Application of Micro-Tubing Reeling System to Serial Femtosecond Crystallography
Abstract
1. Introduction
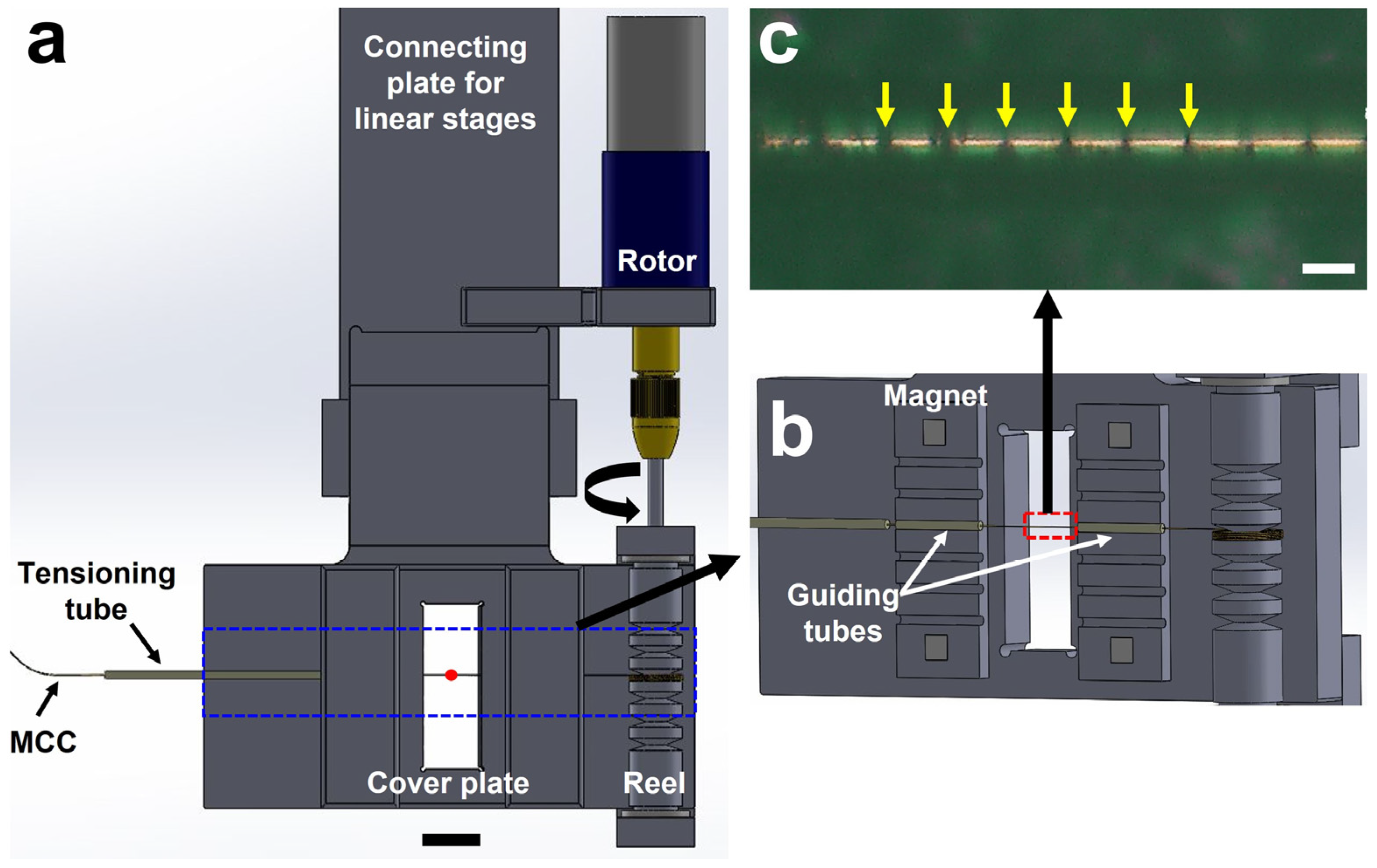
2. Micro-Tubing Reeling System
3. Experiments and Results
3.1. Experimental Conditions and Data Acquisition
3.2. Operation of the MRS
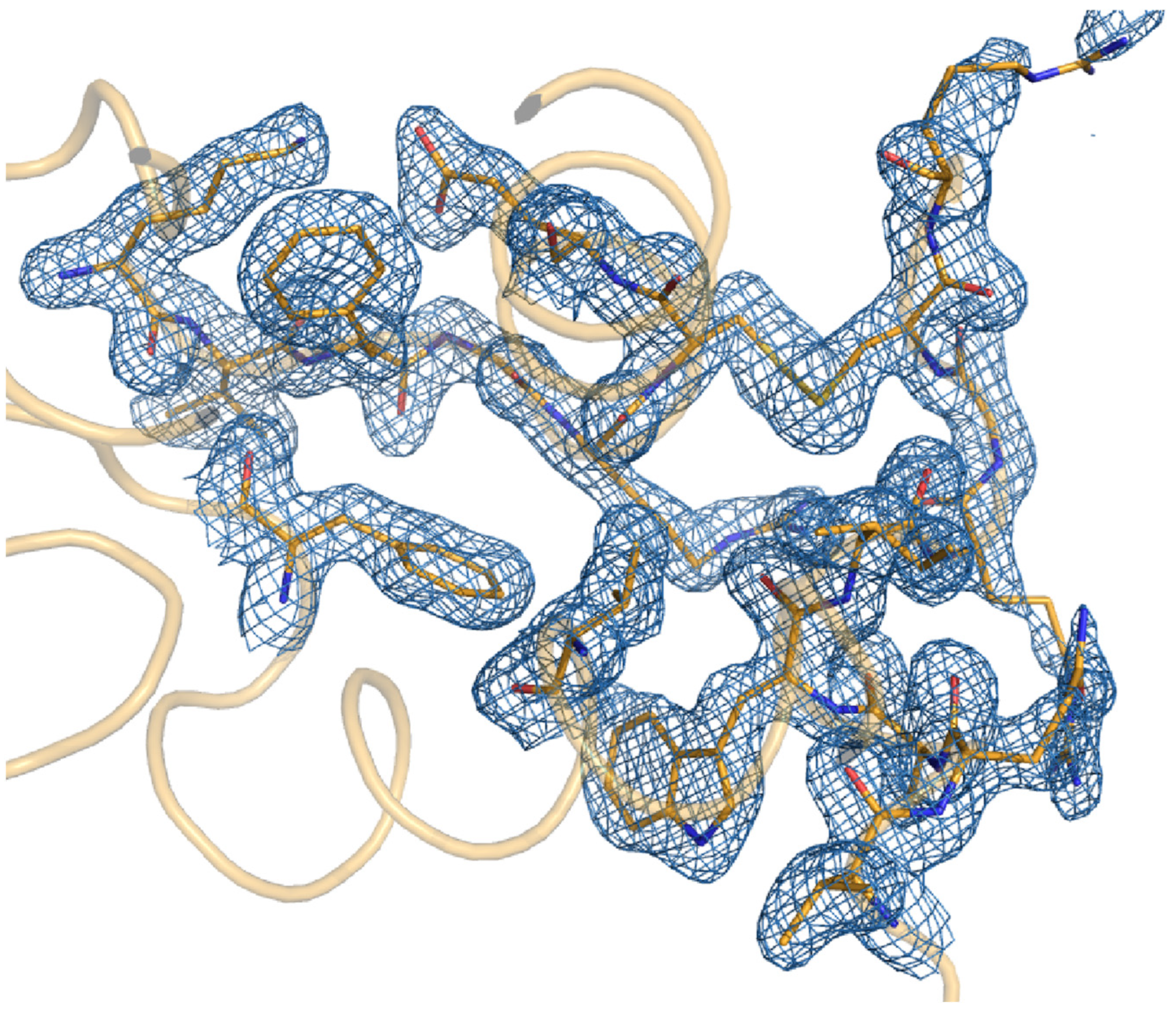
3.3. Structural Analysis of Lysozyme
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neutze, R.; Wouts, R.; van der Spoel, D.; Weckert, E.; Hajdu, J. Potential for biomolecular imaging with femtosecond X-ray pulses. Nature 2000, 406, 752–757. [Google Scholar] [CrossRef]
- Boutet, S.; Lomb, L.; Williams, G.J.; Barends, T.R.; Aquila, A.; Doak, R.B.; Weierstall, U.; DePonte, D.P.; Steinbrener, J.; Shoeman, R.L.; et al. High-resolution protein structure determination by serial femtosecond crystallography. Science 2012, 337, 362–364. [Google Scholar] [CrossRef]
- Chapman, H.N.; Fromme, P.; Barty, A.; White, T.A.; Kirian, R.A.; Aquila, A.; Hunter, M.S.; Schulz, J.; DePonte, D.P.; Weierstall, U.; et al. Femtosecond X-ray protein nanocrystallography. Nature 2011, 470, 73–77. [Google Scholar] [CrossRef]
- Liu, W.; Wacker, D.; Gati, C.; Han, G.W.; James, D.; Wang, D.; Nelson, G.; Weierstall, U.; Katritch, V.; Barty, A.; et al. Serial femtosecond crystallography of G protein-coupled receptors. Science 2013, 342, 1521–1524. [Google Scholar] [CrossRef]
- Spence, J.C.H.; Weierstall, U.; Chapman, H.N. X-ray lasers for structural and dynamic biology. Rep. Prog. Phys. 2012, 75, 102601. [Google Scholar] [CrossRef]
- DePonte, D.P.; Doak, R.B.; Hunter, M.; Liu, Z.; Weierstall, U.; Spence, J.C. SEM imaging of liquid jets. Micron 2009, 40, 507–509. [Google Scholar] [CrossRef]
- Deponte, D.P.; McKeown, J.T.; Weierstall, U.; Doak, R.B.; Spence, J.C. Towards ETEM serial crystallography: Electron diffraction from liquid jets. Ultramicroscopy 2011, 111, 824–827. [Google Scholar] [CrossRef]
- Weierstall, U.; James, D.; Wang, C.; White, T.A.; Wang, D.; Liu, W.; Spence, J.C.; Bruce Doak, R.; Nelson, G.; Fromme, P.; et al. Lipidic cubic phase injector facilitates membrane protein serial femtosecond crystallography. Nat. Commun. 2014, 5, 3309. [Google Scholar] [CrossRef]
- Sugahara, M.; Nakane, T.; Masuda, T.; Suzuki, M.; Inoue, S.; Song, C.; Tanaka, R.; Nakatsu, T.; Mizohata, E.; Yumoto, F.; et al. Hydroxyethyl cellulose matrix applied to serial crystallography. Sci. Rep. 2017, 7, 703. [Google Scholar] [CrossRef]
- Oghbaey, S.; Sarracini, A.; Ginn, H.M.; Pare-Labrosse, O.; Kuo, A.; Marx, A.; Epp, S.W.; Sherrell, D.A.; Eger, B.T.; Zhong, Y.; et al. Fixed target combined with spectral mapping: Approaching 100% hit rates for serial crystallography. Acta Crystallogr. D Struct. Biol. 2016, 72 Pt 8, 944–955. [Google Scholar] [CrossRef]
- Roedig, P.; Ginn, H.M.; Pakendorf, T.; Sutton, G.; Harlos, K.; Walter, T.S.; Meyer, J.; Fischer, P.; Duman, R.; Vartiainen, I.; et al. High-speed fixed-target serial virus crystallography. Nat. Methods 2017, 14, 805–810. [Google Scholar] [CrossRef]
- Sherrell, D.A.; Foster, A.J.; Hudson, L.; Nutter, B.; O’Hea, J.; Nelson, S.; Pare-Labrosse, O.; Oghbaey, S.; Miller, R.J.; Owen, R.L. A modular and compact portable mini-endstation for high-precision, high-speed fixed target serial crystallography at FEL and synchrotron sources. J. Synchrotron Radiat. 2015, 22, 1372–1378. [Google Scholar] [CrossRef] [PubMed]
- Doak, R.B.; Nass Kovacs, G.; Gorel, A.; Foucar, L.; Barends, T.R.M.; Grunbein, M.L.; Hilpert, M.; Kloos, M.; Roome, C.M.; Shoeman, R.L.; et al. Crystallography on a chip—without the chip: Sheet-on-sheet sandwich. Acta Crystallogr. D Struct. Biol. 2018, 74 Pt 10, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Hunter, M.S.; Segelke, B.; Messerschmidt, M.; Williams, G.J.; Zatsepin, N.A.; Barty, A.; Benner, W.H.; Carlson, D.B.; Coleman, M.; Graf, A.; et al. Fixed-target protein serial microcrystallography with an x-ray free electron laser. Sci. Rep. 2014, 4, 6026. [Google Scholar] [CrossRef]
- Lee, D.; Baek, S.; Park, J.; Lee, K.; Kim, J.; Lee, S.J.; Chung, W.K.; Lee, J.L.; Cho, Y.; Nam, K.H. Nylon mesh-based sample holder for fixed-target serial femtosecond crystallography. Sci. Rep. 2019, 9, 6971. [Google Scholar] [CrossRef] [PubMed]
- Sierra, R.G.; Gati, C.; Laksmono, H.; Dao, E.H.; Gul, S.; Fuller, F.; Kern, J.; Chatterjee, R.; Ibrahim, M.; Brewster, A.S.; et al. Concentric-flow electrokinetic injector enables serial crystallography of ribosome and photosystem II. Nat. Methods 2016, 13, 59–62. [Google Scholar] [CrossRef]
- Fuller, F.D.; Gul, S.; Chatterjee, R.; Burgie, E.S.; Young, I.D.; Lebrette, H.; Srinivas, V.; Brewster, A.S.; Michels-Clark, T.; Clinger, J.A.; et al. Drop-on-demand sample delivery for studying biocatalysts in action at X-ray free-electron lasers. Nat. Methods 2017, 14, 443–449. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Zhang, B.; Yan, E.K.; Sun, B.; Wang, Z.J.; He, J.H.; Yin, D.C. A guide to sample delivery systems for serial crystallography. FEBS J. 2019, 286, 4402–4417. [Google Scholar] [CrossRef]
- Sui, S.; Mulichak, A.; Kulathila, R.; McGee, J.; Filiatreault, D.; Saha, S.; Cohen, A.; Song, J.; Hung, H.; Selway, J.; et al. A capillary-based microfluidic device enables primary high-throughput room-temperature crystallographic screening. J. Appl. Crystallogr. 2021, 54 Pt 4, 1034–1046. [Google Scholar] [CrossRef]
- Nogly, P.; James, D.; Wang, D.; White, T.A.; Zatsepin, N.; Shilova, A.; Nelson, G.; Liu, H.; Johansson, L.; Heymann, M.; et al. Lipidic cubic phase serial millisecond crystallography using synchrotron radiation. IUCrJ 2015, 2 Pt 2, 168–176. [Google Scholar] [CrossRef]
- Botha, S.; Nass, K.; Barends, T.R.M.; Kabsch, W.; Latz, B.; Dworkowski, F.; Foucar, L.; Panepucci, E.; Wang, M.T.; Shoeman, R.L.; et al. Room-temperature serial crystallography at synchrotron X-ray sources using slowly flowing free-standing high-viscosity microstreams. Acta Crystallogr. D 2015, 71, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Beyerlein, K.R.; Dierksmeyer, D.; Mariani, V.; Kuhn, M.; Sarrou, I.; Ottaviano, A.; Awel, S.; Knoska, J.; Fuglerud, S.; Jonsson, O.; et al. Mix-and-diffuse serial synchrotron crystallography. IUCrJ 2017, 4 Pt 6, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, K.A.; Prester, A.; Andaleeb, H.; Bui, S.; Yefanov, O.; Catapano, L.; Henkel, A.; Wiedorn, M.O.; Lorbeer, O.; Crosas, E.; et al. Rapid and efficient room-temperature serial synchrotron crystallography using the CFEL TapeDrive. IUCrJ 2022, 9 Pt 6, 778–791. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Park, S.; Lee, K.; Kim, J.; Park, G.; Nam, K.H.; Baek, S.; Chung, W.K.; Lee, J.L.; Cho, Y.; et al. Application of a high-throughput microcrystal delivery system to serial femtosecond crystallography. J. Appl. Crystallogr. 2020, 53 Pt 2, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z. Dynamic analysis of center-driven web winder controls. In Conference Record of the 1999 IEEE Industry Applications Conference. In Proceedings of the Thirty-Forth IAS Annual Meeting (Cat. No.99CH36370), Phoenix, AZ, USA, 3–7 October 1999; pp. 1388–1396. [Google Scholar]
- Park, J.; Kim, S.; Nam, K.H.; Kim, B.; Ko, I.S. Current status of the CXI beamline at the PAL-XFEL. J. Korean Phys. Soc. 2016, 69, 1089–1093. [Google Scholar] [CrossRef]
- Kang, H.S.; Min, C.K.; Heo, H.; Kim, C.; Yang, H.; Kim, G.; Nam, I.; Baek, S.Y.; Choi, H.J.; Mun, G.; et al. Hard X-ray free-electron laser with femtosecond-scale timing jitter. Nat. Photonics 2017, 11, 708. [Google Scholar] [CrossRef]
- Ko, I.S.; Kang, H.S.; Heo, H.; Kim, C.; Kim, G.; Min, C.K.; Yang, H.; Baek, S.Y.; Choi, H.J.; Mun, G.; et al. Construction and Commissioning of PAL-XFEL Facility. Appl. Sci 2017, 7, 479. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.Y.; Park, J.; Kim, S.; Kim, S.; Rah, S.; Lim, J.; Nam, K.H. Focusing X-ray free-electron laser pulses using Kirkpatrick-Baez mirrors at the NCI hutch of the PAL-XFEL. J. Synchrotron Radiat. 2018, 25, 289–292. [Google Scholar] [CrossRef]
- Park, J.; Kim, S.; Kim, S.; Nam, K.H. Multifarious injection chamber for molecular structure study (MICOSS) system: Development and application for serial femtosecond crystallography at Pohang Accelerator Laboratory X-ray Free-Electron Laser. J. Synchrotron Radiat. 2018, 25, 323–328. [Google Scholar] [CrossRef]
- White, T.A. Post-refinement method for snapshot serial crystallography. Philos. Trans. R Soc. B 2014, 369, 20130330. [Google Scholar] [CrossRef]
- White, T.A.; Barty, A.; Liu, W.; Ishchenko, A.; Zhang, H.T.; Gati, C.; Zatsepin, N.A.; Basu, S.; Oberthür, D.; Metz, M.; et al. Serial femto-second crystallography datasets from G protein-coupled receptors. Sci. Data 2016, 3, 160057. [Google Scholar] [CrossRef] [PubMed]
- White, T.A.; Barty, A.; Stellato, F.; Holton, J.M.; Kirian, R.A.; Zatsepin, N.A.; Chapman, H.N. Crystallographic data processing for free-electron laser sources. Acta Crystallogr. D 2013, 69, 1231–1240. [Google Scholar] [CrossRef] [PubMed]
- White, T.A.; Kirian, R.A.; Martin, A.V.; Aquila, A.; Nass, K.; Barty, A.; Chapman, H.N. CrystFEL: A software suite for snapshot serial crystallography. J. Appl. Crystallogr. 2012, 45, 335–341. [Google Scholar] [CrossRef]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkóczi, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.W.; Jain, S.; McCoy, A.J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. D 2019, 75, 861–877. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 486–501. [Google Scholar] [CrossRef]
- Available online: http://www2.mrc-lmb.cam.ac.uk/personal/pemsley/coot/ (accessed on 13 December 2023).
- Available online: https://www.matweb.com/search/datasheet.aspx?matguid=99c680bc28dd409fb7e8fd3ddbcee537 (accessed on 7 September 2023).

| Data Collection | Lysozyme |
|---|---|
| PDB code | 8JQV |
| Wavelength (Å) | 1 Å |
| Exposure time | 25 fs |
| Space group | P 43 21 1 |
| Cell dimensions | |
| a,b,c (Å) | 79.3, 79.3, 38.3 |
| α, β, γ (°) | 90.0, 90.0, 90.0 |
| No. of collected images | 44,272 |
| No. of hit images | 21,633 |
| No. of indexed images | 9132 |
| Resolution (Å) | 50–1.80 (1.87–1.80) a |
| No. of unique reflections | 12,462 (1224) |
| Completeness (%) | 100 (100) |
| Redundancy | 335.6 (233.8) |
| SNR | 3.62 (1.36) |
| Rsplit b | 25.98 (78.87) |
| CC1/2 | 0.91 (0.42) |
| CC* c | 0.98 (0.77) |
| Wilson B-factor (Å2) | 32.17 |
| Refinement statistics | |
| Resolution (Å) | 39.65–1.80 (1.94–1.80) |
| R-work | 0.2040 (0.3772) |
| R-free c | 0.2302 (0.3911) |
| RMS deviations | |
| Bond (Å) | 0.006 |
| Angle (°) | 0.879 |
| Average B-factor (Å2) | |
| Protein | 29.07 |
| Water | 35.78 |
| Clash score | 3.06 |
| Ramachandran (%) | |
| Favored | 97.64 |
| Allowed | 2.36 |
| Rotamer outlier (%) | 0.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Park, S.; Cho, Y.; Park, J. Application of Micro-Tubing Reeling System to Serial Femtosecond Crystallography. Photonics 2024, 11, 95. https://doi.org/10.3390/photonics11010095
Kim J, Park S, Cho Y, Park J. Application of Micro-Tubing Reeling System to Serial Femtosecond Crystallography. Photonics. 2024; 11(1):95. https://doi.org/10.3390/photonics11010095
Chicago/Turabian StyleKim, Jihan, Sehan Park, Yunje Cho, and Jaehyun Park. 2024. "Application of Micro-Tubing Reeling System to Serial Femtosecond Crystallography" Photonics 11, no. 1: 95. https://doi.org/10.3390/photonics11010095
APA StyleKim, J., Park, S., Cho, Y., & Park, J. (2024). Application of Micro-Tubing Reeling System to Serial Femtosecond Crystallography. Photonics, 11(1), 95. https://doi.org/10.3390/photonics11010095





