Facile Fabrication of Mixed–Cation FA1−XCsXPbI3 Perovskites Thin Films for Photodetector Applications
Abstract
1. Introduction
2. Experimental Section
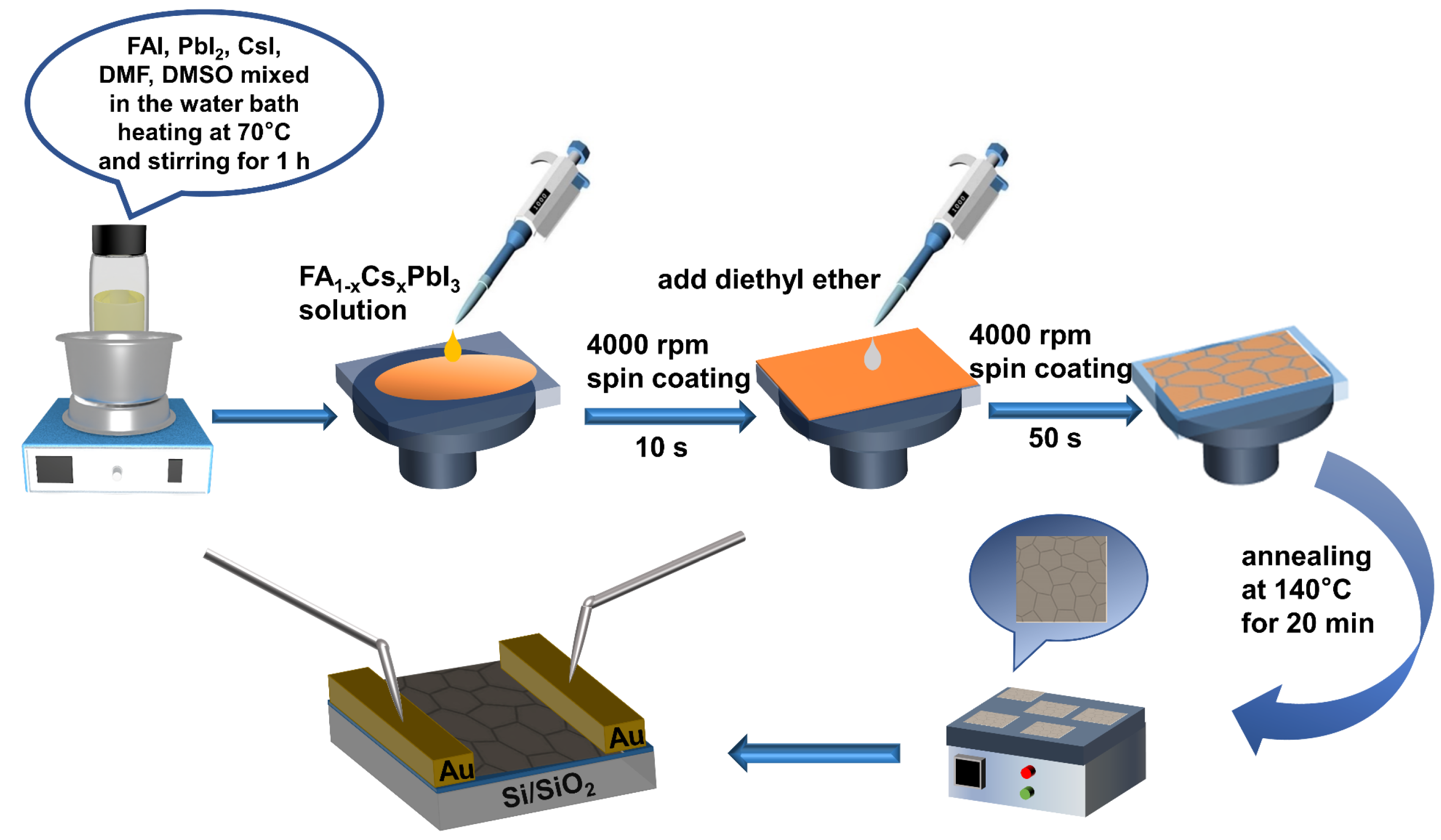
2.1. Synthesis of Perovskite Precursor Solution
2.2. Preparation of FA1−xCsxPbI3 Thin Films
2.3. Characterizations
2.4. Fabrication of FA1−xCsxPbI3 Thin-Film Photodetectors
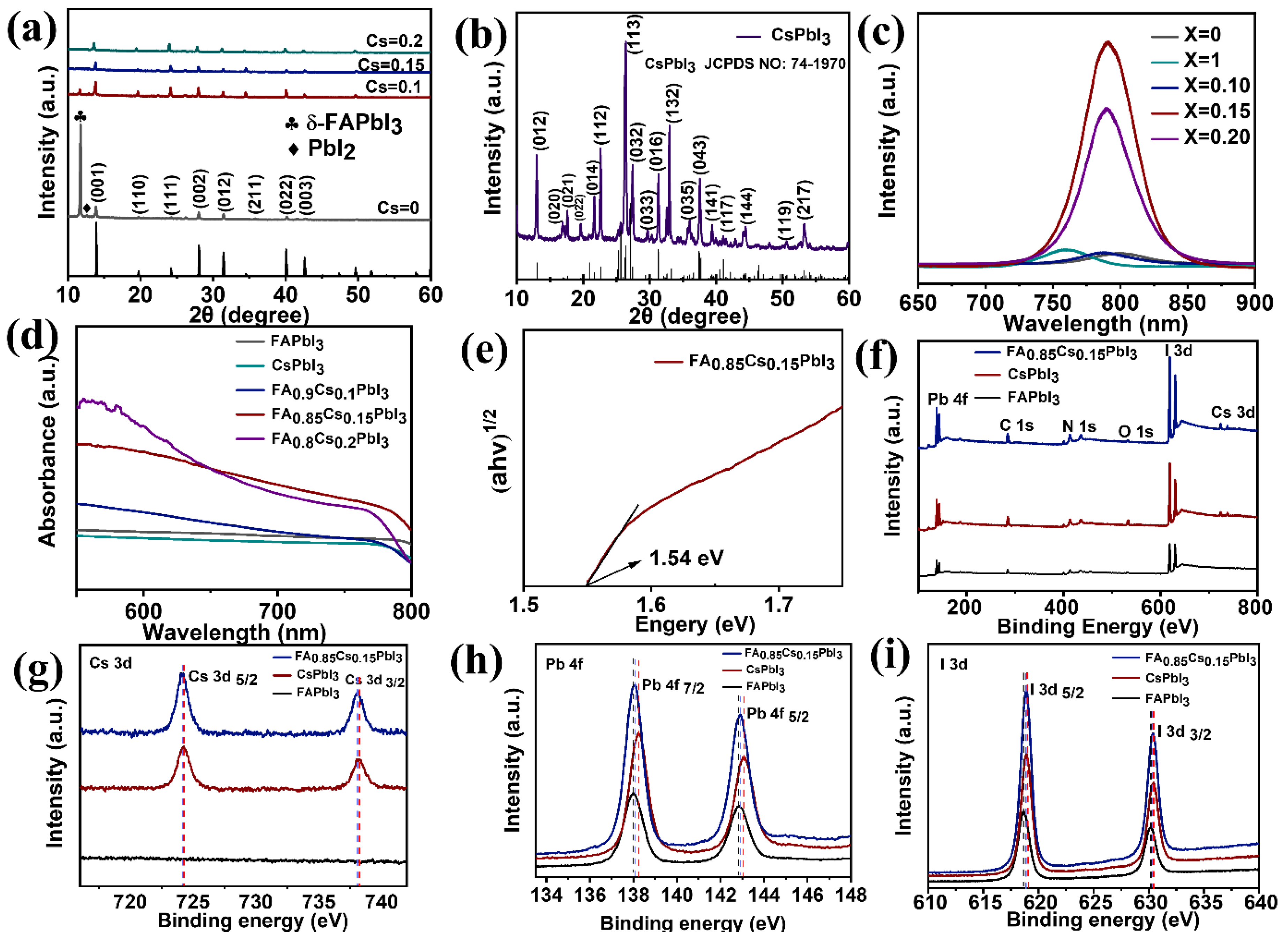
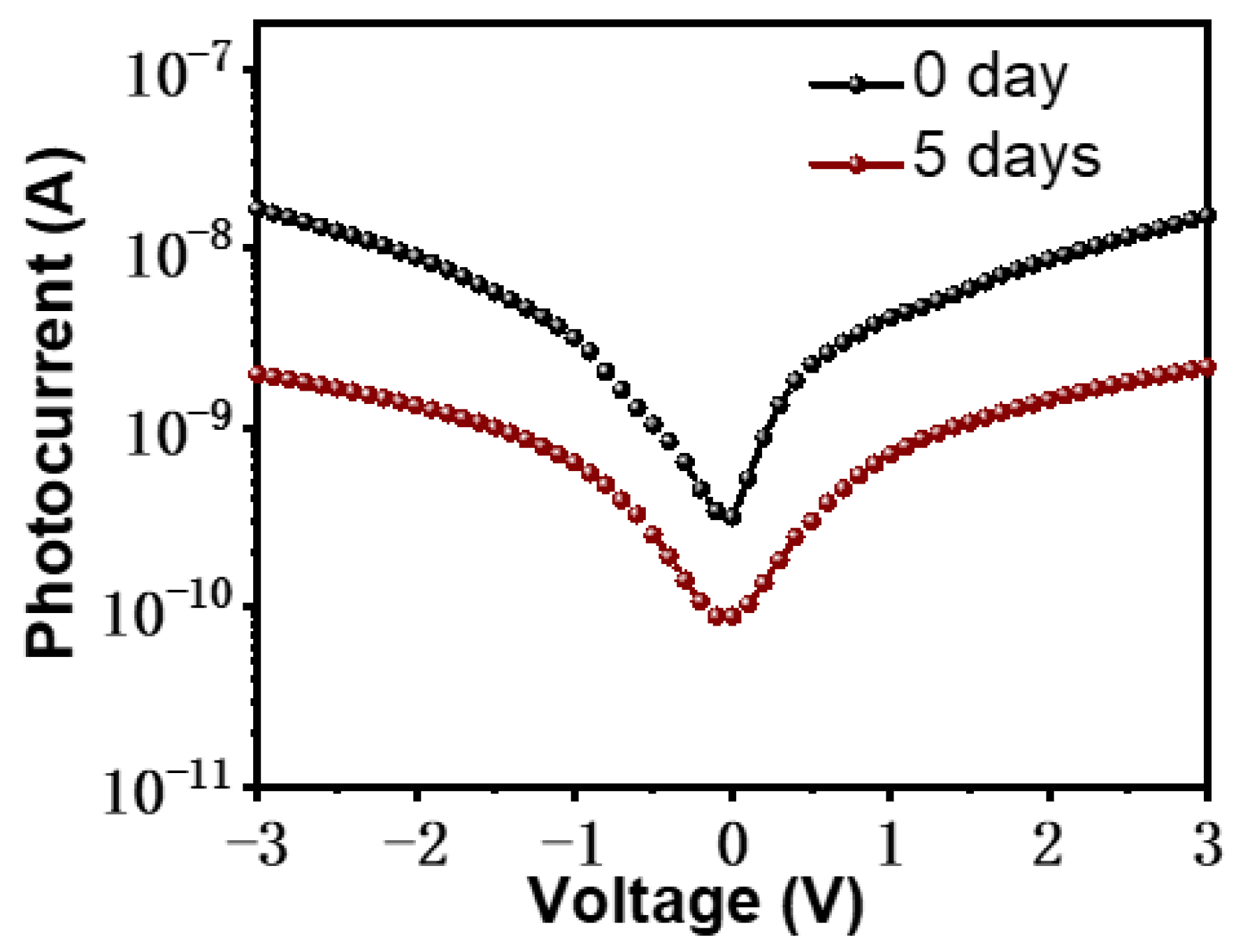
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zheng, Z.; Wang, S.; Hu, Y.; Rong, Y.; Mei, A.; Han, H. Development of formamidinium lead iodide-based perovskite solar cells: Efficiency and stability. Chem. Sci. 2022, 13, 2167–2183. [Google Scholar] [CrossRef] [PubMed]
- Murugan, P.; Hu, T.; Hu, X.; Chen, Y. Advancements in organic small molecule hole-transporting materials for perovskite solar cells: Past and future. J. Mater. Chem. A 2022, 10, 5044–5081. [Google Scholar] [CrossRef]
- Murugan, P.; Hu, T.; Hu, X.; Chen, Y. Current Development toward Commercialization of Metal-Halide Perovskite Photovoltaics. Adv. Opt. Mater. 2021, 9, 2100390. [Google Scholar] [CrossRef]
- Zhang, X.; Han, D.; Wang, C.; Muhammad, I.; Zhang, F.; Shmshad, A.; Xue, X.; Ji, W.; Chang, S.; Zhong, H. Highly Efficient Light Emitting Diodes Based on In Situ Fabricated FAPbI3 Nanocrystals: Solvent Effects of On-Chip Crystallization. Adv. Opt. Mater. 2019, 7, 1900774. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, S.; Ni, Z.; Van Brackle, C.H.; Zhao, L.; Xiao, X.; Dai, X.; Huang, J. Highly Efficient Pure-Blue Light-Emitting Diodes Based on Rubidium and Chlorine Alloyed Metal Halide Perovskite. Adv. Mater. 2021, 33, e2100783. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhu, H.; Schrader, A.W.; Liang, D.; Ding, Q.; Joshi, P.; Hwang, L.; Zhu, X.Y.; Jin, S. Nanowire Lasers of Formamidinium Lead Halide Perovskites and Their Stabilized Alloys with Improved Stability. Nano Lett. 2016, 16, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Song, L.; Chen, Y.; Huang, W. Emerging New-Generation Photodetectors Based on Low-Dimensional Halide Perovskites. ACS Photonics 2019, 7, 10–28. [Google Scholar] [CrossRef]
- Mei, F.; Sun, D.; Mei, S.; Feng, J.; Zhou, Y.; Xu, J.; Xiao, X. Recent progress in perovskite-based photodetectors: The design of materials and structures. Adv. Phys. X 2019, 4, 1592709. [Google Scholar] [CrossRef]
- Arya, S.; Mahajan, P.; Gupta, R.; Srivastava, R.; kumar Tailor, N.; Satapathi, S.; Sumathi, R.R.; Datt, R.; Gupta, V. A comprehensive review on synthesis and applications of single crystal perovskite halides. Prog. Solid State Chem. 2020, 60, 100286. [Google Scholar] [CrossRef]
- Yang, S.; Xu, Y.; Hao, Z.; Qin, S.; Zhang, R.; Han, Y.; Du, L.; Zhu, Z.; Du, A.; Chen, X.; et al. Recent Advances in High-Efficiency Perovskite for Medical Sensors. Acta Phys. Chim. Sin. 2022, 39, 2211025. [Google Scholar] [CrossRef]
- Zhang, G.; Xu, Y.; Yang, S.; Ren, S.; Jiao, Y.; Wang, Y.; Ma, X.; Li, H.; Hao, W.; He, C. Robust mica perovskite photoelectric resistive switching memory. Nano Energy 2023, 106, 108074. [Google Scholar] [CrossRef]
- Xu, X.; Ma, C.; Xie, Y.-M.; Cheng, Y.; Tian, Y.; Li, M.; Ma, Y.; Lee, C.-S.; Tsang, S.-W. Air-processed mixed-cation Cs0.15FA0.85PbI3 planar perovskite solar cells derived from a PbI2–CsI–FAI intermediate complex. J. Mater. Chem. A 2018, 6, 7731–7740. [Google Scholar] [CrossRef]
- Leijtens, T.; Bush, K.; Cheacharoen, R.; Beal, R.; Bowring, A.; McGehee, M.D. Towards enabling stable lead halide perovskite solar cells; interplay between structural, environmental, and thermal stability. J. Mater. Chem. A 2017, 5, 11483–11500. [Google Scholar] [CrossRef]
- Xiao, J.W.; Liu, L.; Zhang, D.; De Marco, N.; Lee, J.W.; Lin, O.; Chen, Q.; Yang, Y. The Emergence of the Mixed Perovskites and Their Applications as Solar Cells. Adv. Energy Mater. 2017, 7, 1700491. [Google Scholar] [CrossRef]
- Tai, Q.; You, P.; Sang, H.; Liu, Z.; Hu, C.; Chan, H.L.; Yan, F. Efficient and stable perovskite solar cells prepared in ambient air irrespective of the humidity. Nat. Commun. 2016, 7, 11105. [Google Scholar] [CrossRef] [PubMed]
- Asuo, I.M.; Gedamu, D.; Doumon, N.Y.; Ka, I.; Pignolet, A.; Cloutier, S.G.; Nechache, R. Ambient condition-processing strategy for improved air-stability and efficiency in mixed-cation perovskite solar cells. Mater. Adv. 2020, 1, 1866–1876. [Google Scholar] [CrossRef]
- Krishna, B.G.; Ghosh, D.S.; Tiwari, S. Progress in ambient air-processed perovskite solar cells: Insights into processing techniques and stability assessment. Solar Energy 2021, 224, 1369–1395. [Google Scholar] [CrossRef]
- Gratzel, M. The Rise of Highly Efficient and Stable Perovskite Solar Cells. Acc. Chem. Res. 2017, 50, 487–491. [Google Scholar] [CrossRef]
- Luo, P.; Zhou, S.; Zhou, Y.; Xia, W.; Sun, L.; Cheng, J.; Xu, C.; Lu, Y. Fabrication of Cs(x)FA(1−x)PbI(3) Mixed-Cation Perovskites via Gas-Phase-Assisted Compositional Modulation for Efficient and Stable Photovoltaic Devices. ACS Appl. Mater. Interfaces 2017, 9, 42708–42716. [Google Scholar] [CrossRef]
- Cao, X.; Hao, L.; Liu, Z.; Su, G.; He, X.; Zeng, Q.; Wei, J. All green solvent engineering of organic–inorganic hybrid perovskite layer for high-performance solar cells. Chem. Eng. J. 2022, 437, 135458. [Google Scholar] [CrossRef]
- Li, P.; Cheng, Y.; Zhou, L.; Yu, X.; Jiang, J.; He, M.; Liang, X.; Xiang, W. Photoluminescence properties and device application of CsPb2Br5 quantum dots in glasses. Mater. Res. Bull. 2018, 105, 63–67. [Google Scholar] [CrossRef]
- Wang, F.; Yu, H.; Xu, H.; Zhao, N. HPbI3: A New Precursor Compound for Highly Efficient Solution-Processed Perovskite Solar Cells. Adv. Funct. Mater. 2015, 25, 1120–1126. [Google Scholar] [CrossRef]
- Li, G.; Zhang, T.; Guo, N.; Xu, F.; Qian, X.; Zhao, Y. Ion-Exchange-Induced 2D-3D Conversion of HMA(1−x) FA(x) PbI(3) Cl Perovskite into a High-Quality MA(1−x) FA(x) PbI(3) Perovskite. Angew. Chem. Int. Ed. Engl. 2016, 55, 13460–13464. [Google Scholar] [CrossRef]
- Pool, V.L.; Dou, B.; Van Campen, D.G.; Klein-Stockert, T.R.; Barnes, F.S.; Shaheen, S.E.; Ahmad, M.I.; van Hest, M.F.; Toney, M.F. Thermal engineering of FAPbI(3) perovskite material via radiative thermal annealing and in situ XRD. Nat. Commun. 2017, 8, 14075. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, Z.; Cui, D.; Ren, X.; Sun, J.; Liu, X.; Zhang, J.; Wei, Q.; Fan, H.; Yu, F.; et al. Two-Inch-Sized Perovskite CH3NH3PbX3 (X = Cl, Br, I) Crystals: Growth and Characterization. Adv. Mater. 2015, 27, 5176–5183. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yang, M.; Park, J.-S.; Wei, S.-H.; Berry, J.J.; Zhu, K. Stabilizing Perovskite Structures by Tuning Tolerance Factor: Formation of Formamidinium and Cesium Lead Iodide Solid-State Alloys. Chem. Mater. 2015, 28, 284–292. [Google Scholar] [CrossRef]
- Tang, X.; Han, S.; Zu, Z.; Hu, W.; Zhou, D.; Du, J.; Hu, Z.; Li, S.; Zang, Z. All-Inorganic Perovskite CsPb2Br5 Microsheets for Photodetector Application. Front. Phys. 2018, 5, 69. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, D.-H.; Kim, H.-S.; Seo, S.-W.; Cho, S.M.; Park, N.-G. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, Y.; Wang, W.; Zheng, W.; Lin, R.; Huang, F. Growth, characterization and optoelectronic applications of pure-phase large-area CsPb2Br5 flake single crystals. J. Mater. Chem. C 2018, 6, 446–451. [Google Scholar] [CrossRef]
- Peng, Z.; Wei, Q.; Chen, H.; Liu, Y.; Wang, F.; Jiang, X.; Liu, W.; Zhou, W.; Ling, S.; Ning, Z. Cs0.15FA0.85PbI3/CsxFA1−xPbI3 core/shell heterostructure for highly stable and efficient perovskite solar cells. Cell Rep. Phys. Sci. 2020, 1, 100224. [Google Scholar] [CrossRef]
- Cheng, Y.; Xu, X.; Xie, Y.; Li, H.-W.; Qing, J.; Ma, C.; Lee, C.-S.; So, F.; Tsang, S.-W. 18% High-Efficiency Air-Processed Perovskite Solar Cells Made in a Humid Atmosphere of 70% RH. Solar RRL 2017, 1, 1700097. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Pang, S.; Xiao, Z.; Zhang, J.; Chai, W.; Xu, H.; Liu, Z.; Padture, N.P.; Cui, G. Additive-Modulated Evolution of HC(NH2)2PbI3 Black Polymorph for Mesoscopic Perovskite Solar Cells. Chem. Mater. 2015, 27, 7149–7155. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, C.R.; Im, J.H.; Lee, K.B.; Moehl, T.; Marchioro, A.; Moon, S.J.; Humphry-Baker, R.; Yum, J.H.; Moser, J.E.; et al. Lead iodide perovskite sensitized all-solid-state submicron thin film mesoscopic solar cell with efficiency exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Y.; Gao, F.; Chen, H.; Chen, Y.; Wang, X.; Liu, X.; Zhu, K. Sequential Cesium Incorporation for Highly Efficient Formamidinium-Cesium Perovskite Solar Cells. Chem 2022. [Google Scholar] [CrossRef]
- Lindblad, R.; Jena, N.K.; Philippe, B.; Oscarsson, J.; Bi, D.; Lindblad, A.; Mandal, S.; Pal, B.; Sarma, D.D.; Karis, O.; et al. Electronic Structure of CH3NH3PbX3 Perovskites: Dependence on the Halide Moiety. J. Phys. Chem. C 2015, 119, 1818–1825. [Google Scholar] [CrossRef]
- Ren, L.; Gao, K.; Tan, Q.; Qing, C.; Wang, Q.; Yang, P.; Liu, Y. High-performance perovskite photodetectors based on CsPbBr(3) microwire arrays. Appl. Opt. 2021, 60, 8896–8903. [Google Scholar] [CrossRef]
- Chen, G.; Yu, Y.; Zheng, K.; Ding, T.; Wang, W.; Jiang, Y.; Yang, Q. Fabrication of Ultrathin Bi2 S3 Nanosheets for High-Performance, Flexible, Visible-NIR Photodetectors. Small 2015, 11, 2848–2855. [Google Scholar] [CrossRef]
- Tian, W.; Min, L.; Cao, F.; Li, L. Nested Inverse Opal Perovskite toward Superior Flexible and Self-Powered Photodetection Performance. Adv. Mater. 2020, 32, 1906974. [Google Scholar] [CrossRef]
- Bao, C.; Yang, J.; Bai, S.; Xu, W.; Yan, Z.; Xu, Q.; Liu, J.; Zhang, W.; Gao, F. High performance and stable all-inorganic metal halide perovskite-based photodetectors for optical communication applications. Adv. Mater. 2018, 30, 1803422. [Google Scholar] [CrossRef]
- Eze, V.O.; Adams, G.R.; Braga Carani, L.; Simpson, R.J.; Okoli, O.I. Enhanced inorganic CsPbIBr2 perovskite film for a sensitive and rapid response self-powered photodetector. J. Phys. Chem. C 2020, 124, 20643–20653. [Google Scholar] [CrossRef]
- Li, D.; Zhou, D.; Xu, W.; Chen, X.; Pan, G.; Zhou, X.; Ding, N.; Song, H. Plasmonic photonic crystals induced two-order fluorescence enhancement of blue perovskite nanocrystals and its application for high-performance flexible ultraviolet photodetectors. Adv. Funct. Mater. 2018, 28, 1804429. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, D.; Zhang, J.; Qiao, J.; Min, C.; Yuan, X.; Somekh, M.; Feng, F. High-Performance and Stable Plasmonic-Functionalized Formamidinium-Based Quasi-2D Perovskite Photodetector for Potential Application in Optical Communication. Adv. Funct. Mater. 2022, 32, 2208694. [Google Scholar] [CrossRef]
- Yun, Y.; Han, G.S.; Park, G.N.; Kim, J.; Park, J.; Vidyasagar, D.; Jung, J.; Choi, W.C.; Choi, Y.J.; Heo, K. A Wide Bandgap Halide Perovskite Based Self-Powered Blue Photodetector with 84.9% of External Quantum Efficiency. Adv. Mater. 2022, 34, 2206932. [Google Scholar] [CrossRef]
- Cheng, W.; He, X.; Wang, J.G.; Tian, W.; Li, L. N-(2-aminoethyl) Acetamide Additive Enables Phase-Pure and Stable α-FAPbI3 for Efficient Self-Powered Photodetectors. Adv. Mater. 2022, 34, 2208325. [Google Scholar] [CrossRef] [PubMed]
- Adams, G.R.; Eze, V.O.; Shohag, M.A.S.; Simpson, R.; Parker, H.; Okoli, O.I. Fabrication of rapid response self-powered photodetector using solution-processed triple cation lead-halide perovskite. Eng. Res. Express 2020, 2, 015043. [Google Scholar] [CrossRef]
- Vuong, V.-H.; Pammi, S.; Ippili, S.; Jella, V.; Thi, T.N.; Pasupuleti, K.S.; Kim, M.-D.; Jeong, M.J.; Jeong, J.-R.; Chang, H.S. Flexible, Stable, and Self-Powered Photodetectors Embedded with Chemical Vapor Deposited Lead-Free Bismuth Mixed Halide Perovskite Films. Chem. Eng. J. 2023, 458, 141473. [Google Scholar] [CrossRef]
- Pasupuleti, K.S.; Chougule, S.S.; Jung, N.; Yu, Y.-J.; Oh, J.-E.; Kim, M.-D. Plasmonic Pt nanoparticles triggered efficient charge separation in TiO2/GaN NRs hybrid heterojunction for the high performance self-powered UV photodetectors. Appl. Surf. Sci. 2022, 594, 153474. [Google Scholar] [CrossRef]
- Meng, J.; Li, Q.; Huang, J.; Pan, C.; Li, Z. Self-powered photodetector for ultralow power density UV sensing. Nano Today 2022, 43, 101399. [Google Scholar] [CrossRef]
- Lin, D.; Shi, T.; Xie, H.; Wan, F.; Ren, X.; Liu, K.; Zhao, Y.; Ke, L.; Lin, Y.; Gao, Y.; et al. Ion Migration Accelerated Reaction between Oxygen and Metal Halide Perovskites in Light and Its Suppression by Cesium Incorporation. Adv. Energy Mater. 2021, 11, 2002552. [Google Scholar] [CrossRef]
- Niu, G.; Li, W.; Meng, F.; Wang, L.; Dong, H.; Qiu, Y. Study on the stability of CH3NH3PbI3films and the effect of post-modification by aluminum oxide in all-solid-state hybrid solar cells. J. Mater. Chem. A 2014, 2, 705–710. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; Liu, P.; Wang, W.; Ran, R.; Zhou, W.; Shao, Z. Improving Moisture/Thermal Stability and Efficiency of CH3NH3PbI3 -Based Perovskite Solar Cells via Gentle Butyl Acrylate Additive Strategy. Solar RRL 2020, 5, 2000621. [Google Scholar] [CrossRef]
- Liu, X.; Ren, S.; Li, Z.; Guo, J.; Yi, S.; Yang, Z.; Hao, W.; Li, R.; Zhao, J. Flexible transparent high-efficiency photoelectric perovskite resistive switching memory. Adv. Funct. Mater. 2022, 32, 2202951. [Google Scholar] [CrossRef]
- Yuan, Y.; Huang, J. Ion Migration in Organometal Trihalide Perovskite and Its Impact on Photovoltaic Efficiency and Stability. Acc. Chem. Res. 2016, 49, 286–293. [Google Scholar] [CrossRef]
- Yan, X.; Fan, W.; Cheng, F.; Sun, H.; Xu, C.; Wang, L.; Kang, Z.; Zhang, Y. Ion migration in hybrid perovskites: Classification, identification, and manipulation. Nano Today 2022, 44, 101503. [Google Scholar] [CrossRef]
- Doherty, T.A.; Nagane, S.; Kubicki, D.J.; Jung, Y.-K.; Johnstone, D.N.; Iqbal, A.N.; Guo, D.; Frohna, K.; Danaie, M.; Tennyson, E.M. Stabilized tilted-octahedra halide perovskites inhibit local formation of performance-limiting phases. Science 2021, 374, 1598–1605. [Google Scholar] [CrossRef] [PubMed]
- Nan, Z.-A.; Chen, L.; Liu, Q.; Wang, S.-H.; Chen, Z.-X.; Kang, S.-Y.; Ji, J.-B.; Tan, Y.-Y.; Hui, Y.; Yan, J.-W.; et al. Revealing phase evolution mechanism for stabilizing formamidinium-based lead halide perovskites by a key intermediate phase. Chem 2021, 7, 2513–2526. [Google Scholar] [CrossRef]
- Cheng, Y.; Ding, L. Pushing commercialization of perovskite solar cells by improving their intrinsic stability. Energy Environ. Sci. 2021, 14, 3233–3255. [Google Scholar] [CrossRef]
- Li, B.; Jiao, Y.; Qin, S.; Wang, Y.; Liu, H.; Li, R.; Hao, W.; Li, H.; Xia, Y.; Li, X. Photoinduced Strain in Organometal Halide Perovskites. J. Phys. Chem. Lett. 2023, 14, 1343–1353. [Google Scholar] [CrossRef]
- Liu, D.; Liu, F.; Liu, Y.; Pang, Z.; Zhuang, X.; Yin, Y.; Dong, S.; He, L.; Tan, Y.; Liao, L. Schottky-Contacted High-Performance GaSb Nanowires Photodetectors Enabled by Lead-Free All-Inorganic Perovskites Decoration. Small 2022, 18, 2200415. [Google Scholar] [CrossRef]
- Xia, G.; Huang, B.; Zhang, Y.; Zhao, X.; Wang, C.; Jia, C.; Zhao, J.; Chen, W.; Li, J. Nanoscale Insights into Photovoltaic Hysteresis in Triple-Cation Mixed-Halide Perovskite: Resolving the Role of Polarization and Ionic Migration. Adv. Mater. 2019, 31, 1902870. [Google Scholar] [CrossRef]
- Cacovich, S.; Cina, L.; Matteocci, F.; Divitini, G.; Midgley, P.A.; Di Carlo, A.; Ducati, C. Gold and iodine diffusion in large area perovskite solar cells under illumination. Nanoscale 2017, 9, 4700–4706. [Google Scholar] [CrossRef]
- Shlenskaya Natalia, N.; Belich, N.A.; Grätzel, M.; Goodilin, E.A.; Tarasov, A.B. Light-induced reactivity of gold and hybrid perovskite as a new possible degradation mechanism in perovskite solar cells. J. Mater. Chem. A 2018, 6, 1780–1786. [Google Scholar] [CrossRef]
- Jacobsson, T.J.; Schwan, L.J.; Ottosson, M.; Hagfeldt, A.; Edvinsson, T. Determination of Thermal Expansion Coefficients and Locating the Temperature-Induced Phase Transition in Methylammonium Lead Perovskites Using X-ray Diffraction. Inorg. Chem. 2015, 54, 10678–10685. [Google Scholar] [CrossRef] [PubMed]
- Gratia, P.; Grancini, G.; Audinot, J.N.; Jeanbourquin, X.; Mosconi, E.; Zimmermann, I.; Dowsett, D.; Lee, Y.; Gratzel, M.; De Angelis, F.; et al. Intrinsic Halide Segregation at Nanometer Scale Determines the High Efficiency of Mixed Cation/Mixed Halide Perovskite Solar Cells. J. Am. Chem. Soc. 2016, 138, 15821–15824. [Google Scholar] [CrossRef] [PubMed]
- Philippe, B.; Saliba, M.; Correa-Baena, J.-P.; Cappel, U.B.; Turren-Cruz, S.-H.; Grätzel, M.; Hagfeldt, A.; Rensmo, H. Chemical Distribution of Multiple Cation (Rb+, Cs+, MA+, and FA+) Perovskite Materials by Photoelectron Spectroscopy. Chem. Mater. 2017, 29, 3589–3596. [Google Scholar] [CrossRef]
- Zhu, C.; Niu, X.; Fu, Y.; Li, N.; Hu, C.; Chen, Y.; He, X.; Na, G.; Liu, P.; Zai, H.; et al. Strain engineering in perovskite solar cells and its impacts on carrier dynamics. Nat. Commun. 2019, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Brennan, M.C.; Draguta, S.; Kamat, P.V.; Kuno, M. Light-Induced Anion Phase Segregation in Mixed Halide Perovskites. ACS Energy Lett. 2017, 3, 204–213. [Google Scholar] [CrossRef]
- Bush, K.A.; Rolston, N.; Gold-Parker, A.; Manzoor, S.; Hausele, J.; Yu, Z.J.; Raiford, J.A.; Cheacharoen, R.; Holman, Z.C.; Toney, M.F.; et al. Controlling Thin-Film Stress and Wrinkling during Perovskite Film Formation. ACS Energy Lett. 2018, 3, 1225–1232. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, C.; Jha, S.K.; Li, Z.; Zhu, K.; Priya, S. Improved Phase Stability of Formamidinium Lead Triiodide Perovskite by Strain Relaxation. ACS Energy Lett. 2016, 1, 1014–1020. [Google Scholar] [CrossRef]
- Prasanna, R.; Gold-Parker, A.; Leijtens, T.; Conings, B.; Babayigit, A.; Boyen, H.G.; Toney, M.F.; McGehee, M.D. Band Gap Tuning via Lattice Contraction and Octahedral Tilting in Perovskite Materials for Photovoltaics. J. Am. Chem. Soc. 2017, 139, 11117–11124. [Google Scholar] [CrossRef] [PubMed]
- Murali, B.; Yengel, E.; Peng, W.; Chen, Z.; Alias, M.S.; Alarousu, E.; Ooi, B.S.; Burlakov, V.; Goriely, A.; Eddaoudi, M.; et al. Temperature-Induced Lattice Relaxation of Perovskite Crystal Enhances Optoelectronic Properties and Solar Cell Performance. J. Phys. Chem. Lett. 2017, 8, 137–143. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, D.P.; Wang, Z.; Rehman, W.; Pulvirenti, F.; Patel, J.B.; Noel, N.K.; Johnston, M.B.; Marder, S.R.; Herz, L.M.; Snaith, H.J. Crystallization Kinetics and Morphology Control of Formamidinium-Cesium Mixed-Cation Lead Mixed-Halide Perovskite via Tunability of the Colloidal Precursor Solution. Adv. Mater. 2017, 29, 1607039. [Google Scholar] [CrossRef] [PubMed]



| Binding Energy (eV) | FAPbI3 | CsPbI3 | FA0.85Cs0.15PbI3 |
|---|---|---|---|
| I 3d3/2 | 630.2 | 630.5 | 630.4 |
| I 3d5/2 | 618.7 | 619.0 | 618.9 |
| Pb 4f5/2 | 142.8 | 143.1 | 142.9 |
| Pb 4f7/2 | 138.0 | 138.2 | 138.1 |
| Cs 3d3/2 | _ | 738.2 | 738.1 |
| Cs 3d5/2 | _ | 724.2 | 724.1 |
| Device Structure | Method | Wavelength (nm) | Ilight/Idark | Rλ (A W−1) | EQE (%) | D* (Jones) | Rise/Decay (ms) | Ref |
|---|---|---|---|---|---|---|---|---|
| Au/(BA)2FAPb2I7/ | - | 405 | - | 0.95 | - | - | 62/57 | [42] |
| ITO/MA1−xFAx(Br0.65Cl0.35)3 thin film/Au | A-site compositional | 450 | 5.72 × 105 | 0.28 | 77.4 | 5.08 × 1012 | 585/531 | [43] |
| ITO/SnO2/FAPbI3/Spiro- OMeTAD/Ag | Additive engineering | 700 | - | 0.48 | 95 | 2 × 1011 | 0.00394/0.00476 | [44] |
| FTO/Cs0.05 MA0.16 FA0.79 Pb(I0.9Br0.1)3/Spiro/Au | - | - | 7.3 × 105 | 0.52 | - | 8.8 × 1012 | 0.019/0.021 | [45] |
| PET/ITO/SnO2/Active layer/CuI/Au Active layer: MBI, MBIB MBIC films | chemical-vapor-deposited | 382 | - | 0.30(MBI) 0.63(MBIB) 0.92(MBIC) | - | 0.9 × 1013 2 × 1013 2.9 × 1013 | - | [46] |
| Pt NP@TiO2/GaN NRs | - | 382 | 44.6 | 8.65 × 103 | 1.57 × 1014 | 180/200 | [47] | |
| Au NPs@ZnO NWs | hydrothermal | 325 | - | 0.000485 | - | 27.49 × 1010 | - | [48] |
| SiO2/FA0.85Cs0.15PbI3 thin film/Au | solution | 790 | 1.58 × 103 | 0.42 | 66.5 | 2.2 × 109 | 1500/1200 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Murugan, P.; Dong, S.; Zheng, X.; Man, J.; Liu, Z.; Zhang, W.; Zhu, T.; Wang, H.-E. Facile Fabrication of Mixed–Cation FA1−XCsXPbI3 Perovskites Thin Films for Photodetector Applications. Photonics 2023, 10, 312. https://doi.org/10.3390/photonics10030312
Wang F, Murugan P, Dong S, Zheng X, Man J, Liu Z, Zhang W, Zhu T, Wang H-E. Facile Fabrication of Mixed–Cation FA1−XCsXPbI3 Perovskites Thin Films for Photodetector Applications. Photonics. 2023; 10(3):312. https://doi.org/10.3390/photonics10030312
Chicago/Turabian StyleWang, Fenyun, Pachaiyappan Murugan, Shunhong Dong, Xiaolu Zheng, Jiaxiu Man, Zhiyong Liu, Weibin Zhang, Ting Zhu, and Hong-En Wang. 2023. "Facile Fabrication of Mixed–Cation FA1−XCsXPbI3 Perovskites Thin Films for Photodetector Applications" Photonics 10, no. 3: 312. https://doi.org/10.3390/photonics10030312
APA StyleWang, F., Murugan, P., Dong, S., Zheng, X., Man, J., Liu, Z., Zhang, W., Zhu, T., & Wang, H.-E. (2023). Facile Fabrication of Mixed–Cation FA1−XCsXPbI3 Perovskites Thin Films for Photodetector Applications. Photonics, 10(3), 312. https://doi.org/10.3390/photonics10030312






