Retinal Laser Therapy Mechanisms, Innovations, and Clinical Applications
Abstract
1. Introduction
1.1. History
1.2. Principles of Laser
1.3. Laser–Tissue Interactions
- Photochemical interactions: In photodynamic therapy (PDT), exogenous photosensitizers preferentially accumulate in target tissues and are then activated by light to drive non-thermal photochemistry [15]. Therapeutic photochemical interactions used in PDT are typically performed at very low irradiances (<1 W/cm2) and with long exposure ranging from seconds to tens of minutes [16].
- Photothermal interactions: Absorbed light is converted to heat. The temperature rise and exposure time determine outcomes ranging from coagulation/necrosis to carbonization, melting, or vaporization. Conventional retinal photocoagulation operates in this thermal regime [17].Clinically important retinal laser effects include sublethal, adaptive thermal hormesis in viable retinal pigment epithelium (RPE). Brief, low-dose heating activates heat-shock responses (e.g., HSP70) and downstream cytoprotective signaling that modulate permeability and retinal homeostasis without coagulative necrosis. The intended endpoint of nondamaging paradigms (e.g., subthreshold/endpoint management approaches) is to treat large areas at high density while explicitly sparing tissue destruction [18,19].
- Photomechanical interactions: With short, high-peak-power pulses, energy is deposited faster than it can dissipate, producing thermoelastic pressure transients, cavitation/microbubbles, and shock waves that enable photodisruption and scalpel-like tissue breakup. This regime underlies microcavitation-based selectivity, distinct from thermal micropulse (100–300 µs), which operates in a photothermal regime (see ‘Subthreshold diode micropulse (SDM)’ section) [2].
1.4. Retinal Laser Therapy
- Peripheral scatter laser (e.g., panretinal photocoagulation, or PRP) to treat proliferative diabetic retinopathy, proliferative sickle cell retinopathy, and retinal venous occlusive diseases with associated neovascularization.
- Macular focal or grid laser photocoagulation to treat diabetic macular edema or macular edema from branch retinal vein occlusion.
- Laser therapy of focal chorioretinal lesions, including extrafoveal choroidal neovascularization and retinal and choroidal tumors.
- Laser to create adhesions for retinal tears, holes, lattice degeneration, and retinal detachment.
1.5. Effect of Pulse Duration
2. Conventional Retinal Laser Therapy
3. Selective Retinal Therapy
3.1. Mechanism: Cavitation Dominates in the Lower-Microsecond Range
3.2. Implementation and Real-Time Dosimetry
3.3. Dosing, Tissue Variability, and Modeling
3.4. Distinguishing SRT from Subthreshold Micropulse (SDM)
3.5. Historical Development
3.6. Clinical Evidence and Safety
3.7. Cautions and Limitations
4. Innovations in Retinal Laser Therapy
4.1. Pattern Scanning Laser
4.2. Subthreshold Diode Micropulse Laser
4.3. Recent Advancements in Retinal Laser Therapy
4.3.1. Endpoint Management
4.3.2. Navigated Laser (NAVILAS)
4.3.3. OCT Monitoring with Automatic Dosimetry
4.3.4. Sub-Nanosecond Rejuvenation Laser (2RT)
4.3.5. Remote/Teleguided Photocoagulation
5. Discussion
6. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAO | American Academy of Ophthalmology |
| AMD | Age-related macular degeneration |
| BCVA | Best-corrected visual acuity |
| BRVO | Branch retinal vein occlusion |
| CCT | Controlled clinical trial |
| CI | Confidence interval |
| CME | Cystoid macular edema |
| CMT | Central macular thickness |
| CNV | Choroidal neovascularization |
| CSCR | Central serous chorioretinopathy |
| CSME | Clinically significant macular edema |
| CST | Central subfield thickness |
| DME | Diabetic macular edema |
| DRS | Diabetic Retinopathy Study |
| DR | Diabetic retinopathy |
| DRCR.net | Diabetic Retinopathy Clinical Research Network |
| EPM | Endpoint Management |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| FAZ | Foveal avascular zone |
| HSP | Heat shock protein |
| iAMD | Intermediate age-related macular degeneration |
| ILM | Inner limiting membrane |
| IVI | Intravitreal injection |
| LIO | Laser indirect ophthalmoscope |
| MD | Mean deviation (visual field index) |
| MMG | Mild macular grid |
| NPDR | Non-proliferative diabetic retinopathy |
| OCT | Optical coherence tomography |
| PASCAL | PAttern SCAn Laser |
| PDT | Photodynamic therapy |
| PDR | Proliferative diabetic retinopathy |
| PRP | Pan-retinal photocoagulation |
| RCT | Randomized controlled trial |
| RD | Retinal detachment |
| RPE | Retinal pigment epithelium |
| RP | Retinitis pigmentosa |
| SDM | Subthreshold diode micropulse (laser) |
| SML | Subthreshold micropulse laser |
| SMLP | Subthreshold micropulse laser photocoagulation |
| SNL | Sub-nanosecond laser |
| SRF | Subretinal fluid |
| SRT | Selective retinal therapy |
| VF | Visual field |
| VEGF | Vascular endothelial growth factor |
| VPN | Virtual private network |
| WF-SML | Wide field subthreshold micropulse laser |
References
- Munnerlyn, C.R. Lasers in ophthalmology: Past, present and future. J. Mod. Opt. 2003, 50, 2351–2360. [Google Scholar] [CrossRef]
- Vogel, A.; Venugopalan, V. Mechanisms of pulsed laser ablation of biological tissues. Chem. Rev. 2003, 103, 577–644. [Google Scholar] [CrossRef]
- Birngruber, R.; Hillenkamp, F.; Gabel, V.P. Theoretical investigations of laser thermal retinal injury. Health Phys. 1985, 48, 781–796. [Google Scholar] [CrossRef]
- Delibasic, H.; Petrovic, V.; Petrovic, I. Laser Breakdown in Water Induced by λ = 532 nm Nanosecond Pulses: Analytical Calculation of the Number Density of Free Electrons. J. Phys. Soc. Jpn. 2020, 89, 114501. [Google Scholar] [CrossRef]
- Bianco, L.; Gawęcki, M.; Antropoli, A.; Arrigo, A.; Bandello, F.; Battaglia Parodi, M. Laser Treatment for Retinal Arterial Macroaneurysm. Photonics 2022, 9, 851. [Google Scholar] [CrossRef]
- Hecht, J. A short history of laser development. Appl. Opt. 2010, 49, F99–F122. [Google Scholar] [CrossRef]
- Meyer-Schwickerath, G.R. The history of photocoagulation. Aust. N. Z. J. Ophthalmol. 1989, 17, 427–434. [Google Scholar] [CrossRef]
- Bridges, W.B. Laser Oscillation in Singly Ionized Argon in the Visible Spectrum. Appl. Phys. Lett. 1964, 4, 128–130. [Google Scholar] [CrossRef]
- Beetham, W.P.; Aiello, L.M.; Balodimos, M.C.; Koncz, L. Ruby-laser photocoagulation of early diabetic neovascular retinopathy: Preliminary report of a long-term controlled study. Trans. Am. Ophthalmol. Soc. 1969, 67, 39–67. [Google Scholar] [CrossRef] [PubMed]
- L’Esperance, F.A. An ophthalmic argon laser photocoagulation system: Design, construction, and laboratory investigations. Trans. Am. Ophthalmol. Soc. 1968, 66, 827–904. [Google Scholar] [PubMed]
- Palanker, D.V.; Blumenkranz, M.S.; Marmor, M.F. Fifty years of ophthalmic laser therapy. Arch. Ophthalmol. 2011, 129, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Palanker, D.V. Ophthalmic Laser Therapy: Mechanisms and Applications. Master’s Thesis, University of New South Wales, Sydney, Australia, 2014. [Google Scholar]
- Yun, S.H.; Adelman, R.A. Recent developments in laser treatment of diabetic retinopathy. Middle East. Afr. J. Ophthalmol. 2015, 22, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Blumenkranz, M.S. The Evolution of Laser Therapy in Ophthalmology: A Perspective on the Interactions Between Photons, Patients, Physicians, and Physicists: The LXX Edward Jackson Memorial Lecture. Am. J. Ophthalmol. 2014, 158, 12–25.E1. [Google Scholar] [CrossRef]
- Ion, R.M. Photodynamic therapy (PDT): A photochemical concept with medical applications. Rev. Roum. Chim. 2007, 52, 1093–1102. [Google Scholar]
- Woodburn, K.W.; Engelman, C.J.; Blumenkranz, M.S. Photodynamic therapy for choroidal neovascularization: A review. Retina 2002, 22, 391–405. [Google Scholar] [CrossRef]
- Knappe, V.; Frank, F.; Rohde, E. Principles of lasers and biophotonic effects. Photomed. Laser Surg. 2004, 22, 411–417. [Google Scholar] [CrossRef]
- Wang, J.; Quan, Y.; Dalal, R.; Palanker, D. Comparison of Continuous-Wave and Micropulse Modulation in Retinal Laser Therapy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 4722–4732. [Google Scholar] [CrossRef][Green Version]
- Sramek, C.; Mackanos, M.; Spitler, R.; Leung, L.; Nomoto, H.; Contag, C.H. Non-damaging retinal phototherapy: Dynamic range of heat shock protein expression. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1780–1787. [Google Scholar] [CrossRef]
- Keunen, J.E.E.; Battaglia-Parodi, M.; Vujosevic, S.; Luttrull, J.K. International Retinal Laser Society Guidelines for Subthreshold Laser Treatment. Trans. Vis. Sci. Technol. 2020, 9, 15. [Google Scholar] [CrossRef]
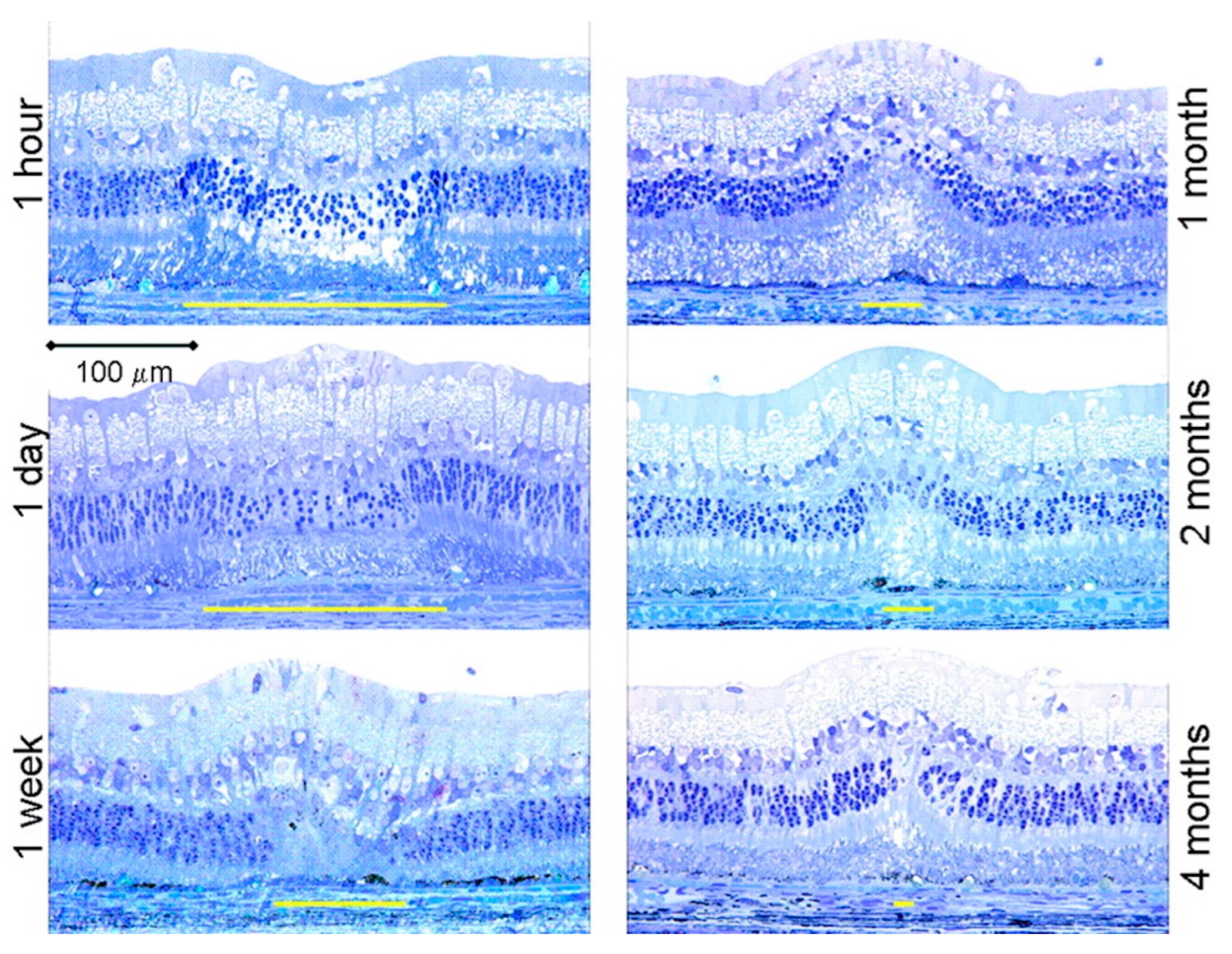
- Paulus, Y.M.; Jain, A.; Gariano, R.F.; Stanzel, B.V.; Marmor, M.; Blumenkranz, M.S. Healing of retinal photocoagulation lesions. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5540–5545. [Google Scholar] [CrossRef] [PubMed]
- Koinzer, S.; Saeger, M.; Hesse, C.; Portz, L.; Kleemann, S.; Schlott, K. Correlation with OCT and histology of photocoagulation lesions in patients and rabbits. Acta Ophthalmol. 2013, 91, e603–e611. [Google Scholar] [CrossRef] [PubMed]
- Maeshima, K.; Utsugi-Sutoh, N.; Otani, T.; Kishi, S. Progressive enlargement of scattered photocoagulation scars in diabetic retinopathy. Retina 2004, 24, 507–511. [Google Scholar] [CrossRef]
- Subash, M.; Comyn, O.; Samy, A.; Qatarneh, D.; Antonakis, S.; Mehat, M.; Tee, J.; Mansour, T.; Xing, W.; Bunce, C.; et al. The Effect of Multispot Laser Panretinal Photocoagulation on Retinal Sensitivity and Driving Eligibility in Patients with Diabetic Retinopathy. JAMA Ophthalmol. 2016, 134, 666–672. [Google Scholar] [CrossRef]
- Stefánsson, E. The therapeutic effects of retinal laser treatment and vitrectomy. A theory based on oxygen and vascular physiology. Acta Ophthalmol. Scand. 2001, 79, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Ascaso, F.J.; Huerva, V.; Grzybowski, A. The role of inflammation in the pathogenesis of macular edema secondary to retinal vascular diseases. Mediat. Inflamm. 2014, 2014, 432685. [Google Scholar] [CrossRef] [PubMed]
- Paulus, Y.M.; Kuo, C.; Morohoshi, K.; Nugent, A.; Zheng, L.L.; Nomoto, H.; Blumenkranz, M.S.; Palanker, D.; Ono, S.J. Serum inflammatory markers after rupture retinal laser injury in mice. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 362–368. [Google Scholar] [CrossRef]
- Matsumoto, M.; Yoshimura, N.; Honda, Y. Increased production of transforming growth factorbeta 2 from cultured human retinal pigment epithelial cells by photocoagulation. Investig. Ophthalmol. Vis. Sci. 1994, 35, 4245–4252. [Google Scholar]
- Prskavec, F.H.; Fulmek, R.; Klemen, C.; Stelzer, N. Changes in the visual field and dark adaptation following panretinal photocoagulation in diabetic retinopathy. Klin. Monbl. Augenheilkd. 1986, 189, 385–387. [Google Scholar] [CrossRef]
- Russell, P.W.; Sekuler, R.; Fetkenhour, C. Visual Function After Pan-Retinal Photocoagulation: A Survey. Diabetes Care 1985, 8, 57–63. [Google Scholar] [CrossRef]
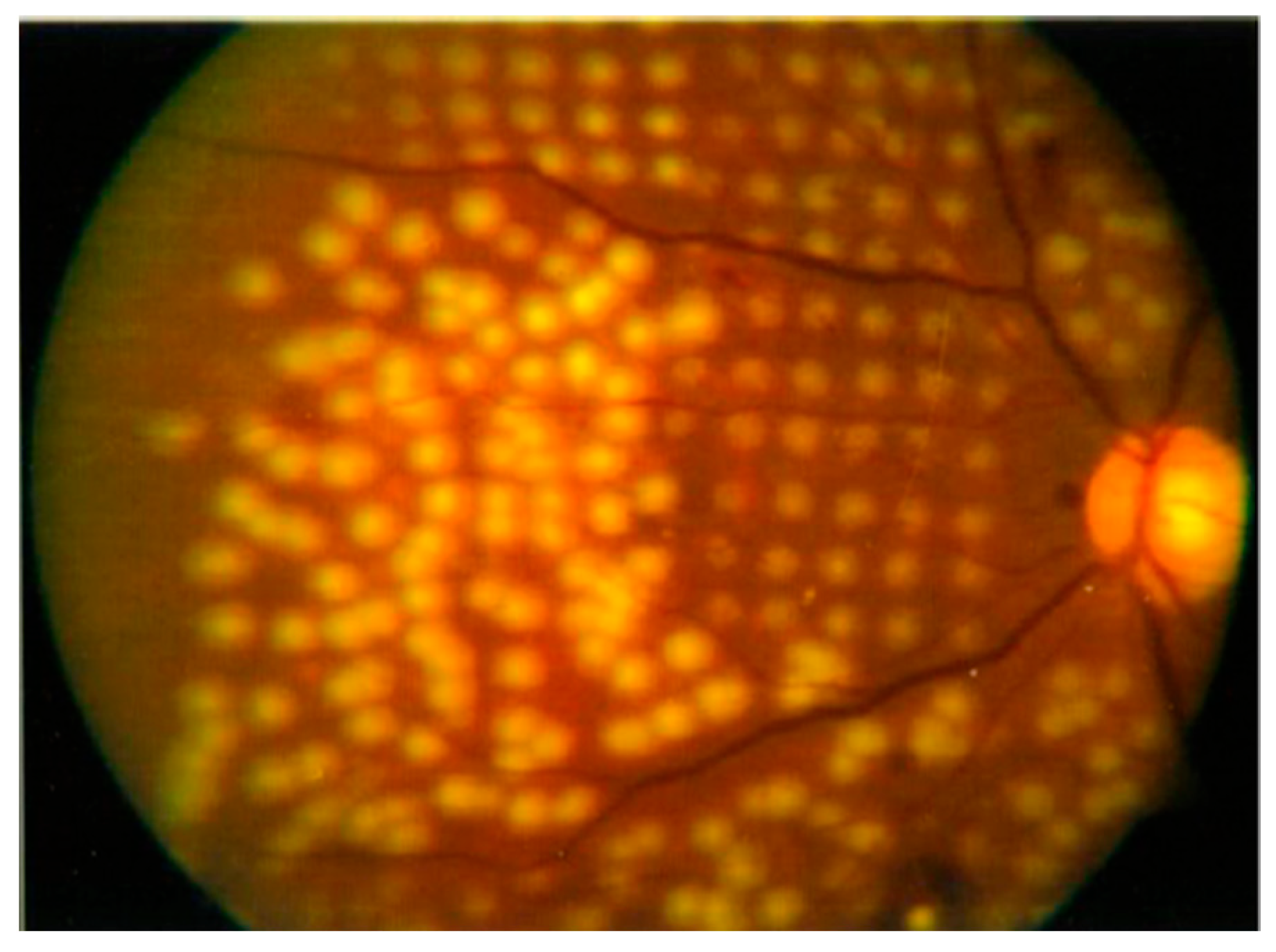
- Blumenkranz, M.S.; Yellachich, D.; Andersen, D.E.; Wiltberger, M.W.; Mordaunt, D.; Marcellino, G.R. Semiautomated patterned scanning laser for retinal photocoagulation. Retina 2006, 26, 370–376. [Google Scholar] [CrossRef]
- Chappelow, A.V.; Tan, K.; Waheed, N.K.; Kaiser, P.K. Panretinal Photocoagulation for Proliferative Diabetic Retinopathy: Pattern Scan Laser Versus Argon Laser. Am. J. Ophthalmol. 2012, 153, 137–142.e2. [Google Scholar] [CrossRef] [PubMed]
- Roider, J.; Michaud, N.A.; Flotte, T.J.; Birngruber, R. Response of the retinal pigment epithelium to selective photocoagulation. Arch. Ophthalmol. 1992, 110, 1786–1792. [Google Scholar] [CrossRef]
- Sinclair, S.H.; Mainster, M.A. Seminars in ophthalmology. Semin. Ophthalmol. 1999, 14, 197–199. [Google Scholar] [CrossRef]
- Sramek, C.; Paulus, Y.; Nomoto, H.; Huie, P.; Brown, J.; Palanker, D. Dynamics of retinal photocoagulation and rupture. J. Biomed. Opt. 2009, 14, 034007. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Blumenkranz, M.S.; Paulus, Y.; Wiltberger, M.W.; Andersen, D.E.; Huie, P.; Palanker, D. Effect of pulse duration on size and character of the lesion in retinal photocoagulation. Arch. Ophthalmol. 2008, 126, 78–85. [Google Scholar] [CrossRef]
- Schuele, G.; Rumohr, M.; Huettmann, G.; Brinkmann, R. RPE damage thresholds and mechanisms for laser exposure in the microsecond-to-millisecond time regimen. Investig. Ophthalmol. Vis. Sci. 2005, 46, 714–719. [Google Scholar] [CrossRef]
- Lee, H.; Alt, C.; Pitsillides, C.M.; Lin, C.P. Optical detection of intracellular cavitation during selective laser targeting of the retinal pigment epithelium: Dependence of cell death mechanism on pulse duration. J. Biomed. Opt. 2007, 12, 064034. [Google Scholar] [CrossRef]
- Seifert, E.; Sonntag, S.R.; Kleingarn, P.; Theisen-Kunde, D.; Grisanti, S.; Birngruber, R.; Miura, Y.; Brinkmann, R. Investigations on Retinal Pigment Epithelial Damage at Laser Irradiation in the Lower Microsecond Time Regime. Investig. Ophthalmol. Vis. Sci. 2021, 62, 32. [Google Scholar] [CrossRef]
- Schuele, G.; Elsner, H.; Framme, C.; Roider, J.; Birngruber, R.; Brinkmann, R. Optoacoustic real-time dosimetry for selective retina treatment. J. Biomed. Opt. 2005, 10, 064022. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Považay, B.; Brinkmann, R.; Stoller, M.; Kessler, R. Selective Retina Therapy. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Switzerland, 2019; pp. 237–259. [Google Scholar]
- Seifert, E.; Tode, J.; Pielen, A.; Theisen-Kunde, D.; Framme, C.; Roider, J.; Miura, Y.; Birngruber, R.; Brinkmann, R. Algorithms for optoacoustically controlled selective retina therapy (SRT). Photoacoustics 2022, 25, 100316. [Google Scholar] [CrossRef]
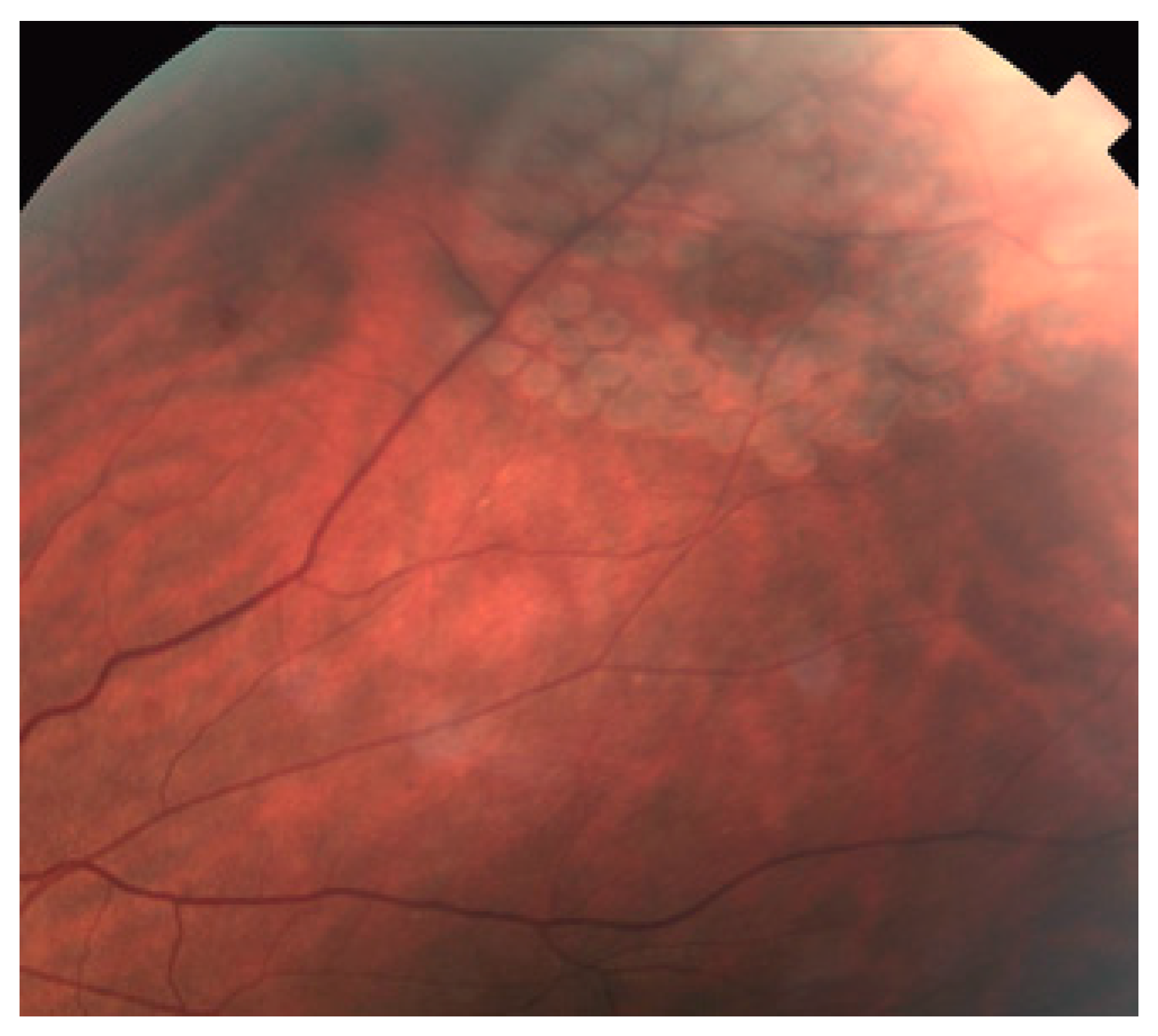
- Lavinsky, D.; Wang, J.; Huie, P.; Dalal, R.; Lee, S.J.; Lee, D.Y.; Palanker, D. Nondamaging Retinal Laser Therapy: Rationale and Applications to the Macula. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2488–2500. [Google Scholar] [CrossRef]
- Scholz, P.; Altay, L.; Fauser, S. A Review of Subthreshold Micropulse Laser for Treatment of Macular Disorders. Adv. Ther. 2017, 34, 1528–1555. [Google Scholar] [CrossRef]
- Chhablani, J.; Alshareef, R.; Kim, D.T.; Narayanan, R.; Goud, A.; Mathai, A. Comparison of different settings for yellow subthreshold laser treatment in diabetic macular edema. BMC Ophthalmol. 2018, 18, 168. [Google Scholar] [CrossRef]
- Wang, J.; Lavinsky, D.; Huie, P.; Dalal, R.; Lee, D.Y.; Lee, S.J.; Palanker, D.V. Determining the therapeutic window of non-damaging retinal laser therapy by protein expression. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5845. [Google Scholar]
- Little, H.L.; Zweng, H.C.; Peabody, R.R. Argon laser slit-lamp retinal photocoagulation. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1970, 74, 85–97. [Google Scholar]
- Law, N.M.; Fan, R.F. Clinical experience with the laser indirect ophthalmoscope. Ann. Acad. Med. Singap. 1991, 20, 750–754. [Google Scholar]
- Smiddy, W.E. Diode endolaser photocoagulation. Arch. Ophthalmol. 1992, 110, 1172–1174. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Treatment Techniques and Clinical Guidelines for Photocoagulation of Diabetic Macular Edema: Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology 1987, 94, 761–774. [Google Scholar] [CrossRef] [PubMed]
- The Diabetic Retinopathy Study Research Group. Photocoagulation Treatment of Proliferative Diabetic Retinopathy: Clinical Application of Diabetic Retinopathy Study (DRS) Findings. Ophthalmology 1981, 88, 583–600. [Google Scholar] [CrossRef]
- Huang, T.; Li, X.; Xie, J.; Zhang, L.; Zhang, G.; Zhang, A.; Chen, X.; Cui, Y.; Meng, Q. Long-Term Retinal Neurovascular and Choroidal Changes After Panretinal Photocoagulation in Diabetic Retinopathy. Front. Med. 2021, 8, 752538. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Early Photocoagulation for Diabetic Retinopathy. Ophthalmology 1991, 98, 766–785. [Google Scholar] [CrossRef]
- Ogata, N.; Ando, A.; Uyama, M.; Matsumura, M. Expression of cytokines and transcription factors in photocoagulated human retinal pigment epithelial cells. Graefes Arch. Clin. Exp. Ophthalmol. 2001, 239, 87–95. [Google Scholar] [CrossRef]
- The Diabetic Retinopathy Study Research Group. Photocoagulation for Diabetic Macular Edema: Early Treatment Diabetic Retinopathy Study report number 1. Arch. Ophthalmol. 1985, 103, 1796–1806. [Google Scholar] [CrossRef]
- Writing Committee for the Diabetic Retinopathy Clinical Research Network; Fong, D.S.; Strauber, S.F.; Aiello, L.P.; Beck, R.W.; Callanan, D.G.; Danis, R.P.; Davis, M.D.; Feman, S.S.; Ferris, F.; et al. Comparison of the modified Early Treatment Diabetic Retinopathy Study and mild macular grid laser photocoagulation strategies for diabetic macular edema. Arch. Ophthalmol. 2007, 125, 469–480. [Google Scholar] [CrossRef]
- Diabetic Retinopathy Clinical Research Network (DRCR.net). A Randomized Trial Comparing Intravitreal Triamcinolone Acetonide and Focal/Grid Photocoagulation for Diabetic Macular Edema. Ophthalmology 2008, 115, 1447–1459. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, M.; Ando, R.; Kimura, T.; Kato, F.; Yasukawa, T. The Role of Laser Photocoagulation in Treating Diabetic Macular Edema in the Era of Intravitreal Drug Administration: A Descriptive Review. Medicina 2023, 59, 1319. [Google Scholar] [CrossRef] [PubMed]
- Colyear, B.H.; Pischel, D.K. Preventive treatment of retinal detachment by means of light coagulation. Trans. Pac. Coast. Otoophthalmol. Soc. Annu. Meet. 1960, 41, 193–217. [Google Scholar] [PubMed]
- Glaser, B.M.; Vidaurri-Leal, J.; Michels, R.G.; Campochiaro, P.A. Cryotherapy during Surgery for Giant Retinal Tears and Intravitreal Dispersion of Viable Retinal Pigment Epithelial Cells. Ophthalmology 1993, 100, 466–470. [Google Scholar] [CrossRef]
- Brod, R.D.; Flynn, H.W.; Lightman, D.A. Asymptomatic rhegmatogenous retinal detachments. Arch. Ophthalmol. 1995, 113, 1030–1032. [Google Scholar] [CrossRef]
- Morris, R.E.; Kuhn, F.; Richardson, C. Preventing Retinal Detachment: The Encircling Laser Retinopexy Technique. Clin. Ophthalmol. 2023, 17, 1505–1513. [Google Scholar] [CrossRef]
- Pollak, A.; Oliver, M. Argon laser photocoagulation of symptomatic flap tears and retinal breaks of fellow eyes. Br. J. Ophthalmol. 1981, 65, 469–472. [Google Scholar] [CrossRef]
- Mainster, M.A.; Crossman, J.L.; Erickson, P.J.; Heacock, G.L. Retinal laser lenses: Magnification, spot size, and field of view. Br. J. Ophthalmol. 1990, 74, 177–179. [Google Scholar] [CrossRef]
- Silva, R.A.; Blumenkranz, M.S. Prophylaxis for Retinal Detachments. Available online: https://www.aao.org/education/current-insight/prophylaxis-retinal-detachments (accessed on 15 September 2025).
- Gabel, V.; Birngruber, R.; Hillenkamp, F. Visible and near infrared light absorption in pigment epithelium and choroid. In Proceedings of the 23rd Consilium Ophthalmologicum, Kyoto) Excerpta Medic, Kyoto, Japan, 14–20 May 1978; pp. 658–662. [Google Scholar]
- Richert, E.; Papenkort, J.; von der Burchard, C.; Klettner, A.; Arnold, P.; Lucius, R.; Brinkmann, R.; Framme, C.; Roider, J.; Tode, J. Selective retina therapy and thermal stimulation of the retina: Different regenerative properties—Implications for AMD therapy. BMC Ophthalmol. 2021, 21, 412. [Google Scholar] [CrossRef]
- Jacques, S.L.; McAuliffe, D.J. The melanosome: Threshold temperature for explosive vaporization and internal absorption coefficient during pulsed laser irradiation. Photochem. Photobiol. 1991, 53, 769–775. [Google Scholar] [CrossRef]
- Burri, C.; Salzmann, S.; Wandel, J.; Hoffmann, L.; Považay, B.; Meier, C.; Frenz, M. Real-time OCT feedback-controlled RPE photodisruption in ex vivo porcine eyes using 8 microsecond laser pulses. Biomed. Opt. Express 2023, 14, 6328–6349. [Google Scholar] [CrossRef]
- Steiner, P.; Ebneter, A.; Berger, L.E.; Zinkernagel, M.; Považay, B.; Meier, C.; Kowal, J.H.; Framme, C.; Brinkmann, R.; Wolf, S.; et al. Time-Resolved Ultra–High Resolution Optical Coherence Tomography for Real-Time Monitoring of Selective Retina Therapy. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6654–6662. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, L.; Ness, S.; Yi, J. Wavelength-dependent optical properties of melanosomes in retinal pigmented epithelium and their changes with melanin bleaching: A numerical study. Biomed. Opt. Express 2017, 8, 3966–3980. [Google Scholar] [CrossRef] [PubMed]
- Shimojo, Y.; Nishimura, T.; Ozawa, T.; Tsuruta, D.; Awazu, K. Nonlinear absorption-based analysis of energy deposition in melanosomes for 532-nm short-pulsed laser skin treatment. Lasers Surg. Med. 2023, 55, 305–315. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, H.F. Photoacoustic imaging of the eye: A mini review. Photoacoustics 2016, 4, 112–123. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Sandhu, R.; Tandon, A.; Sayed-Ahmed, K.; Mchugh, D.A. Subthreshold micropulse diode laser photocoagulation for clinically significant diabetic macular oedema: A three-year follow up. Clin. Exp. Ophthalmol. 2007, 35, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Brader, H.S.; Young, L. Subthreshold Diode Micropulse Laser: A Review. Semin. Ophthalmol. 2016, 31, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Roider, J.; Brinkmann, R.; Wirbelauer, C.; Laqua, H.; Birngruber, R. Subthreshold (retinal pigment epithelium) photocoagulation in macular diseases: A pilot study. Br. J. Ophthalmol. 2000, 84, 40–47. [Google Scholar] [CrossRef]
- Framme, C.; Alt, C.; Schnell, S.; Sherwood, M.; Brinkmann, R.; Lin, C.P. Selective targeting of the retinal pigment epithelium in rabbit eyes with a scanning laser beam. Investig. Ophthalmol. Vis. Sci. 2007, 48, 1782–1792. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paulus, Y.M.; Jain, A.; Nomoto, H.; Sramek, C.; Gariano, R.F.; Andersen, D.; Schuele, G.; Leung, L.S.; Leng, T.; Palanker, D. Selective retinal therapy with microsecond exposures using a continuous line scanning laser. Retina 2011, 31, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.H.; Kim, M.; Roh, Y. Use of a Fundus Image-Based Titration Strategy for Selective Retina Therapy for Central Serous Chorioretinopathy. J. Clin. Med. 2024, 13, 5230. [Google Scholar] [CrossRef]
- Yoon, C.K.; Yu, H.G. Selective retina therapy with real-time feedback-controlled technology in central serous chorioretinopathy: A 24-month follow-up real-world prospective study. BMJ Open Ophthalmol. 2024, 9, e001517. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Jeon, S.; Lee, S.; Roh, Y. The Effect of Selective Retina Therapy with Automatic Real-Time Feedback-Controlled Dosimetry for Chronic Central Serous Chorioretinopathy: A Randomized, Open-Label, Controlled Clinical Trial. J. Clin. Med. 2021, 10, 4295. [Google Scholar] [CrossRef]
- Getahun, H.; Apte, R.S. Therapeutic interventions for chronic central serous chorioretinopathy: A comprehensive assessment of systematic reviews. Int. J. Retin. Vitr. 2025, 11, 34. [Google Scholar] [CrossRef]
- Roider, J.; Liew, S.H.M.; Klatt, C.; Elsner, H.; Poerksen, E.; Hillenkamp, J.; Brinkmann, R.; Birngruber, R. Selective retina therapy (SRT) for clinically significant diabetic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2010, 248, 1263–1272. [Google Scholar] [CrossRef]
- Park, Y.G.; Kim, J.R.; Kang, S.; Seifert, E.; Theisen-Kunde, D.; Brinkmann, R.; Roh, Y. Safety and efficacy of selective retina therapy (SRT) for the treatment of diabetic macular edema in Korean patients. Graefes Arch. Clin. Exp. Ophthalmol. 2016, 254, 1703–1713. [Google Scholar] [CrossRef]
- Guymer, R.H.; Chen, F.K.; Hodgson, L.A.B.; Caruso, E.; Harper, C.A.; Wickremashinghe, S.S.; Cohn, A.C.; Sivarajah, P.; Tindill, N.; Luu, C.D.; et al. Subthreshold Nanosecond Laser in Age-Related Macular Degeneration: Observational Extension Study of the LEAD Clinical Trial. Ophthalmol. Retin. 2021, 5, 1196–1203. [Google Scholar] [CrossRef]
- Chichan, H.; Maus, M.; Heindl, L.M. Subthreshold Nanosecond Laser, from Trials to Real-Life Clinical Practice: A Cohort Study. Clin. Ophthalmol. 2021, 15, 1887–1895. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Feuer, W.J. Warning: Do Not Treat Intermediate AMD with Laser Therapy. Ophthalmology 2019, 126, 839–840. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Gray, G.; Bhavan, S.V. Vision protection therapy for prevention of neovascular age-related macular degeneration. Sci. Rep. 2023, 13, 16710. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K.; Gray, G. Real World Data Comparison of Standard Care vs SDM Laser Vision Protection Therapy for Prevention of Neovascular AMD. Clin. Ophthalmol. 2022, 16, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Paulus, Y.M.; Kaur, K.; Egbert, P.R.; Blumenkranz, M.S.; Moshfeghi, D.M. Human histopathology of PASCAL laser burns. Eye 2013, 27, 995–996. [Google Scholar] [CrossRef]
- Hassanpoor, N.; Ahoor, M.; Latifi, A.; Niyousha, M. Conventional and Pattern Scanning Pan-Retinal Photocoagulation Laser in Diabetic Patients’ Visual Field. J. Lasers Med. Sci. 2022, 13, e40. [Google Scholar] [CrossRef]
- Palanker, D.; Lavinsky, D.; Blumenkranz, M.S.; Marcellino, G. The impact of pulse duration and burn grade on size of retinal photocoagulation lesion: Implications for pattern density. Retina 2011, 31, 1664–1669. [Google Scholar] [CrossRef]
- Muqit, M.; Marcellino, G.R.; Gray, J.; Mclauchlan, R.; Henson, D.B.; Young, L.B. Pain responses of Pascal 20 ms multi-spot and 100 ms single-spot panretinal photocoagulation. Br. J. Ophthalmol. 2010, 2, 1493–1498. [Google Scholar] [CrossRef]
- Azarcon, C.P.; Artiaga, J.C.M. Comparison of Pain Scores Among Patients Undergoing Conventional and Novel Panretinal Photocoagulation for Diabetic Retinopathy: A Systematic Review. Clin. Ophthalmol. 2021, 15, 953–971. [Google Scholar] [CrossRef]
- Koca, S.; Kılıç, D. Long-term longitudinal retinal changes after conventional and pattern scan laser panretinal photocoagulation in diabetic retinopathy. Photodiagnosis Photodyn. Ther. 2023, 44, 103845. [Google Scholar] [CrossRef] [PubMed]
- Kozak, I.; Luttrull, J.K. Modern retinal laser therapy. Saudi J. Ophthalmol. 2015, 29, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.; Bhuckory, M.; Li, H.; Hattori, J.; Pham-Howard, D.; Veysset, D.; Ling, T.; Palanker, D. Retinal thermal deformations measured with phase-sensitive optical coherence tomography in vivo. Light Sci. Appl. 2025, 14, 151. [Google Scholar] [CrossRef]
- Amoroso, F.; Pedinielli, A.; Astroz, P.; Semoun, O.; Capuano, V.; Miere, A.; Souied, E.H. Comparison of pain experience and time required for pre-planned navigated peripheral laser versus conventional multispot laser in the treatment of diabetic retinopathy. Acta Diabetol. 2020, 57, 535–541. [Google Scholar] [CrossRef]
- Inan, S.; Polat, O.; Yıgıt, S.; Inan, U.U. PASCAL laser platform produces less pain responses compared to conventional laser system during the panretinal photocoagulation: A randomized clinical trial. Afr. Health Sci. 2018, 18, 1010–1017. [Google Scholar] [CrossRef]
- Nagpal, M.; Marlecha, S.; Nagpal, K. Comparison of laser photocoagulation for diabetic retinopathy using 532-nm standard laser versus multispot pattern scan laser. Retina 2010, 30, 452–458. [Google Scholar] [CrossRef]
- Palanker, D.; Blumenkranz, M.S. Panretinal photocoagulation for proliferative diabetic retinopathy. Am. J. Ophthalmol. 2012, 153, 780–781. [Google Scholar] [CrossRef]
- Paulus, Y.M.; Palanker, D.; Blumenkranz, M.S. Short-pulse Laser Treatment: Redefining Retinal Therapy. Retin. Physician 2010, 7, 56–59. [Google Scholar]
- Muqit, M.; Gray, J.; Marcellino, G.R.; Henson, D.B.; Young, L.B.; Patton, N. Barely Visible 10-Millisecond Pascal Laser Photocoagulation for Diabetic Macular Edema: Observations of Clinical Effect and Burn Localization. Am. J. Ophthalmol. 2010, 149, 979–986.e2. [Google Scholar] [CrossRef]
- Muqit, M.; Sanghvi, C.; Mclauchlan, R.; Delgado, C.; Young, L.B.; Charles, S.J. Study Study of clinical applications and safety for Pascal® laser photocoagulation in retinal vascular disorders. Acta Ophthalmol. 2012, 90, 155–161. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Sinclair, S.H. Safety of transfoveal subthreshold diode micropulse laser for fovea-involving diabetic macular edema in eyes with good visual acuity. Retina 2014, 34, 2010–2020. [Google Scholar] [CrossRef]
- Pankratov, M.M. Pulsed delivery of laser energy in experimental thermal retinal photocoagulation. In Proceedings of the SPIE, Laser-Tissue Interaction, Los Angeles, CA, USA, 1 June 1990; Volume 1202, pp. 1202–1209. [Google Scholar]
- Chang, D.B.; Luttrull, J.K. Comparison of Subthreshold 577 and 810 nm Micropulse Laser Effects on Heat-Shock Protein Activation Kinetics: Implications for Treatment Efficacy and Safety. Transl. Vis. Sci. Technol. 2020, 9, 23. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Shuo, T.; Katakura, K.; Ebihara, N.; Murakami, A.; Ohkoshi, K. Sublethal Photothermal Stimulation with a Micropulse Laser Induces Heat Shock Protein Expression in ARPE-19 Cells. J. Ophthalmol. 2015, 2015, 729792. [Google Scholar] [CrossRef] [PubMed]
- Flaxel, C.; Bradle, J.; Acott, T.; Samples, J.R. Retinal pigment epithelium produces matrix metalloproteinases after laser treatment. Retina 2007, 27, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Hattenbach, L.O.; Beck, K.F.; Pfeilschifter, J.; Koch, F.; Ohrloff, C.; Schacke, W. Pigment-epitheliumderived factor is upregulated in photocoagulated human retinal pigment epithelial cells. Ophthalmic Res. 2005, 37, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Mainster, M.A. Decreasing Retinal Photocoagulation Damage: Principles and Techniques. Semin. Ophthalmol. 1999, 14, 200–209. [Google Scholar] [CrossRef]
- Frizziero, L.; Calciati, A.; Midena, G.; Torresin, T.; Parrozzani, R.; Pilotto, E.; Midena, E. Subthreshold Micropulse Laser Modulates Retinal Neuroinflammatory Biomarkers in Diabetic Macular Edema. J. Clin. Med. 2021, 10, 3134. [Google Scholar] [CrossRef]
- Sivaprasad, S.; Elagouz, M.; Mchugh, D.; Shona, O.; Dorin, G. Micropulsed Diode Laser Therapy: Evolution and Clinical Applications. Surv. Ophthalmol. 2010, 55, 516–530. [Google Scholar] [CrossRef]
- Kiire, C.; Sivaprasad, S.; Chong, V. Subthreshold micropulse laser therapy for retinal disorders. Retina Today 2011, 1, 67–70. [Google Scholar]
- Hu, X.; Cao, L.; Gao, Y.; Luan, J.; Xu, X. Comparative Efficacy of Subthreshold Micropulse Laser Photocoagulation versus Conventional Laser Photocoagulation for Diabetic Macular Edema: A Meta-Analysis. Ophthalmic Res. 2023, 66, 611–619. [Google Scholar] [CrossRef]
- Su, D.; Hubschman, J. A Review of Subthreshold Micropulse Laser and Recent Advances in Retinal Laser Technology. Ophthalmol. Ther. 2017, 6, 1–6. [Google Scholar] [CrossRef][Green Version]
- Luttrull, J.K. Improved retinal and visual function following panmacular subthreshold diode micropulse laser for retinitis pigmentosa. Eye 2018, 32, 1099–1110. [Google Scholar] [CrossRef]
- Bressler, S.B.; Almukhtar, T.; Aiello, L.P.; Bressler, N.M.; Ferris, F.L.; Glassman, A.R. Green or yellow laser treatment for diabetic macular edema: Exploratory assessment within the Diabetic Retinopathy Clinical Research Network. Retina 2013, 33, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Luttrull, J.K.; Bhavan, S.V. Visual Field Improvement by Standardized Automated Perimetry Following Panmacular Subthreshold Diode Micropulse Laser (SDM) in Open-Angle Glaucoma and Other Optic Atrophies. Diagnostics 2025, 15, 912. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Zhu, T.; Huang, L.; Wang, X.; Chen, M. Clinical efficacy of subthreshold micropulse laser combined with anti-VEGF drugs in the treatment of diabetic macular edema: A meta-analysis. Medicine 2024, 103, e34583. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Dorin, G. Subthreshold diode micropulse laser photocoagulation (SDM) as invisible retinal phototherapy for diabetic macular edema: A review. Curr. Diabetes Rev. 2012, 8, 274–284. [Google Scholar] [CrossRef]
- Figueira, J.; Khan, J.; Nunes, S.; Sivaprasad, S.; Rosa, A.; De Abreu, J.F. Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br. J. Ophthalmol. 2009, 93, 1341–1344. [Google Scholar] [CrossRef]
- Lavinsky, D.; Cardillo, J.A.; Melo, L.; Dare, A.; Farah, M.E.; Belfort, R. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4314–4323. [Google Scholar] [CrossRef] [PubMed]
- Lois, N.; Campbell, C.; Waugh, N.; Azuara-Blanco, A.; Maredza, M.; Mistry, H.; McAuley, D.; Acharya, N.; Aslam, T.M.; Bailey, C.; et al. Diabetic Macular Edema and Diode Subthreshold Micropulse Laser: A Randomized Double-Masked Noninferiority Clinical Trial. Ophthalmology 2023, 130, 14–27. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Musch, D.C.; Spink, C.A. Subthreshold diode micropulse panretinal photocoagulation for proliferative diabetic retinopathy. Eye 2008, 22, 607–612. [Google Scholar] [CrossRef]
- Li, Z.; Lu, T.; Zhou, L.; Huang, C.; Zhao, H.; Liang, J.; Li, C.; Cong, Q.; Lan, Y.; Jin, C. Retinal and Choroidal Alterations in Diabetic Retinopathy Treatment using Subthreshold Panretinal Photocoagulation with Endpoint Management Algorithm: A Secondary Analysis of a Randomized Clinical Trial. Ophthalmol. Ther. 2023, 12, 1867–1880. [Google Scholar] [CrossRef]
- Lavinsky, D.; Sramek, C.; Wang, J.; Huie, P.; Dalal, R.; Mandel, Y.; Palanker, D. Subvisible Retinal Laser Therapy: Titration Algorithm and Tissue Response. Retina 2014, 34, 87–97. [Google Scholar] [CrossRef]
- Önen, M.; Zor, K.R.; Doğan, L.; Küçük, E.; Yıldırım Biçer, G.; Özer, Ö. The results of subthreshold 577 nm yellow laser application using pascal laser system in patients with chronic central serous chorioretinopathy. Lasers Med. Sci. 2025, 40, 262. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.Ç.; Çitirik, M.; Yilmaz, M.; Şensoy, E. Short-term efficacy and safety of nondamaging retinal laser therapy for retinitis pigmentosa-associated cystoid macular edema. Turk. J. Med. Sci. 2025, 55, 652–657. [Google Scholar] [CrossRef]
- Veysset, D.; Zhuo, Y.; Hattori, J.; Bhuckory, M.B.; Pandiyan, V.P.; Sabesan, R.; Palanker, D.V. Retinal absorption measurements for laser therapy through interferometric imaging of the thermal expansion. Investig. Ophthalmol. Vis. Sci. 2022, 63, 1062-F0309. [Google Scholar]
- Kernt, M.; Cheuteu, R.; Vounotrypidis, E.; Haritoglou, C.; Kampik, A.; Ulbig, M.W. Focal and panretinal photocoagulation with a navigated laser (NAVILAS). Acta Ophthalmol. 2011, 89, e662–e664. [Google Scholar] [CrossRef] [PubMed]
- Schlott, K.; Koinzer, S.; Ptaszynski, L.; Bever, M.; Baade, A.; Roider, J. Automatic temperature controlled retinal photocoagulation. J. Biomed. Opt. 2012, 17, 061223. [Google Scholar] [CrossRef]
- Kleyman, V.; Eggert, S.; Schmidt, C.; Schaller, M.; Worthmann, K.; Brinkmann, R.; Müller, M.A. Model Predictive Temperature Control for Retinal Laser Treatments. Transl. Vis. Sci. Technol. 2024, 13, 28. [Google Scholar] [CrossRef]
- Oh, J.; Yoon, C.K.; Kim, B.H.; Yu, H.G. Evaluation of the Safety and Efficacy of Selective Retina Therapy Laser Treatment in Patients with Central Serous Chorioretinopathy. Korean J. Ophthalmol. 2021, 35, 51–63. [Google Scholar] [CrossRef]
- Park, Y.G.; Kang, S.; Kim, M.; Yoo, N.; Roh, Y.J. Selective retina therapy with automatic real-time feedback-controlled dosimetry for chronic central serous chorioretinopathy in Korean patients. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 1375–1383. [Google Scholar] [CrossRef]
- Cusumano, A.; Ross, R.; Falsini, B.; Lombardo, M. Teleguided photocoagulation treatments across continents with a remotely programmed laser for retinal diseases. Ther. Adv. Ophthalmol. 2025, 17, 2515–8414. [Google Scholar] [CrossRef]
- Chen, H.; Pan, X.; Yang, J.; Fan, J.; Qin, M.; Sun, H.; Liu, J.; Li, N.; Ting, D.S.W.; Chen, Y. Application of 5G Technology to Conduct Real-Time Teleretinal Laser Photocoagulation for the Treatment of Diabetic Retinopathy. JAMA Ophthalmol. 2021, 139, 975–982. [Google Scholar] [CrossRef]
- Miura, Y.; Inagaki, K.; Hutfilz, A.; Seifert, E.; Schmarbeck, B.; Murakami, A.; Ohkoshi, K.; Brinkmann, R. Temperature Increase and Damage Extent at Retinal Pigment Epithelium Compared between Continuous Wave and Micropulse Laser Application. Life 2022, 12, 1313. [Google Scholar] [CrossRef]
- Gross, J.G.; Glassman, A.R.; Liu, D.; Sun, J.K.; Antoszyk, A.N.; Baker, C.W.; Bressler, N.M.; Elman, M.J.; Ferris, F.L., III; Gardner, T.W.; et al. Five-Year Outcomes of Panretinal Photocoagulation vs Intravitreous Ranibizumab for Proliferative Diabetic Retinopathy: A Randomized Clinical Trial. JAMA Ophthalmol. 2018, 136, 1138–1148. [Google Scholar] [CrossRef]
- Beaulieu, W.T.; Bressler, N.M.; Melia, M.; Owsley, C.; Mein, C.E.; Gross, J.G.; Jampol, L.M.; Glassman, A.R. Panretinal Photocoagulation Versus Ranibizumab for Proliferative Diabetic Retinopathy: Patient-Centered Outcomes from a Randomized Clinical Trial. Am. J. Ophthalmol. 2016, 170, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Glassman, A.R.; Beaulieu, W.T.; Stockdale, C.R.; Bressler, N.M.; Flaxel, C.; Gross, J.G.; Shami, M.; Jampol, L.M. Rationale and Application of the Protocol S Anti–Vascular Endothelial Growth Factor Algorithm for Proliferative Diabetic Retinopathy. Ophthalmology 2019, 126, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chhablani, J.; Mathai, A.; Rani, P.; Gupta, V.; Arevalo, J.F.; Kozak, I. Comparison of Conventional Pattern and Novel Navigated Panretinal Photocoagulation in Proliferative Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2014, 55, 3432–3438. [Google Scholar] [CrossRef][Green Version]
- Polat, O.; Inan, S.; Baysal, Z.; Yigit, S.; Inan, U.U. Comparison of navigated laser and conventional single-spot laser system for induced pain during panretinal photocoagulation. Lasers Med. Sci. 2020, 35, 687–693. [Google Scholar] [CrossRef]
- Kozak, I.; Oster, S.F.; Cortes, M.A.; Dowell, D.; Hartmann, K.; Kim, J.S.; Freeman, W.R. Clinical Evaluation and Treatment Accuracy in Diabetic Macular Edema Using Navigated Laser Photocoagulator NAVILAS. Ophthalmology 2011, 118, 1119–1124. [Google Scholar] [CrossRef]
- Palanker, D. The Scientific Rationale for Non-Damaging Retinal Laser Therapy. Insert to Retina Today 2015, Nov/Dec, 2–4. Available online: https://assets.bmctoday.net/retinatoday/pdfs/1215_insert3.pdf (accessed on 20 October 2025).
- Mistry, H.; Maredza, M.; Campbell, C.; Lois, N. Subthreshold micropulse laser versus standard laser for the treatment of central-involving diabetic macular oedema with central retinal thickness of <400µ: A cost-effectiveness analysis from the DIAMONDS trial. BMJ Open 2023, 13, e067684. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, H.; Ueta, T.; Hirasawa, K.; Toyama, T.; Shiraya, T. Subthreshold micropulse laser combined with anti-vascular endothelial growth factor therapy for diabetic macular edema: A systematic review and meta-analysis. Graefes Arch. Clin. Exp. Ophthalmol. 2024, 262, 3073–3083. [Google Scholar] [CrossRef] [PubMed]
- Kim, L.A.; D’Amore, P.A. A brief history of anti-VEGF for the treatment of ocular angiogenesis. Am. J. Pathol. 2012, 181, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Nomoto, H.; Lavinsky, D.; Paulus, Y.M.; Leung, L.; Dalal, R.; Blumenkranz, M.S. Effect of intravitreal triamcinolone acetonide on healing of retinal photocoagulation lesions. Retina 2013, 33, 63–70. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Munir, L.; Paulus, Y.M. Retinal Laser Therapy Mechanisms, Innovations, and Clinical Applications. Photonics 2025, 12, 1043. https://doi.org/10.3390/photonics12111043
Xie X, Munir L, Paulus YM. Retinal Laser Therapy Mechanisms, Innovations, and Clinical Applications. Photonics. 2025; 12(11):1043. https://doi.org/10.3390/photonics12111043
Chicago/Turabian StyleXie, Xinyi, Luqman Munir, and Yannis Mantas Paulus. 2025. "Retinal Laser Therapy Mechanisms, Innovations, and Clinical Applications" Photonics 12, no. 11: 1043. https://doi.org/10.3390/photonics12111043
APA StyleXie, X., Munir, L., & Paulus, Y. M. (2025). Retinal Laser Therapy Mechanisms, Innovations, and Clinical Applications. Photonics, 12(11), 1043. https://doi.org/10.3390/photonics12111043




