Abstract
This work studies the features of the formation of isometric polyhedral ZnO microcrystals that provide stimulated emission and whispering-gallery-mode (WGM) lasing in the near-UV range. For this purpose, the growth stages of such crystals in the process of gas-transport synthesis and the luminescent properties of the structures obtained at each stage were investigated. It was shown that the growth of laser microcrystals begins with the formation of microspheroids with thin ZnO shells. Such spheroids exhibit mainly white luminescence with a small contribution of near-UV emission. Increasing the synthesis duration results in thickening and faceting of the spheroid shells, as well as a decrease in the contribution of the yellow–red component to the luminescence spectrum. At the same time, ZnO microcrystallites nucleate and grow inside the spheroids, using as a material the remains of a liquid zinc drop and oxygen entering the spheroids through their shells. Such growth conditions allow them to take on an equilibrium polyhedral shape. Eventually, upon destruction of the spheroid shell, a polyhedral ZnO microcrystal supporting WGMs is observed.
1. Introduction
The development of short-wavelength optoelectronics and laser physics is associated with the search for new, effective light sources and microcavities. Such devices are required today to solve a variety of application problems, from the development of compact optical integrated circuits and high-density optical memory devices to optical sensing and medical therapy [1,2,3,4,5].
Due to its wide and direct bandgap, high luminescence yield, efficient doping possibilities, and relatively low fabrication cost, ZnO is considered a suitable material for such applications [6,7,8]. In addition, ZnO has a high morphological diversity, which makes it possible to grow a large number of different micro- and nanostructures. Due to the wurtzite type of crystal lattice and the high refractive index of ZnO, many of these structures possess optical microcavity properties after their synthesis without the need for post-growth processing. The possibility of self-organization of such objects on a substrate provides additional advantages of using them in practice. Among such naturally formed ZnO crystal cavities, a special place is occupied by whispering-gallery-mode (WGM) microcavities. It is notorious that WGM microlasers, including those based on ZnO, provide higher Q-factors, lower thresholds, and higher radiation intensities in comparison with Fabry–Perot-mode micro-/nanolasers [6,9,10].
The most actively studied ZnO-based WGM microcavities/microlasers are microrod cavities due to their relatively simple production provided by the natural growth of ZnO crystallites along the c-axis. Among the disadvantage of such microlasers are the difficulty of their post-growth orientation on a substrate and, as a consequence, the difficulty of obtaining the necessary spatial characteristics of laser emission; optical losses as a result of the possible contacts of the active parts of such microlasers with substrates or other crystals (in particular, when arrays of crystals are formed); and the difficulty of forming electrical contacts with such crystals. From this point of view, other interesting WGM structures are isometric (quasi-isometric), symmetrical ZnO microcrystals. Such microcrystals include, in particular, crystals in the form of spheres/spheroids and polyhedral microcrystals [11,12,13,14,15]. Among the advantages of such microcrystals, one can note the convenience of arrangement of such objects on a substrate due to their spatial symmetry, the convenience of manufacturing electrical contacts, and their mechanical strength compared with elongated micro-/nanocrystals, e.g., whisker- or rod-like crystals. In view of these considerations, the development of technologies for producing such microcavities and studying their optical properties is an urgent task.
In our recent study [16], the possibility of synthesizing fairly large (10–80 μm in size) polyhedral ZnO microcrystals with laser properties was demonstrated. WGM lasing was observed in relatively small microcrystals with diameters up to 15 μm; larger crystals exhibited only amplified spontaneous emission (ASE)—a laser effect was not achieved due to strong optical losses. The purpose of the present work was to elucidate the formation mechanisms for such polyhedral microcrystals. By varying the synthesis duration, we showed that such crystals grow within microspheroids with thin ZnO shells, which in turn are formed at the initial stage of synthesis from zinc microdroplets. The evolution of the luminescent properties of the obtained microstructures in the near-UV and visible ranges was investigated.
2. Materials and Methods
ZnO growth on Si (111) substrates was carried out by gas-transport synthesis. The laboratory setup included a flow-type quartz reactor and external resistive heaters. Zinc powder in a quartz boat was placed at the end of a quartz ampoule sealed on one side. The ampoule was placed in a horizontal quartz reactor in such a way that the zinc was in the evaporation zone (T2) and the substrate was in the growth zone (T1). The process was carried out at reduced pressure, for which the reactor was evacuated using a forepump. Then, with pumping continued, argon was fed to the reactor. When stationary pressure was achieved, the temperature T1 was brought to the operating value of 520 °C. Next, the temperature T2 was raised to 620 °C. When the operating mode was reached, oxygen was fed in. Oxygen consumption was 10–20% of the total gas consumption. To study the stages of crystal formation, three samples were prepared, differing in synthesis time: 5 min (S1), 20 min (S2), and 30 min (S3).
Microscopic studies of the samples were carried out via scanning electron microscopy (SEM) using a Jeol Neoscope 2 (JCM-6000) microscope equipped with an energy-dispersive X-ray (EDX) microanalyzer. Structural studies of the films were performed using the X-ray diffraction (XRD) method. XRD patterns were obtained using a PANalytical X’Pert Pro MRD diffractometer in the Bragg–Brentano geometry. Radiation from a copper anode (CuKα2, λ = 1.54 Å) was used (voltage, 40 kV; current, 40 mA). During the XRD measurements, the samples were rotated around their axis at a rate of 2 rps. XRD patterns were analyzed by means of the High Score Plus program, using the ICSD database.
Photoluminescence (PL) of the samples was studied at low and high excitation intensities. To obtain PL spectra at low continuous-wave (cw) excitation intensity, we used light with a wavelength of 315 nm, spectrally selected in a SOLAR CM2203 spectrofluorometer equipped with a xenon lamp and a photomultiplier detector. Relatively high excitation intensities were provided by a third harmonic (355 nm) of a pulsed Nd:YAG laser (pulse duration, 10 ns; pulse repetition rate, 15 Hz). The emission of the samples was collimated to the entrance slit of an MDR-206 monochromator coupled to a Peltier-cooled charge-coupled device camera. The size of the excitation spot at the sample’s surface was ~200 μm in the case of laser excitation and several millimeters in the case of cw excitation.
3. Results and Discussion
Figure 1 shows micrographs of the synthesized ZnO samples. Sample S1 (Figure 1a) was characterized by the presence of spheroid microcrystals with a smooth thin shell and cavities inside. The sizes of the microspheroids were within a wide range, from several micrometers to ~0.25 mm. Many spheroids had cracked or torn shells. The morphology of sample S2 was more diverse (Figure 1b). Similar to sample S1, spheroids were also observed in this sample. However, their surfaces were not as smooth, and in many cases, they were partially faceted. The hexagonal shape of the facets indicated the wurtzite structure of such crystals. In addition to spheroids, polyhedral microcrystals were also observed in the micrographs of sample S2. Such crystals had 14 faces: 2 faces were end faces and 12 faces were side faces (six pairs of faces each located at an angle of 60° relative to each other and forming a hexagonal cross-section of the crystal). In the case of sample S3, spheroids were no longer observed—only polyhedral microcrystals were present. At the same time, many of them were covered with fine-grated structures—smaller microcrystallites. Over almost the entire surface of the sample, fragments of a thin-walled structure were observed, with which the polyhedral microcrystals were partially covered.
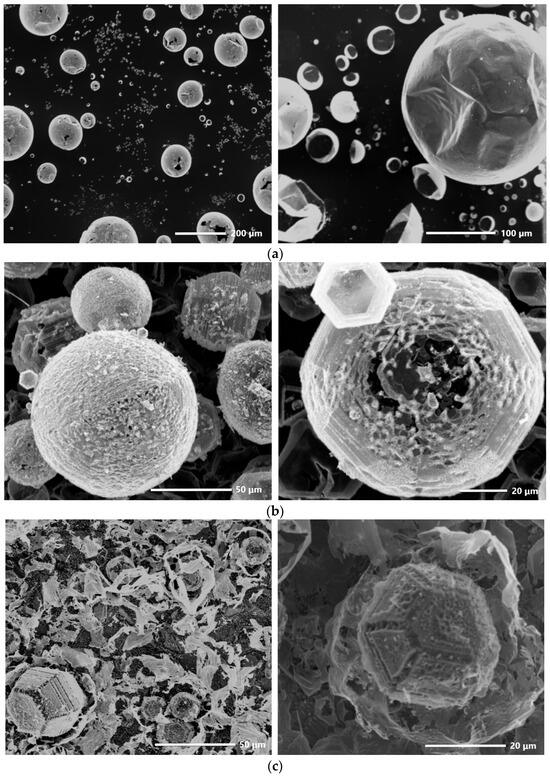
Figure 1.
SEM-images of samples S1 (a), S2 (b), and S3 (c).
Figure 2 shows the XRD patterns of samples S1 and S3, which were in fact representatives of two different types of ZnO morphologies—spheroids and polyhedral microcrystals. The XRD analysis confirmed that the ZnO structures under study crystallized in the wurtzite phase. The ZnO shells of the spheroids (sample S1) had a predominantly (002) orientation—despite the fact that reflections from the (100) and (101) planes were also observed, their intensity was significantly lower than that of the (002) peak. The peak at 2θ = 28.56° corresponded to the reflection from the (111) plane of a Si substrate. At the same time, the crystal structure of sample S3, in addition to the indicated peaks, was characterized by the appearance of reflections from the ZnO (102), (110), (103), (112), and (201) planes. Besides that, the (100) and (101) reflections increased significantly and amounted to ~1/3 of the (002) peak in intensity.
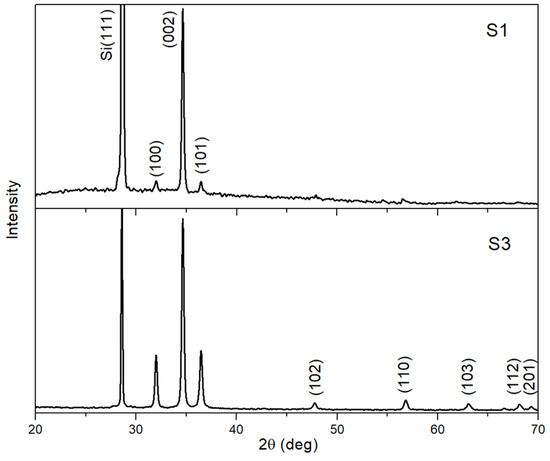
Figure 2.
XRD patterns of samples S1 (top) and S3 (bottom).
Analysis of the morphological and structural features of the samples suggested the following mechanisms for the formation of the observed ZnO structures. At the beginning of the synthesis process, when the flow of oxygen and argon is turned on, Zn microdroplets are transferred from the zinc source to the substrate. Zinc belongs to the group of fusible metals with melting point of ~420 °C. In addition, zinc is quite volatile—its evaporation temperature is ~906 °C at atmospheric pressure. As a result, at the optimal temperature of gas-phase synthesis and at reduced pressure, a sufficiently high concentration of Zn vapor can be achieved, which is necessary for condensation on the substrate of liquid metal droplets. At low crystallization temperatures, when kinetic restrictions are strong, the liquid phase significantly increases the growth rate, bringing the regime closer to the diffusion one. This leads to rapid overgrowth of the droplet surface with solid oxide in an oxygen flow, forming spheroids covered with a thin ZnO shell. Here, one can talk about the vapor–liquid–solid growth mechanism, which in this case led to the implementation of the energetically most-favorable growth direction, <001> (see Figure 2, top part). At the same time, liquid zinc still remains inside the spheroids. Due to the higher partial pressure of zinc vapor inside the spheroid compared to the pressure of the gas flow outside, cracks may form in the spheroid’s thin shell. In some cases, the spheroids even burst, with their shells significantly destroyed, and the zinc remaining inside the spheroids in a liquid state completely evaporates. In such cases, partially broken spheroids or only remnants of their shells remain on the substrate. All this can be seen in micrographs of sample S1 (Figure 1a).
If only relatively small cracks appear in the shell, only partial evaporation of zinc from the inner part of the spheroid occurs. This takes place in addition to the mechanism of Zn vapor diffusion through the gas-permeable shell of the spheroid, when it is still relatively thin [17]. This results in a decrease in the volume of liquid zinc in the spheroid and the formation of a cavity inside the spheroid, separating the remaining part of the liquid zinc droplet and the ZnO shell of the spheroid. On the other hand, oxygen, which is still purging the reactor, enters the spheroid through cracks in its shell from the external flow. This oxygen reacts with the zinc remaining inside the spheroid. However, the rate of such oxygen supply to the inner region of the spheroid is limited. As a result, the crystallization process of ZnO inside the spheroid occurs slowly, in a regime close to thermodynamic equilibrium. This contributes to the formation of a ZnO microcrystal with an equilibrium shape, which in the case of hexagonal packing is an isometric polyhedron [18,19]. In such a polyhedron, in addition to the <001> crystallographic direction, other directions are also developed, in particular, <100> and <101>, which is reflected in the results of the XRD studies (see Figure 2, bottom part). During long-term synthesis (more than 20–25 min), almost all polyhedral microcrystals formed inside their spheroids are freed from them—the spheroid shells are completely destroyed (sample S3). For example, in Figure 1c, the remains of the spheroid shell are clearly visible around the polyhedral microcrystals. Only small fragments of shells remain on the surface of such microcrystals. Thus, the shell of the spheroid serves as a cocoon for the polyhedral microcrystals, promoting their thermodynamic equilibrium growth. Continuation of the synthesis after the destruction of the spheroid shell leads to the rapid overgrowth of the microcrystal’s surface with smaller microcrystallites due to Zn microdroplets settling onto it and the rapid influx of oxygen, which is no longer retained by the spheroid shell. This leads to the formation of a fine-grained ZnO structure on the surface of the polyhedral microcrystals of sample S3.
The case of sample S2 is an intermediate stage between samples S1 and S3. Here, we observed both undamaged and cracked spheroids, as well as polyhedral microcrystals, already freed from their spheroids but not yet overgrown with smaller microcrystallites (Figure 1b). The example of this sample clearly shows what happens to the spheroids and their surfaces during a synthesis process lasting longer than 5 min. After the initial formation of spheroids with ZnO shells and a liquid zinc phase inside, the surface of the spheroid begins to thicken as a result of the continued flow of reagents into the growth zone. The faceting of the spheroids occurs at high concentrations of the reagents during an exothermic reaction (Zn + O2 → ZnO). In this case, the formation of active structural elements on the surface—steps and kinks—can be observed (see the right side of Figure 1b). Layer-by-layer growth on a faceted spheroid is a sign of two-dimensional nucleation. The formation of two-dimensional nuclei is facilitated by reducing the surface energy of the liquid–crystal interface and low temperatures [20,21]. Of note, in the present work, the temperature in the growth zone was significantly lower than that used by us in the synthesis of whisker- and plate-like crystals (T1 was around 580 °C) using the same experimental equipment. Apparently, the described process occurred in parallel with the formation of polyhedral ZnO microcrystals inside the spheroids.
The results of EDX analysis of the samples revealed a significant change in the relative zinc and oxygen contents with increasing synthesis time. Thus, in the case of sample S1, the zinc/oxygen ratio on the surface of the spheroids was on average 37/63 at.%, demonstrating a significant excess of oxygen and a lack of zinc. Similar data were obtained for both the outer and inner surfaces of spheroid shells. As the synthesis time increased, the relative zinc content increased. In particular, for sample S3, the zinc/oxygen ratio reached 80/20 at.% on the surface of some microcrystals.
The PL of the samples was studied in the UV and visible ranges under cw low-intensity excitation and pulsed laser excitation. Due to the small laser excitation spot on the sample surfaces and the heterogeneity of the samples, a different number of crystals of different sizes fell into the excitation zone, depending on the location on the sample. This resulted in significant variation in the PL intensity of the samples under this excitation type. In addition, an increase in synthesis time was reflected in a significant increase in the amount of material in the samples. This also greatly affected the intensity of the luminescent signals. In particular, the UV emission intensity for sample S1 turned out to be more than an order of magnitude lower than that for samples S2 and S3, already under low-intensity cw excitation. In this regard, it is advisable to analyze only the shape of the PL properties of the samples and the wavelengths and intensity ratios of individual components in these spectra, as well as the behavior of PL with respect to the excitation intensity.
Figure 3 compares the PL spectra of the samples obtained under low-intensity cw excitation (Figure 3a,b) and pulsed laser excitation at a relatively low power density, ρexc ≈ 15 kW/cm2 (Figure 3c). For the convenience of comparative analysis, the PL spectra were normalized to the maximum of the UV band and placed one below the other. Figure 3b additionally shows the visible parts of the PL spectra of the samples (cw excitation) vs. photon energy.
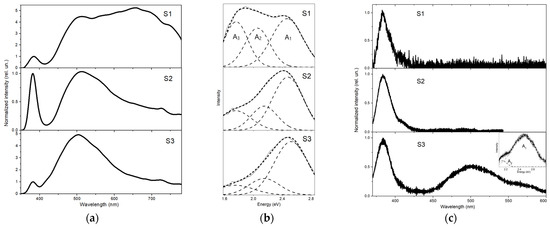
Figure 3.
PL spectra of samples registered in the near-UV and visible ranges under low-intensity cw excitation (a,b) and pulsed laser excitation with power density, ρexc ≈ 15 kW/cm2 (c). The spectra are normalized to the NBEL band maximum. Figures (b,c) show the Gaussian decomposition of the visible part of the PL spectra (DL) plotted vs. photon energy.
In the PL spectra of all samples under low-intensity cw excitation, two emission bands were observed (Figure 3a). The one of shorter wavelength, located in the region of 381–382 nm for all samples, was the near-band-edge luminescence (NBEL) of ZnO. The full width at half maximum (FWHM) of the band was ~25 nm (0.2 eV). The wide band, located in the visible part of the spectrum, represented the defect (or deep-level) luminescence (DL) of ZnO. While the wavelengths and shapes of the NBEL bands for all the samples were approximately the same, such parameters of the DL band were different. In particular, the shape of the DL band was significantly transformed when the synthesis time was more than 5 min. In the case of sample S1, the DL band was very wide (FWHM reached ~310 nm or 1.1 eV), occupying the entire visible range (white luminescence could be observed from the surface of the sample by the naked eye). This band was clearly made up of several components. The same can be said for the case of samples S2 and S3, taking into account the flat long-wavelength tail of the main maximum at 505–510 nm. In this regard, it may be convenient to decompose the DL part of the spectrum into the Gaussian components. In the case studied, satisfactory fitting could be achieved using three Gaussian components peaking at 2.48–2.52 eV, 2.05–2.15 eV, and 1.75 eV, which are designated as A1, A2 and A3 in Figure 3b. It can be seen that in the DL spectrum of sample S1, all three components have comparable intensities. Meanwhile, in the spectra of samples S2 and S3, the longer wavelength components A2 and A3 significantly lost intensity compared to the component A1, which gave the PL of these samples a green tint. Moreover, in the case of sample S3, this effect was even more pronounced. This was reflected in a noticeable decrease in the FWHM of the DL band from 1.1 eV (sample S1) to 0.75 eV (170 nm) for sample S2 and 0.70 eV (150 nm) for sample S3.
At the same time, it is instructive to compare these data with the EDX results. A weakening of the yellow–red parts of the DL (components A2 and A3) of the samples, which was observed with increasing synthesis duration, was accompanied by a significant increase in the zinc/oxygen ratio. Thus, there is a clear correlation between the PL data of the samples and their elemental composition. In particular, we can associate a yellow–red part of the DL (components A2 and A3), which was rather strong in sample S1, mainly with excess oxygen. This is consistent with the results of some studies in which the corresponding optical transitions were associated with the participation of zinc vacancies (VZn) and oxygen interstitials (Oi), including their complexes with other defects [22,23,24,25,26,27]. In particular, in [23], it was assumed that Oi provided an emission band around 1.95 eV. At the same time, some authors argue that Oi complexes with other elements rather than isolated Oi are responsible for such red–orange emission [26]. The authors of [22,25] suggest the participation of VZn in the radiative transition with an energy of 2.0–2.1 eV. The green luminescence peaking at around 2.5 eV (described by the component A1 in our case) is more often associated with oxygen vacancies and their complexes [25,27,28].
A somewhat different picture was observed under laser excitation (Figure 3c). In this case, the ratio of the DL and NBEL bands changed sharply. In particular, the PL spectra of samples S1 and S2 showed no visible parts. There was only the NBEL band peaking at 382–384 nm. The PL spectrum of sample S3 contained both luminescence bands with maxima at 383 and 500 nm, respectively. The FWHM of the NBEL band of the samples remained virtually unchanged, comparable in value to the case of low-intensity cw excitation. At the same time, the DL band of sample S3 narrowed by almost two times, revealing the FWHM of 80 nm (0.4 eV). The Gaussian decomposition in this case showed only two components remaining—A1 and A2 (see the inset in the bottom part of Figure 3c). Here, the component A2 formed a noticeable long-wavelength shoulder to the main maximum provided by the component A1.
Such a redistribution of NBEL and DL intensities upon a significant increase in excitation power (see Figure 3a,c) was also observed earlier for ZnO structures [29,30,31] and can be explained by at least two effects: saturation of DL emission centers at the high-density excitation and a decrease in the thickness of the depletion layer as a result of flattening of energy bands. Particularly in the second case, electron–hole pairs, intensively created by photons with an energy greater than the bandgap, neutralized the excess charge on the semiconductor surface (the existence of this charge, in the absence of illumination, causes bending of the energy bands and formation of the depletion layer [32]). The straightening of the bands was accompanied by a partial deactivation of DL emission centers located near the surface and an increase in the probability of interband transitions. All these factors resulted in a relative increase in the UV component as compared to the visible one.
Figure 4 shows the evolution of the NBEL spectra of the samples with increasing ρexc. In the case of sample S1 (Figure 4a), the characteristics of the NBEL band (shape, FWHM, and wavelength) did not change substantially at different ρexc values and were practically independent of the sample’s irradiated region. The dependence of the integral intensity of the NBEL band on ρexc (see inset in Figure 4a) demonstrated a linear character at initial ρexc values and a tendency to fast saturation at elevated ρexc. The weak NBEL and the rapid saturation of its intensity in this case were apparently associated with the low crystalline quality and the small thickness of the oxide shells of the spheroids. The small reservoir of NBEL centers of such structures is supplemented by a large number of crystal lattice defects, which serve as effective centers for nonradiative recombination of electron–hole pairs [33,34]. At the same time, a thin ZnO layer forming the spheroid shell cannot provide significant absorption of exciting radiation. All this results in the low efficiency of conversion of excitation light into luminescence and its rapid saturation.
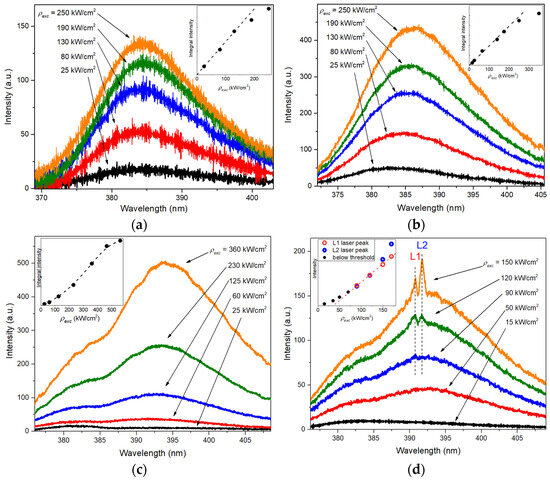
Figure 4.
The examples of NBEL spectrum evolution with increasing excitation power density, ρexc: (a) spontaneous emission of sample S1; (b) spontaneous emission of sample S2; (c) ASE in sample S3; (d) lasing in sample S3.
In the case of samples S2 and S3, the heterogeneity of the samples’ structures significantly manifested itself in the NBEL, since crystals of different morphology, shape, and size were present. This was reflected not only in the intensity of the NBEL band, but also in its shape and behavior at different ρexc values. In particular, three types of evolution of NBEL spectra were observed with increasing ρexc values. The first type was similar to the case of sample S1—the NBEL spectrum represented a single band of spontaneous emission, which became saturated with increasing ρexc values. The main difference in this case was the much more significant variability in the band intensity depending on the sample’s region due to the more diverse morphology. In regions where the PL intensity was quite high (more than an order of magnitude higher than in the case of sample S1), a redshift of the NBEL band could also be observed. An example of such spectra is shown in Figure 4b. In this case, the redshift was ~1.5 nm, with an increase in ρexc by a factor of 3. The other two types of evolution of the NBEL spectra of samples S2 and S3 exhibited stimulated emission properties. In particular, in the first case, amplified spontaneous emission (ASE) was observed. ASE manifested itself by a change in the shape of the NBEL band and a superlinear dependence of the NBEL integral intensity on ρexc. Figure 4c shows an example of such a case. Here, when a certain value of ρexc was reached, in addition to the main maximum at 382–383 nm, a long-wavelength shoulder appeared in the spectrum. With a further increase in ρexc, this shoulder formed into a separate band peaking at 391–392 nm at lower excitation levels, which began to dominate over the short-wavelength component and subsequently formed the main part of the NBEL. As ρexc increased, the maximum of the long-wavelength NBEL component intensively redshifted. The redshift was 3.5 nm as ρexc increased by a factor of 3 in the case presented in Figure 4c. The appearance of the second, long-wavelength NBEL component was accompanied by a superlinear increase in the NBEL integral intensity. When the ρexc was more than ~350 kW/cm2, the integrated intensity tended to saturate (see inset in Figure 4c). The third type of NBEL evolution revealed the laser properties of the samples. In this case, with the initial increase in ρexc, in addition to the spontaneous emission band at 382–383 nm, which is the main one at low excitation intensities, a long-wavelength component appeared, demonstrating ASE, similar to the second type of spectra (Figure 4c). The laser effect manifested itself in the appearance of narrow peaks in the region of the long-wavelength component of the NBEL and a rapid increase in their intensity with a further increase in ρexc. This can be seen in Figure 4d, which shows the evolution of the NBEL spectra of sample S3’s region with a single laser microcrystal. In this case, laser peaks appeared at ρexc ~ 90 kW/cm2; their average FWHM was ~0.4 nm. As ρexc rose, the intensity of these peaks increased, and new peaks with lower energies appeared. The spacing between adjacent laser peaks was 0.9–1 nm. As in the case of ASE, there was a noticeable redshift of the entire spectral region of lasing. In addition to the appearance of increasingly longer wavelength peaks, this redshift manifested itself in a redistribution of the intensities of the laser peaks. This is well reflected by the dependence of the intensity of two neighboring laser peaks with wavelengths of 390.8 nm (the L1 laser peak) and 391.7 nm (the L2 laser peak) on ρexc plotted in the inset of Figure 4d. For subthreshold pumping intensities, the dependence of the NBEL intensity (measured in the region λ = 391.2 nm, i.e., in the range of the long-wavelength component) on ρexc was superlinear, suggesting the presence of ASE. When the L1 and L2 laser peaks appeared, their intensities were almost the same. However, with increasing ρexc, the intensity of the L2 peak increased faster than that of the L1 peak. In this case, the dependence of the L2 peak intensity on ρexc turned out to be superlinear, which, generally speaking, is not typical for classical microlasers. This superlinearity was obviously related to the contribution of ASE to the spectrum in addition to the lasing. Apparently, the ASE and the laser emission were formed either in different regions of the microcrystal or, more likely, in different microcrystals that fell in the excitation region. This is evidenced by the absence of a noticeable narrowing of the long-wavelength band with increasing ρexc after overcoming the lasing threshold, which should have occurred due to the transfer of its energy into optical modes—this situation is usually observed for single micro- and nanocrystal ZnO cavities [35,36], including polyhedral microcrystals studied earlier [16].
In the case under study, WGM lasing is excited, as was shown in our previous work [16], where such polyhedral microcrystals were studied for the first time. In particular, the wavelengths of the laser peaks are described well within the framework of a planar model of a hexagonal WGM cavity:
where D is the diameter of the hexagonal cavity, and ( and are the refractive indices for TE and TM polarizations), and N is the mode number (interference order) [37,38]. We considered only TE modes, which are predominantly excited in the near-UV range in ZnO WGM microcavities [38,39].
A separate issue is the nature of the long-wavelength NBEL component that appeared in the ASE and lasing spectra of the samples. There are reports on luminescence associated with shallow defects in the ZnO lattice that can appear in this spectral region [40,41,42]. However, the appearance of the long-wavelength NBEL band in only some places of the samples, its redshift, and finally its accompaniment by a superlinear increase in the NBEL integral intensity and lasing allowed us to exclude the defect origin of this band in the case under study and, instead, to assume the participation of processes leading to optical gain. Although the gain mechanisms in ZnO have been studied for quite a long time, in many cases, doubts and disputes still remain—this applies to a greater extent to room-temperature gain, when the interpretation of emission spectra is difficult due to the strong spectral broadening and overlapping of the different emission bands. The spectral pattern of ASE and lasing with two emission bands, one of which (short-wavelength) has a spontaneous nature and the second of which (long-wavelength) is stimulated (laser) emission, is often observed for WGM ZnO microstructures [9,31,35,43,44,45]. In this case, the spectral behavior of the stimulated emission bands can be different, depending on the excitation power. In some cases, there is an intense redshift, as observed in the present work. In other cases, the redshift is either weakly pronounced or completely absent. Until recently, it was widely believed that in WGM ZnO microstructures exhibiting intense redshift of the ASE/lasing band, optical gain is formed as a result of population inversion in the electron–hole plasma (EHP) [9,35,43,45]. However, in a recent study of low-threshold ZnO microrod lasers [46], we showed that the mechanism of redshifting gain at room temperature is exciton scattering on free electrons rather than an inverted EHP.
The threshold pumping intensities required to excite lasing in the ZnO polyhedral microcrystals under study were not as low as for the microrods in [46]. A simple estimate of the density of electron–hole pairs created by optical pumping [16] does not exclude reaching values on the order of the threshold Mott density (~1018 cm−3 for ZnO [47]), already at near-threshold pumping intensities. Nevertheless, the estimates of the dynamic change in the bandgap energy due to renormalization [16] show that for microcrystalline ZnO, the range of 390–395 nm is too long-wavelength for stimulated emission due to recombination in an inverted EHP. At the same time, it was noted in [46] that the spectral picture, corresponding to the case of a redshifting gain profile, does not change upon transition from the excitonic regime to the EHP regime, even with a significant increase in excitation intensity. From this, we can conclude that above the Mott threshold, scattering processes similar to excitonic ones are realized, i.e., population inversion in EHP does not occur. In particular, in [48], the scattering of Coulomb-correlated electron–hole pairs by free carriers in the EHP was suggested as a replacement for the exciton–electron scattering process. Moreover, we believe that the true pair density created in the crystals under study was less than the estimated one. This was due to the reduced efficiency of pumping energy absorption as a result of the presence of fragments of spheroid shells on the microcrystals’ surfaces, which created additional scattering and absorption in the path of exciting light. In this regard, we believe that the gain mechanism similar to exciton–electron scattering is realized in polyhedral microcrystals, which can transform into an analogous process in EHP with increasing pumping intensity.
The spontaneous emission component of the NBEL peaking at 382–384 nm (see, e.g., Figure 4a,b) can be associated with phonon replicas of free exciton emission. Moreover, in the case of relatively low excitation intensities, the main contribution to this band is made by the first phonon replica [28]. The redshift of this band with increasing excitation intensity, observed in the case of samples S2 and S3 (Figure 4b), indicates an increase in the fraction of the second phonon replica, the maximum of which falls in the range of approximately 390–392 nm in the case of microcrystalline ZnO at room temperature [46,49].
4. Conclusions
In this work, we investigated the stages of formation of laser polyhedral ZnO microcrystals with whispering-gallery modes. Such microcrystals have an isometric shape and sizes from 10 to 80 μm and support both amplified spontaneous emission and lasing with the gain provided by WGMs. The study showed that the formation of such microcrystals begins inside spheroids, which are formed at the initial stage of synthesis from zinc microdroplets covered with a ZnO shell. The dimensions of some spheroids reach a quarter of a millimeter. As the synthesis continues, the oxide shell is destroyed, and polyhedral microcrystals remain on the substrate. The studies also revealed the luminescence characteristics of the grown structures and allowed us to identify a correlation with their morphology and elemental composition. In particular, as the synthesis duration increases, the relative oxygen content in the structure of growing crystals decreases, accompanied by a weakening of the red–yellow part of luminescence and an increase in the green component. At the same time, the intensity of the near-band-edge emission increases and the excitation of amplified spontaneous emission and lasing becomes possible. The process of exciton–electron scattering and an analogous mechanism in electron-hole plasma are suggested to be responsible for optical gain in polyhedral ZnO microcrystals.
Author Contributions
Conceptualization, L.A.Z. and A.P.T.; methodology, L.A.Z. and A.P.T.; software, A.P.T.; validation, L.A.Z. and A.P.T.; formal analysis, L.A.Z. and A.P.T.; investigation, L.A.Z. and A.P.T.; resources, L.A.Z., A.P.T. and V.M.K.; data curation, A.P.T.; writing—original draft preparation, A.P.T.; writing—review and editing, L.A.Z. and A.P.T.; visualization, A.P.T.; supervision, A.P.T. and V.M.K.; project administration, A.P.T.; funding acquisition, A.P.T. All authors have read and agreed to the published version of the manuscript.
Funding
The study was supported by a grant from the Russian Science Foundation (grant no. 23-29-00535; https://rscf.ru/en/project/23-29-00535/ (accessed on 27 November 2023)).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data included in the article can be made available upon reasonable request.
Acknowledgments
The authors thank I.S. Volchkov for assistance in performing the XRD studies.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kneissl, M.; Seong, T.Y.; Han, J.; Amano, H. The emergence and prospects of deep-ultraviolet light-emitting diode technologies. Nat. Photon. 2019, 13, 233–244. [Google Scholar] [CrossRef]
- Zhang, Y.; Saxena, D.; Aagesen, M.; Liu, H. Toward electrically driven semiconductor nanowire lasers. Nanotechnology 2019, 30, 192002. [Google Scholar] [CrossRef] [PubMed]
- Zollner, C.J.; DenBaars, S.P.; Speck, J.S.; Nakamura, S. Germicidal ultraviolet LEDs: A review of applications and semiconductor technologies. Semicond. Sci. Technol. 2021, 36, 123001. [Google Scholar] [CrossRef]
- Yu, D.; Humar, M.; Meserve, K.; Bailey, R.C.; Chormaic, S.N.; Vollmer, F. Whispering-gallery-mode sensors for biological and physical sensing. Nat. Rev. Methods Primers 2021, 1, 83. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Sun, H. Advances and prospects for whispering gallery mode microcavities. Adv. Opt. Mater. 2015, 3, 1136–1162. [Google Scholar] [CrossRef]
- Dong, H.; Zhou, B.; Li, J.; Zhan, J.; Zhang, L. Ultraviolet lasing behavior in ZnO optical microcavities. J. Mater. 2017, 3, 255–266. [Google Scholar] [CrossRef]
- Borysiewicz, M.A. ZnO as a Functional Material, a Review. Crystals 2019, 9, 505. [Google Scholar] [CrossRef]
- Sharma, D.K.; Shukla, S.; Sharma, K.K.; Kumar, V. A review on ZnO: Fundamental properties and applications. Mater. Today Proc. 2022, 49, 3028–3035. [Google Scholar] [CrossRef]
- He, L.; Özdemir, Ş.K.; Yang, L. Whispering gallery microcavity lasers. Laser Photon. Rev. 2013, 7, 60–82. [Google Scholar] [CrossRef]
- Yang, Y.D.; Tang, M.; Wang, F.L.; Xiao, Z.X.; Xiao, J.L.; Huang, Y.Z. Whispering-gallery mode hexagonal micro-/nanocavity lasers. Photon. Res. 2019, 7, 594–607. [Google Scholar] [CrossRef]
- Okazaki, K.; Shimogaki, T.; Fusazaki, K.; Higashihata, M.; Nakamura, D.; Koshizaki, N.; Okada, T. Ultraviolet whispering-gallery-mode lasing in ZnO micro/nano sphere crystal. Appl. Phys. Lett. 2012, 101, 211105. [Google Scholar] [CrossRef]
- Ngo, T.H.B.; Chang, Y.C. Whispering gallery modes in hybrid Au-ZnO microsphere resonators: Experimental and theoretical investigations. Opt. Mater. Express 2017, 7, 2962–2967. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; Zhu, Z.; You, D.; Wang, R.; Qin, F.; Wang, X.; Cui, Q.; Shi, Z. Controllable fabrication of ZnO microspheres for whispering gallery mode microcavity. Cryst. Growth Des. 2018, 18, 5279–5286. [Google Scholar] [CrossRef]
- Fabitha, K.; Rao, R. Biocompatible miniature temperature sensor based on whispering gallery modes of Sm3+ activated ZnO optical micro-resonators. Appl. Phys. Lett. 2021, 118, 163104. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Wang, R.; Zhu, G.; Qin, F.; Chen, J.; Wang, J.; Shi, Z.; Cui, Q.; Xu, C. Electrically driven optical resonance of spherical ZnO whispering gallery mode microcavity. Appl. Phys. Lett. 2021, 119, 021101. [Google Scholar] [CrossRef]
- Tarasov, A.P.; Zadorozhnaya, L.A.; Muslimov, A.E.; Briskina, C.M.; Kanevsky, V.M. Stimulated emission and lasing in polyhedral ZnO microcrystals. JETP Lett. 2021, 114, 517–523. [Google Scholar] [CrossRef]
- Abduev, A.K.; Asvarov, A.S.; Akhmedov, A.K.; Baryshnikov, V.G. Vapor phase synthesis of ZnO structures. Tech. Phys. Lett. 2002, 28, 952–954. [Google Scholar] [CrossRef]
- Givargizov, E.I. Artificial Epitaxy Is a Promising Technology for the Microelectronics Element Base; Nauka: Moscow, Russia, 1988; 176p. (In Russian) [Google Scholar]
- Givargizov, E.I. Oriented Crystallization on Amorphous Substrates; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013. [Google Scholar]
- Givargizov, E.I. Fundamental aspects of VLS growth. In Vapour Growth and Epitaxy; Elsevier: Amsterdam, The Netherlands, 1975; pp. 20–30. [Google Scholar]
- Givargizov, E.I. Growth of Whisker and Plate-like Crystals from Steam; Nauka: Moscow, Russia, 1977. (In Russian) [Google Scholar]
- Dong, Y.; Tuomisto, F.; Svensson, B.G.; Kuznetsov, A.Y.; Brillson, L.J. Vacancy defect and defect cluster energetics in ion-implanted ZnO. Phys. Rev. B 2010, 81, 081201. [Google Scholar] [CrossRef]
- Kukreja, L.M.; Misra, P.; Fallert, J.; Phase, D.M.; Kalt, H. Correlation of spectral features of photoluminescence with residual native defects of ZnO thin films annealed at different temperatures. J. Appl. Phys. 2012, 112, 013525. [Google Scholar] [CrossRef]
- Chen, Y.N.; Xu, S.J.; Zheng, C.C.; Ning, J.Q.; Ling, F.C.C.; Anwand, W.; Brauer, G.; Skorupa, W. Nature of red luminescence band in research-grade ZnO single crystals: A “self-activated” configurational transition. Appl. Phys. Lett. 2014, 105, 041912. [Google Scholar] [CrossRef]
- Uklein, A.V.; Multian, V.V.; Kuz’micheva, G.M.; Linnik, R.P.; Lisnyak, V.V.; Popov, A.I.; Gayvoronsky, V.Y. Nonlinear optical response of bulk ZnO crystals with different content of intrinsic defects. Opt. Mater. 2018, 84, 738–747. [Google Scholar] [CrossRef]
- Lv, J.; Li, C.; Chai, Z. Defect luminescence and its mediated physical properties in ZnO. J. Lumin. 2019, 208, 225–237. [Google Scholar] [CrossRef]
- Galdámez-Martinez, A.; Santana, G.; Güell, F.; Martínez-Alanis, P.R.; Dutt, A. Photoluminescence of ZnO Nanowires: A Review. Nanomaterials 2020, 10, 857. [Google Scholar] [CrossRef] [PubMed]
- Ozgur, U.; Alivov, Y.I.; Liu, C.; Teke, A.; Reshchikov, M.A.; Dogan, S.; Avrutin, V.; Cho, S.J.; Morkoc, H. A comprehensive review of ZnO materials and devices. J. Appl. Phys. 2005, 98, 41301. [Google Scholar] [CrossRef]
- Guo, B.; Qiu, Z.R.; Wong, K.S. Intensity dependence and transient dynamics of donor–acceptor pair recombination in ZnO thin films grown on (001) silicon. Appl. Phys. Lett. 2003, 82, 2290–2292. [Google Scholar] [CrossRef]
- Djurišić, A.B.; Choy, W.C.; Roy, V.A.L.; Leung, Y.H.; Kwong, C.Y.; Cheah, K.W.; Rao, T.K.G.; Chan, W.K.; Lui, H.F.; Surya, C. Photoluminescence and electron paramagnetic resonance of ZnO tetrapod structures. Adv. Funct. Mater. 2004, 14, 856–864. [Google Scholar] [CrossRef]
- Tarasov, A.P.; Muslimov, A.E.; Kanevsky, V.M. Stimulated Emission in Vertically Aligned Hexagonal ZnO Microcrystals Synthesized by Magnetron Sputtering Method. Photonics 2022, 9, 871. [Google Scholar] [CrossRef]
- Zhang, Z.; Yates, J.T., Jr. Band Bending in Semiconductors: Chemical and Physical Consequences at Surfaces and Interfaces. Chem. Rev. 2012, 112, 5520–5551. [Google Scholar] [CrossRef]
- Morkoc, H.; Ozgur, U. Zinc Oxide: Fundamentals, Materials and Device Technology; John Wiley & Sons: Hoboken, NJ, USA, 2008; 488p. [Google Scholar]
- Klingshirn, C.F. Semiconductor Optics, 4th ed.; Springer: Berlin, Germany, 2012. [Google Scholar]
- Xu, C.; Dai, J.; Zhu, G.; Zhu, G.; Lin, Y.; Li, J.; Shi, Z. Whispering-gallery mode lasing in ZnO microcavities. Laser Photon. Rev. 2014, 8, 469–494. [Google Scholar] [CrossRef]
- Wille, M.; Sturm, C.; Michalsky, T.; Röder, R.; Ronning, C.; Schmidt-Grund, R.; Grundmann, M. Carrier density driven lasing dynamics in ZnO nanowires. Nanotechnology 2016, 27, 225702. [Google Scholar] [CrossRef]
- Sun, L.; Dong, H.; Xie, W.; An, Z.; Shen, X.; Chen, Z. Quasi-whispering gallery modes of exciton-polaritons in a ZnO microrod. Opt. Express 2010, 18, 15371–15376. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lee, S.; Ahn, Y.; Park, J.Y.; Koh, K.H.; Park, K.H. Identification of dispersion-dependent hexagonal cavity modes of an individual ZnO nanonail. Appl. Phys. Lett. 2008, 92, 263102. [Google Scholar] [CrossRef]
- Czekalla, C.; Sturm, C.; Schmidt-Grund, R.; Cao, B.; Lorenz, M.; Grundmann, M. Whispering gallery mode lasing in zinc oxide microwires. Appl. Phys. Lett. 2008, 92, 241102. [Google Scholar] [CrossRef]
- Su, S.C.; Lu, Y.M.; Zhang, Z.Z.; Li, B.H.; Shen, D.Z.; Yao, B.; Zhang, J.Y.; Zhao, D.X.; Fan, X.W. Structural, optical, and hydrogenation properties of ZnO nanowall networks grown on a Si (1 1 1) substrate by plasma-assisted molecular beam epitaxy. Phys. B Cond. Mater. 2008, 403, 2590–2593. [Google Scholar] [CrossRef]
- Ryzhkov, M.V.; Rumyantsev, S.I.; Markushev, V.M.; Briskina, C.M.; Tarasov, A.P. Edge luminescence of ZnO films. J. Appl. Spectrosc. 2014, 81, 877–880. [Google Scholar] [CrossRef]
- Gandhi, A.C.; Liao, C.H.; Yeh, W.L.; Huang, Y.L. Non-monotonous size-dependent photoluminescence and excitonic relaxations in nanostructured ZnO thin films. RSC Adv. 2019, 9, 2180–2188. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Xu, C.X.; Wu, P.; Guo, J.Y.; Li, Z.H.; Shi, Z.L. Exciton and electron-hole plasma lasing in ZnO dodecagonal whispering-gallery-mode microcavities at room temperature. Appl. Phys. Lett. 2010, 97, 011101. [Google Scholar] [CrossRef]
- Chen, R.; Ling, B.; Sun, X.W.; Sun, H.D. Room temperature excitonic whispering gallery mode lasing from high-quality hexagonal ZnO microdisks. Adv. Mater. 2011, 23, 2199–2204. [Google Scholar] [CrossRef]
- Dai, J.; Xu, C.; Nakamura, T.; Wang, Y.; Li, J.; Lin, Y. Electron–hole plasma induced band gap renormalization in ZnO microlaser cavities. Opt. Express 2014, 22, 28831–28837. [Google Scholar] [CrossRef]
- Tarasov, A.P.; Muslimov, A.E.; Kanevsky, V.M. Excitonic Mechanisms of Stimulated Emission in Low-Threshold ZnO Microrod Lasers with Whispering Gallery Modes. Materials 2022, 15, 8723. [Google Scholar] [CrossRef] [PubMed]
- Versteegh, M.A.; Kuis, T.; Stoof, H.T.C.; Dijkhuis, J.I. Ultrafast screening and carrier dynamics in ZnO: Theory and experiment. Phys. Rev. B 2011, 84, 035207. [Google Scholar] [CrossRef]
- Klingshirn, C.; Hauschild, R.; Fallert, J.; Kalt, H. Room-temperature stimulated emission of ZnO: Alternatives to excitonic lasing. Phys. Rev. B 2007, 75, 115203. [Google Scholar] [CrossRef]
- Vasilyev, N.N.; Borisov, E.N.; Novikov, B.V. Exciton-phonon stimulated emission in ZnO crystalline film at room temperature. Phys. Solid State 2020, 62, 1774–1779. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).