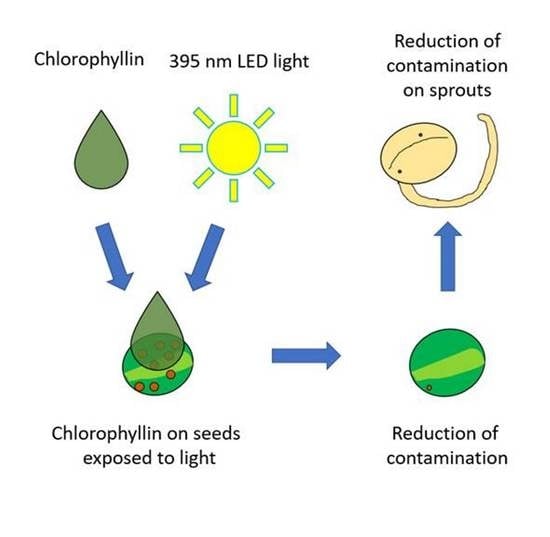
Towards Microbial Food Safety of Sprouts: Photodynamic Decontamination of Seeds
Abstract
1. Introduction
2. Material and Methods
2.1. Chemicals, Bacteria, and Seeds
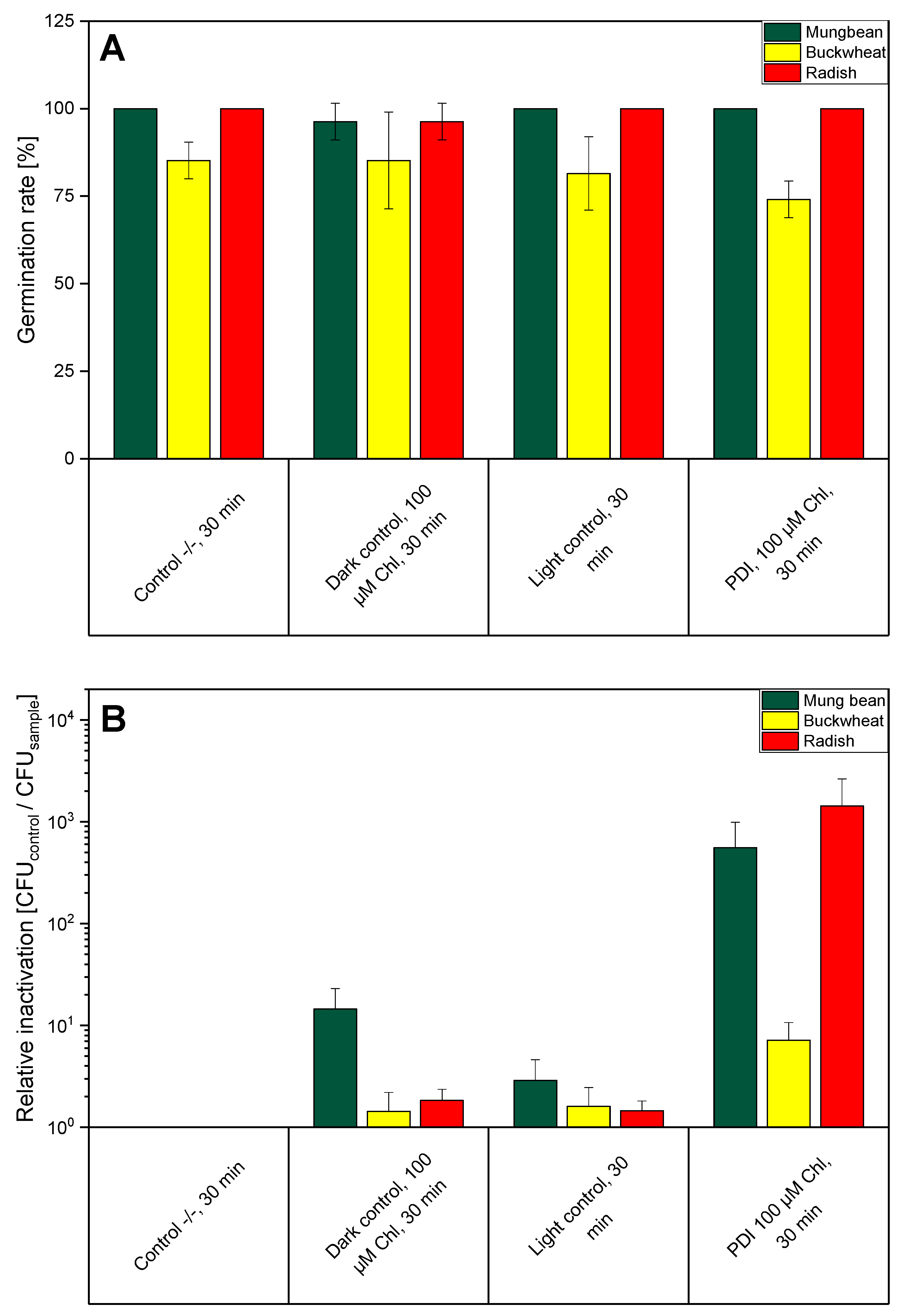
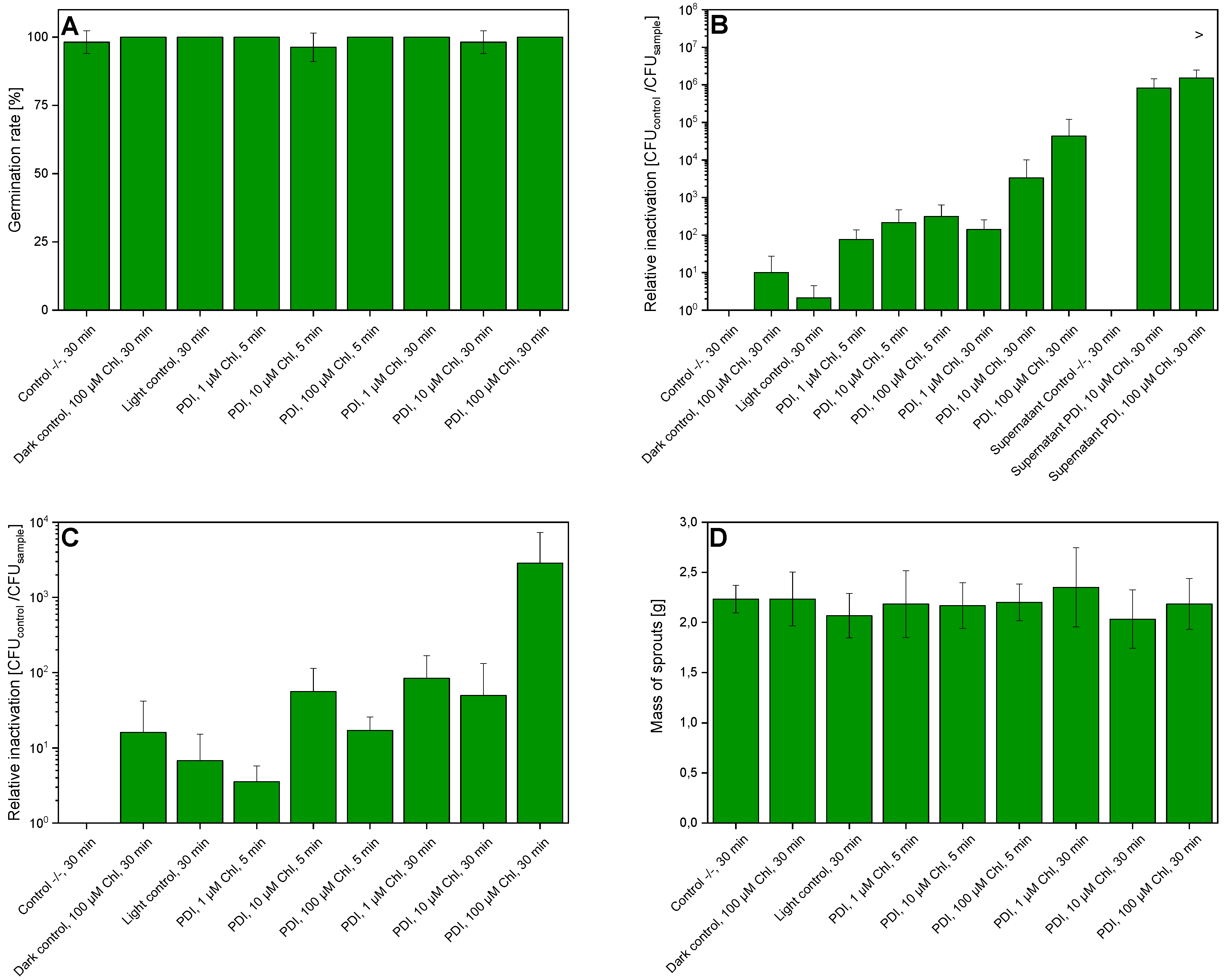
2.2. PDI-Disinfection of Seeds
2.3. PDI Parameters
2.4. Germination of Seeds and Determination of Germination Rate
2.5. Storage and Treatment of Sprouts
2.6. Determination of Sprout Mass
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Tubiello, F.N.; Rosenzweig, C.; Conchedda, G.; Karl, K.; Gütschow, J.; Xueyao, P.; Obli-Laryea, G.; Wanner, N.; Qiu, S.Y.; de Barros, J.; et al. Greenhouse Gas Emissions from Food Systems: Building the Evidence Base. Environ. Res. Lett. 2021, 16, 65007. [Google Scholar] [CrossRef]
- Westhoek, H.; Lesschen, J.P.; Rood, T.; Wagner, S.; de Marco, A.; Murphy-Bokern, D.; Leip, A.; van Grinsven, H.; Sutton, M.A.; Oenema, O. Food Choices, Health and Environment: Effects of Cutting Europe’s Meat and Dairy Intake. Glob. Environ. Chang. 2014, 26, 196–205. [Google Scholar] [CrossRef]
- Irz, X.; Jensen, J.D.; Leroy, P.; Réquillart, V.; Soler, L.G. Promoting Climate-Friendly Diets: What Should We Tell Consumers in Denmark, Finland and France? Environ. Sci. Policy 2019, 99, 169–177. [Google Scholar] [CrossRef]
- Ebert, A.W. Sprouts and Microgreens—Novel Food Sources for Healthy Diets. Plants 2022, 11, 571. [Google Scholar] [CrossRef]
- Poore, J.; Nemecek, T. Reducing Food’s Environmental Impacts through Producers and Consumers. Science 2018, 360, 987–992. [Google Scholar] [CrossRef]
- Robert-Koch-Institut Abschließende Darstellung Und Bewertung Der Epidemiologischen Erkenntnisse Im EHEC O104:H4 Ausbruch, Deutschland.2011.1–23. Available online: https://www.rki.de/DE/Content/InfAZ/E/EHEC/EHEC_O104/EHEC-Abschlussbericht.pdf (accessed on 30 January 2023).
- EFSA. Tracing Seeds, in Particular Fenugreek (Trigonella Foenum-graecum) Seeds, in Relation to the Shiga Toxin-producing E. Coli (STEC) O104:H4 2011 Outbreaks in Germany and France. EFSA Support. Publ. 2011, 8, 7. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Tompkin, R.B. Control of Listeria Monocytogenes in the Food-Processing Environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef]
- Farkas, J. Irradiation as a Method for Decontaminating Food: A Review. Int. J. Food Microbiol. 1998, 44, 189–204. [Google Scholar] [CrossRef]
- Brodowska, A.J.; Nowak, A.; Śmigielski, K. Ozone in the Food Industry: Principles of Ozone Treatment, Mechanisms of Action, and Applications: An Overview. Crit. Rev. Food Sci. Nutr. 2018, 58, 2176–2201. [Google Scholar] [CrossRef]
- Millan-Sango, D.; Sammut, E.; van Impe, J.F.; Valdramidis, V.P. Decontamination of Alfalfa and Mung Bean Sprouts by Ultrasound and Aqueous Chlorine Dioxide. LWT—Food Sci. Technol. 2017, 78, 90–96. [Google Scholar] [CrossRef]
- Sy, K.V.; Murray, M.B.; Harrison, M.D.; Beuchat, L.R. Evaluation of Gaseous Chlorine Dioxide as a Sanitizer for Killing Salmonella, Escherichia Coli O157:H7, Listeria Monocytogenes, and Yeasts and Molds on Fresh and Fresh-Cut Produce. J. Food Prot. 2005, 68, 1176–1187. [Google Scholar] [CrossRef]
- Allende, A.; Selma, M.V.; López-Gálvez, F.; Villaescusa, R.; Gil, M.I. Role of Commercial Sanitizers and Washing Systems on Epiphytic Microorganisms and Sensory Quality of Fresh-Cut Escarole and Lettuce. Postharvest Biol. Technol. 2008, 49, 155–163. [Google Scholar] [CrossRef]
- EFSA; ECDC. The European Union One Health 2020 Zoonoses Report. EFSA J. 2021, 19, e06971. [Google Scholar] [CrossRef]
- Kirbis, A.; Krizman, M. Spread of Antibiotic Resistant Bacteria from Food of Animal Origin to Humans and Vice Versa. Procedia Food Sci. 2015, 5, 148–151. [Google Scholar] [CrossRef]
- Founou, L.L.; Founou, R.C.; Essack, S.Y. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front. Microbiol. 2016, 7, 1881. [Google Scholar] [CrossRef]
- Buchanan, R.L.; Gorris, L.G.M.; Hayman, M.M.; Jackson, T.C.; Whiting, R.C. A Review of Listeria Monocytogenes: An Update on Outbreaks, Virulence, Dose-Response, Ecology, and Risk Assessments. Food Control. 2017, 75, 1–13. [Google Scholar] [CrossRef]
- Ramaswamy, V.; Cresence, V.M.; Rejitha, J.S.; Lekshmi, U.; Dharsana, K.S.; Prasad, P.; Vijila, M. Listeria-Review of Epidemiology and Pathogenesis. J. Microbiol. Immunol. Infect. 2007, 40, 4–13. Available online: www.academia.edu/download/31249641/listeria.pdf (accessed on 20 February 2023).
- Halbedel, S.; Wilking, H.; Holzer, A.; Kleta, S.; Fischer, M.A.; Lüth, S.; Pietzka, A.; Huhulescu, S.; Lachmann, R.; Krings, A.; et al. Large Nationwide Outbreak of Invasive Listeriosis Associated with Blood Sausage, Germany, 2018–2019. Emerg. Infect. Dis. 2020, 26, 1456–1464. [Google Scholar] [CrossRef]
- EFSA; ECDC. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2015. EFSA J. 2016, 14, e04634. [Google Scholar] [CrossRef]
- Mogren, L.; Windstam, S.; Boqvist, S.; Vågsholm, I.; Söderqvist, K.; Rosberg, A.K.; Lindén, J.; Mulaosmanovic, E.; Karlsson, M.; Uhlig, E.; et al. The Hurdle Approach-A Holistic Concept for Controlling Food Safety Risks Associated with Pathogenic Bacterial Contamination of Leafy Green Vegetables. A Review. Front. Microbiol. 2018, 9, 1965. [Google Scholar] [CrossRef]
- Barth, M.; Hankinson, T.R.; Zhuang, H.; Breidt, F. Microbiological Spoilage of Fruits and Vegetables. In Compendium of the Microbiological Spoilage of Foods and Beverages; Springer: New York, NY, USA, 2009; pp. 135–183. [Google Scholar]
- Nousiainen, L.L.; Joutsen, S.; Lunden, J.; Hänninen, M.L.; Fredriksson-Ahomaa, M. Bacterial Quality and Safety of Packaged Fresh Leafy Vegetables at the Retail Level in Finland. Int. J. Food Microbiol. 2016, 232, 73–79. [Google Scholar] [CrossRef]
- Plaetzer, K.; Krammer, B.; Berlanda, J.; Berr, F.; Kiesslich, T. Photophysics and Photochemistry of Photodynamic Therapy: Fundamental Aspects. Lasers Med. Sci. 2009, 24, 259–268. [Google Scholar] [CrossRef]
- Hamminger, C.; Glueck, M.; Fefer, M.; Ckurshumova, W.; Liu, J.; Tenhaken, R.; Plaetzer, K. Photodynamic Inactivation of Plant Pathogens Part II: Fungi. Photochem. Photobiol. Sci. 2022, 21, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Pereira Gonzales, F.; Maisch, T. Photodynamic Inactivation for Controlling Candida Albicans Infections. Fungal Biol. 2012, 116, 1–10. [Google Scholar] [CrossRef]
- Wimmer, A.; Glueck, M.; Ckurshumova, W.; Liu, J.; Fefer, M.; Plaetzer, K. Breaking the Rebellion: Photodynamic Inactivation against Erwinia Amylovora Resistant to Streptomycin. Antibiotics 2022, 11, 544. [Google Scholar] [CrossRef]
- Glueck, M.; Hamminger, C.; Fefer, M.; Liu, J.; Plaetzer, K. Save the Crop: Photodynamic Inactivation of Plant Pathogens I: Bacteria. Photochem. Photobiol. Sci. 2019, 18, 1700–1708. [Google Scholar] [CrossRef]
- Glueck, M.; Schamberger, B.; Eckl, P.; Plaetzer, K. New Horizons in Microbiological Food Safety: Photodynamic Decontamination Based on a Curcumin Derivative. Photochem. Photobiol. Sci. 2017, 16, 1784–1791. [Google Scholar] [CrossRef]
- Ryu, A.R.; Han, C.S.; Oh, H.K.; Lee, M.Y. Chlorin E6-Mediated Photodynamic Inactivation with Halogen Light against Microbes and Fungus. Toxicol. Environ. Health Sci. 2015, 7, 231–238. [Google Scholar] [CrossRef]
- Hanakova, A.; Bogdanova, K.; Tomankova, K.; Pizova, K.; Malohlava, J.; Binder, S.; Bajgar, R.; Langova, K.; Kolar, M.; Mosinger, J.; et al. The Application of Antimicrobial Photodynamic Therapy on S. Aureus and E. Coli Using Porphyrin Photosensitizers Bound to Cyclodextrin. Microbiol. Res. 2014, 169, 163–170. [Google Scholar] [CrossRef]
- Tortik, N.; Spaeth, A.; Plaetzer, K. Photodynamic Decontamination of Foodstuff from Staphylococcus Aureus Based on Novel Formulations of Curcumin. Photochem. Photobiol. Sci. 2014, 13, 1402–1409. [Google Scholar] [CrossRef]
- El-Tayeb, T.A.; El-Aziz, N.M.A.; Awad, H.H. A Study on the Dynamics of Aedes Caspius Larval Uptake and Release of Novel Haematoporphyrin. Afr. Entomol. 2013, 21, 15–23. [Google Scholar] [CrossRef]
- Lima, A.R.; Silva, C.M.; Caires, C.S.A.; Prado, E.D.; Rocha, L.R.P.; Cabrini, I.; Arruda, E.J.; Oliveira, S.L.; Caires, A.R.L. Evaluation of Eosin-Methylene Blue as a Photosensitizer for Larval Control of Aedes Aegypti by a Photodynamic Process. Insects 2018, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T. Resistance in Antimicrobial Photodynamic Inactivation of Bacteria. Photochem. Photobiol. Sci. 2015, 14, 1518–1526. [Google Scholar] [CrossRef]
- Žudyte, B.; Lukšiene, Ž. Toward Better Microbial Safety of Wheat Sprouts: Chlorophyllin-Based Photosensitization of Seeds. Photochem. Photobiol. Sci. 2019, 18, 2521–2530. [Google Scholar] [CrossRef]
- Lukseviciute, V.; Luksiene, Z. Inactivation of Molds on the Surface of Wheat Sprouts by Chlorophyllin-Chitosan Coating in the Presence of Visible LED-Based Light. J. Photochem. Photobiol. B 2020, 202, 111721. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, Z.; Shao, C.; Xie, J. Chlorophyllin-Based 405 Nm Light Photodynamic Improved Fresh-Cut Pakchoi Quality at Postharvest and Inhibited the Formation of Biofilm. Foods 2022, 11, 2541. [Google Scholar] [CrossRef]
- Luksiene, Z.; Paskeviciute, E. Microbial Control of Food-Related Surfaces: Na-Chlorophyllin-Based Photosensitization. J. Photochem. Photobiol. B 2011, 105, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Hammer, P.; Bockelmann, W.; Hoffmann, W. Fate of Listeria Innocua during Production and Ripening of Smeared Hard Cheese Made from Raw Milk. J. Dairy Sci. 2017, 100, 7846–7856. [Google Scholar] [CrossRef]
- Girardin, H.; Morris, C.E.; Albagnac, C.; Dreux, N.; Glaux, C.; Nguyen-The, C. Behaviour of the Pathogen Surrogates Listeria Innocua and Clostridium Sporogenes during Production of Parsley in Fields Fertilized with Contaminated Amendments. FEMS Microbiol. Ecol. 2005, 54, 287–295. [Google Scholar] [CrossRef]
- Friedly, E.C.; Crandall, P.G.; Ricke, S.; O’Bryan, C.A.; Martin, E.M.; Boyd, L.M. Identification of Listeria Innocua Surrogates for Listeria Monocytogenes in Hamburger Patties. J. Food Sci. 2008, 73, M174–M178. [Google Scholar] [CrossRef]
- Sommers, C.H.; Cooke, P.H.; Fan, X.; Sites, J.E. Ultraviolet Light (254 Nm) Inactivation of Listeria Monocytogenes on Frankfurters That Contain Potassium Lactate and Sodium Diacetate. J. Food Sci. 2009, 74, M114–M119. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Miliauskienė, J.; Vaštakaitė-Kairienė, V.; Sutulienė, R.; Laužikė, K.; Duchovskis, P.; Małek, S. Effect of Different Ratios of Blue and Red Led Light on Brassicaceae Microgreens under a Controlled Environment. Plants 2021, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, Thermal and Chemical Degradation of Riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. [Google Scholar] [CrossRef]
- Neo, S.Y.; Lim, P.Y.; Phua, L.K.; Khoo, G.H.; Kim, S.J.; Lee, S.C.; Yuk, H.G. Efficacy of Chlorine and Peroxyacetic Acid on Reduction of Natural Microflora, Escherichia Coli O157: H7, Listeria Monocyotgenes and Salmonella Spp. on Mung Bean Sprouts. Food Microbiol. 2013, 36, 475–480. [Google Scholar] [CrossRef]
- Trząskowska, M.; Dai, Y.; Delaquis, P.; Wang, S. Pathogen Reduction on Mung Bean Reduction of Escherichia Coli O157:H7, Salmonella Enterica and Listeria Monocytogenes on Mung Bean Using Combined Thermal and Chemical Treatments with Acetic Acid and Hydrogen Peroxide. Food Microbiol. 2018, 76, 62–68. [Google Scholar] [CrossRef]
- Puligundla, P.; Kim, J.W.; Mok, C. Effects of Nonthermal Plasma Treatment on Decontamination and Sprouting of Radish (Raphanus Sativus L.) Seeds. Food Bioproc. Technol. 2017, 10, 1093–1102. [Google Scholar] [CrossRef]
- Wohllebe, S.; Ulbrich, C.; Grimm, D.; Pietsch, J.; Erzinger, G.; Richter, R.; Lebert, M.; Richter, P.R.; Häder, D.P. Photodynamic Treatment of Chaoborus Crystallinus Larvae with Chlorophyllin Induces Necrosis and Apoptosis. Photochem. Photobiol. 2011, 87, 1113–1122. [Google Scholar] [CrossRef]
- de Menezes, H.D.; Rodrigues, G.B.; Teixeira, S.d.P.; Massola, N.S.; Bachmann, L.; Wainwright, M.; Braga, G.U.L. In Vitro Photodynamic Inactivation of Plant-Pathogenic Fungi Colletotrichum Acutatum and Colletotrichum Gloeosporioides with Novel Phenothiazinium Photosensitizers. Appl. Environ. Microbiol. 2014, 80, 1623–1632. [Google Scholar] [CrossRef]
- Ding, H.; Fu, T.J.; Smith, M.A. Microbial Contamination in Sprouts: How Effective Is Seed Disinfection Treatment? J. Food Sci. 2013, 78, R495–R501. [Google Scholar] [CrossRef]
- European Commission Commission Regulation (EC). No 889/2008 of 5 September 2008 Laying Down Detailed Rules for the Implementation of Council Regulation (EC) No 834/2007 on Organic Production and Labelling of Organic Products With Regard to Organic Production, Labelling and Control; European Commission Commission Regulation (EC): Brussels, Belgium, 2008. [Google Scholar]
- Ölmez, H.; Kretzschmar, U. Potential Alternative Disinfection Methods for Organic Fresh-Cut Industry for Minimizing Water Consumption and Environmental Impact. LWT—Food Sci. Technol. 2009, 42, 686–693. [Google Scholar] [CrossRef]
- Lehto, M.; Sipilä, I.; Alakukku, L.; Kymäläinen, H.-R. Water Consumption and Wastewaters in Fresh-Cut Vegetable Production. Agric. Food Sci. 2014, 23, 246–256. [Google Scholar] [CrossRef]
- Gómez-López, V.M.; Ragaert, P.; Debevere, J.; Devlieghere, F. Decontamination Methods to Prolong the Shelf-Life of Minimally Processed Vegetables, State-of-the-Art. Crit. Rev. Food Sci. Nutr. 2008, 48, 487–495. [Google Scholar] [CrossRef]
- European Commission Commission Regulation (EC). No 1441/2007 of 5 December 2007 Amending Regulation (EC) No 2073/2005 on Microbiological Criteria for Foodstuffs; European Commission Commission Regulation (EC): Brussels, Belgium, 2007. [Google Scholar]
- European Commission Commission Regulation (EC). No 2073/2005 of 15 November 2005 on Microbiological Criteria for Foodstuffs; European Commission Commission Regulation (EC): Brussels, Belgium, 2005. [Google Scholar]




| Sample | Incubation (min) | Illumination (min) | Chl Concentration (µM) |
|---|---|---|---|
| Control −/− | 30 | 0 | 0 |
| Dark control | 30 | 0 | 100 |
| Light control | 30 | 10/20/40 | 0 |
| PDI, 1 µM Chl, 5 min | 5 | 10/20/40 | 1 |
| PDI, 10 µM Chl, 5 min | 5 | 10/20/40 | 10 |
| PDI, 100 µM Chl, 5 min | 5 | 10/20/40 | 100 |
| PDI, 1 µM Chl, 30 min | 30 | 10/20/40 | 1 |
| PDI, 10 µM Chl, 30 min | 30 | 10/20/40 | 10 |
| PDI, 100 µM Chl, 30 min | 30 | 10/20/40 | 100 |
| Supernatant Control −/− | 30 | 0 | 0 |
| Supernatant PDI, 10 µM Chl, 30 min | 30 | 10/20/40 | 10 |
| Supernatant PDI, 100 µM Chl, 30 min | 30 | 10/20/40 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fellner, A.; Hamminger, C.; Fefer, M.; Liu, J.; Plaetzer, K. Towards Microbial Food Safety of Sprouts: Photodynamic Decontamination of Seeds. Photonics 2023, 10, 239. https://doi.org/10.3390/photonics10030239
Fellner A, Hamminger C, Fefer M, Liu J, Plaetzer K. Towards Microbial Food Safety of Sprouts: Photodynamic Decontamination of Seeds. Photonics. 2023; 10(3):239. https://doi.org/10.3390/photonics10030239
Chicago/Turabian StyleFellner, Andreas, Christoph Hamminger, Michael Fefer, Jun Liu, and Kristjan Plaetzer. 2023. "Towards Microbial Food Safety of Sprouts: Photodynamic Decontamination of Seeds" Photonics 10, no. 3: 239. https://doi.org/10.3390/photonics10030239
APA StyleFellner, A., Hamminger, C., Fefer, M., Liu, J., & Plaetzer, K. (2023). Towards Microbial Food Safety of Sprouts: Photodynamic Decontamination of Seeds. Photonics, 10(3), 239. https://doi.org/10.3390/photonics10030239