SVM-Based Optical Detection of Retinal Ganglion Cell Apoptosis
Abstract
1. Introduction
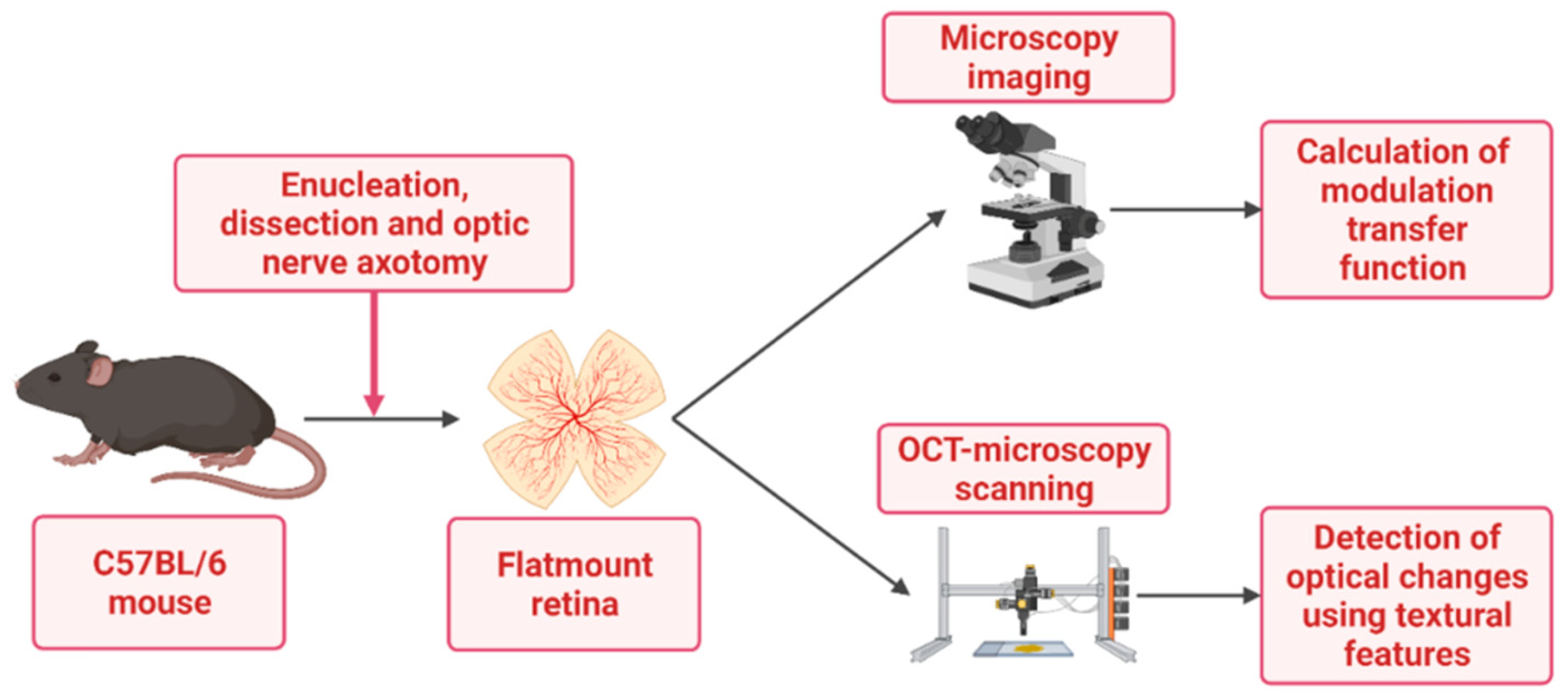
2. Materials and Methods
2.1. Tissue Preparation
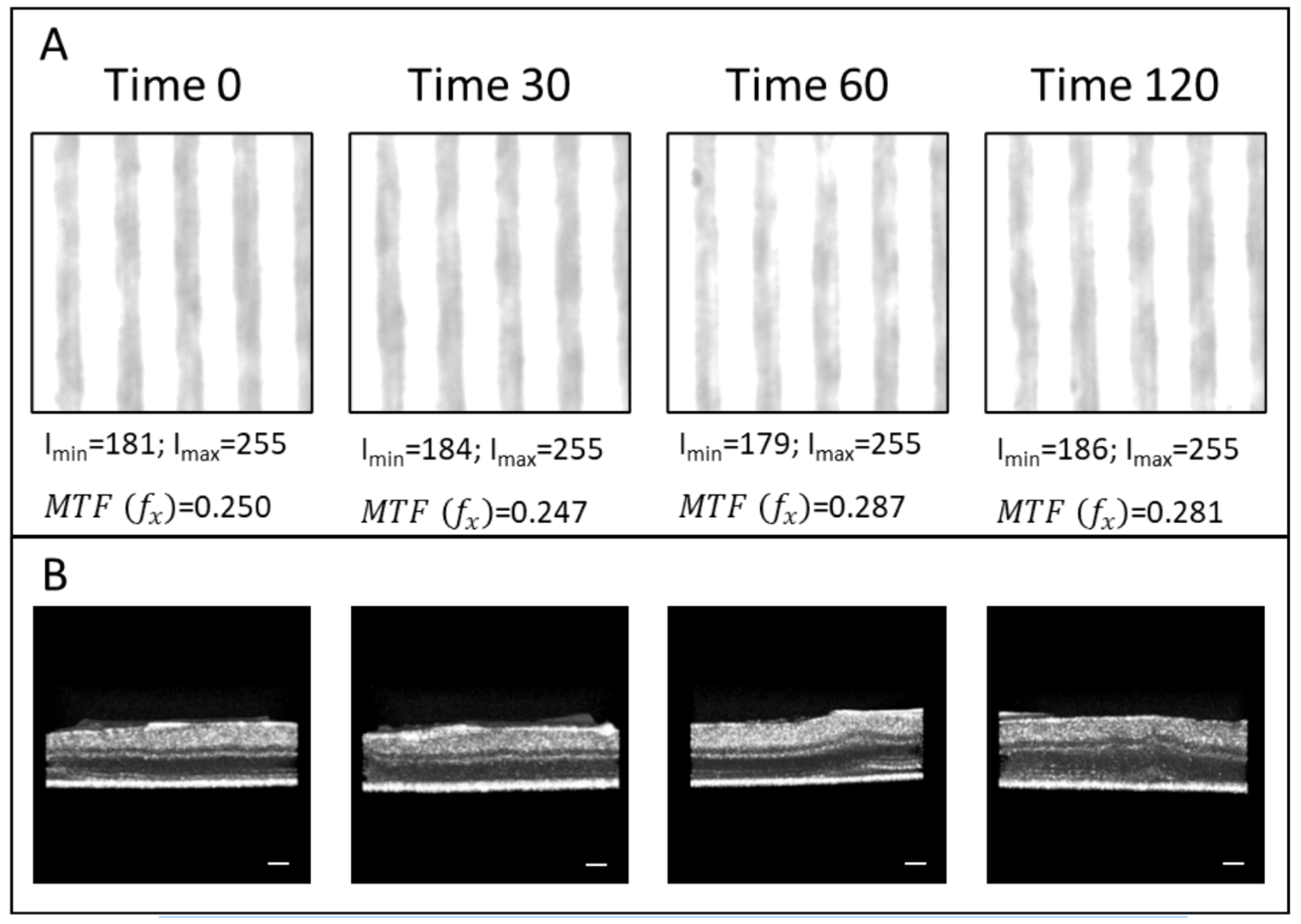
2.2. Retinal Transparency Post Axotomy: Analysis of the Modulation Transfer Function
2.3. Retinal Atrophy Post Axotomy: Texture Analysis of RGC Dendritic Tree
3. Results
3.1. Transparency of the Retinal Explants
3.2. IPL Texture Analysis Post Axotomy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELM | External limiting membrane |
| ESF | Edge spread function |
| FFT | Fourier transform |
| FPR | False positive rate |
| FWHM | Full width at half maximum |
| GCL | Ganglion cell layer |
| GLCM | Grey-level co-occurrence matrix |
| HBSS | Hank’s balanced salt solution |
| INL | Inner nuclear layer |
| IPL | Inner plexiform layer |
| IS/OS | Junction between the photoreceptor outer and inner segments |
| LSF | Line spread function |
| ML | Machine learning |
| MTF | Modulation transfer function |
| NB | Neurobasal |
| OCT | Optical coherence tomography |
| ONL | Outer nuclear layer |
| OPL | Outer plexiform layer |
| PCA | Principal component analysis |
| PR | Photoreceptor |
| PSF | Point spread function |
| RGC | Retinal ganglion cell |
| RNFL | Retinal nerve fibre layer |
| ROI | Region of interest |
| RPE | Retinal pigment epithelium |
| SVM | Support vector machine |
| TPR | True positive rate |
References
- Almasieh, M.; Wilson, A.M.; Morquette, B.; Cueva Vargas, J.L.; Di Polo, A. The molecular basis of retinal ganglion cell death in glaucoma. Prog. Retin. Eye Res. 2012, 31, 152–181. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.; Vianna, J.; Chauhan, B. Assessing retinal ganglion cell damage. Eye 2017, 31, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Khatib, T.; Martin, K. Protecting retinal ganglion cells. Eye 2017, 31, 218–224. [Google Scholar] [CrossRef]
- Vernazza, S.; Oddone, F.; Tirendi, S.; Bassi, A.M. Risk Factors for Retinal Ganglion Cell Distress in Glaucoma and Neuroprotective Potential Intervention. Int. J. Mol. Sci. 2021, 22, 7994. [Google Scholar] [CrossRef]
- DePina, A.S.; Langford, G.M. Vesicle transport: The role of actin filaments and myosin motors. Microsc. Res. Tech. 1991, 47, 93–106. [Google Scholar] [CrossRef]
- Munemasa, Y.; Kitaoka, Y. Molecular mechanisms of retinal ganglion cell degeneration in glaucoma and future prospects for cell body and axonal protection. Front. Cell. Neurosci. 2013, 6, 60. [Google Scholar] [CrossRef]
- Williams, P.A.; Tribble, J.R.; Pepper, K.W.; Cross, S.D.; Morgan, B.P.; Morgan, J.E.; John, S.W.M.; Howell, G.R. Inhibition of the classical pathway of the complement cascade prevents early dendritic and synaptic degeneration in glaucoma. Mol. Neurodegener. 2016, 11, 26. [Google Scholar] [CrossRef]
- Williams, P.A.; Howell, G.R.; Barbay, J.M.; Braine, C.E.; Sousa, G.L.; John, S.W.M.; Morgan, J.E. Retinal Ganglion Cell Dendritic Atrophy in DBA/2J Glaucoma. PLoS ONE 2013, 8, e72282. [Google Scholar] [CrossRef]
- Fan, T.-J.; Han, L.-H.; Cong, R.-S.; Liang, J. Caspase Family Proteases and Apoptosis. Acta Biochim. Biophys. Sin. 2005, 37, 719–727. [Google Scholar] [CrossRef]
- Kumar, S. Caspase function in programmed cell death. Cell Death Differ. 2006, 14, 32. [Google Scholar] [CrossRef]
- Hoshino, A.; Ratnapriya, R.; Brooks, M.J.; Chaitankar, V.; Wilken, M.S.; Zhang, C.; Starostik, M.R.; Gieser, L.; La Torre, A.; Nishio, M.; et al. Molecular Anatomy of the Developing Human Retina. Dev. Cell. 2017, 43, 763–779. [Google Scholar] [CrossRef] [PubMed]
- Tribble, J.R.; Vasalauskaite, A.; Redmond, T.; Young, R.D.; Hassan, S.; Fautsch, M.P.; Sengpiel, F.; Williams, P.A.; Morgan, J.E. Midget retinal ganglion cell dendritic and mitochondrial degeneration is an early feature of human glaucoma. Brain Commun. 2019, 1, fcz035. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Knoops, K.; Boesten, I.; Berendschot, T.T.J.M.; van Zandvoort, M.A.M.J.; Benedikter, B.J.; Webers, C.A.B.; Reutelingsperger, C.P.M.; Gorgels, T.G.M.F. A time window for rescuing dying retinal ganglion cells. Cell Commun. Signal. 2024, 22, 88. [Google Scholar] [CrossRef] [PubMed]
- Reche, J.; Stocker, A.B.; Henchoz, V.; Habra, O.; Escher, P.; Wolf, S.; Zinkernagel, M.S. High-Resolution Optical Coherence Tomography in Healthy Individuals Provides Resolution at the Cellular and Subcellular Levels. Transl. Vis. Sci. Technol. 2023, 12, 12. [Google Scholar] [CrossRef]
- Hamza, A.; Hayward, T.M.; Majumder, A.; Brimhall, N.; Menon, R.; Ha, I. High-resolution optical coherence tomography using a multi-level diffractive lens. Opt. Express. 2024, 32, 27748–27755. [Google Scholar] [CrossRef]
- Erchova, I.; Tumlinson, A.R.; Fergusson, J.; White, N.; Drexler, W.; Sengpiel, F.; Morgan, J.E. Optophysiological Characterisation of Inner Retina Responses with High-Resolution Optical Coherence Tomography. Sci. Rep. 2018, 8, 1813. [Google Scholar] [CrossRef]
- Wilson, J.D.; Cottrell, W.J.; Foster, T.H. Index-of-refraction-dependent subcellular light scattering observed with organelle-specific dyes. J. Biomed. Opt. 2007, 12, 014010. [Google Scholar] [CrossRef]
- Mourant, J.R.; Canpolat, M.; Brocker, C.; Esponda-Ramos, O.; Johnson, T.M.; Matanock, A.; Stetter, K.; Freyer, J.P. Light scattering from cells: The contribution of the nucleus and the effects of proliferative status. J. Biomed. Opt. 2000, 5, 131–137. [Google Scholar] [CrossRef]
- Beuthan, J.; Minet, O.; Helfmann, J.; Herrig, M.; Müller, G. The spatial variation of the refractive index in biological cells. Phys. Med. Biol. 1996, 41, 369–382. [Google Scholar] [CrossRef]
- Gourley, P.L.; Hendricks, J.K.; McDonald, A.E.; Copeland, R.G.; Barrett, K.E.; Gourley, C.R.; Singh, K.K.; Naviaux, R.K. Mitochondrial Correlation Microscopy and Nanolaser Spectroscopy—New Tools for Biophotonic Detection of Cancer in Single Cells. Technol. Cancer Res. Treat 2005, 4, 585–592. [Google Scholar] [CrossRef]
- Tudor, D.; Kajić, V.; Rey, S.; Erchova, I.; Považay, B.; Hofer, B.; Powell, K.A.; Marshall, D.; Rosin, P.L.; Drexler, W.; et al. Non-Invasive Detection of Early Retinal Neuronal Degeneration by Ultrahigh Resolution Optical Coherence Tomography. PLoS ONE 2014, 9, e93916. [Google Scholar] [CrossRef] [PubMed]
- Kulmaganbetov, M.; Morgan, J.E. Application of Texture Analysis in Retinal OCT Imaging. In Handbook of Texture Analysis; CRC Press: Boca Raton, FL, USA, 2024; pp. 154–179. [Google Scholar]
- Williams, C.S.; Becklund, O.A. Introduction to the Optical Transfer Function; SPIE Press: Bellingham, WA, USA, 2002; pp. 23–64. [Google Scholar]
- Boreman, G.D. Modulation Transfer Function in Optical and Electro-Optical Systems; SPIE Press: Bellingham, WA, USA, 2001; pp. 1–50. [Google Scholar]
- Kulmaganbetov, M.; Bevan, R.J.; Anantrasirichai, N.; Achim, A.; Erchova, I.; White, N.; Albon, J.; Morgan, J.E. Textural Feature Analysis of Optical Coherence Tomography Phantoms. Electronics 2022, 11, 669. [Google Scholar] [CrossRef]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural Features for Image Classification. IEEE Trans. Syst. Man Cybern. 1973, 3, 610–621. [Google Scholar] [CrossRef]
- Conners, R.W.; Trivedi, M.M.; Harlow, C.A. Segmentation of a high-resolution urban scene using texture operators. Comput. Vis. Graph. Image Process. 1984, 25, 273–310. [Google Scholar] [CrossRef]
- Anantrasirichai, N.; Achim, A.; Morgan, J.E.; Erchova, I.; Nicholson, L. SVM-based texture classification in Optical Coherence Tomography. In Proceedings of the 2013 IEEE 10th International Symposium on Biomedical Imaging, San Francisco, CA, USA, 7–11 April 2013. [Google Scholar]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef]
- Koch, I.; Naito, K. Dimension selection for feature selection and dimension reduction with principal and independent component analysis. Neur. Comput. 2007, 19, 513–545. [Google Scholar] [CrossRef]
- Litts, K.M.; Zhang, Y.; Freund, K.B.; Curcio, C.A. Optical coherence tomography and histology of age-related macular degeneration support mitochondria as reflectivity sources. Retina. 2018, 38, 445–461. [Google Scholar] [CrossRef]
- Scholler, J.; Groux, K.; Goureau, O.; Sahel, J.A.; Fink, M.; Reichman, S.; Boccara, C.; Grieve, K. Dynamic full-field optical coherence tomography: 3D live-imaging of retinal organoids. Light Sci. Appl. 2020, 9, 140. [Google Scholar] [CrossRef]
- Chauhan, P.; Kho, A.M.; FitzGerald, P.; Shibata, B.; Srinivasan, V.J. Subcellular Comparison of Visible-Light Optical Coherence Tomography and Electron Microscopy in the Mouse Outer Retina. Invest. Ophthalmol. Vis. Sci. 2022, 63, 10. [Google Scholar] [CrossRef]
- Schultheiss, M.; Schnichels, S.; Miteva, K.; Warstat, K.; Szurman, P.; Spitzer, M.S.; Van Linthout, S. Staurosporine-induced differentiation of the RGC-5 cell line leads to apoptosis and cell death at the lowest differentiating concentration. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 1221–1229. [Google Scholar] [CrossRef]
- Gil, J.; Almeida, S.; Oliveira, C.R.; Rego, A.C. Cytosolic and mitochondrial ROS in staurosporine-induced retinal cell apoptosis. Free Radic. Biol. Med. 2003, 35, 1500–1514. [Google Scholar] [CrossRef]
- Cordeiro, M.F.; Guo, L.; Luong, V.; Harding, G.; Wang, W.; Jones, H.E.; Moss, S.E.; Sillito, A.M.; Fitzke, F.W. Real-time imaging of single nerve cell apoptosis in retinal neurodegeneration. Proc. Natl. Acad. Sci. USA 2004, 101, 13352–13356. [Google Scholar] [CrossRef] [PubMed]
- Israelsen, N.M.; Petersen, C.R.; Barh, A.; Jain, D.; Jensen, M.; Hannesschläger, G.; Tidemand-Lichtenberg, P.; Pedersen, C.; Podoleanu, A.; Bang, O. Real-time high-resolution mid-infrared optical coherence tomography. Light Sci. Appl. 2019, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Nishizawa, N.; Kawagoe, H.; Yamanaka, M.; Matsushima, M.; Mori, K.; Kawabe, T. Wavelength Dependence of Ultrahigh-Resolution Optical Coherence Tomography Using Supercontinuum for Biomedical Imaging. IEEE J. Sel. Top. Quantum Electron. 2019, 25, 1–15. [Google Scholar] [CrossRef]
- Wachulak, P.; Bartnik, A.; Fiedorowicz, H. Optical coherence tomography (OCT) with 2 nm axial resolution using a compact laser plasma soft X-ray source. Sci. Rep. 2018, 8, 8494. [Google Scholar] [CrossRef]
- Lee, S.W.; Jeong, H.W.; Kim, B.M.; Ahn, Y.C.; Jung, W.; Chen, Z. Optimization for Axial Resolution, Depth Range, and Sensitivity of Spectral Domain Optical Coherence Tomography at 1.3 µm. J. Korean Phys. Soc. 2009, 55, 2354–2360. [Google Scholar] [CrossRef]
- Lee, B.; Chen, S.; Moult, E.M.; Yu, Y.; Alibhai, A.Y.; Mehta, N.; Baumal, C.R.; Waheed, N.K.; Fujimoto, J.G. High-Speed, Ultrahigh-Resolution Spectral-Domain OCT with Extended Imaging Range Using Reference Arm Length Matching. Trans. Vis. Sci. Tech. 2020, 9, 12. [Google Scholar] [CrossRef]
- Sato, M.; Wakaki, I.; Watanabe, Y.; Tanno, N. Fundamental characteristics of a synthesized light source for optical coherence tomography. Appl. Opt. 2005, 44, 2471–2481. [Google Scholar] [CrossRef]
- Bousi, E.; Charalambous, I.; Pitris, C. Optical coherence tomography axial resolution improvement by step-frequency encoding. Opt. Express. 2010, 18, 11877–11890. [Google Scholar] [CrossRef]
- Makita, S.; Fabritius, T.; Yasuno, Y. Full-range, high-speed, high-resolution 1-µm spectral-domain optical coherence tomography using BM-scan for volumetric imaging of the human posterior eye. Opt. Express. 2008, 16, 8406–8420. [Google Scholar] [CrossRef]
- Tsai, C.Y.; Shih, C.H.; Chu, H.S.; Hsieh, Y.T.; Huang, S.L.; Chen, W.L. Submicron spatial resolution optical coherence tomography for visualising the 3D structures of cells cultivated in complex culture systems. Sci. Rep. 2021, 11, 3492. [Google Scholar] [CrossRef]



| SVM Classification | |||||||
|---|---|---|---|---|---|---|---|
| Predicted Class (% Correct) | TPR | FPR | |||||
| Time Points | Time 0 | 30 min | 60 min | 120 min | |||
| True Class | time 0 | 75% | 25% | 0% | 0% | 75% | 25% |
| 30 min | 23% | 77% | 0% | 0% | 77% | 23% | |
| 60 min | 0% | 0% | 97% | 3% | 97% | 3% | |
| 120 min | 0% | 0% | 4% | 96% | 96% | 4% | |
| PCA and SVM Classification | |||||||
| Predicted Class (% Correct) | TPR | FPR | |||||
| Time Points | Time 0 | 30 min | 60 min | 120 min | |||
| True Class | time 0 | 70% | 30% | 0% | 0% | 70% | 30% |
| 30 min | 28% | 72% | 0% | 0% | 72% | 28% | |
| 60 min | 0% | 0% | 92% | 8% | 92% | 8% | |
| 120 min | 0% | 0% | 7% | 93% | 93% | 7% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulmaganbetov, M.; Bevan, R.; Want, A.; Anantrasirichai, N.; Achim, A.; Albon, J.; Morgan, J. SVM-Based Optical Detection of Retinal Ganglion Cell Apoptosis. Photonics 2025, 12, 128. https://doi.org/10.3390/photonics12020128
Kulmaganbetov M, Bevan R, Want A, Anantrasirichai N, Achim A, Albon J, Morgan J. SVM-Based Optical Detection of Retinal Ganglion Cell Apoptosis. Photonics. 2025; 12(2):128. https://doi.org/10.3390/photonics12020128
Chicago/Turabian StyleKulmaganbetov, Mukhit, Ryan Bevan, Andrew Want, Nantheera Anantrasirichai, Alin Achim, Julie Albon, and James Morgan. 2025. "SVM-Based Optical Detection of Retinal Ganglion Cell Apoptosis" Photonics 12, no. 2: 128. https://doi.org/10.3390/photonics12020128
APA StyleKulmaganbetov, M., Bevan, R., Want, A., Anantrasirichai, N., Achim, A., Albon, J., & Morgan, J. (2025). SVM-Based Optical Detection of Retinal Ganglion Cell Apoptosis. Photonics, 12(2), 128. https://doi.org/10.3390/photonics12020128







