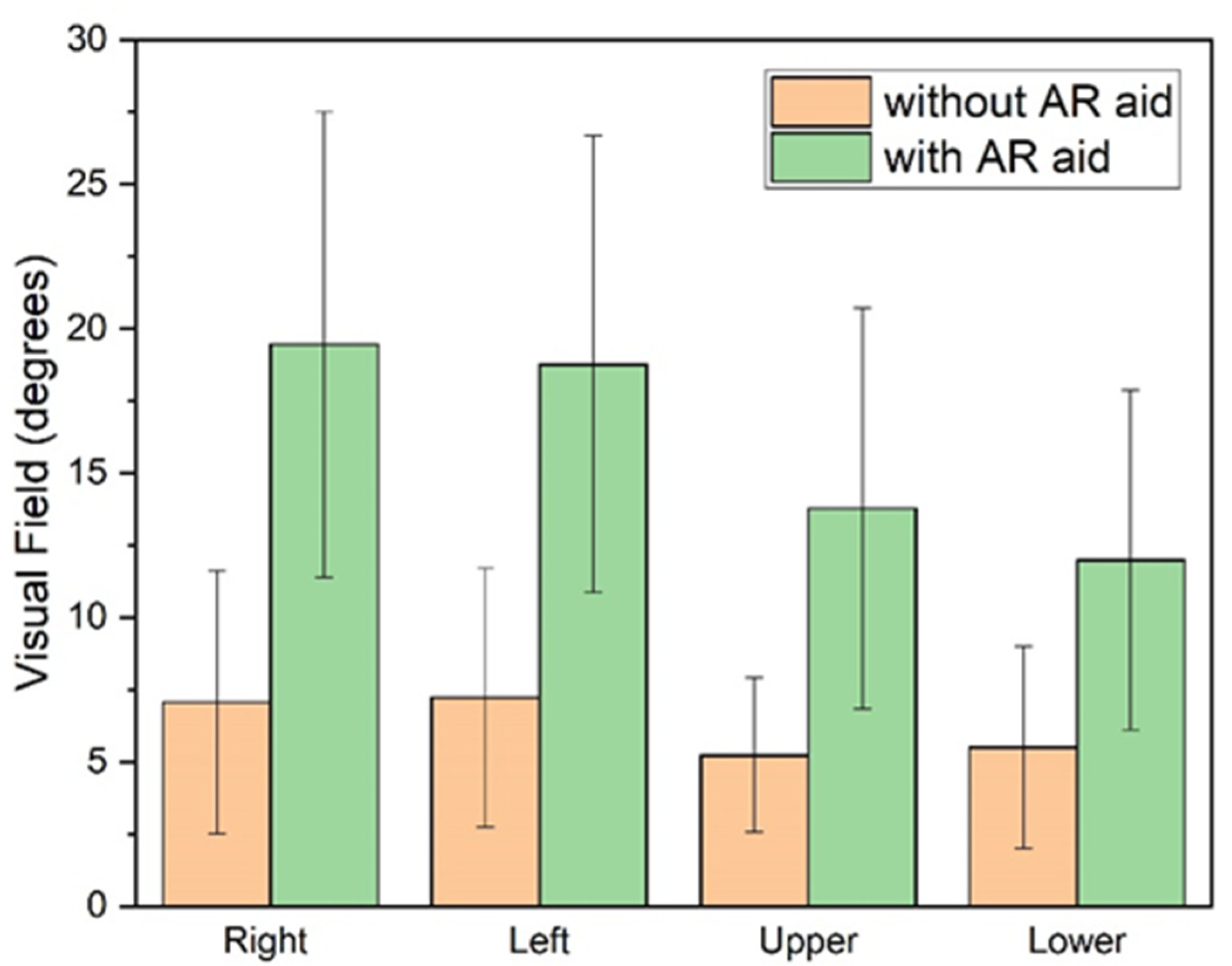
The expected enhancement in the visual field was observed with the use of the AR aid (
Table 3,
Figure 3). Specifically, when the smart glasses were worn, there was a significant expansion of the visual field across all four quadrants analyzed (right, left, upper, and lower;
p < 0.001). The greatest expansion was observed in the right quadrant, followed by the upper, left, and finally, the lower quadrant (with approximate increases of 175%, 163%, 160%, and 118%, respectively).
Furthermore, when the patients used the Retiplus device, both the average horizontal and vertical VF diameters were significantly enlarged (
Figure 3). The horizontal diameter enlargement (21.38° ± 12.94°) was greater than the vertical diameter enlargement (15° ± 10.08°), with a statistically significant difference (t = 2.70;
p = 0.010).
Overall, the results indicated an expansion of the visual field by a factor of 2.2 to 2.75, demonstrating that the AR device positively impacted the visual field performance under the tested conditions.
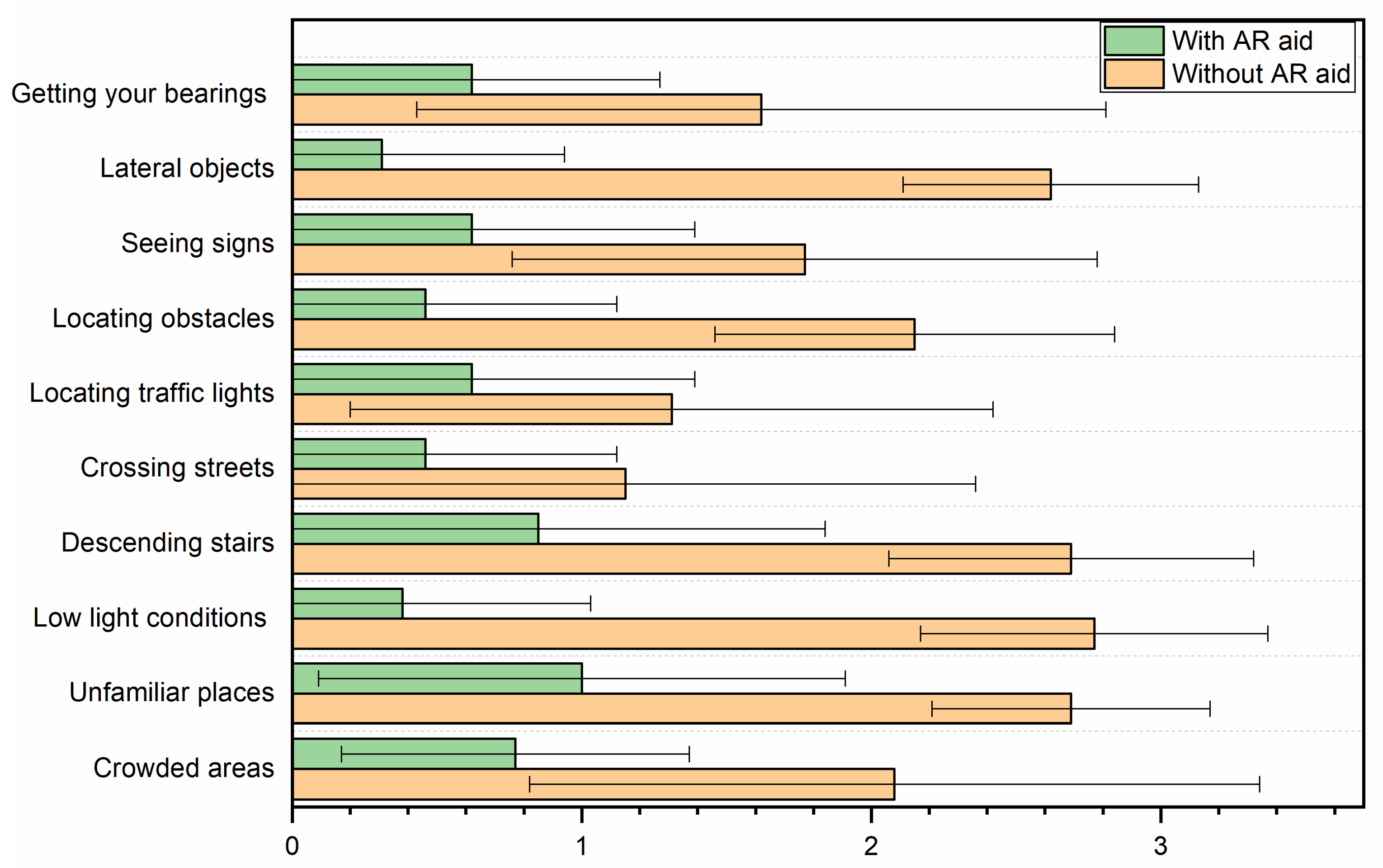
The increase in the visual field was perceived as beneficial for participants’ ability to perform ADL, as demonstrated by the results of the peripheral vision and mobility questionnaire (
Table 4,
Figure 4). Notably, crossing streets and locating traffic lights were the only activities in which the patients did not exhibit significant differences when using the AR aid (
p = 0.119 and
p = 0.133, respectively). In contrast, patients reported the greatest benefits from the AR aid in tasks performed at dusk or under low-light conditions, as well as in the detection of laterally positioned objects. Regarding the improvement in sign perception, this can be explained by the device’s adjustable brightness, contrast, and zoom settings. These allow for a controlled reduction in minification, preventing a significant loss of visual acuity. As a result, participants may experience an enhanced perception of signs despite the expected VA reduction.
A correlation analysis revealed a significant association between perceived difficulty in dusk or low-light environments and the degree of visual field expansion across quadrants. Specifically, a strong negative correlation was observed between perceived difficulty and visual field expansion in the right and left quadrants (ρ = −0.7, p = 0.008 for both), indicating that patients with greater visual field expansion experienced less difficulty under low-light conditions in these regions. Additionally, similar significant negative correlations were found in the upper (ρ = −0.56, p = 0.045) and lower quadrants (ρ = −0.7, p = 0.011). These findings suggest that augmented reality aids, which facilitate visual field expansion, could significantly alleviate the challenges faced by patients with low vision, particularly in visually demanding environments, such as at dusk or in dim light.
After completing a training program consisting of five weekly one-hour sessions, the participants’ responses to the satisfaction questionnaire indicated that they perceived notable benefits from using the AR low-vision aid. All participants rated their ability to describe objects in their surroundings as significantly improved (mean score: 4.0/4.0). They also reported a better overview of their environment (mean score: 3.9 ± 0.3) and improved vision under low-illumination conditions (mean score: 3.6 ± 0.6). Additionally, most participants indicated that the AR aid enhanced their orientation abilities (mean score: 3.5 ± 0.9) and their capacity for ambulation (mean score: 3.7 ± 0.6).
However, due to the 5x minification of the display image, the use of the AR aid significantly worsened binocular visual acuity in all subjects compared with the baseline conditions (
Table 3). This deterioration was observed under both photopic and mesopic conditions and for both high- and low-contrast stimuli. Under photopic conditions, the most pronounced deterioration occurred for high-contrast stimuli, with an increase of 0.52 logMAR (
p < 0.001). Under mesopic conditions, binocular visual acuity worsened by 0.36 logMAR (
p < 0.001) for both high- and low-contrast stimuli.
The increase in logMAR values indicates a significant decline in visual acuity, corresponding to a 186% worsening for high-contrast stimuli and a 78% worsening for low-contrast stimuli under photopic conditions. Similarly, under mesopic (low light) conditions, binocular visual acuity declined by over 82% for high-contrast stimuli and by 46% for low-contrast stimuli when using the AR aid.
4. Discussion
The present study demonstrated that Retiplus, a new commercial optical-see-through AR HMD, uses a reduced image display mode that can effectively increase the visual field size by approximately three times and improve visual search performance in patients with peripheral vision loss. The results revealed that the use of this AR aid significantly enhanced participants’ ability to perform various daily activities. Participants reported the greatest benefit from using the AR aid in tasks performed at dusk or under low-light conditions, such as descending steps or stairs, and in locating laterally positioned objects. Additionally, a significant association was found between perceived difficulty in dim or low-light environments and the degree of visual field expansion, with less difficulty observed as the visual field expanded.
However, despite these gains, a reduction in visual acuity was observed, primarily due to the image minification introduced by the AR system. To the best of our knowledge, studies evaluating the clinical effectiveness of AR aids based on HMDs in patients with peripheral visual field loss are limited.
Our findings align with those of previous research, indicating that AR-based interventions incorporating minification techniques can be beneficial for low-vision rehabilitation by enhancing visual field expansion tailored to the needs of different patients. For instance, a study involving two patients with RP and severely restricted visual fields (5° and 10°) reported that using various combinations of off-the-shelf cameras and see-through HMDs significantly improved their ability to navigate, perceive objects, and avoid hazards by displaying a minified contour image, resulting in a threefold visual field expansion [
16]. Another study demonstrated that augmented view implementation using an optoelectronic platform with the use of a see-through HMD (Nomad ND2000, Microvision, Washington, DC, USA) was able to expand the visual field by a 3.5 factor, thus aiding mobility tasks for patients with tunnel vision [
18]. The prototype developed by Bowers et al. consists of a pair of glasses integrated with an optical-see-through monocular display. The device, which presented a 4x minified grayscale view of the real world, was tested on six patients with night blindness. Their results also showed that the minified view significantly expanded the subjects’ visual field (by 178% at 16 lux and 287% at 2 lux); however, the insufficient light sensitivity of the camera and the limited resolution of both the camera and the display limited the prototype’s performance [
19]. Our study found that Retiplus significantly enlarged the horizontal and vertical VF in patients with RP. The horizontal VF diameter was extended to 38°, and the vertical VF diameter was extended to approximately 25°. Similar results were found by Xu and colleagues, who reported that patients with tunnel vision wearing Acesight, a new head-mounted AR aid that reduces the image by 0.5 or 0.25 times, experienced similar VF expansion [
20].
Individuals with peripheral field loss often face significant mobility challenges, particularly under low-illumination conditions. These challenges hinder the detection and avoidance of obstacles during navigation, which increases dependence on daily life and increases the risk of accidents, such as falls and injuries. Various studies have explored mobility testing in patients with VFL to evaluate the potential benefits of AR-based technologies in addressing these challenges.
A notable study assessed the impact of augmented vision on collision judgments in both normally sighted individuals (n = 12) and patients with tunnel vision (n = 7). This study demonstrated that superimposing 5x minified edge images did not significantly alter the participants’ collision-judgment capabilities. According to the authors, this outcome can be attributed to the fact that central vision provides sufficient information for collision judgment. They concluded that an augmented-vision HMD device could serve as a useful mobility aid for individuals with severely restricted peripheral vision [
21].
Building on these findings, Ikeda et al. evaluated a custom device comprising a see-through display, camera, and control box in patients diagnosed with typical RP. Their results revealed that using the device significantly reduced trial failures and travel times, supporting the conclusion that minified images could help RP patients overcome mobility issues [
22,
23].
More recently, Sayed et al. tested a new AR see-through digital spectacle prototype equipped with a customizable visual field expansion method in a simulated walking environment. Among 21 patients with peripheral VF defects in both eyes, the device improved walking maneuverability, with 90.5% of participants showing enhanced shape identification scores and more natural eye movement behavior [
24].
Angelopoulos et al. reported further advances, evaluating the efficacy of a Microsoft Hololens headset combined with a mixed-reality toolkit. This system uses high-contrast pseudocolor mapping overlays to assist patients with RP. Their findings indicated that participants experienced an average 50% reduction in collisions and improved object grasping [
25].
Despite these promising developments, some limitations remain. For example, the HoloLens system, while capable of providing low-latency self-motion information, is hindered by several factors, including lag, a limited range of mapping, the restricted field of view covered by the see-through display, and reduced brightness. These constraints highlight the need for further refinement to optimize the utility of AR-based devices for individuals with peripheral vision loss [
26]. In our study, Retiplus includes the Epson Moverio BT-350 system, which uses Si-OLED transparent displays. While both devices offer augmented reality experiences, their design goals and technological implementations differ significantly. The Moverio BT-350 excels in transparency, brightness, and low-latency overlays, making it suitable for tasks requiring continuous real-world awareness. On the other hand, the HoloLens provides a more immersive experience with advanced interaction but at the cost of some limitations in brightness, contrast, and latency.
As anticipated with the 5x minification, the Retiplus AR system significantly impaired visual acuity under both photopic and mesopic conditions compared with unaided vision. Our findings demonstrated that binocular visual acuity worsened by 0.52 logMAR under photopic conditions and 0.36 logMAR under mesopic conditions when patients used Retiplus. These results align with the inherent trade-offs of image minification, which enhances the field of view but reduces visual detail. According to our results, a moderate visual acuity ≤ 0.5 logMAR is necessary to process the minified image display [
16,
20].
To date, studies evaluating the effects of AR aids on visual function in patients with peripheral VF loss are limited. For example, Bowers et al. evaluated the prototype LV-3, which integrates glasses with an optical-see-through monocular display. Their study reported a reduction of 0.24 logMAR in visual acuity at 16 lux compared with unaided conditions [
19]. However, in contrast to the current study, subjective ratings from participants indicated that the LV-3 system did not improve or even worsened their perceived difficulty in recognizing and avoiding obstacles.
Recently, Xu et al. evaluated the Acesight device in its VF expansion mode and similarly found that VA was statistically decreased when using the device (0.89 ± 0.53 logMAR) compared with unaided vision (0.62 ± 0.50 logMAR). The observed reduction was attributed to image minification, which altered the patients’ spatial perception. Despite these visual limitations, more than half of the participants reported that Acesight was helpful, particularly for tasks involving mobility. Notably, younger patients exhibited greater receptivity to AR HMD visual aids, suggesting that age and adaptability may play a critical role in the utility of these systems [
20].
These findings demonstrate that AR-based HMD systems that provide VF expansion through image minification may be beneficial for activities that rely heavily on peripheral vision, such as mobility, obstacle detection, and spatial orientation. It is important to note that the reduction in visual acuity observed in this study was primarily due to the image minification process. However, with the integrated remote controller, patients can easily adjust the display mode to suit their individual preferences, allowing them to modify the digital zoom, brightness, luminosity, and contrast. This flexibility allows Retiplus to display enlarged images, making it suitable for tasks requiring detailed vision.
Another key advantage of Retiplus is its display transparency, which is a critical feature for patients with night blindness when navigating under dim illumination. Unlike some previously cited prototypes [
18,
21,
22], Retiplus provides augmented vision binocularly, further enhancing its utility. Additionally, its portability and autonomy, with approximately six hours of battery life, are fundamental features that support improved mobility and spatial orientation.
Despite these advantages, our study has several limitations. The sample size was relatively small, limiting the generalizability of the findings to a broader population with peripheral vision loss. However, other studies evaluating the efficacy of AR-based HMD technology in people with low vision have included a similar number of participants [
18,
19,
21,
22,
27]. Regarding misalignment, we acknowledge that geometric discrepancies between the real-world view and the AR overlay are unavoidable due to the offset between the camera’s position and the user’s eyes, as well as system processing delays. However, the goal of the minification mode is not precise spatial registration. Instead, it aims to provide complementary situational awareness by alerting the user to the presence of objects beyond their natural field rather than to replace or perfectly align with their direct view. Users are instructed to interpret the minified image as an additional, generalized spatial cue rather than an exact overlay of reality.
Furthermore, all participants underwent a structured training program consisting of five weekly sessions, each lasting for one hour. This duration is significantly longer than that reported in other studies, where training sessions ranged from 2 to 30 min [
2,
19,
21,
27]. Future research should explore different vision enhancement combinations and conduct long-term follow-up studies with extended training periods. Also, it could be interesting to explore alternative display technologies that might overcome the misalignment challenges associated with OST designs. Such efforts would help assess the durability of the visual benefits and gather more comprehensive subjective feedback from patients.